Abstract
Circulating bone-marrow-derived cells, named endothelial progenitor cells (EPCs), are capable of maintaining, generating, and replacing terminally differentiated cells within their own specific tissue as a consequence of physiological cell turnover or tissue damage due to injury. Endothelium maintenance and restoration of normal endothelial cell function is guaranteed by a complex physiological procedure in which EPCs play a significant role. Decreased number of peripheral blood EPCs has been associated with endothelial dysfunction and high cardiovascular risk. In this review, we initially report current knowledge with regard to the role of EPCs in healthy subjects and the clinical value of EPCs in different disease populations such as arterial hypertension, obstructive sleep-apnea syndrome, obesity, diabetes mellitus, peripheral arterial disease, coronary artery disease, pulmonary hypertension, and heart failure. Recent studies have introduced the novel concept that physical activity, either performed as a single exercise session or performed as part of an exercise training program, results in a significant increase of circulating EPCs. In the second part of this review we provide preliminary evidence from recent studies investigating the effects of acute and long-term exercise in healthy subjects and athletes as well as in disease populations.
Keywords: Circulating endothelial cells, Circulating progenitor cells, Exercise, Cardiovascular disease
CIRCULATING ENDOTHELIAL AND PROGENITOR CELLS
Τhe process of blood vessel formation occurs by two different mechanisms, angiogenesis and vasculogenesis. Angiogenesis describes blood vessel growth from existing blood vessels by sprouting of differentiated endothelial cells or intussusceptions of existing capillaries[1,2]. In contrast, vasculogenesis is defined as blood vessel growth from in situ differentiating angioblasts[3]. Until recently, it has been assumed that vasculogenesis is limited to embryogenesis. For the first time in 1997, Asahara et al[4] described the existence of endothelial cells in the peripheral blood of adults derived from the bone marrow, and confirmed the role of vasculogenesis during the process of postnatal neovascularization. Hematopoietic stem cells that give rise to blood cells and move between bone marrow and peripheral blood are the best-characterized adult stem cells in humans. This circulating bone-marrow-derived cell population has been named endothelial progenitor cells (EPCs)[5]. There are at least two different types of EPCs population, early and late. Early EPCs are usually referred to as the angiogenic EPC population obtained from short-term cultures of 4-7 d. Late EPCs, often called outgrowth EPCs, have different growth patterns and are usually obtained from long-term cultures of at least 2-4 wk[6]. These cells are capable of maintaining, generating, and replacing terminally differentiated cells within their own specific tissue as a consequence of physiological cell turnover or tissue damage due to injury[7].
Endothelial cell injury, after tissue ischemia or vascular injury, initiates physiological processes of reparation and regeneration. The initial step is the mobilization of EPCs from the bone marrow into peripheral blood, which is followed by the recruitment of EPCs to the site of tissue ischemia or vascular injury (Figure 1). Locally, vasculogenesis occurs after EPC adhesion and migration into the newly formed vascular network and differentiation into mature endothelial cells[8]. The pathophysiology mentioned above is critical in terms of endothelium maintenance and restoration of normal endothelial cell function.
Figure 1.
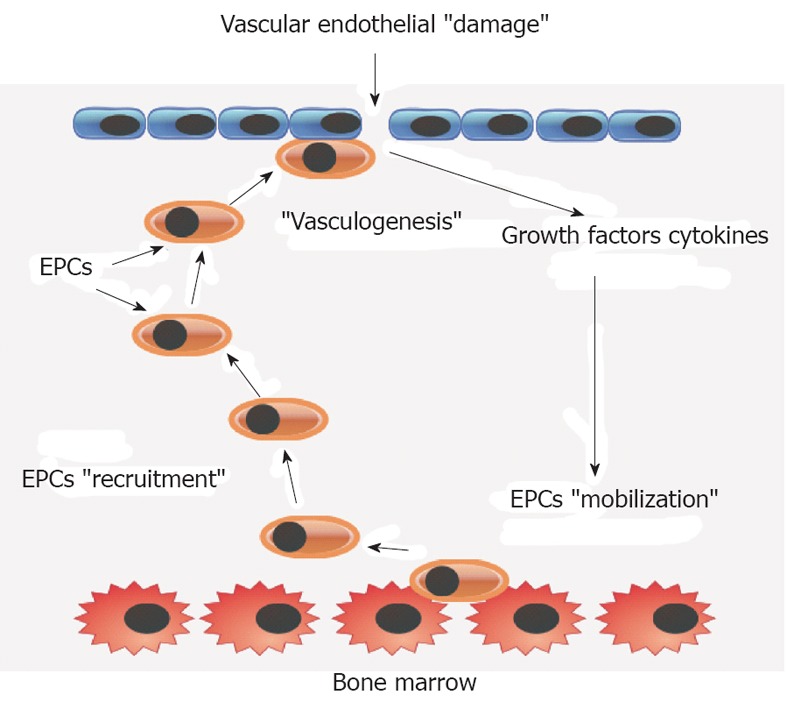
An illustrative model of vasculogenesis after vascular endothelial damage mediated by bone marrow endothelial progenitor cell mobilization and recruitment. EPC: Endothelial progenitor cell.
The current literature suggests that adult stem cells generate differentiated cells beyond their own tissue boundaries, a process termed “developmental plasticity”. Circulating EPCs play two major roles, endothelial healing and neoangiogenesis[9,10].
EPCs AND ENDOTHELIAL DYSFUNCTION
For more than 10 years researchers as well as clinicians have focused on understanding the physiological and pathophysiological role of the EPCs in the cardiovascular system and in cardiovascular disease, because endothelial dysfunction has been established as an independent prognostic risk factor for cardiovascular disease[11,12]. Although there are no clear definition criteria for accurate identification of EPCs so far, there have been several studies indicating the important role of EPCs in restoring endothelial damage[13]. Decreased numbers of EPCs have been associated with endothelial dysfunction and high cardiovascular risk[5,14].
Promotion of recruited bone-marrow-derived EPCs is the primary mechanism for endothelial replacement in areas of vascular damage as demonstrated in animal models. In these experimental ischemic injuries, bone-marrow-derived cells play an important role in vascular repair and regeneration enhancing tissue recovery[15-17].
EPCs are present in peripheral blood circulation of healthy adult subjects, although in small numbers and are responsible for vascular and endothelial repair after tissue damage[18]. A decrease in EPC levels was shown to inversely correlate with the occurrence of endothelial dysfunction[5,19].
EPCs AND AGING
It has been also documented that EPCs are significantly reduced in elderly populations[20], and appear to have elevated apoptotic susceptibility[21]. The number and function of early EPCs isolated from the peripheral blood of 20 healthy young and 20 old (61 ± 2 years) individuals have been investigated by Heiss et al[22]. Early EPCs from the old subjects were found to be significant impaired in terms of fundamental functional features like proliferation, migration and survival, although no quantitative differences in EPCs were observed. Chronic treatment with bone-marrow-derived progenitor cells from young non-atherosclerotic ApoE-/- mice prevents atherosclerosis progression in ApoE-/- mice recipients despite persistent hypercholesterolemia. In contrast, treatment with bone marrow cells derived from older ApoE-/-mice is much less effective[23]. Similarly, transplantation of bone-marrow-derived EPCs from young, but not old donor mice prevented a decline in the angiogenic platelet-derived growth factor signaling and cardiac angiogenesis in an aging murine model[24]. Despite these well-documented experimental studies on EPCs in aging populations, the mechanisms are still unknown and further research is needed.
EPCs AND CORONARY HEART DISEASE
In human studies, circulating EPC levels have been investigated as surrogate markers of coronary artery disease (CAD) severity and indices of clinical outcome, with significant results[25-28].
The activity and migratory capacity of EPCs is reduced in patients with coronary heart disease[29]. Furthermore it has been shown that EPCs enhance angiogenesis, promote vascular repair, improve endothelial function, inhibit atherosclerosis and increase ventricular function after myocardial infarction[5,9,30-32]. All these findings indicate that the number of EPCs may be a marker of cardiovascular risk. EPCs levels were also inversely correlated with the occurrence of in-stent restenosis[33].
EPCs AND CHRONIC HEART FAILURE
Very few research groups have studied circulating EPCs levels in chronic heart failure (CHF). Specifically, it has been shown that EPC mobilization occurs in a biphasic pattern in CHF; increased in mild stages, and depressed in advanced CHF[34,35]; possibly due to the myelosuppressive role of cytokines such as tumor necrosis factor-α in severe CHF. A significant progressive increase of EPCs was noted in patients admitted with acute exacerbation concomitantly with their clinical amelioration during hospitalization[36].
Interestingly, Geft et al[35] have studied early and late apoptotic progenitor cells in CHF, showing that severe heart failure patients exhibited higher numbers of late apoptotic progenitors, and there was a significant association of the latter cells with disease severity. However, in another clinical study, the authors reported that there was no correlation between CD34+ circulating cells and New York Heart Association (NYHA) functional class[37].
Further research is needed to clarify the clinical significance of EPCs levels and their role in CHF patients in relation to acute exacerbations and disease severity.
EPCs AND LEFT VENTRICULAR ASSIST DEVICES
Over the past 4 years a few studies investigated the role of left ventricular assist device (LVAD) implantation in the number of EPCs in patients with end-stage CHF (NYHA class III or IV). In a recent study, Jahanyar et al[38] have demonstrated that LVADs cause a significant increase of stem cell factor and its receptor (c-kit) gene expression, which coincided with a surge of mast cells after ventricular unloading. In another study[39], a significant increase in EPCs was also reported after LVAD implantation.
Both studies were limited by the small number of participants and the lack of an adequate control group. In the study of Jahanyar et al[38] the patients’ EPCs were compared to tru-cut biopsies of donor hearts, while in the study of Manginas et al[39], the control group comprised patients who were ineligible for LVAD implantation.
In addition, in an experimental study[40], the research group found that the intramuscular injection of EPCs [in particular KLS cells (Lin-–/c-kit+/Sca1+)] in the LV-unloading heart of rodents, may have a protective effect against cardiac systolic dysfunction and myocardial atrophy. The latter study suggests that this intervention may have a clinical potential as a combination therapy together with LVAD implantation.
Besides the nature of the study populations with the methodological flow limiting results, it seems that LVAD implantation increases EPC levels.
EPCs AND PULMONARY ARTERIAL HYPERTENSION
Endothelial dysfunction is strongly involved in the pathophysiology of pulmonary arterial hypertension (PAH)[41]. For this reason a growing interest has been recently raised in the role of EPCs in PAH. In a study of Smadja et al[42], the number of circulating endothelial cells was significantly higher in 10 patients with irreversible PAH than in 16 patients with reversible PAH and five control subjects, while progenitor cells did not differ among the groups. To make things more confusing it has also been reported that a significant decrease was observed in circulating EPCs in 20 patients with idiopathic PAH compared to 20 healthy controls[43]. Accordingly, Hansmann et al[44] reported that EPC numbers were 50% lower in PAH subjects versus matched controls.
In contrast with previous studies mentioned above it has been reported that EPCs were increased in patients with PAH compared with controls[45]. In a recent clinical trial, where nine patients with PAH, nine patients with chronic thromboembolic pulmonary hypertension (CTEPH), and seven subjects with normal pulmonary arterial pressure were enrolled, the researchers concluded that EPCs were significantly increased in PAH patients compared to CTEPH and controls[46].
The results from the above studies appear conflicting, thus, further research is needed to clarify the role of EPCs in PAH. Research on EPCs should target on the different PAH stages, and the PAH diagnosis duration that might differentiate in terms of number and function.
EPCs AND CRITICAL ILLNESS
Recent innovative studies have shown that mobilization of EPCs occur in critically ill patients and are significantly associated with clinical outcome and prognosis[47-51].
In 2001, an increased number of EPCs in patients with sepsis and septic shock was found compared to healthy subjects[49]. These findings were confirmed in a more recent study by Rafat et al[47]. They reported that after studying 32 intensive care unit patients with sepsis, 15 patients without sepsis and 15 healthy controls, the number of EPCs was not only significantly higher in septic patients compared to non-septic patients and controls, but was also associated with survival. In a methodologically similar study, Burnham et al[48] have shown that increased number of EPCs was highly correlated with improved survival in patients with early acute lung injury. In addition, in a prospective study enrolling 44 patients with ventilator-associated pneumonia and sepsis, the research group concluded that EPCs count on day 1 was correlated with survival[50]. These studies suggest that patients with sepsis appear to present with elevated EPCs numbers compared to healthy controls, and furthermore, that EPCs levels are strongly correlated with outcome.
EPCs AND CHRONIC OBSTRUCTIVE PULMONARY DISEASE
To the best of our knowledge, only a few studies[52-54] have investigated the number of EPCs in patients with chronic obstructive pulmonary disease (COPD). Methodological differences might have played a significant role in the fact that the studies had conflicting results. Peinado et al[52] conducted dissection on pulmonary arteries of lung specimens of 15 patients undergoing lung resection because of carcinoma and compared the EPC numbers in nine subjects with moderate to severe COPD to the six subjects free of COPD. The authors concluded that EPCs were significantly greater in number in COPD patients.
In contrast to that study, two other controlled studies[53,54] reported lower levels of EPCs in blood samples from COPD patients compared to controls. The number of EPCs was also strongly correlated to disease severity for both studies. In a study by Sala et al[55], the authors investigated the response of EPCs to episodes of exacerbation of COPD (ECOPD). After studying 35 patients hospitalized because of ECOPD, 44 COPD patients, 10 smokers free of COPD and 10 healthy non-smokers, they found a higher level of EPCs in the ECOPD group compared to the other groups. Just recently, it has been shown that there is an impaired EPC mobilization and colony-forming capacity in COPD patients with stage I and II lung cancer undergoing thoracic surgery[56]. It seems that more evidence is necessary to understand better the role of EPCs in COPD due to the inconclusive results mentioned above.
EPCs AND OBSTRUCTIVE SLEEP APNEA
Four controlled studies[57-60] have been conducted to investigate the number of EPCs in patients with obstructive sleep apnea (OSA), who lacked cardiovascular disease and were free of any other known cardiovascular risk factor. All studies had a small number of participants and reported conflicting results. El Solh et al[60] by studying 14 subjects with OSA documented higher numbers of EPCs compared to 10 healthy controls. They also reported that an 8-wk treatment with continuous positive airway pressure (CPAP) therapy significantly reduced the levels of EPCs in the OSA patient group. The same research group a year later confirmed the above outcome by enrolling 35 OSA patients on 8 wk nasal CPAP therapy[58].
In another study that included 13 OSA patients and 13 controls, there was a reduced number of EPCs in patients with OSA compared to controls[59]. However, other researchers found no significant differences in EPCs between OSA patients and controls[57].
The conflicting results of the above studies need further investigation with larger numbers of study participants before conclusions can be made for the possible role of EPCs in OSA. Disease severity might play a significant role in the EPCs level and should be further assessed.
EPCs AND DIABETES
In diabetic patients the EPC levels are significantly reduced compared to non-diabetic controls. Loomans and colleagues reported that the EPCs numbers were decreased in patients with type I diabetes, compared to EPCs levels in healthy subjects[61]. Enrolling 74 patients with type I diabetes, EPCs counts were significantly lower in these patients compared to 80 healthy controls[62].
Similarly, other studies have also demonstrated that EPCs levels were lower in patients with diabetes mellitus type II compared to healthy controls[63-65]. Treatment with strict glycemic control and total cholesterol improvement increased EPCs numbers[65]. There is strong evidence that patients with diabetes types I and II appear to have lower EPCs numbers compared to healthy controls. Further research is needed demonstrating the beneficial effects of the potential increase in EPCs by certain therapeutic measures (diet, exercise training, antidiabetic drug treatment, etc.).
EPCs AND CEREBROVASCULAR DISEASE
In a study of Ghani et al[66], there was a significant difference in EPC counts between stroke patients (acute stroke: median 4.75, range 0-33; stable stroke: median 7.25, range 0-43) and control subjects (median 15.5, range 4.3-50), independent of age. In contrast, Yip et al[67] demonstrated that the levels of circulating EPCs did not differ between patients with ischemic stroke and normal control subjects. The authors also reported that in patients with acute ischemic stroke, the EPCs levels were significantly higher than in subjects at high cerebrovascular risk, but appeared to be lower in patients with severe neurological impairment. The research group stated that the inconsistency of their findings with the results of Ghani et al[66] was due to the difference in the time interval for blood sampling between the studies.
There was a significant decrease in circulating CD34+ cell levels in 25 patients with a history of atherothrombotic cerebral ischemic events compared with age-matched controls[68]. Colony forming units (CFUs) and outgrowth cell population as a subset of EPCs were significantly reduced in 75 patients with acute stroke, compared with 45 patients with chronic stroke and 40 age-matched control subjects. Moreover, patients with large artery atherosclerosis had much lower CFU numbers and functional activities than the ones with cardioembolism[69].
It has also been reported that the increase in circulating EPC levels after acute ischemic stroke is associated with good functional outcome and reduced infarct growth, suggesting that EPCs might participate in neurorepair after ischemic stroke[70]. There are convincing data that ischemic stroke is strongly correlated with low EPC levels and that levels are strongly correlated with functional outcome in acute ischemic stroke.
EPCs AND PERIPHERAL ARTERIAL DISEASE
Little information is available on EPC mobilization in patients with peripheral arterial disease (PAD). In 2008, Delva et al[71] studied the number of EPCs by different methods in a carefully selected group of 45 patients with CAD along with 24 healthy subjects. In patients with PAD, by utilizing the dual-binding method, the number of circulating EPCs was significantly increased compared to that in healthy controls. On the contrary, while using fluorescence-activated cell sorting analysis the results were different, both CD34+ and CD133+ cell counts were significantly decreased compared to controls. The use of different methods in data collection may explain the discrepancy. Finally, CFUs were significantly increased in PAD compared to healthy subjects[71].
After studying 48 PAD patients and 22 patients without PAD the researchers concluded that EPC mobilization occurred in PAD and showed a biphasic response, with elevated EPC levels in the moderate phase and reduced EPC levels in the advanced phase. EPC levels were also associated with the levels of novel circulating biomarkers and several aspects of PAD, including the severity, progression and disease outcome[72].
As a result of the limited number of studies, further research is needed on the levels of EPCs in patients with PAD. It seems however, that there is an increase of EPC levels in the moderate phase and a decrease in the advance phase of PAD.
EPCs AND ARTERIAL HYPERTENSION
In the very early stage of hypertension a significant increase in circulating progenitor cells is associated with reactive oxygen species and oxidative stress[73]. Vasa et al[19], after studying patients with CAD concluded that the migratory capacity of EPCs was reduced in those patients with hypertension, although their total number did not change significantly.
Additionally, only recently it has been demonstrated that the in vivo endothelial repair capacity of early EPCs is substantially impaired in patients with newly diagnosed pre-hypertension and hypertension as their only cardiovascular risk factor[74]. Increased senescence of early EPCs in pre-hypertensive and hypertensive patients was related to abnormal EPCs endothelial repair capacity. In the same study there was not a significant difference in the numbers of EPCs and endothelial apoptotic microparticles in pre-hypertensive and hypertensive patients when compared with healthy controls. From this study emerges evidence that the endothelial repair capacity of early EPCs is substantially impaired in hypertensive patients.
EPCs AND OBESITY
It is well established that obesity is associated with decreased numbers of EPCs[75-79]. The number of circulating EPCs is lower in obese compared with overweight and normal weight subjects, while EPCs colony forming capacity is blunted in overweight and obese compared with normal weight subjects[76]. Tobler et al[77] also stated that reduced numbers of EPCs along with their premature senescence might contribute to the development and progression of vascular dysfunction in obesity. Furthermore, the function of EPCs in obesity is impaired either by the impaired ability of circulating EPCs to release proangiogenic growth factors compared with normal weight adults and by the lower EPC resistance to an apoptotic stimulus in overweight and obese compared to normal weight[78]. Interestingly, low EPCs levels[75] and their functional deficiency[79] can be reversed after significant weight reduction.
A summary of the above studies of EPCs in different disease populations is reported in Table 1.
Table 1.
Summary of endothelial progenitor cell levels in different disease populations
| Disease | Results | Comments | Ref. |
| Aging | ↓ EPCs proliferation | [20,22] | |
| ↓ EPCs migration | |||
| ↓ EPCs survival | |||
| Coronary heart disease | ↓ EPC activity | [29] | |
| ↓ EPC migratory capacity | |||
| CHF | ↑ EPC levels in mild stages[34,35] | [34-36] | |
| ↑ EPC levels in acute exacerbation[36] | |||
| ↑ Late apoptotic progenitors[35] | |||
| ↓ EPC levels in advanced stages[34,35] | |||
| LVADs | ↑ EPC levels | [38,39] | |
| PAH | ↑ EPC levels[42,45,46] | Conflicting results | [42-46] |
| ↓ EPC levels[43,44] | |||
| Critical illness | ↑ EPC levels in sepsis and septic shock[47,49] | [47-49] | |
| ↑ EPC levels in early acute lung injury[48] | |||
| COPD | ↑ EPC levels[52] | Conflicting results | [52-56] |
| ↓ EPC levels[53,54] | |||
| ↑ EPC levels in exacerbation of COPD[55] | |||
| ↓ EPC mobilization and colony-forming capacity[56] | |||
| OSA | ↑ EPC levels[58,60] | Conflicting results | [57-60] |
| ↓ EPC levels[59] | |||
| - No difference[57] | |||
| Diabetes mellitus type Iand II | ↓ EPC levels | [61-65] | |
| Cerebrovascular disease | ↓ EPC levels in stroke[66] | [66-69] | |
| - No difference in ischemic stroke[67] | |||
| ↑ EPC levels in acute ischemic stroke[67] | |||
| ↓ EPC levels in atherothrombotic cerebral ischemic event[68] | |||
| ↓ EPC colony forming in acute stroke[69] | |||
| PAD | ↑ EPC levels in moderate phase | [71,72] | |
| ↑ EPC levels in advance phase | |||
| Arterial hypertension | ↑ EPC levels in early stage of hypertension[73] | [19,73,74] | |
| ↓ EPC migratory capacity in CAD and hypertension[19] | |||
| - No difference on EPC levels in pre-hypertensive and hypertensive[74] | |||
| Obesity | ↓ EPC levels[75-77] | [75-79] | |
| ↓ EPC colony forming capacity[76] |
EPC: Endothelial progenitor cell; CHF: Chronic heart failure; LVAD: Left ventricular assist device; PAH: Pulmonary arterial hypertension; COPD: Chronic obstructive pulmonary disease; OSA: Obstructive sleep apnea; PAD: Peripheral arterial disease; CAD: Coronary artery disease.
PHYSICAL ACTIVITY, EXERCISE TRAINING AND ENDOTHELIAL FUNCTION
It is well recognised that physical activity has significant beneficial effects on overall health, and especially on cardiovascular morbidity and mortality. Physical inactivity is an independent risk factor for the development of coronary heart disease, stroke and peripheral vascular disease[80].
Regular physical activity significantly attenuates the atherosclerotic process by reducing atherosclerotic risk factors, retarding arterial wall aging[81,82], delaying development of endothelial dysfunction and preserving vascular function[83]. Furthermore, physical training reduces vascular oxidative stress, increases the activity of endothelial nitric oxide synthase (eNOS)[84] and results in increased blood flow to oxidative skeletal muscle fibers[85].
Accordingly, the beneficial effects of exercise have been reported in patients with CAD as part of the secondary prevention[86,87]. It is well known that physical training improves endothelial function and exercise capacity in patients with CAD[88], CHF[89] and PAD[90]. Exercise is also associated with improved body weight, blood pressure, insulin sensitivity and hemostatic and inflammatory variables[91,92]. High-intensity interval training is a relative novel alternative modality of exercise in metabolic syndrome[93], coronary heart disease[94] and in CHF patients[95-97], allowing more intense stimuli in the peripheral muscles. The addition of strength training has been also shown to confer significant improvement in endothelial function[98,99] and peripheral microcirculation[100] in CHF patients.
It has become common knowledge that exercise training has a significant therapeutic role in a vast majority of diseases. A structured exercise training program, and more specifically a combination of aerobic exercise (continuous or high-intensity interval training) with or without the addition of strength training can significantly attenuate the atherosclerotic process through its beneficial effects on endothelial function[93,95,98-100]. However, there is growing interest concerning the role of exercise training on EPCs that may interact with endothelium function; this issue is currently under investigation from several research groups in both healthy subjects and diseases.
EFFECTS OF EXERCISE TRAINING ON EPCs
Laufs et al[101] in 2004 were the first research group to demonstrate that physical activity leads to an increased number of circulating EPCs in mice after 28 wk of running wheels. This effect occurred rapidly (7 d after training) and was sustained for at least 4 wk, providing evidence that EPC numbers can be increased by nearly threefold by exercise training. These findings were confirmed by another research group in a human study in which middle aged and older subjects following a 3 mo training program of walking, at moderate intensity, increased circulating angiogenic cell (CAC) migratory capacity by 50%[20]. Accordingly, Yang et al[102] showed that the number and activity of circulating EPCs of 10 older and 10 young sedentary healthy men were increased after 3 mo regular exercise. However, the increased number and activity of circulating EPCs of older sedentary healthy men was higher compared to the younger group. In contrast, in a study of 20 healthy men who followed a 6-wk interval exercise training program, [moderate (9 subjects) or high intensity (11 subjects)], there was no significant effect on EPC number in both groups, even though there was an improvement of vasoconstrictor function[103].
Furthermore, a study of 182 children (aged 11.1 ± 0.7 years) showed that physical activity by means of daily school exercise lessons can increase the number of circulating progenitor cells[104]. More interestingly, EPC numbers decreased significantly after 10 d of detraining in highly active older men, even lower than the level of low-active (sedentary) men[105]. These findings agree with the fact that sustained physical activity is necessary to preserve improved endothelial function for maintaining long-term training effects[106].
In an animal study, 8 wk of aerobic training in mice with advanced atherosclerotic lesions showed no improvement in atherosclerosis, whereas mice with early lesions benefited. The authors suggest that the impact of exercise on atherogenesis is primarily to retard the progression to advanced lesions, rather than reversing advanced lesions. Interestingly, the level of EPCs decreased, along with proinflammatory cytokines in response to exercise[107].
In another experimental study conducted in the early phase following traumatic brain injury in rats, the exercise group compared to the control group enhanced significantly proliferation of neural stem cells (NSCs) around the damaged area[108].
Despite the significant experimental study of Laufs et al[101], Luk et al[109] showed that there was no significant increase in CD34/KDR+ EPCs after an 8-wk exercise training programme on 32 patients with CAD compared to controls. In contrast, in a recent uncontrolled study by Cesari et al[110], there was a significant increase in EPCs and a significant decrease in proinflammatory biomarkers, in patients with acute coronary syndrome, occurring after a 30-d period of cardiac rehabilitation (3 sessions per week of endurance training on a cycle ergometer, at 60%-70% of individual VO2 level obtained at peak exercise during baseline symptom-limited cardiopulmonary exercise testing). Because of the conflicting results and the small number of studies, more human studies are needed to clarify the possible beneficial effects of exercise on EPCs in CAD.
EPC levels [assessed as CD34+ cells coexpressing AC133 and vascular endothelial growth factor receptor 2 (VEGFR2), and as endothelial CFUs (e-CFUs)] were also evaluated in chronic renal failure patients on hemodialysis after a 6-mo walking exercise program. This study showed that there was not a significant change in CD34+ or CD34+/AC133+/VEGFR2+ cell numbers, but there was a significant change in e-CFUs[111].
A significant enhancement of circulating EPCs after 8 wk of supervised exercise training has also been demonstrated in CHF patients. In that study, 8 wk of detraining led to baseline EPC levels[112]. In a recent study by Van Craenenbroeck et al[113] similar results have emerged after investigating the effect of 6 mo exercise training in CHF, compared with a no exercise CHF group and a no exercise group of healthy subjects. The authors reported a reverse effect of exercise training on circulating angiogenic cell dysfunction and an increase in EPCs. An analogous increase in EPC numbers was found, after short-term exercise training (3 wk) in 14 CHF patients[114]. The exercise program was a combination of calisthenics and aerobic exercise with an intensity up to 75%-85% of the maximum heart rate attained in the exercise test. The authors reported that even a relatively short-term exercise training program significantly improved the serum ability to support viability of EPCs as well as upregulation of proteins participating in LV remodeling.
Similarly, 37 CHF patients (LV ejection fraction 24% ± 2%) were randomly assigned to 12 wk of exercise training (n = 18) or sedentary lifestyle (n = 19). Exercise training increased the number of CD34+ progenitor cells from 1094 ± 677 to 1450 ± 798 cells/mL blood in the training group (P = 0.032 vs control). The number of CD34+/KDR+ EPCs was found to be augmented from 100 ± 127 to 183 ± 156 cells (P = 0.014 vs control) and their migratory capacity by 224 ± 263 vs -12 ± 159 CPCs/1000 plated CPCs in controls (P = 0.03)[115].
The most recent paper on the effect of exercise training in patients with CHF is the one published by Mezzani et al[116]. Patients (n = 30) with NYHA class II were allocated to either an aerobic 3-mo exercise training group or a control group, while seven age-matched healthy subjects were also studied. In addition to the previous studies, the authors reported that the numbers of EPCs, even though they did not differ between patient groups at baseline, significantly increased in the CHF training group, reaching values similar to those of healthy controls, while the difference between CHF controls and healthy controls did not reach statistical significance. Additionally, the levels of EPCs remained unchanged in the control patient group.
In a randomized controlled clinical trial, 40 patients with PAD were allocated to either an exercise or a control group. The intervention group followed a standardized training program twice a week for 6 mo. The initial duration included 35 min of intermittent walking, which was increased by 5 min each session until 50 min of intermittent walking was achieved. EPC levels were significantly increased and asymmetric dimethylarginine levels were decreased in the exercise group compared with the controls. The authors suggested an enhanced angiogenesis and improved endothelial function that might contribute to cardiovascular risk reduction[117].
The first study on the effect of exercise training in overweight and obese patients has recently been published by Cesari et al[118]. Even though the study had a few methodological limitations, the authors reported a significant increase in all the three groups of EPCs (CD34+/KDR+: +33.3%; CD133/KDR+: +35%; CD34+/CD133+/KDR+: +35.7%) after a 3 mo exercise intervention program, compared to baseline measurements.
The underlying mechanisms of the effect of exercise training on EPC levels needs further investigation, but taken together with the existing data, it has been suggested that physical exercise increases NO bioavailability[119], and in parallel with this fact, the effect of physical activity on EPCs is markedly reduced after inhibition or deletion of eNOS, which suggests an NO-dependent increase in EPCs in response to exercise[101,113]. Additionally, it has been reported that physical exercise may increase EPC numbers by prolonging their lifespan. Data derived from cultured EPCs have indicated that the observed EPCs enhancement in the circulation and the bone marrow could be explained partly by antiapoptotic effects of physical activity on EPCs and potentially their progeny[101]. The extra muscle stress that is additive to hypoxic stress and leads to a hypoxia-induced factor-1-mediated upregulation of both VEGF and stromal-cell-derived factor (SDF)-1 stimulated EPCs mobilization, as demonstrated by Sarto et al[112].
Even though the studies mentioned above followed different methodologies and exercise training protocols, we may assume that regular exercise training increases significantly circulating EPC levels, both in healthy subjects (rodents and humans) and in a variety of diseases. The strongest evidence so far in cardiovascular disease emerges from studies in CHF and PAD. More data are needed, however, to confirm the results of these preliminary studies and to investigate the optimum exercise protocol in order to maximize the possible beneficial effects.
Summaries of the long-term exercise training effects on EPCs in animals and healthy subjects as well in disease populations are illustrated in Tables 2 and 3, respectively.
Table 2.
Effects of exercise training on endothelial progenitor cells in animals and healthy subjects
| Subjects | n | Study group; age | Modality | Exercise prescription | Duration | Results | Limitations | Ref. |
| Mice | 12 | Exercise group; Control group | Aerobic | Exercise group: Voluntary running 5100 ± 800 m 7 d a week; Control group: No intervention | 28 d | ↑ EPCs | [101] | |
| Healthy male | 10 | Exercise (n = 10) 59 ± 2 yr | Home based aerobic | Walking/jogging 60%-75% predicted peak HR 40-50 min-5-7 sessions per week | 3 mo | ↑ EPC colonies about 100% from 10 ± 3 to 22 ± 5; ↑ Migratory activity about 50% from 683 ± 96 to 1022 ± 123 RFUs | Non control study | [20] |
| Healthy male | 47 | Exercise: Elderly (n = 25) 67.8 ± 3.38 yr, young (n = 22) 26.3 ± 3.15 yr | Aerobic | Treadmill 30 min 3 sessions per week | 12 wk | ↑Re-endothelialization capacity of EPCs from 15% ± 4% to 36% ± 9% | Non control study | [102] |
| Healthy male (n = 7), female (n = 13) | 20 | Interval exercise: Moderate (n = 9), heavy (n = 11) | Aerobic | Ergometer moderate interval (10 s @ 120% peak work rate : 20 s @ 20 W); Heavy interval (30 s @ 120% peak work rate : 60 s @ 20 W), 30-40 min 3 sessions per week | 6 wk | No significant change on EPC numbers | Non control study measurements took place 48 h after the last session | [103] |
| Children | 182 | Intervention (n = 109), control (n = 73) | Intervention group: PA at school 45 min + endurance training 15 min per school day; Control group: PA at school 45 min 2 school day per week | 1 school year | ↑ CPCs | [104] |
EPC: Endothelial progenitor cell; PA: Physical activity.
Table 3.
Effects of exercise training on endothelial progenitor cells in different disease populations
| Subjects | n | Study group; age | Disease | Modality | Exercise prescription | Duration | Results | Limitations | Ref. |
| Mice | 12 | Exercise; Control | Advanced atherosclerotic lesions; Early atherosclerotic lesions | Aerobic | Voluntary running | 8 wk | No change on advanced atherosclerotic lesions; ↑ EPC levels on early atherosclerotic lesions | [107] | |
| Mice | 10 | Exercise; Control | After traumatic brain injury | Aerobic | Exercise group: Treadmill 22 m/min 30 min 7 d per week; Control group: No intervention | 1 wk | ↑ Neuronal stem cell | [108] | |
| Male (n = 24); Female (n = 51) | 64 | Exercise (n = 32); Control (n = 32) 67.1 ± 8.4 yr old | Coronary artery disease | Aerobic + resistance exercise | Exercise group: Bicycle ergometer, treadmill, rowing, steps, arm ergometer + dumbbell, weight training 80% of HRmax 50 min + (5 min warm-up and 5 min cool down) 3 sessions per week | 8 wk | No change | [109] | |
| Male (n = 92); Female (n = 20) | 112 | Exercise (n = 112) 58.2 ± 9.5 yr old | After acute coronary syndrome | Aerobic | Exercise group: Bicycle ergometer 60%-70% of peak VO2 30 min + ( 5 min warm-up and 5 min cool down) 3 sessions per week | 30 d | ↑ EPC levels + ↓ pro-inflammatory markers | No controls; No homogeneity of population | [110] |
| Male (n = 20); Female (n = 10) | 30 | Exercise (n = 16); Control (n = 14) 67 ± 12 yr old | Hemodialysis | Aerobic | Exercise group: Treadmill/walking 50% max speed 10 min 2 sessions per day; Control group: No intervention | 6 mo | No change | Small sample size; No standarized work | [111] |
| Male (n = 16); Female (n = 6) | 22 | Exercise (n = 22) 61.4 yr old (SE 1.60) | CHF NYHA II or III | Aerobic | Exercise group: Bicycle ergometer 60% of HR reserve 45 min + (5 min warm-up and 5 min cool down) 3 sessions per week | 8 wk | ↑ EPC levels | No controls; No homogeneity of population | [112] |
| Male (n = 30); Female (n = 8) | 38 | Exercise (n = 21) 61.3 ± 2.2 yr old; Control (n = 17) 63.4 ± 3 yr old | CHF | Aerobic | Exercise group: 90% HR 60 min 3 sessions per week; Control group: No intervention | 6 mo | Improves CAC migratory capacity; ↑ EPC levels | No randomization | [113] |
| Male (n = 16); Female (n = 12) | 28 | Exercise (n = 14) 72 ± 11 yr old; Control (n = 14) 73 ± 11 yr old | CHF Exercise NYHA II; Control NYHAI | Calistenics + aerobic | Exercise group: Bicycle ergometer 75%-85% of HRmax 30 min 2 sessions per day 6 sessions per week; Control group: No intervention | 3 wk | ↑ EPC levels | Small sample size | [114] |
| Male | 37 | Exercise (n = 18) 60 ± 11 yr old; Control (n = 19) 62 ± 10 yr old | NYHA functional class IIIb | Aerobic + calisthenics + noncompetitive ball games | Exercise group: Bicycle ergometer in hospital (50% of VO2max 5-20 min, 3-6 sessions per day, 3 wk) home exercise (60% of VO2max 20-30 min, 7 sessions per week, 12 wk) + 1 supervised session ( 60 min, walking, calisthenics, ball games); Control group: No intervention | 12 wk | ↑ EPC levels | [115] | |
| Male | 37 | Exercise (n = 15) 65 ± 7 yr old; CHF control (n = 15) 63 ± 7 yr old; Healthy control (n = 7) 66 ± 4 yr old | NYHA functional class II | Aerobic | Exercise group: Bicycle ergometer HR of ventilatory threshold 30 min 5 sessions per week; Control groups: No intervention | 3 mo | Exercise group ↑ EPC levels; Control patient group no change | [116] | |
| Male (n = 24); Female (n = 16) | 40 | Exercise (n = 30) 69 ± 8 yr old; Control (n = 20) 70 ± 11 yr old | PAD | Aerobic | Exercise group: Treadmill intermittent walking 5-10 min warm-up 35-50 min 2 sessions per week; Control group: No exercise intervention | 6 mo | ↑ EPC levels | [117] | |
| Male (n = 22); Female (n = 18), median age 48 yr | 40 | Exercise (n = 40); Group A (n = 21) compliant individuals; Group B (n = 19) noncompliant individuals | Overweight and obese BMI ≥ 25 kg/m² | Aerobic | Non supervised self reported walking briskly or moderate running 45 min HR @ the individual anaerobic threshold 3 sessions per week | 3 mo | Group A ↑ EPC levels; Group B no change | Small sample size; Limited duration of follow-up; Non valid compliance measurement; Unable to distinguish which (exercise or weight change) contributed to the increased EPC levels | [118] |
EPC: Endothelial progenitor cell; BMI: Body mass index; NYHA: New York Heart Association; CHF: Chronic heart failure; CAC: Circulating angiogenic cell; PAD: Peripheral arterial disease.
EFFECT OF ACUTE EXERCISE ON EPCs
The effect of a single exercise session on EPC levels has also been investigated. Rehman et al[120] have demonstrated that exercise can acutely (within 5-10 min after completion of exercise) increase EPCs and CACs in 22 volunteer patients that underwent a symptom-limited treadmill or bicycle exercise test. It has been reported that a modified Bruce treadmill acute exercise protocol can significantly contribute to upregulation of circulating EPCs in healthy subjects[121]. In the same study, the plasma NO levels were also increased and there was a significant linear relationship between the enhanced plasma NO levels and increased number of circulating EPCs activity after acute exercise.
Since that study, researchers have sought to investigate the effect of a single bout of strenuous exercise on late outgrowth endothelial cells (OECs). In a non-controlled study of 11 healthy, physically fit subjects, who followed 1 h of high-intensity (80% of the predicted maximum heart rate) aerobic exercise, the numbers of OEC colonies were doubled 1 h after exercise compared to baseline measurements. The OEC levels returned back to baseline levels 48 h after exercise[122]. In another study[123], it was shown that strenuous exercise at 70% of the individual anaerobic threshold for 4 h could lead to a significant rise in progenitor cells (hematopoietic and EPCs).
In a recent study[124], exercise-induced changes in putative EPC gene expression were associated with thrombin production and the authors suggested that they may be increased by long-term exercise training. Moreover, in a study by Van Craenenbroeck et al[125], the authors analyzed the concentration of EPC levels before and immediately after a maximal cardiopulmonary exercise test, showing a significant increase of 50% in EPCs.
The acute effect of different exercise durations and intensities on EPC levels was also studied in healthy individuals[126]. In that study, the authors reported that intensive and moderate aerobic exercise for 30 min but not for 10 min increased circulating levels of EPCs.
Furthermore, the analysis of EPC levels in 68 healthy marathon runners after finishing a marathon race, demonstrated no change compared to baseline levels[127]. Similarly, in a more recent study[128], EPC levels were measured in a group of 10 healthy amateur runners after finishing a marathon and after completing a 1.5-km field test. The researchers observed no change in EPC levels after the marathon, while there was a significant increase after the field test. In addition, Goussetis et al[129] showed a significant increase in inflammatory markers, which correlated with a rise in EPCs levels in amateur spartathlon runners (246 km). An interesting finding of this study was also the observation that endothelial cell colonies derived from mobilized EPCs contained significantly more cells 48 h after the race than those obtained in controls and in athletes before the race.
While studying a group of healthy young subjects, a group of healthy old and a group of patients with PAD after an acute exercise session, Shaffer et al[130] concluded that young healthy individuals have an increased capacity to mobilize EPCs, as compared with older individuals. Interestingly, patients with PAD appeared to be unable to mount a significant increase in circulating EPCs, despite the ischemic stimulus. Van Craenenbroeck et al[131] studied 41 CHF patients (22 mild, 19 severe) and 13 healthy subjects while performing a symptom limited cardiopulmonary exercise test. Even though there was no significant change in circulating CD34+ cells and CD34+/KDR+ progenitor cells, an improvement in CAC migratory capacity was most prominent in severe CHF, increased to levels that were no longer different from healthy controls. The same research group just recently sought to investigate whether the above result reflected an attenuated or delayed mobilization of EPCs, so they measured CD34+/KDR+ EPCs over a longer time period post-graded exercise testing (GXT). Even though the number of subjects was small (7 CHF patients and 8 healthy controls), the authors reported that EPC numbers increased within 10 min following GXT and remained elevated for up to 2 h. In CHF patients, the initial increase was small and normalized within 30 min[132].
From the current literature it emerges that the acute effect of a single exercise session, either performed as an exercise test, or as a single bout of strenuous exercise, can lead to a significant rise in EPCs in healthy subjects. On the contrary, there are still conflicting data with regard to long distance runners (e.g., marathon, spartathlon) and for this reason further investigation is required prior to definite conclusions. In CHF patients it seems that acute exercise might exert a rise in EPCs; however, the small number of studies with different exercise protocols, methodologies and the small number of study participants limit preliminary study results. Further investigational studies targeting on exercise characteristics (modality of exercise, intensity, duration, etc.) are also needed to elucidate the acute exercise effects on EPCs in CHF.
Schematic results from the acute effects of exercise in healthy subjects and disease are presented in Tables 4 and 5.
Table 4.
Acute effects of exercise in healthy subjects
| Subjects | n | Study group; age (yr) | Modality | Exercise | Results | Limitations | Ref. |
| Men | 22 | Healthy 54 ± 10 | Symptom limited exercise test | Treadmill/bicycle | ↑ EPC + ↑ CAC | [120] | |
| Men | 16 | Healthy 25.1 ± 2.7 | Bruce modified | Treadmill | ↑ EPC + ↑ NO level | [121] | |
| Men (n = 2); Women (n = 9) | 11 | Healthy 31 ± 16 | 1 h Spinning session | Bicycle 80% HRmax | ↑ OEC | No accurate peak VO2 measurements, no gender differentiation | [122] |
| Men | 18 | Healthy (sportive) 32.4 ± 2.3 | Ergometer test | Bicycle 70% IAT 240 min | ↑ EPC | No control | [123] |
| Men | 23 | Endurance athletes 62 ± 1.6; Healthy low active 65 ± 1.5 | Exercise test | Treadmill 75% ± 5% VO2max 30 min (5 min ramp-up + 25 min + 3 min cool down) | No change in both groups, no change between groups | [124] | |
| Men; Women | 25 | GroupI(n = 11) 23.9 ± 1.4; Group II (n = 14) 36.2 ± 9.3 | Symptom limited cardiopulmonary exercise test | Bicycle ergometer: 40 W warm up; 20 W incremental test | ↑ EPC | Small sample size | [125] |
| Men | 25 | Healthy | Running exercise | Protocol 1 30 min 100% IAT; Protocol 2 30 min 80% IAT; Protocol 3 10 min 80% IAT | Protocol 1 ↑ EPC; Protocol 2 ↑ EPC; Protocol 3 no change | [126] | |
| Men | 68 | Marathon runners 57 ± 6 | Marathon | Race | No change | [127] | |
| Men | 10 | Marathon protocol (n = 9) 43.6 ± 11.6; Field test protocol (n = 8) 43.4 ± 10.9 | Running exercise | Marathon protocol marathon race; Field test protocol 1500 m max speed | Marathon protocol no change; Field test protocol ↑ EPC | [128] | |
| Men | 20 | Spartathlon runners (n = 10) 42.8 ± 1.4 Control sedentary (n = 10) 42.2 ± 10.4 | Spartathlon | Exercise group race (246 km); Control group no intervention | Spartathlon runners ↑ EPC; Control no change | [129] |
EPC: Endothelial progenitor cell; CAC: Circulating angiogenic cell; IAT: Individual anaerobic threshold.
Table 5.
Acute effects of exercise in different disease populations
| Subjects | n | Study group; age (yr) | Disease | Modality | Exercise prescription | Duration | Results | Ref. |
| Men | 37 | Young group (n = 9), mean age 33; Old group (n = 13), mean age 66; PAD group (n = 15), mean age 69 | PAD | Treadmill | Young group bruce protocol; Old group gardner protocol; PAD group gardner protocol | Young group until exhaustion or 15 min; Old group 10 min; PAD group symptom limited or 10 min | Young group ↑ EPCs; Old group no change; PAD group no change | [130] |
| Male (n = 41); Female (n = 13) | 54 | Healthy controls (n = 13) 55.7 ± 1.6; Mild CHF (n = 22) 61.9 ± 2.5; Severe CHF (n = 19) 63 ± 2.6 | CHF | Bicycle ergometer | Cardiopulmonary exercise testing individualized ramp protocol | 8-10 min | EPC no change; Improved CAC migration in mild CHF + severe CHF | [131] |
| Male (n = 13); Female (n = 2) | 15 | Young group (n = 4) mean age 33; Old group (n = 4) mean age 66; CHF group (n = 7) mean age 69 | CHF II/III | Bicycle ergometer | Symptom-limited graded exercise test | ↑EPCs in young group; ↑EPCs in old group; Non significant ↑EPCs in CHF group | [132] |
EPC: Endothelial progenitor cell; CHF: Chronic heart failure; CAC: Circulating angiogenic cell; PAD: Peripheral arterial disease.
The mechanisms behind the exercise-induced mobilization of EPCs are not fully understood. In patients with cardiovascular disease, exercise-induced ischemia seems to stimulate augmentation of EPCs[133]. In healthy subjects, tissue ischemia is not normally expected to occur due to exercise, but strenuous exercise at levels above what is defined as anaerobic levels, might induce oxidative stress and an inflammatory response and thus could stimulate the release of EPCs from the bone marrow[121,122]. Researchers have hypothesized[34,132] that, in an attempt to address vascular damage, EPCs show a compensatory increase in patients with mild to moderate CHF. Interestingly, VEGF and SDF are potent angiogenic factors that have been shown to increase after exercise sessions with concomitant rise in EPCs in cardiovascular disease, indicating a possible pathophysiological link. Therefore, endothelial factors, cytokines, bone-marrow-derived factors and oxidative stress are involved throughout exercise training and may explain exercise beneficial effects in health and cardiovascular disease.
In conclusion, recent studies provide evidence for the novel concept that physical activity, either performed as a single exercise session or performed as part of an exercise training program, results in a significant increase in circulating EPC levels. Moreover, in patients with cardiovascular disease, exercise training markedly improves vascular endothelium. However, future studies with larger sample sizes are required to investigate and confirm possible exercise training-induced EPCs rise in both healthy subjects and specific subsets, such as patients with hypertension, obesity, OSA, dyslipidemia, diabetes, CAD, PAD, or CHF. Studies should aim to characterize the optimum exercise regimen with regard to type, intensity and duration of exercise that may increase EPC levels. Confirming evidence will give the opportunity to the medical community to use a non-pharmacologic intervention, such as exercise, to mobilize EPCs. Revealing the mechanisms of exercise-induced EPC mobilization may result in the development of optimum exercise protocols to improve endothelial function and enhance angiogenesis.
Footnotes
Peer reviewers: Jesus Peteiro, MD, PhD, Unit of Echocardio-graphy and Department of Cardiology, Juan Canalejo Hospital, A Coruna University, A Coruna, P/ Ronda, 5-4º izda, 15011 A Coruña, Spain; Dr. Thomas Hellmut Schindler, Department of Medecine, University Hospitals of Geneva, Rue Gabrielle-Perret-Gentil, 4, 1211 Geneva, Switzerland
S- Editor Cheng JX L- Editor Kerr C E- Editor Li JY
References
- 1.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 2.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 3.Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 4.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 5.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Ingram DA, Murphy MP, Saadatzadeh MR, Mead LE, Prater DN, Rehman J. Release of proinflammatory mediators and expression of proinflammatory adhesion molecules by endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2009;296:H1675–H1682. doi: 10.1152/ajpheart.00665.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 8.Rosenzweig A. Endothelial progenitor cells. N Engl J Med. 2003;348:581–582. doi: 10.1056/NEJMp020175. [DOI] [PubMed] [Google Scholar]
- 9.Werner N, Junk S, Laufs U, Link A, Walenta K, Bohm M, Nickenig G. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res. 2003;93:e17–e24. doi: 10.1161/01.RES.0000083812.30141.74. [DOI] [PubMed] [Google Scholar]
- 10.Tepper OM, Capla JM, Galiano RD, Ceradini DJ, Callaghan MJ, Kleinman ME, Gurtner GC. Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood. 2005;105:1068–1077. doi: 10.1182/blood-2004-03-1051. [DOI] [PubMed] [Google Scholar]
- 11.Schächinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 12.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 13.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt-Lucke C, Rössig L, Fichtlscherer S, Vasa M, Britten M, Kämper U, Dimmeler S, Zeiher AM. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 15.Moreno PR, Sanz J, Fuster V. Promoting mechanisms of vascular health: circulating progenitor cells, angiogenesis, and reverse cholesterol transport. J Am Coll Cardiol. 2009;53:2315–2323. doi: 10.1016/j.jacc.2009.02.057. [DOI] [PubMed] [Google Scholar]
- 16.Pacilli A, Faggioli G, Stella A, Pasquinelli G. An update on therapeutic angiogenesis for peripheral vascular disease. Ann Vasc Surg. 2010;24:258–268. doi: 10.1016/j.avsg.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Tongers J, Roncalli JG, Losordo DW. Role of endothelial progenitor cells during ischemia-induced vasculogenesis and collateral formation. Microvasc Res. 2010;79:200–206. doi: 10.1016/j.mvr.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crosby JR, Kaminski WE, Schatteman G, Martin PJ, Raines EW, Seifert RA, Bowen-Pope DF. Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ Res. 2000;87:728–730. doi: 10.1161/01.res.87.9.728. [DOI] [PubMed] [Google Scholar]
- 19.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 20.Hoetzer GL, Van Guilder GP, Irmiger HM, Keith RS, Stauffer BL, DeSouza CA. Aging, exercise, and endothelial progenitor cell clonogenic and migratory capacity in men. J Appl Physiol. 2007;102:847–852. doi: 10.1152/japplphysiol.01183.2006. [DOI] [PubMed] [Google Scholar]
- 21.Kushner EJ, MacEneaney OJ, Weil BR, Greiner JJ, Stauffer BL, DeSouza CA. Aging is associated with a proapoptotic endothelial progenitor cell phenotype. J Vasc Res. 2011;48:408–414. doi: 10.1159/000324837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol. 2005;45:1441–1448. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 23.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, Wang T, Gregg D, Ramaswami P, Pippen AM, Annex BH, Dong C, Taylor DA. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108:457–463. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- 24.Edelberg JM, Tang L, Hattori K, Lyden D, Rafii S. Young adult bone marrow-derived endothelial precursor cells restore aging-impaired cardiac angiogenic function. Circ Res. 2002;90:E89–E93. doi: 10.1161/01.res.0000020861.20064.7e. [DOI] [PubMed] [Google Scholar]
- 25.Jujo K, Ii M, Losordo DW. Endothelial progenitor cells in neovascularization of infarcted myocardium. J Mol Cell Cardiol. 2008;45:530–544. doi: 10.1016/j.yjmcc.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sekiguchi H, Ii M, Losordo DW. The relative potency and safety of endothelial progenitor cells and unselected mononuclear cells for recovery from myocardial infarction and ischemia. J Cell Physiol. 2009;219:235–242. doi: 10.1002/jcp.21672. [DOI] [PubMed] [Google Scholar]
- 27.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Böhm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 28.Zeoli A, Dentelli P, Brizzi MF. Endothelial progenitor cells and their potential clinical implication in cardiovascular disorders. J Endocrinol Invest. 2009;32:370–382. doi: 10.1007/BF03345729. [DOI] [PubMed] [Google Scholar]
- 29.Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH, Martin H, Zeiher AM, Dimmeler S. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109:1615–1622. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 30.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 31.Assmus B, Schächinger V, Teupe C, Britten M, Lehmann R, Döbert N, Grünwald F, Aicher A, Urbich C, Martin H, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 32.Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rütten H, Fichtlscherer S, Martin H, Zeiher AM. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.George J, Herz I, Goldstein E, Abashidze S, Deutch V, Finkelstein A, Michowitz Y, Miller H, Keren G. Number and adhesive properties of circulating endothelial progenitor cells in patients with in-stent restenosis. Arterioscler Thromb Vasc Biol. 2003;23:e57–e60. doi: 10.1161/01.ATV.0000107029.65274.db. [DOI] [PubMed] [Google Scholar]
- 34.Valgimigli M, Rigolin GM, Fucili A, Porta MD, Soukhomovskaia O, Malagutti P, Bugli AM, Bragotti LZ, Francolini G, Mauro E, et al. CD34+ and endothelial progenitor cells in patients with various degrees of congestive heart failure. Circulation. 2004;110:1209–1212. doi: 10.1161/01.CIR.0000136813.89036.21. [DOI] [PubMed] [Google Scholar]
- 35.Geft D, Schwartzenberg S, Rogowsky O, Finkelstein A, Ablin J, Maysel-Auslender S, Wexler D, Keren G, George J. Circulating apoptotic progenitor cells in patients with congestive heart failure. PLoS One. 2008;3:e3238. doi: 10.1371/journal.pone.0003238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nonaka-Sarukawa M, Yamamoto K, Aoki H, Nishimura Y, Tomizawa H, Ichida M, Eizawa T, Muroi K, Ikeda U, Shimada K. Circulating endothelial progenitor cells in congestive heart failure. Int J Cardiol. 2007;119:344–348. doi: 10.1016/j.ijcard.2006.07.191. [DOI] [PubMed] [Google Scholar]
- 37.Carvalho VO, Ruiz MA, Bocchi EA, Carvalho VO, Guimarães GV. Correlation between CD34+ and exercise capacity, functional class, quality of life and norepinephrine in heart failure patients. Cardiol J. 2009;16:426–431. [PubMed] [Google Scholar]
- 38.Jahanyar J, Youker KA, Torre-Amione G, Koerner MM, Bruckner B, Noon GP, Loebe M. Increased expression of stem cell factor and its receptor after left ventricular assist device support: a potential novel target for therapeutic interventions in heart failure. J Heart Lung Transplant. 2008;27:701–709. doi: 10.1016/j.healun.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 39.Manginas A, Tsiavou A, Sfyrakis P, Giamouzis G, Tsourelis L, Leontiadis E, Degiannis D, Cokkinos DV, Alivizatos PA. Increased number of circulating progenitor cells after implantation of ventricular assist devices. J Heart Lung Transplant. 2009;28:710–717. doi: 10.1016/j.healun.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Koike M, Kojima H, Fujimiya M, Matsubayashi K, Aimi Y, Kimura H, Asai T. Transfer of bone marrow progenitors prevents coronary insufficiency and systolic dysfunction in the mechanical unloaded heart in mice. J Surg Res. 2011;171:47–57. doi: 10.1016/j.jss.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 41.Tuder RM, Cool CD, Yeager M, Taraseviciene-Stewart L, Bull TM, Voelkel NF. The pathobiology of pulmonary hypertension. Endothelium. Clin Chest Med. 2001;22:405–418. doi: 10.1016/s0272-5231(05)70280-x. [DOI] [PubMed] [Google Scholar]
- 42.Smadja DM, Gaussem P, Mauge L, Israël-Biet D, Dignat-George F, Peyrard S, Agnoletti G, Vouhé PR, Bonnet D, Lévy M. Circulating endothelial cells: a new candidate biomarker of irreversible pulmonary hypertension secondary to congenital heart disease. Circulation. 2009;119:374–381. doi: 10.1161/CIRCULATIONAHA.108.808246. [DOI] [PubMed] [Google Scholar]
- 43.Junhui Z, Xingxiang W, Guosheng F, Yunpeng S, Furong Z, Junzhu C. Reduced number and activity of circulating endothelial progenitor cells in patients with idiopathic pulmonary arterial hypertension. Respir Med. 2008;102:1073–1079. doi: 10.1016/j.rmed.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 44.Hansmann G, Plouffe BD, Hatch A, von Gise A, Sallmon H, Zamanian RT, Murthy SK. Design and validation of an endothelial progenitor cell capture chip and its application in patients with pulmonary arterial hypertension. J Mol Med (Berl) 2011;89:971–983. doi: 10.1007/s00109-011-0779-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toshner M, Voswinckel R, Southwood M, Al-Lamki R, Howard LS, Marchesan D, Yang J, Suntharalingam J, Soon E, Exley A, et al. Evidence of dysfunction of endothelial progenitors in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2009;180:780–787. doi: 10.1164/rccm.200810-1662OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smadja DM, Mauge L, Sanchez O, Silvestre JS, Guerin C, Godier A, Henno P, Gaussem P, Israël-Biet D. Distinct patterns of circulating endothelial cells in pulmonary hypertension. Eur Respir J. 2010;36:1284–1293. doi: 10.1183/09031936.00130809. [DOI] [PubMed] [Google Scholar]
- 47.Rafat N, Hanusch C, Brinkkoetter PT, Schulte J, Brade J, Zijlstra JG, van der Woude FJ, van Ackern K, Yard BA, Beck GCh. Increased circulating endothelial progenitor cells in septic patients: correlation with survival. Crit Care Med. 2007;35:1677–1684. doi: 10.1097/01.CCM.0000269034.86817.59. [DOI] [PubMed] [Google Scholar]
- 48.Burnham EL, Taylor WR, Quyyumi AA, Rojas M, Brigham KL, Moss M. Increased circulating endothelial progenitor cells are associated with survival in acute lung injury. Am J Respir Crit Care Med. 2005;172:854–860. doi: 10.1164/rccm.200410-1325OC. [DOI] [PubMed] [Google Scholar]
- 49.Mutunga M, Fulton B, Bullock R, Batchelor A, Gascoigne A, Gillespie JI, Baudouin SV. Circulating endothelial cells in patients with septic shock. Am J Respir Crit Care Med. 2001;163:195–200. doi: 10.1164/ajrccm.163.1.9912036. [DOI] [PubMed] [Google Scholar]
- 50.Tsaganos T, Giamarellos-Bourboulis EJ, Kollias S, Zervakis D, Karagianni V, Pelekanou A, Tampaki EC, Kontogiorgi M, Koroneos A, Drakoulis N, et al. Kinetics of progenitor hemopoetic stem cells in sepsis: correlation with patients survival? BMC Infect Dis. 2006;6:142. doi: 10.1186/1471-2334-6-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burnham EL, Mealer M, Gaydos J, Majka S, Moss M. Acute lung injury but not sepsis is associated with increased colony formation by peripheral blood mononuclear cells. Am J Respir Cell Mol Biol. 2010;43:326–333. doi: 10.1165/rcmb.2009-0015OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peinado VI, Ramírez J, Roca J, Rodriguez-Roisin R, Barberà JA. Identification of vascular progenitor cells in pulmonary arteries of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2006;34:257–263. doi: 10.1165/rcmb.2005-0255OC. [DOI] [PubMed] [Google Scholar]
- 53.Fadini GP, Schiavon M, Cantini M, Baesso I, Facco M, Miorin M, Tassinato M, de Kreutzenberg SV, Avogaro A, Agostini C. Circulating progenitor cells are reduced in patients with severe lung disease. Stem Cells. 2006;24:1806–1813. doi: 10.1634/stemcells.2005-0440. [DOI] [PubMed] [Google Scholar]
- 54.Palange P, Testa U, Huertas A, Calabrò L, Antonucci R, Petrucci E, Pelosi E, Pasquini L, Satta A, Morici G, et al. Circulating haemopoietic and endothelial progenitor cells are decreased in COPD. Eur Respir J. 2006;27:529–541. doi: 10.1183/09031936.06.00120604. [DOI] [PubMed] [Google Scholar]
- 55.Sala E, Villena C, Balaguer C, Ríos A, Fernández-Palomeque C, Cosío BG, García J, Noguera A, Agustí A. Abnormal levels of circulating endothelial progenitor cells during exacerbations of COPD. Lung. 2010;188:331–338. doi: 10.1007/s00408-009-9225-8. [DOI] [PubMed] [Google Scholar]
- 56.Takahashi T, Suzuki S, Kubo H, Yamaya M, Kurosawa S, Kato M. Impaired endothelial progenitor cell mobilization and colony-forming capacity in chronic obstructive pulmonary disease. Respirology. 2011;16:680–687. doi: 10.1111/j.1440-1843.2011.01959.x. [DOI] [PubMed] [Google Scholar]
- 57.Martin K, Stanchina M, Kouttab N, Harrington EO, Rounds S. Circulating endothelial cells and endothelial progenitor cells in obstructive sleep apnea. Lung. 2008;186:145–150. doi: 10.1007/s00408-008-9073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.El Solh AA, Akinnusi ME, Berim IG, Peter AM, Paasch LL, Szarpa KR. Hemostatic implications of endothelial cell apoptosis in obstructive sleep apnea. Sleep Breath. 2008;12:331–337. doi: 10.1007/s11325-008-0182-x. [DOI] [PubMed] [Google Scholar]
- 59.de la Peña M, Barceló A, Barbe F, Piérola J, Pons J, Rimbau E, Ayllón O, Agustí AG. Endothelial function and circulating endothelial progenitor cells in patients with sleep apnea syndrome. Respiration. 2008;76:28–32. doi: 10.1159/000109643. [DOI] [PubMed] [Google Scholar]
- 60.El Solh AA, Akinnusi ME, Baddoura FH, Mankowski CR. Endothelial cell apoptosis in obstructive sleep apnea: a link to endothelial dysfunction. Am J Respir Crit Care Med. 2007;175:1186–1191. doi: 10.1164/rccm.200611-1598OC. [DOI] [PubMed] [Google Scholar]
- 61.Loomans CJ, De Koning EJ, Staal FJ, Rabelink TJ, Zonneveld AJ. Endothelial progenitor cell dysfunction in type 1 diabetes: another consequence of oxidative stress? Antioxid Redox Signal. 2005;7:1468–1475. doi: 10.1089/ars.2005.7.1468. [DOI] [PubMed] [Google Scholar]
- 62.Sibal L, Aldibbiat A, Agarwal SC, Mitchell G, Oates C, Razvi S, Weaver JU, Shaw JA, Home PD. Circulating endothelial progenitor cells, endothelial function, carotid intima-media thickness and circulating markers of endothelial dysfunction in people with type 1 diabetes without macrovascular disease or microalbuminuria. Diabetologia. 2009;52:1464–1473. doi: 10.1007/s00125-009-1401-0. [DOI] [PubMed] [Google Scholar]
- 63.Fadini GP, Avogaro A. It is all in the blood: the multifaceted contribution of circulating progenitor cells in diabetic complications. Exp Diabetes Res. 2012;2012:742976. doi: 10.1155/2012/742976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 65.Reinhard H, Jacobsen PK, Lajer M, Pedersen N, Billestrup N, Mandrup-Poulsen T, Parving HH, Rossing P. Multifactorial treatment increases endothelial progenitor cells in patients with type 2 diabetes. Diabetologia. 2010;53:2129–2133. doi: 10.1007/s00125-010-1843-4. [DOI] [PubMed] [Google Scholar]
- 66.Ghani U, Shuaib A, Salam A, Nasir A, Shuaib U, Jeerakathil T, Sher F, O’Rourke F, Nasser AM, Schwindt B, et al. Endothelial progenitor cells during cerebrovascular disease. Stroke. 2005;36:151–153. doi: 10.1161/01.STR.0000149944.15406.16. [DOI] [PubMed] [Google Scholar]
- 67.Yip HK, Chang LT, Chang WN, Lu CH, Liou CW, Lan MY, Liu JS, Youssef AA, Chang HW. Level and value of circulating endothelial progenitor cells in patients after acute ischemic stroke. Stroke. 2008;39:69–74. doi: 10.1161/STROKEAHA.107.489401. [DOI] [PubMed] [Google Scholar]
- 68.Taguchi A, Matsuyama T, Moriwaki H, Hayashi T, Hayashida K, Nagatsuka K, Todo K, Mori K, Stern DM, Soma T, et al. Circulating CD34-positive cells provide an index of cerebrovascular function. Circulation. 2004;109:2972–2975. doi: 10.1161/01.CIR.0000133311.25587.DE. [DOI] [PubMed] [Google Scholar]
- 69.Chu K, Jung KH, Lee ST, Park HK, Sinn DI, Kim JM, Kim DH, Kim JH, Kim SJ, Song EC, et al. Circulating endothelial progenitor cells as a new marker of endothelial dysfunction or repair in acute stroke. Stroke. 2008;39:1441–1447. doi: 10.1161/STROKEAHA.107.499236. [DOI] [PubMed] [Google Scholar]
- 70.Sobrino T, Hurtado O, Moro MA, Rodríguez-Yáñez M, Castellanos M, Brea D, Moldes O, Blanco M, Arenillas JF, Leira R, et al. The increase of circulating endothelial progenitor cells after acute ischemic stroke is associated with good outcome. Stroke. 2007;38:2759–2764. doi: 10.1161/STROKEAHA.107.484386. [DOI] [PubMed] [Google Scholar]
- 71.Delva P, De Marchi S, Prior M, Degan M, Lechi A, Trettene M, Arosio E. Endothelial progenitor cells in patients with severe peripheral arterial disease. Endothelium. 2008;15:246–253. doi: 10.1080/10623320802487718. [DOI] [PubMed] [Google Scholar]
- 72.Morishita T, Uzui H, Nakano A, Mitsuke Y, Geshi T, Ueda T, Lee JD. Number of endothelial progenitor cells in peripheral artery disease as a marker of severity and association with pentraxin-3, malondialdehyde-modified low-density lipoprotein and membrane type-1 matrix metalloproteinase. J Atheroscler Thromb. 2012;19:149–158. doi: 10.5551/jat.10074. [DOI] [PubMed] [Google Scholar]
- 73.Mandraffino G, Sardo MA, Riggio S, Loddo S, Imbalzano E, Alibrandi A, Saitta C, Cinquegrani M, Mormina EM, Saitta A. Circulating progenitor cells are increased in newly diagnosed untreated hypertensive patients with arterial stiffening but normal carotid intima-media thickness. Hypertens Res. 2011;34:876–883. doi: 10.1038/hr.2011.56. [DOI] [PubMed] [Google Scholar]
- 74.Giannotti G, Doerries C, Mocharla PS, Mueller MF, Bahlmann FH, Horvàth T, Jiang H, Sorrentino SA, Steenken N, Manes C, et al. Impaired endothelial repair capacity of early endothelial progenitor cells in prehypertension: relation to endothelial dysfunction. Hypertension. 2010;55:1389–1397. doi: 10.1161/HYPERTENSIONAHA.109.141614. [DOI] [PubMed] [Google Scholar]
- 75.Müller-Ehmsen J, Braun D, Schneider T, Pfister R, Worm N, Wielckens K, Scheid C, Frommolt P, Flesch M. Decreased number of circulating progenitor cells in obesity: beneficial effects of weight reduction. Eur Heart J. 2008;29:1560–1568. doi: 10.1093/eurheartj/ehn213. [DOI] [PubMed] [Google Scholar]
- 76.MacEneaney OJ, Kushner EJ, Van Guilder GP, Greiner JJ, Stauffer BL, DeSouza CA. Endothelial progenitor cell number and colony-forming capacity in overweight and obese adults. Int J Obes (Lond) 2009;33:219–225. doi: 10.1038/ijo.2008.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tobler K, Freudenthaler A, Baumgartner-Parzer SM, Wolzt M, Ludvik B, Nansalmaa E, Nowotny PJ, Seidinger D, Steiner S, Luger A, et al. Reduction of both number and proliferative activity of human endothelial progenitor cells in obesity. Int J Obes (Lond) 2010;34:687–700. doi: 10.1038/ijo.2009.280. [DOI] [PubMed] [Google Scholar]
- 78.MacEneaney OJ, Kushner EJ, Westby CM, Cech JN, Greiner JJ, Stauffer BL, DeSouza CA. Endothelial progenitor cell function, apoptosis, and telomere length in overweight/obese humans. Obesity (Silver Spring) 2010;18:1677–1682. doi: 10.1038/oby.2009.494. [DOI] [PubMed] [Google Scholar]
- 79.Heida NM, Müller JP, Cheng IF, Leifheit-Nestler M, Faustin V, Riggert J, Hasenfuss G, Konstantinides S, Schäfer K. Effects of obesity and weight loss on the functional properties of early outgrowth endothelial progenitor cells. J Am Coll Cardiol. 2010;55:357–367. doi: 10.1016/j.jacc.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 80.Hambrecht R, Niebauer J, Marburger C, Grunze M, Kälberer B, Hauer K, Schlierf G, Kübler W, Schuler G. Various intensities of leisure time physical activity in patients with coronary artery disease: effects on cardiorespiratory fitness and progression of coronary atherosclerotic lesions. J Am Coll Cardiol. 1993;22:468–477. doi: 10.1016/0735-1097(93)90051-2. [DOI] [PubMed] [Google Scholar]
- 81.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 82.Kozakova M, Palombo C, Mhamdi L, Konrad T, Nilsson P, Staehr PB, Paterni M, Balkau B. Habitual physical activity and vascular aging in a young to middle-age population at low cardiovascular risk. Stroke. 2007;38:2549–2555. doi: 10.1161/STROKEAHA.107.484949. [DOI] [PubMed] [Google Scholar]
- 83.Seals DR, Desouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol. 2008;105:1323–1332. doi: 10.1152/japplphysiol.90553.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kojda G, Cheng YC, Burchfield J, Harrison DG. Dysfunctional regulation of endothelial nitric oxide synthase (eNOS) expression in response to exercise in mice lacking one eNOS gene. Circulation. 2001;103:2839–2844. doi: 10.1161/01.cir.103.23.2839. [DOI] [PubMed] [Google Scholar]
- 85.Jasperse JL, Laughlin MH. Endothelial function and exercise training: evidence from studies using animal models. Med Sci Sports Exerc. 2006;38:445–454. doi: 10.1249/01.mss.0000191187.24525.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Laughlin MH. Joseph B. Wolfe Memorial lecture. Physical activity in prevention and treatment of coronary disease: the battle line is in exercise vascular cell biology. Med Sci Sports Exerc. 2004;36:352–362. doi: 10.1249/01.mss.0000117114.02875.5c. [DOI] [PubMed] [Google Scholar]
- 87.Hambrecht R, Walther C, Möbius-Winkler S, Gielen S, Linke A, Conradi K, Erbs S, Kluge R, Kendziorra K, Sabri O, et al. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: a randomized trial. Circulation. 2004;109:1371–1378. doi: 10.1161/01.CIR.0000121360.31954.1F. [DOI] [PubMed] [Google Scholar]
- 88.Belardinelli R, Paolini I, Cianci G, Piva R, Georgiou D, Purcaro A. Exercise training intervention after coronary angioplasty: the ETICA trial. J Am Coll Cardiol. 2001;37:1891–1900. doi: 10.1016/s0735-1097(01)01236-0. [DOI] [PubMed] [Google Scholar]
- 89.Hornig B, Maier V, Drexler H. Physical training improves endothelial function in patients with chronic heart failure. Circulation. 1996;93:210–214. doi: 10.1161/01.cir.93.2.210. [DOI] [PubMed] [Google Scholar]
- 90.Stewart KJ, Hiatt WR, Regensteiner JG, Hirsch AT. Exercise training for claudication. N Engl J Med. 2002;347:1941–1951. doi: 10.1056/NEJMra021135. [DOI] [PubMed] [Google Scholar]
- 91.Stewart KJ. Exercise training and the cardiovascular consequences of type 2 diabetes and hypertension: plausible mechanisms for improving cardiovascular health. JAMA. 2002;288:1622–1631. doi: 10.1001/jama.288.13.1622. [DOI] [PubMed] [Google Scholar]
- 92.Wannamethee SG, Lowe GD, Whincup PH, Rumley A, Walker M, Lennon L. Physical activity and hemostatic and inflammatory variables in elderly men. Circulation. 2002;105:1785–1790. doi: 10.1161/hc1502.107117. [DOI] [PubMed] [Google Scholar]
- 93.Tjønna AE, Lee SJ, Rognmo Ø, Stølen TO, Bye A, Haram PM, Loennechen JP, Al-Share QY, Skogvoll E, Slørdahl SA, et al. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118:346–354. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moholdt T, Wisløff U, Nilsen TI, Slørdahl SA. Physical activity and mortality in men and women with coronary heart disease: a prospective population-based cohort study in Norway (the HUNT study) Eur J Cardiovasc Prev Rehabil. 2008;15:639–645. doi: 10.1097/HJR.0b013e3283101671. [DOI] [PubMed] [Google Scholar]
- 95.Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, Tjønna AE, Helgerud J, Slørdahl SA, Lee SJ, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115:3086–3094. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 96.Dimopoulos S, Anastasiou-Nana M, Sakellariou D, Drakos S, Kapsimalakou S, Maroulidis G, Roditis P, Papazachou O, Vogiatzis I, Roussos C, et al. Effects of exercise rehabilitation program on heart rate recovery in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2006;13:67–73. doi: 10.1097/01.hjr.0000198449.20775.7c. [DOI] [PubMed] [Google Scholar]
- 97.Meyer K, Foster C, Georgakopoulos N, Hajric R, Westbrook S, Ellestad A, Tilman K, Fitzgerald D, Young H, Weinstein H, et al. Comparison of left ventricular function during interval versus steady-state exercise training in patients with chronic congestive heart failure. Am J Cardiol. 1998;82:1382–1387. doi: 10.1016/s0002-9149(98)00646-8. [DOI] [PubMed] [Google Scholar]
- 98.Beckers PJ, Denollet J, Possemiers NM, Wuyts FL, Vrints CJ, Conraads VM. Combined endurance-resistance training vs. endurance training in patients with chronic heart failure: a prospective randomized study. Eur Heart J. 2008;29:1858–1866. doi: 10.1093/eurheartj/ehn222. [DOI] [PubMed] [Google Scholar]
- 99.Anagnostakou V, Chatzimichail K, Dimopoulos S, Karatzanos E, Papazachou O, Tasoulis A, Anastasiou-Nana M, Roussos C, Nanas S. Effects of interval cycle training with or without strength training on vascular reactivity in heart failure patients. J Card Fail. 2011;17:585–591. doi: 10.1016/j.cardfail.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 100.Gerovasili V, Drakos S, Kravari M, Malliaras K, Karatzanos E, Dimopoulos S, Tasoulis A, Anastasiou-Nana M, Roussos C, Nanas S. Physical exercise improves the peripheral microcirculation of patients with chronic heart failure. J Cardiopulm Rehabil Prev. 2009;29:385–391. doi: 10.1097/HCR.0b013e3181b4ca4e. [DOI] [PubMed] [Google Scholar]
- 101.Laufs U, Werner N, Link A, Endres M, Wassmann S, Jürgens K, Miche E, Böhm M, Nickenig G. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004;109:220–226. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]
- 102.Yang Z, Xia WH, Su C, Wu F, Zhang YY, Xu SY, Liu X, Zhang XY, Ou ZJ, Lai GH, et al. Regular exercise-induced increased number and activity of circulating endothelial progenitor cells attenuates age-related decline in arterial elasticity in healthy men. Int J Cardiol. 2011:Sep 26; Epub ahead of print. doi: 10.1016/j.ijcard.2011.08.055. [DOI] [PubMed] [Google Scholar]
- 103.Rakobowchuk M, Harris E, Taylor A, Baliga V, Cubbon RM, Rossiter HB, Birch KM. Heavy and moderate interval exercise training alters low-flow-mediated constriction but does not increase circulating progenitor cells in healthy humans. Exp Physiol. 2012;97:375–385. doi: 10.1113/expphysiol.2011.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Walther C, Gaede L, Adams V, Gelbrich G, Leichtle A, Erbs S, Sonnabend M, Fikenzer K, Körner A, Kiess W, et al. Effect of increased exercise in school children on physical fitness and endothelial progenitor cells: a prospective randomized trial. Circulation. 2009;120:2251–2259. doi: 10.1161/CIRCULATIONAHA.109.865808. [DOI] [PubMed] [Google Scholar]
- 105.Witkowski S, Lockard MM, Jenkins NT, Obisesan TO, Spangenburg EE, Hagberg JM. Relationship between circulating progenitor cells, vascular function and oxidative stress with long-term training and short-term detraining in older men. Clin Sci (Lond) 2010;118:303–311. doi: 10.1042/CS20090253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Haram PM, Kemi OJ, Wisloff U. Adaptation of endothelium to exercise training: insights from experimental studies. Front Biosci. 2008;13:336–346. doi: 10.2741/2683. [DOI] [PubMed] [Google Scholar]
- 107.Ajijola OA, Dong C, Herderick EE, Ma Q, Goldschmidt-Clermont PJ, Yan Z. Voluntary running suppresses proinflammatory cytokines and bone marrow endothelial progenitor cell levels in apolipoprotein-E-deficient mice. Antioxid Redox Signal. 2009;11:15–23. doi: 10.1089/ars.2008.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Itoh T, Imano M, Nishida S, Tsubaki M, Hashimoto S, Ito A, Satou T. Exercise increases neural stem cell proliferation surrounding the area of damage following rat traumatic brain injury. J Neural Transm. 2011;118:193–202. doi: 10.1007/s00702-010-0495-3. [DOI] [PubMed] [Google Scholar]
- 109.Luk TH, Dai YL, Siu CW, Yiu KH, Chan HT, Lee SW, Li SW, Fong B, Wong WK, Tam S, et al. Effect of exercise training on vascular endothelial function in patients with stable coronary artery disease: a randomized controlled trial. Eur J Prev Cardiol. 2012;19:830–839. doi: 10.1177/1741826711415679. [DOI] [PubMed] [Google Scholar]
- 110.Cesari F, Marcucci R, Gori AM, Burgisser C, Francini S, Sofi F, Gensini GF, Abbate R, Fattirolli F. Impact of a cardiac rehabilitation program and inflammatory state on endothelial progenitor cells in acute coronary syndrome patients. Int J Cardiol. 2012:May 22; Epub ahead of print. doi: 10.1016/j.ijcard.2012.04.157. [DOI] [PubMed] [Google Scholar]
- 111.Manfredini F, Rigolin GM, Malagoni AM, Catizone L, Mandini S, Sofritti O, Mauro E, Soffritti S, Boari B, Cuneo A, et al. Exercise training and endothelial progenitor cells in haemodialysis patients. J Int Med Res. 2009;37:534–540. doi: 10.1177/147323000903700229. [DOI] [PubMed] [Google Scholar]
- 112.Sarto P, Balducci E, Balconi G, Fiordaliso F, Merlo L, Tuzzato G, Pappagallo GL, Frigato N, Zanocco A, Forestieri C, et al. Effects of exercise training on endothelial progenitor cells in patients with chronic heart failure. J Card Fail. 2007;13:701–708. doi: 10.1016/j.cardfail.2007.06.722. [DOI] [PubMed] [Google Scholar]
- 113.Van Craenenbroeck EM, Hoymans VY, Beckers PJ, Possemiers NM, Wuyts K, Paelinck BP, Vrints CJ, Conraads VM. Exercise training improves function of circulating angiogenic cells in patients with chronic heart failure. Basic Res Cardiol. 2010;105:665–676. doi: 10.1007/s00395-010-0105-4. [DOI] [PubMed] [Google Scholar]
- 114.Gatta L, Armani A, Iellamo F, Consoli C, Molinari F, Caminiti G, Volterrani M, Rosano GM. Effects of a short-term exercise training on serum factors involved in ventricular remodelling in chronic heart failure patients. Int J Cardiol. 2012;155:409–413. doi: 10.1016/j.ijcard.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 115.Erbs S, Höllriegel R, Linke A, Beck EB, Adams V, Gielen S, Möbius-Winkler S, Sandri M, Kränkel N, Hambrecht R, et al. Exercise training in patients with advanced chronic heart failure (NYHA IIIb) promotes restoration of peripheral vasomotor function, induction of endogenous regeneration, and improvement of left ventricular function. Circ Heart Fail. 2010;3:486–494. doi: 10.1161/CIRCHEARTFAILURE.109.868992. [DOI] [PubMed] [Google Scholar]
- 116.Mezzani A, Grassi B, Jones AM, Giordano A, Corrà U, Porcelli S, Della Bella S, Taddeo A, Giannuzzi P. Speeding of pulmonary VO(2) on-kinetics by light-to-moderate-intensity aerobic exercise training in chronic heart failure: Clinical and pathophysiological correlates. Int J Cardiol. 2012:Jun 15; Epub ahead of print. doi: 10.1016/j.ijcard.2012.05.124. [DOI] [PubMed] [Google Scholar]
- 117.Schlager O, Giurgea A, Schuhfried O, Seidinger D, Hammer A, Gröger M, Fialka-Moser V, Gschwandtner M, Koppensteiner R, Steiner S. Exercise training increases endothelial progenitor cells and decreases asymmetric dimethylarginine in peripheral arterial disease: a randomized controlled trial. Atherosclerosis. 2011;217:240–248. doi: 10.1016/j.atherosclerosis.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 118.Cesari F, Sofi F, Gori AM, Corsani I, Capalbo A, Caporale R, Abbate R, Gensini GF, Casini A. Physical activity and circulating endothelial progenitor cells: an intervention study. Eur J Clin Invest. 2012;42:927–932. doi: 10.1111/j.1365-2362.2012.02670.x. [DOI] [PubMed] [Google Scholar]
- 119.Fukai T, Siegfried MR, Ushio-Fukai M, Cheng Y, Kojda G, Harrison DG. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J Clin Invest. 2000;105:1631–1639. doi: 10.1172/JCI9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rehman J, Li J, Parvathaneni L, Karlsson G, Panchal VR, Temm CJ, Mahenthiran J, March KL. Exercise acutely increases circulating endothelial progenitor cells and monocyte-/macrophage-derived angiogenic cells. J Am Coll Cardiol. 2004;43:2314–2318. doi: 10.1016/j.jacc.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 121.Yang Z, Wang JM, Chen L, Luo CF, Tang AL, Tao J. Acute exercise-induced nitric oxide production contributes to upregulation of circulating endothelial progenitor cells in healthy subjects. J Hum Hypertens. 2007;21:452–460. doi: 10.1038/sj.jhh.1002171. [DOI] [PubMed] [Google Scholar]
- 122.Thorell D, Borjesson M, Larsson P, Ulfhammer E, Karlsson L, DuttaRoy S. Strenuous exercise increases late outgrowth endothelial cells in healthy subjects. Eur J Appl Physiol. 2009;107:481–488. doi: 10.1007/s00421-009-1144-0. [DOI] [PubMed] [Google Scholar]
- 123.Möbius-Winkler S, Hilberg T, Menzel K, Golla E, Burman A, Schuler G, Adams V. Time-dependent mobilization of circulating progenitor cells during strenuous exercise in healthy individuals. J Appl Physiol. 2009;107:1943–1950. doi: 10.1152/japplphysiol.00532.2009. [DOI] [PubMed] [Google Scholar]
- 124.Lockard MM, Witkowski S, Jenkins NT, Spangenburg EE, Obisesan TO, Hagberg JM. Thrombin and exercise similarly influence expression of cell cycle genes in cultured putative endothelial progenitor cells. J Appl Physiol. 2010;108:1682–1690. doi: 10.1152/japplphysiol.00884.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Van Craenenbroeck EM, Vrints CJ, Haine SE, Vermeulen K, Goovaerts I, Van Tendeloo VF, Hoymans VY, Conraads VM. A maximal exercise bout increases the number of circulating CD34+/KDR+ endothelial progenitor cells in healthy subjects. Relation with lipid profile. J Appl Physiol. 2008;104:1006–1013. doi: 10.1152/japplphysiol.01210.2007. [DOI] [PubMed] [Google Scholar]
- 126.Laufs U, Urhausen A, Werner N, Scharhag J, Heitz A, Kissner G, Böhm M, Kindermann W, Nickenig G. Running exercise of different duration and intensity: effect on endothelial progenitor cells in healthy subjects. Eur J Cardiovasc Prev Rehabil. 2005;12:407–414. doi: 10.1097/01.hjr.0000174823.87269.2e. [DOI] [PubMed] [Google Scholar]
- 127.Adams V, Linke A, Breuckmann F, Leineweber K, Erbs S, Kränkel N, Bröcker-Preuss M, Woitek F, Erbel R, Heusch G, et al. Circulating progenitor cells decrease immediately after marathon race in advanced-age marathon runners. Eur J Cardiovasc Prev Rehabil. 2008;15:602–607. doi: 10.1097/HJR.0b013e328309c756. [DOI] [PubMed] [Google Scholar]
- 128.Bonsignore MR, Morici G, Riccioni R, Huertas A, Petrucci E, Veca M, Mariani G, Bonanno A, Chimenti L, Gioia M, et al. Hemopoietic and angiogenetic progenitors in healthy athletes: different responses to endurance and maximal exercise. J Appl Physiol. 2010;109:60–67. doi: 10.1152/japplphysiol.01344.2009. [DOI] [PubMed] [Google Scholar]
- 129.Goussetis E, Spiropoulos A, Tsironi M, Skenderi K, Margeli A, Graphakos S, Baltopoulos P, Papassotiriou I. Spartathlon, a 246 kilometer foot race: effects of acute inflammation induced by prolonged exercise on circulating progenitor reparative cells. Blood Cells Mol Dis. 2009;42:294–299. doi: 10.1016/j.bcmd.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 130.Shaffer RG, Greene S, Arshi A, Supple G, Bantly A, Moore JS, Parmacek MS, Mohler ER. Effect of acute exercise on endothelial progenitor cells in patients with peripheral arterial disease. Vasc Med. 2006;11:219–226. doi: 10.1177/1358863x06072213. [DOI] [PubMed] [Google Scholar]
- 131.Van Craenenbroeck EM, Beckers PJ, Possemiers NM, Wuyts K, Frederix G, Hoymans VY, Wuyts F, Paelinck BP, Vrints CJ, Conraads VM. Exercise acutely reverses dysfunction of circulating angiogenic cells in chronic heart failure. Eur Heart J. 2010;31:1924–1934. doi: 10.1093/eurheartj/ehq058. [DOI] [PubMed] [Google Scholar]
- 132.Van Craenenbroeck EM, Bruyndonckx L, Van Berckelaer C, Hoymans VY, Vrints CJ, Conraads VM. The effect of acute exercise on endothelial progenitor cells is attenuated in chronic heart failure. Eur J Appl Physiol. 2011;111:2375–2379. doi: 10.1007/s00421-011-1843-1. [DOI] [PubMed] [Google Scholar]
- 133.Sandri M, Adams V, Gielen S, Linke A, Lenk K, Kränkel N, Lenz D, Erbs S, Scheinert D, Mohr FW, et al. Effects of exercise and ischemia on mobilization and functional activation of blood-derived progenitor cells in patients with ischemic syndromes: results of 3 randomized studies. Circulation. 2005;111:3391–3399. doi: 10.1161/CIRCULATIONAHA.104.527135. [DOI] [PubMed] [Google Scholar]


