sodium absorption across epithelia in the kidney is critical for the maintenance of extracellular volume and for the regulation of blood pressure. Excessive Na+ absorption is responsible for the majority of inherited forms of hypertension, whereas inadequate Na+ absorption causes Na+ wasting and hypotension (10). In the lung, Na+ absorption maintains the quantity and composition of airway surface liquid, which is critical for gas exchange and host defense. In the kidney and lung, the epithelial Na+ channel, ENaC, plays a key role in Na+ absorption (reviewed in Refs. 15, 17). ENaC is located at the apical membrane, where it conducts Na+ from the extracellular environment into the cell. Coupled with Na+ exit at the basolateral membrane via the Na+-K+-ATPase, ENaC functions as a pathway for Na+ absorption.
To respond to enormous fluctuations in Na+ and volume intake, ENaC activity must vary over a wide range to maintain Na+ balance. This has focused attention on the signaling pathways that regulate ENaC and the mechanisms by which disruption of these pathways results in disease. In this issue, Bao et al. (2) report the surprising discovery that ethanol enhances ENaC-mediated Na+ current. Using a Xenopus model of the kidney collecting duct (A6 cells), they recorded single-channel ENaC currents by patch clamp. Acute exposure of the cells to ethanol had dual effects; it increased ENaC open-state probability and it increased ENaC abundance at the apical membrane. Both would be predicted to increase renal Na+ absorption. These effects were mimicked by acetaldehyde, the first metabolic product of ethanol, and by 1-propanol and 1-butanol. In contrast, iso-alcohols and acetone had no effect.
There is precedent in the literature for ethanol modulation of ion channel activity. In the central nervous system, ethanol and other alcohols potentiate the activity of pentameric ligand-gated channels [e.g., glycine receptors, γ-aminobutyric acid type A receptors, nicotinic acetylcholine receptors (3, 12, 13)], which is in part responsible for their intoxicating effects. The mechanism underlying this potentiation is thought to involve the binding of ethanol to transmembrane cavities within these channels (8). However, in the case of ENaC, the dual effects of ethanol on gating and surface abundance suggest that the mechanism might be more complex.
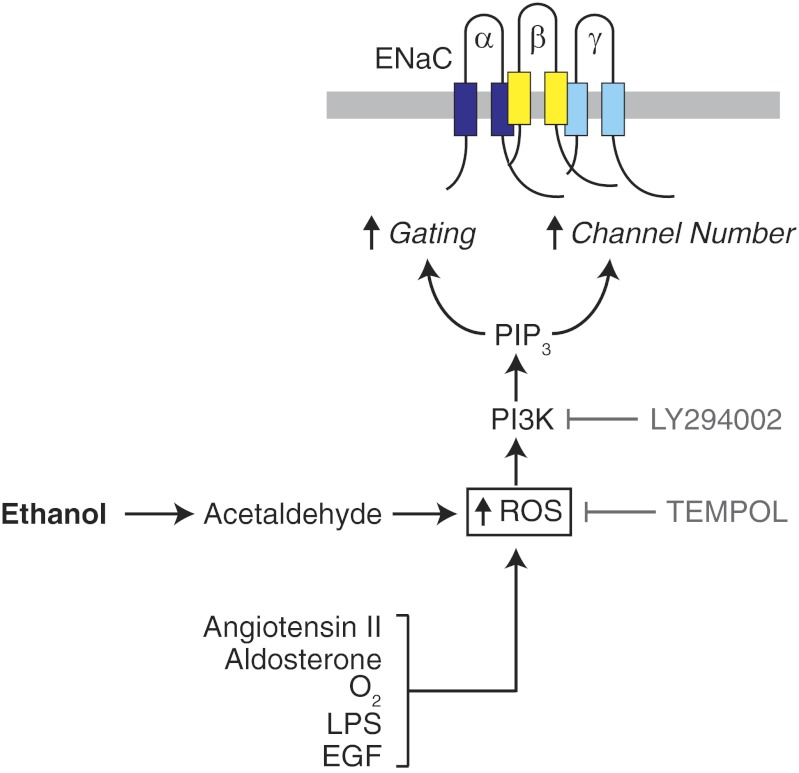
There is growing evidence that reactive oxygen species (ROS) are important regulators of ENaC activity, and hence, of epithelial Na+ absorption. The paper by Bao et al. suggests that ethanol feeds into this signaling pathway (Fig. 1). In the kidney and lung, a variety of stimuli increase production of superoxide (O2−) and hydrogen peroxide (H2O2). Both have been shown to increase ENaC activity. For example, in kidney A6 cells, aldosterone increased O2−, which in turn increased ENaC NPo (the product of channel number and open probability) (20). Additional work reported that H2O2 increased ENaC Po (11). Angiotensin II also increased ENaC NPo through a ROS-dependent mechanism in rat collecting duct (18). In the lung, oxygen tension increases at the time of birth (3% to 21%) when the lung transitions from a fluid-filled to an air-filled organ. This increases O2− production and leads to increased ENaC activity, which enhances lung liquid clearance (5, 14). The inflammatory mediator lipopolysaccharide (LPS) and epithelial growth factor also increase O2− production and ENaC activity, functioning via a pathway that includes Rac1 and the NADPH oxidase Nox2 (5, 19). In contrast, there are other situations in which ROS have the opposite effect on ENaC activity. For example, the influenza virus M2 protein and Mycoplasma pneumoniae increased ROS, but they paradoxically reduced ENaC current and fluid absorption by decreasing ENaC abundance at the cell membrane (7, 9).
Fig. 1.
Model of epithelial sodium channel (ENaC) regulation by ethanol and reactive oxygen species (ROS). Ethanol and a variety of other stimuli increase intracellular ROS. By activating phosphatidylinositol 3-kinase (PI3K), this increases phosphatidylinositol 3,4,5-trisphosphate (PIP3). Previous work indicates that PIP3 enhances ENaC gating and cell surface abundance, resulting in increased Na+ absorption.
The mechanisms that underlie these opposing effects of ROS on ENaC are not yet clear. Work from Ma indicates that the stimulatory effects of ROS are mediated, at least in part, through phosphatidylinositol 3-kinase (PI3K) (Fig. 1); H2O2 stimulates PI3K resulting in increased apical membrane localization of phosphatidylinositol 3,4,5-trisphosphate (PIP3), a mediator known to regulate ENaC gating (11). In addition to its effects on gating, PI3K also increases ENaC cell surface abundance. This occurs through a well-characterized pathway in which PIP3 activates serum and glucocorticoid-regulated kinase (SGK) via 3-phosphoinositide-dependent kinase. SGK phosphorylates the E3 ubiquitin ligase Nedd4-2, reducing its targeting of ENaC for endocytosis and degradation (16). Bao et al. (2) provide evidence that ethanol regulates ENaC via this signaling pathway. First, they found that ethanol increased intracellular ROS in A6 cells. Second, the O2− scavenger TEMPOL abolished ENaC stimulation by ethanol (Fig. 1). Finally, ENaC stimulation by ethanol was prevented by inhibition of PI3K (Fig. 1).
The effects of ethanol on ENaC may have important consequences for total body Na+ and water homeostasis. Acute ethanol exposure reduces Na+ excretion in rats independent of changes in renin and aldosterone secretion (1). This may counter the diuretic effect of ethanol, thought to occur through decreased responsiveness of osmoreceptors in the hypothalamus, which results in reduced levels of circulating vasopressin (4). In the rat lung, chronic ethanol ingestion causes alveolar fluid leak by disrupting formation of tight junctions (6). In this location, enhanced ENaC activity might function as a compensatory mechanism to minimize alveolar liquid to maintain adequate gas exchange.
Because ethanol consumption and ethanol abuse are common, it is important to understand how ethanol alters epithelial salt and water transport. The work by Bao et al. provides an intriguing first step. However, several questions remain. First, although ENaC gating and surface expression were altered by relatively high concentrations of ethanol (0.5–5%), do lower concentrations, as found in the blood after ethanol ingestion (∼0.1%), have similar effects? Second, the current work focused on acute effects of ethanol. Does chronic ethanol exposure alter ENaC expression and activity? Third, why does ROS increase ENaC activity under some circumstances (e.g., ethanol) but inhibit ENaC under others (e.g., influenza)? Fourth, are the ethanol effects observed in Xenopus A6 cells generalizable to other epithelia (e.g., lung) and to other species? Finally, it will be important to extend these in vitro studies to determine whether ethanol alters ENaC activity in the whole organism.
GRANTS
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute Grants HL-058812 and HL-072256 (to P. M. Snyder).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
P.M.S. conception and design of research; prepared the figure; drafted the manuscript; edited and revised the manuscript; approved the final version of the manuscript.
REFERENCES
- 1. Assadi FK. Acute effect of ethanol on renal electrolyte excretion in rats. Alcohol 6: 257–260, 1989 [DOI] [PubMed] [Google Scholar]
- 2. Bao HF, Song JZ, Duke BJ, Ma HP, Denson DD, Eaton DC. Ethanol stimulates epithelial sodium channels by elevating reactive oxygen species. Am J Physiol Cell Physiol (August 15, 2012). doi:10.1152/ajpcell.00139.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bradley RJ, Sterz R, Peper K. The effects of alcohols and diols at the nicotinic acetylcholine receptor of the neuromuscular junction. Brain Res 295: 101–112, 1984 [DOI] [PubMed] [Google Scholar]
- 4. Eisenhofer G, Johnson RH. Effect of ethanol ingestion on plasma vasopressin and water balance in humans. Am J Physiol Regul Integr Comp Physiol 242: R522–R527, 1982 [DOI] [PubMed] [Google Scholar]
- 5. Goodson P, Kumar A, Jain L, Kundu K, Murthy N, Koval M, Helms MN. Nadph oxidase regulates alveolar epithelial sodium channel activity and lung fluid balance in vivo via O2− signaling. Am J Physiol Lung Cell Mol Physiol 302: L410–L419, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guidot DM, Modelska K, Lois M, Jain L, Moss IM, Pittet JF, Brown LA. Ethanol ingestion via glutathione depletion impairs alveolar epithelial barrier function in rats. Am J Physiol Lung Cell Mol Physiol 279: L127–L135, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Hickman-Davis JM, McNicholas-Bevensee C, Davis IC, Ma HP, Davis GC, Bosworth CA, Matalon S. Reactive species mediate inhibition of alveolar type II sodium transport during mycoplasma infection. Am J Respir Crit Care Med 173: 334–344, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Howard RJ, Murail S, Ondricek KE, Corringer PJ, Lindahl E, Trudell JR, Harris RA. Structural basis for alcohol modulation of a pentameric ligand-gated ion channel. Proc Natl Acad Sci USA 108: 12149–12154, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lazrak A, Iles KE, Liu G, Noah DL, Noah JW, Matalon S. Influenza virus M2 protein inhibits epithelial sodium channels by increasing reactive oxygen species. FASEB J 23: 3829–3842, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell 104: 545–556, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Ma HP. Hydrogen peroxide stimulates the epithelial sodium channel through a phosphatidylinositide 3-kinase-dependent pathway. J Biol Chem 286: 32444–32453, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mascia MP, Machu TK, Harris RA. Enhancement of homomeric glycine receptor function by long-chain alcohols and anaesthetics. Br J Pharmacol 119: 1331–1336, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakahiro M, Arakawa O, Nishimura T, Narahashi T. Potentiation of GABA-induced Cl− current by a series of n-alcohols disappears at a cutoff point of a longer-chain n-alcohol in rat dorsal root ganglion neurons. Neurosci Lett 205: 127–130, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Rafii B, Tanswell AK, Otulakowski G, Pitkanen O, Belcastro-Taylor R, O'Brodovich H. O2-induced ENaC expression is associated with NF-κB activation and blocked by superoxide scavenger. Am J Physiol Lung Cell Mol Physiol 275: L764–L770, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Schild L. The epithelial sodium channel: from molecule to disease. Rev Physiol Biochem Pharmacol 151: 93–107, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Snyder PM. Down-regulating destruction: phosphorylation regulates the E3 ubiquitin ligase Nedd4–2. Sci Signal 2: pe41, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Snyder PM. Minireview: regulation of epithelial Na+ channel trafficking. Endocrinology 146: 5079–5085, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Sun P, Yue P, Wang WH. Angiotensin II stimulates epithelial sodium channels in the cortical collecting duct of the rat kidney. Am J Physiol Renal Physiol 302: F679–F687, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takemura Y, Goodson P, Bao HF, Jain L, Helms MN. Rac1-mediated NADPH oxidase release of O2− regulates epithelial sodium channel activity in the alveolar epithelium. Am J Physiol Lung Cell Mol Physiol 298: L509–L520, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu L, Bao HF, Self JL, Eaton DC, Helms MN. Aldosterone-induced increases in superoxide production counters nitric oxide inhibition of epithelial Na channel activity in A6 distal nephron cells. Am J Physiol Renal Physiol 293: F1666–F1677, 2007 [DOI] [PubMed] [Google Scholar]