Abstract
Several non-β-lactam compounds were active against various gram-positive and gram-negative bacterial strains. The MICs of arylalkylidene rhodanines and arylalkylidene iminothiazolidin-4-ones were lower than those of ampicillin and cefotaxime for methicillin-resistant Staphylococcus aureus MI339 and vancomycin-resistant Enterococcus faecium EF12. Several compounds were found to inhibit the cell wall synthesis of S. aureus and the last two steps of peptidoglycan biosynthesis catalyzed by ether-treated cells of Escherichia coli or cell wall membrane preparations of Bacillus megaterium. The effects of the arylalkylidene rhodanines and arylalkylidene iminothiazolidin-4-one derivatives on E. coli PBP 3 and PBP 5, Streptococcus pneumoniae PBP 2xS (PBP 2x from a penicillin-sensitive strain) and PBP 2xR (PBP 2x from a penicillin-resistant strain), low-affinity PBP 2a of S. aureus, and the Actinomadura sp. strain R39 and Streptomyces sp. strain R61 dd-peptidases were studied. Some of the compounds exhibited inhibitory activities in the 10 to 100 μM concentration range. The inhibition of PBP 2xS by several of them appeared to be noncompetitive. The dissociation constant for the best inhibitor (Ki = 10 μM) was not influenced by the presence of the substrate.
The emergence of bacterial strains resistant to present antibiotics highlights the need for new antibacterial compounds (4, 12, 17). The last two steps of peptidoglycan biosynthesis are particularly attractive targets for potential antibacterial compounds since they take place on the external surface of the cytoplasmic membrane and are therefore readily accessible and peptidoglycan is specific to prokaryotic cells. Moenomycin (5), a competitive inhibitor of the transglycosylation reaction, and inactivators of the transpeptidation reaction (β-lactam antibiotics) (12, 13) have been studied in detail over the years. Furthermore, several enzymes of this biosynthetic pathway have been isolated and described. These have mainly been the dd-transpeptidases, also called penicillin-binding proteins (PBPs), which catalyze the last step of peptidoglycan biosynthesis (13).
Several PBPs from Escherichia coli have been isolated, and their roles in peptidoglycan biosynthesis and cell division have been investigated. Among the high-molecular mass PBPs from E. coli, bifunctional enzymes such as PBP 1b exhibit both transglycosylase and transpeptidase activities (29), while monofunctional enzymes such as PBP 3 (2, 3, 18) behave only as transpeptidases. The low-molecular mass PBPs, such as PBP 4 and PBP 5 (31) as well as PBP 6 (13), are responsible for most of the carboxypeptidase activity. These enzymes appear to be involved in maintaining the correct balance between the various precursors in such a way that cells can elongate or divide.
Bacterial strains have developed different strategies to escape the lethal actions of antibiotics. The targets can be modified in a way that allows them to retain their physiological activity but decreases their sensitivity to the aggressor. Various intrinsic penicillin-resistant strains have thus been isolated (22, 27). Another possibility is the protection afforded by efficient permeability barriers or the destruction of the antibiotics. Many β-lactamases have been isolated in the past, and new enzymes which are responsible for important clinical problems are continuously detected (26). Although class A β-lactamases can be inactivated by clavulanic acid, sulbactam, and tazobactam, no efficient inhibitors of the members of the three other classes (classes B, C, and D) are available. Recently, the inhibition of class C β-lactamases by rhodanines similar to those used in this study has been described (19). In the present work, we have shown that several non-β-lactam compounds, arylalkylidene rhodanines and arylalkylidene iminothiazolidin-4-ones (Fig. 1), can interfere with bacterial growth. Their effects on peptidoglycan biosynthesis in vitro and on the activities of several dd-peptidases and PBPs were studied.
FIG. 1.
Structures of the arylalkylidene rhodanines studied (a) and the arylalkylidene iminothiazolidin-4-ones (b) studied. OMe, methoxy.
MATERIALS AND METHODS
Chemicals
All compounds tested (Fig. 1) were identified and provided by Procter & Gamble Pharmaceuticals through high-throughput screening and medicinal chemistry efforts. The discovery and preparation of these compounds will be reported separately. UDP-MurNAc-pentapeptide was a gift from M. Nguyen-Distèche (University of Liège, Liège, Belgium). Fluorescein-labeled ampicillin (Flu-AMP) was given by M. Galleni (University of Liège, Liège, Belgium). The thiolesters S2c and S2d were synthesized as described by Adam et al. (1). UDP-GlcNAc and cephalexin were purchased from Sigma (St. Louis, Mo.). Uridine-diphospho-N-acetyl-d-[14C]glucosamine was supplied by Amersham Biosciences Europe (Roosendaal, The Netherlands). 4,4′-Dithiodipyridine was from Acros Organics (Springfield, N.J.). All buffer materials were reagent grade.
Enzymes
PBP 2xS (PBP 2x from a penicillin-sensitive strain of Streptococcus pneumoniae) and PBP 2xR (PBP 2x from a penicillin-resistant strain of S. pneumoniae) were purified previously (20, 25). PBP 3 from E. coli was given to us by C. Fraipont (University of Liège). PBP 5 from E. coli was a gift from J.-M. Wilkin. The R61 and R39 dd-peptidases were purified as described by Granier et al. (18).
Testing of antibacterial activities
Bacterial strains were obtained from the American Type Culture Collection (Manassas, Va.) or were recent clinical isolates. MICs were determined as follows: twofold serial dilutions of the test compounds were prepared in 100 μl of cation-adjusted Mueller-Hinton broth in each well of 96-well microtiter plates. Bacterial inocula of ∼5 × 105 CFU/ml were delivered to each well. Organisms were incubated at 37°C for 20 to 24 h before visual determination of the end point of no bacterial growth in the wells.
Cell wall synthesis assay
Cell wall synthesis was tested by monitoring the incorporation of [3H]glycine into the cell wall of Staphylococcus aureus. The test compounds were diluted in cation-adjusted Mueller-Hinton broth as 10-point dose-response curves in 96-well microtiter plates. A penicillin-sensitive strain of S. aureus (strain Mi246) was grown to an A600 of 0.05 to 0.08, and the cells were then treated with tetracycline to block protein biosynthesis. Subsequently, this cell suspension was added to 96-well microtiter plates containing serial dilutions of the test compounds. The S. aureus cells were incubated in the presence of inhibitors and [3H]glycine for 30 min at 37°C. The cell wall synthesis reaction was stopped by the addition of 15% trichloroacetic acid, which caused lysis of the cells and precipitation of the cellular macromolecules. Unbound radiolabel was removed by filtering and subsequent washing of the filter plates twice with 5% trichloroacetic acid and once with 100% ethanol. Scintillant was then added to each well and the plates were subjected to scintillation counting on a Packard Topcount counter. The raw counts per minute data were then imported into an Excel spreadsheet for analysis. Percent inhibition values were calculated by comparing the counts per minute of the wells with the test compounds to the average counts per minute for the negative control (0% inhibition) and the background control. Regression analysis was used to calculate the concentration at which cell wall synthesis was inhibited by 50% (IC50).
Inhibition of peptidoglycan biosynthesis
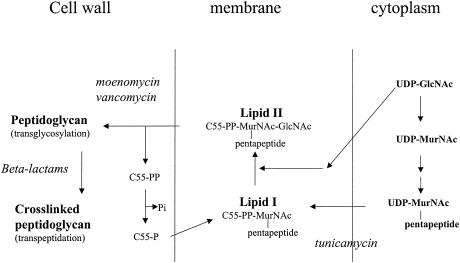
The inhibition of peptidoglycan biosynthesis was determined by starting with the synthesis of the lipid intermediates from UDP-MurNAc-pentapeptide and UDP-GlcNAc (Fig. 2). For the in vitro experiments, Bacillus megaterium membranes and ether-treated E. coli cells were used as the enzyme sources and undecaprenol phosphate was used as the substrate.
FIG. 2.
Synthesis of peptidoglycan. UDP-[14C]GlcNAc and UDP-MurNAc-pentapeptide were used as substrates for the in vitro synthesis of peptidoglycan with B. megaterium membranes and ether-treated E. coli cells.
(i) In vitro experiments with B. megaterium membranes
B. megaterium was grown at 37°C to an A600 of 1.2 in 250 ml of medium, and the cells were harvested and washed as described by Broetz et al. (7). The cells were resuspended in 15 ml of 50 mM Tris-HCl buffer (pH 7.8) containing 10 mM MgCl2. The resuspended cells were broken with 15 ml of glass beads (diameter, 0.1 mm) in a BeadBeater (Biospec Products Inc.). The cells were broken five times for 30 s each time on ice. The runs were performed at 1-min intervals in order to cool the solution. After the disruption the glass beads were washed twice with 5 ml of buffer. The membranes were separated from the buffer by centrifugation (6,000 × g, 10 min, 4°C). The pellet was washed with 7 ml of buffer, and the final precipitate was resuspended in 2 ml of buffer. A protein concentration of 0.8 mg/ml was detected, as determined by the method described by Bradford (6).
Membrane suspensions (20 μl) were incubated in the presence of 0.4 mM UDP-MurNAc-pentapeptide and 0.4 μM UDP-GlcNAc (2 μCi/μmol) in 50 mM Tris-HCl (pH 7.8)-10 mM MgCl2 at 25°C for 1.5 h (total volume, 30 μl), as described by Broetz et al. (7). In the inhibition experiments antibiotics were added at a concentration of 100 μg/ml. The reaction was stopped by heating the sample to 100°C for 1 min. The substrates and the reaction products were separated by paper chromatography, as described by Terrak et al. (29). A total of 20 μl of the assay solution was deposited on the paper. A Bio-Rad FX molecular imager was used for detection.
(ii) In vitro experiments with ether-treated E. coli cells
E. coli DH5α was grown in 250 ml of Luria-Bertani medium at 37°C to an A600 of 0.7. Ether-permeabilized E. coli cells were prepared as described by Vosberg and Hoffmann-Berling (30). A 7.2-ml suspension of ether-permeabilized E. coli cells was stored at −20°C.
A total of 40 μl of the ether-permeabilized E. coli cells was incubated at 30°C with 0.05 mM UDP-MurNAc-pentapeptide and 0.05 μM UDP-GlcNAc (2 μCi/μmol) in 50 mM Tris-HCl (pH 8.3)-MgCl2-50 mM NH4Cl-5% dimethyl sulfoxide-0.5 mM mercaptoethanol, as described by Ge et al. (17). In the inhibition experiments the antibiotics were added at concentrations of 100 μg/ml. The reaction was stopped by centrifugation (4,500 × g, Eppendorf centrifuge, 8 min). The pellet was resuspended in 20 μl of 4% sodium dodecyl sulfate and heated at 100°C for 15 min. A total of 20 μl of the suspension was deposited on the paper, and paper chromatography was performed as described above.
Inhibition of PBP 2a
The PBP 2a gene was cloned from methicillin-resistant S. aureus (MRSA) strain MI339 (24). The recombinant PBP 2a gene was overexpressed in E. coli and purified. The IC50s for PBP 2a were determined by a competitive binding assay with [3H]penicillin G as the reporter and a streptavidin binding membrane as the substrate capture surface (B. D. Keck, R. A. Reilman, and W.-P. Lu, unpublished data). The intensities of binding of [3H]penicillin G to the PBPs were quantified by use of phosphor imaging technology.
Inhibition of S. pneumoniae PBP 2xR
The inhibitors were studied in a competition experiment with Flu-AMP, whose properties have been described by Lakaye et al. (23). The assay system was that of Galleni et al. (16). The enzyme and the substrate concentrations were chosen so that the time course of PBP labeling by Flu-AMP was linear, and the decrease in the rate of labeling was determined with increasing concentrations of inhibitors. A control experiment was performed with benzylpenicillin.
The accumulation of labeled protein was linear for about 20 min when 0.8 μM PBP 2xR and 10 μM Flu-AMP were incubated at 37°C in 10 mM Tris-HCl with 100 mM NaCl (pH 7.5). In the inhibition experiments the inhibitor concentrations were in the 1 to 100 μM range.
The reaction was stopped by the addition of 12.5 μl of denaturation buffer, as described by Galleni et al. (16), and the sample was heated at 100°C for 1 min. A total of 20 μl of the assay mixture was loaded onto a 12% acrylamide-sodium dodecyl sulfate gel (9 by 7 cm). A Bio-Rad FX molecular imager was used for detection.
Inhibition of S. pneumoniae PBP 2xS
The residual activity of 0.2 μM PBP 2xS was determined after preincubation for 20 min at 37°C in the presence of 20 or 50 μM inhibitor in 10 mM sodium phosphate (pH 7.0). The initial rate of hydrolysis of 1 mM S2d thiolester in the presence of 2 mM 4,4′-dithiodipyridine was determined by monitoring the increase in the absorbance at 324 nm (change in ɛ [Δɛ] = 20,000 M−1 cm−1) (Fig. 3). The rate of spontaneous hydrolysis of S2d was also determined in the absence of the enzyme.
FIG. 3.
Thioesterase activities of PBPs. The hydrolysis of thioesters S2d and S2c is shown and can be directly monitored at 250 nm. The test becomes more sensitive by the addition of 4,4′-dithiodipyridine, which reacts with the free SH group of the product.
The inhibition of PBP 2xS by arylalkylidene rhodanine derivatives 2 and 3, arylalkylidene iminothiazolidin-4-one derivative 6, and cephalexin was studied directly by the reporter substrate method in the presence of the S2c thiolester. The first-order rate constant (ki) was determined in the presence of different substrate concentrations and a fixed inhibitor concentration. The spontaneous hydrolysis of the substrate was linear for up to 30 min and had no influence on the ki value. In all cases the level of substrate utilization was less than 10%. The rate of 0.32 μM PBP 2xS inactivation was measured at 37°C in 10 mM sodium phosphate (pH 7.0) in the presence of 1.2 mM 4,4′-dithiodipyridine and different concentrations of S2c. The increase in the absorbance at 324 nm was measured with a Uvikon 860 spectrometer connected to a microcomputer, and the kinetic parameters were computed by analyzing the complete time courses (10).
Steady-state rates of hydrolysis (vss) were also measured with arylalkylidene rhodanine derivative 2. The time courses of S2c hydrolysis, as shown in Fig. 4, were characterized by a burst followed by the steady state after about 20 min. The ki values were determined by use of the data obtained during the burst; and the vss values were analyzed with the help of the general equation for reversible inhibition
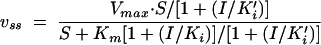 |
where S is the substrate concentration, ki is the dissociation constant of EI and ki′ is that of ESI, and I is the inhibitor concentration, and the program GRAFIT.
FIG. 4.
Time courses of S2c hydrolysis in the presence and absence of 20 μM arylalkylidene rhodanine derivative 2 (I) catalyzed by 0.2 μM PBP 2xS. v0, control hydrolysis rate; vi,0, initial hydolysis rate in the presence of I.
Inhibition of E. coli PBP 3
The residual activity of 1.1 μM PBP 3 after 20 of min preincubation at 37°C in the presence of 20 or 50 μM inhibitor in 0.5 mM Tris and 10 mM sodium phosphate buffer (pH 7.4) containing 10% (vol/vol) dimethyl sulfoxide and 0.5 M NaCl was determined by monitoring the initial rate of hydrolysis of 2 mM S2d thiolester in the presence of 1.2 mM 4,4′-dithiodipyridine.
Inhibition of E. coli PBP 5
The residual activity of 0.8 μM PBP 5 after a 20-min preincubation at 37°C in the presence of 20 or 50 μM inhibitor in 10 mM morpholinepropanesulfonic acid-NaOH buffer (pH 7.0) was determined by monitoring the initial rate of hydrolysis of 0.5 mM S2d thiolester. The decrease in absorbance was monitored at 250 nm (Δɛ = −2,200 M−1cm−1).
Inhibition of Streptomyces sp. strain R61 and Actinomadura sp. strain R39 dd-peptidases
The enzymes were preincubated with 20 or 50 μM inhibitor in 10 mM sodium phosphate (3.4 μM R61) or 10 mM sodium phosphate containing 100 mM NaCl (0.7 μM R39). After 20 min at 37°C in a total volume of 30 μl the tripeptide substrate Nα,Nɛ-diacetyl-l-lysyl-d-alanyl-d-alanine was added at a final concentration of 10 mM (for strain R61) or 7 mM (for strain R39). After 15 min at 37°C, the reactions were stopped by addition of 10 μl of 100 μM penicillin G. The d-alanine produced was quantified with the help of the d-amino acid-oxidase test (18).
RESULTS AND DISCUSSION
Effects of inhibitors on cell growth
The in vivo activities of the arylalkylidene rhodanines and arylalkylidene iminothiazolidin-4-ones were evaluated against six gram-positive bacteria, including MRSA MI339, penicillin-resistant S. pneumoniae (PRSP) STP51, and vancomycin-resistant Enterococcus faecium (VRE) EF12, and three gram-negative strains. The compounds were active against several gram-positive and gram-negative, nonresistant strains, S. aureus ATCC 29213, Enterococcus faecalis ATCC 29212, Moraxella catarrhalis BC2, and Haemophilus influenzae HI26. The MICs are summarized in Table 1. Furthermore, no activity against S. pneumoniae ATCC 6301 or E. coli ATCC 25922 was observed. The compounds were active against MRSA MI339 and VRE EF12, but some compounds had only a low level of activity against PRSP STP51. In contrast, ampicillin and cefotaxime were quite active against penicillin-resistant S. pneumoniae, while the ampicillin and cefotaxime MICs for the other resistant strains, MRSA MI339 and E. faecium EF12 (VRE), were high: 92 and >200 μM, respectively. In particular, the MICs of arylalkylidene rhodanine derivative 1 were lower than those of ampicillin for several strains.
TABLE 1.
MICs of test compounds
| Strain | MIC (μM)
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | Ampi- cillin | Cefo- taxime | |
| S. aureus ATCC 29213 | 0.3 | 8 | 3 | 25 | 4 | 5 | NTa | 32 | 6 | NT | 4 | 17 | 4 | 4 | 4 | 9 | 8 | 7 | 6 | 2 |
| S. aureus MI339 (MRSA) | 1 | 4 | 2 | 12 | 8 | 5 | 4 | 16 | 6 | 4 | 4 | 8 | 4 | 4 | 2 | 9 | 4 | 7 | 92 | >200 |
| S. pneumoniae ATCC 6301 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | 0.2 | ≤0.1 |
| S. pneumoniae STP51 (PRSP) | >200 | >200 | >200 | >200 | 128 | 37 | >200 | 32 | >200 | >200 | >200 | >200 | >200 | >200 | 110 | 140 | >200 | >200 | 23 | 4 |
| E. faecalis ATCC 29212 | 1 | 4 | 8 | 3 | 32 | 74 | 7 | 64 | 6 | 18 | 15 | 34 | 16 | 15 | 7 | 18 | 15 | 7 | 1 | 140 |
| E. faecium EF12 (VRE) | 1 | 4 | 4 | 12 | 16 | 18 | 4 | 64 | 13 | 35 | 29 | 34 | 32 | 29 | 7 | 35 | 15 | 15 | >92 | >200 |
| E. coli ATCC 25922 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | 23 | 0.1 |
| M. catarrhalis BC2 | 0.5 | 1 | 1 | NT | 1 | 2 | 1 | 4 | 6 | 2 | ≤0.2 | 0.5 | 1 | 1 | 1 | 0.6 | 0.5 | 0.5 | 11 | 0.6 |
| H. influenzae HI26 | NT | 8 | NT | NT | NT | 9 | 4 | NT | NT | 140 | 59 | NT | NT | NT | NT | >200 | NT | NT | NT | NT |
NT, not tested.
Effects on peptidoglycan synthesis in vitro
The effects of the compounds on cell wall synthesis were investigated by monitoring the level of [3H]glycine incorporation into the cell wall of a penicillin-sensitive S. aureus strain. The IC50s (Table 2) showed that cell wall synthesis was inhibited in the presence of these compounds. Furthermore, the effects of the compounds on peptidoglycan biosynthesis by B. megaterium and E. coli were studied. In vitro cell wall membrane preparations from B. megaterium and ether-treated E. coli cells catalyze the synthesis of cross-linked peptidoglycan from soluble UDP-linked cytoplasmic precursors (UDP-GlcNAc and UDP-MurNAc-pentapeptide). By using UDP-[14C]GlcNAc, the assay permits the following enzymatic reactions to be distinguished: (i) the formation of lipid II by the translocase I MraY and the glycosyltransferase MurG and (ii) the formation of the polymeric glycan strands by glycosyltransferase and transpeptidase activities (7) (Fig. 2). At −20°C the cell wall membrane preparations of B. megaterium exhibiting a specific activity of 1.2 nmol/mg/min were stable for 1 month and the ether-treated E. coli cells were stable for several months. The effects of various inhibitors on in vitro synthesis with cell wall membrane preparations of B. megaterium are shown in Table 3, and the effects of various inhibitors on in vitro synthesis with ether-treated E. coli cells are shown in Fig. 5. In the presence of tunicamycin (100 μg/ml), an inhibitor of lipid I biosynthesis, no lipid II or peptidoglycan was formed (Table 3). With increasing concentrations of vancomycin, which inhibits the glycosyltransferase activity by complexing the d-Ala-d-Ala of lipid II, increased levels of inhibition of peptidoglycan biosynthesis by ether-treated E. coli cells was observed (Fig. 5), while in the presence of cefoxitin (100 μg/ml), an inhibitor of the transpeptidase, the amount of peptidoglycan synthesized by ether-treated E. coli cells decreased to 77% (absolute error, 15%). These results are in agreement with previous observations (7, 17). In the presence of our compounds no inhibition of the synthesis of lipid II was observed, so that an interaction between these compounds and translocase I and MurG could be excluded. In vitro they behaved as inhibitors of the synthesis of polymeric strands of gram-positive and gram-negative bacteria. In vivo no influence on E. coli cells was observed. Therefore, the compounds tested probably cannot permeate the outer membrane of E. coli.
TABLE 2.
IC50s of test compounds
| Inhibited component | IC50 (μM)
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | Ampicillin | Cefotaxime | |
| PBP 2a | 18 | 5 | 5 | 24 | 4 | 11 | 19 | 12 | 22 | 4 | 38 | 11 | 9 | 5 | 4 | 26 | 12 | 23 | 6 | NTa | NT |
| Cell wall | 5 | 2 | 1 | 15 | 9 | 5 | 27 | 2 | 24 | 1 | 7 | 5 | 4 | 4 | 3 | 4 | 7 | 5 | 7 | NT | NT |
NT, not tested.
TABLE 3.
In vitro peptidoglycan biosynthesis with membrane preparations from B. megateriuma
| Inhibitorb | Peptidoglycan synthesis (%) by B. megateriumc |
|---|---|
| None (control) | 100 |
| Tunicamycin | 0 |
| Moenomycin | 17 |
| Arylalkylidene rhodanine derivative 1 | 61 |
| Arylalkylidene iminothiazolidin-4-ones | |
| 11 | 57 |
| 13 | 64 |
| 16 | 63 |
Peptidoglycan synthesis started with UDP-MurNAc-pentapeptide and UDP-[14C]-GlcNAc as substrates.
Each inhibitor was used at a concentration of 100 μg/ml.
The total incorporation of radioactive S in peptidoglycan in the control experiment without inhibitor was 1.5 pmol (12%).
FIG. 5.
Inhibition of peptidoglycan synthesis with ether-treated E. coli cells. Peptidoglycan (1.3 μM) was synthesized from 0.5 mM UDP-GlcNAc and 0.5 mM UDP-MurNAc-pentapeptide without inhibitor. Solid bars, vancomycin; open bars, compound 13; hatched bars, compound 3; shaded bars, compound 17.
Effects on PBPs
The formation of mature peptidoglycan is catalyzed by PBPs, which cross-link the nascent chains via peptide bonds. The effects of the inhibitors on different PBPs from different species were studied. PBP 3 (2, 3, 18) and PBP 5 (31) from E. coli have different roles in vivo. High-molecular-mass PBP 3 appears to act as a transpeptidase and exhibits a thiolesterase activity. A similar thiolesterase activity is observed with low-molecular-mass PBP 5, which also acts as a dd-carboxypeptidase with peptide substrates. It is possible to directly monitor the hydrolysis of the thiolester substrates by UV spectrophotometry. Table 4 shows the residual activities against thiolester S2d. The effects of various potential inhibitors were studied by measuring the residual hydrolytic activities against thiolester S2d after 20 min of incubation with the compounds (Fig. 3).
TABLE 4.
Results of the inhibition experiments with different PBPsa
| Inhibitor | Residual activity (%)
|
||||
|---|---|---|---|---|---|
| PBP2xS | PBP5 | PBP3 | R61 | R39 | |
| Penicillin G | 0 | 0 | 0 | 0 | 0 |
| Arylalkylidene rhodanines | |||||
| 1 | 4 | 12 | 0 | 75 | 60 |
| 2 | 4 | 6 | 13 | 25 | 5 |
| 3 | 2 | 17 | 8 | 61 | 89 |
| 4 | 39 | 0 | 72 | 85 | NDb |
| Arylalkylidene imino- thiazolidin-4-ones | |||||
| 5 | 61 | 11 | 46 | 100 | ND |
| 6 | 26 | 8 | 24 | 83 | 91 |
| 7 | 68 | 71 | 52 | 97 | ND |
| 8 | 33 | 1 | 21 | 75 | 93 |
| 9 | 87 | 15 | 69 | 100 | ND |
| 10 | 53 | 14 | 32 | 100 | ND |
| 11 | 57 | 18 | 6 | 100 | ND |
| 12 | 72 | 36 | 0 | 100 | ND |
| 13 | 69 | 17 | 14 | 56 | 45 |
| 14 | 92 | 56 | ND | 100 | ND |
| 15 | 92 | 53 | 40 | 100 | ND |
| 16 | 16 | 0 | 3 | 97 | 99 |
| 17 | 8 | 0 | 51 | 76 | 59 |
| 18 | 69 | 4 | 36 | 90 | ND |
| 19 | 12 | 16 | 26 | 100 | 90 |
The residual activities of PBP 2xR, PBP 5, and PBP 3 were determined by monitoring the vi,0 of the S2d thiolester in the presence of 50 μM inhibitor after a 20-min preincubation. The dd-peptidase activities of R61 and R39 were used to determine the residual acitivities of these enzymes in the presence of 50 μM inhibitor. The activity in the absence of the inhibitors was 100%. The absolute error was 10% for all experiments.
ND, not determined.
Some inhibitors exhibited residual activities lower than 20% in the presence of 50 μM inhibitor and residual activities higher than 80% with 20 μM inhibitor. Even with an absolute error of 10%, the decrease in residual activities between 20 and 50 μM seems to be too high for some inhibitors. Furthermore, an unexpected increase in the ki of PBP 2xS in the presence of high concentrations of inhibitor 2 was observed. The unexpected behaviors of some inhibitors could be the result of a cooperative effect. The inhibition of PBP 5 with compounds 2 and 13 was time dependent.
The role of PBP 2 in S. pneumoniae is still unknown (20). The enzyme exhibits esterase and thiolesterase activities in vitro. As described above, the thiolesterase activity of PBP 2xS was used to determine the residual activity after preincubation of the enzyme with different inhibitors (Table 4). The thiolesterase activity of PBP 2xR (21, 25), which was from S. pneumoniae clinical isolate C(S109), was too low to allow easy screening. PBP 2xR was studied in a competition experiment with Flu-AMP. The enzyme and substrate concentrations and the incubation time were optimized in order to find assay conditions that resulted in a linear time course of the labeling reaction. The results in Tables 4 and 5 show that both enzymes were inhibited in the presence of compounds 2, 4, and 13. In assays with whole cells, no activities against S. pneumoniae ATCC 6301 or only low levels of activity of some compounds were observed against PRSP strain STP51. The absence of activity in the whole-cell assay was probably the result of the interaction of these hydrophobic compounds with plasma proteins from the lysed horse blood used in the assay, as described by Roychoudhury et al. (28).
TABLE 5.
Results of the inhibition experiments with PBP 2xR
| Inhibitor | Residual activity (%) in presence of inhibitor at concn (μM)a
|
|
|---|---|---|
| 100 | 10 | |
| Arylalkylidene rhodanines | ||
| 2 | 10 | 60 |
| 4 | 20 | 100 |
| Arylalkylidene iminothiazolidin-4-ones | ||
| 7 | 80 | 100 |
| 9 | 10 | 80 |
| 13 | 10 | 60 |
The absolute error was 15% for the competition experiment with PBP 2xR.
Low-affinity PBP 2a from S. aureus is responsible for β-lactam antibiotic resistance in staphylococci (8). Recombinant PBP 2a was used to determine the IC50s of the different compounds in a competitive binding assay with [3H]penicillin G as the reporter substrate. PBP 2a was inhibited by the compounds tested (Table 2). This result was compatible with those of the whole-cell assay, indicating that the compounds have high levels of activity against MRSA MI339.
The soluble, extracellular PBPs from Streptomyces sp. strain R61 (11, 15) and Actinomadura sp. strain R39 (14) have dd-peptidase, esterase, and thiolesterase activities. The dd-peptidase activity was used to determine the residual activity of the enzyme after preincubation with the various compounds. The results in Table 4 show that significant inhibition of both enzymes was observed only in the presence of arylalkylidene rhodanine derivative 2. In conclusion, several inhibitors of PBPs have been identified. Arylalkylidene rhodanine derivative 2 inhibited all PBPs and dd-peptidases tested.
Characterization of interaction between some inhibitors and PBP 2xS by kinetic studies
The inhibition of PBP 2xS by arylalkylidene rhodanine derivatives 2 and 3 and arylalkylidene iminothiazolidin-4-one derivative 6 was studied in detail in the presence of the thiolester S2c. Thiolester S2c is not the best substrate of PBP 2xS, but it is possible to measure initial rates near the Km value and up to 10 times the Km value. The following values have been determined by Jamin et al. (20): kcat, 0.4 ± 0.04 s−1; Km, 0.13 ± 0.03 mM; and kcat/Km, 3,200 ± 1,000 M−1 s−1). Figure 6 shows the enzymatic activity and the spontaneous hydrolysis of S2c in the presence and absence of PBP 2xS, respectively. In the enzymatic reaction, the kcat value of 0.2 ± 0.01 s−1 and the Km value of 0.18 ± 0.03 mM were found to be in good agreement with the values of Jamin et al. (20). The inhibition of PBP 2xS was time dependent (Fig. 7). For the slowly binding inhibitors the relationship between ki and the substrate concentration with fixed inhibitor concentrations is characteristic of the interaction between the inhibitor and the enzyme (9). The spontaneous hydrolysis of the substrate is linear for up to 30 min and has no influence on the ki value. Cephalexin, a cephalosporin, has a kcat/Km value of 1,600 M−1 s−1. The rate of inhibition by 10 μM cephalexin was slow, so it was possible to investigate the reaction in the presence of S2c as the reporter substrate under the same conditions used with compounds 2, 3, and 6.
FIG. 6.
Hydrolysis of S2c in the presence and the absence of PBP 2xS. The hydrolysis rate (v0) was measured in 10 mM sodium phosphate buffer (pH 7.0)-1.2 mM 4,4′-dithiodipyridine at 37°C. The increase in absorbance was monitored at 324 nm (Δɛ = 20,000 M−1 cm−1). The following values were determined in the presence of 0.32 μM PBP 2xS: kcat, 0.2 ± 0.01 s−1; Km, 0.18 ± 0.03 mM.
FIG. 7.
Time dependence of inhibition of PBP 2xS by arylalkylidene rhodanine derivative 2. PBP 2xS (0.32 μM) was incubated at 37°C in 10 mM sodium phosphate (pH 7.0) in the presence of 50 μM arylalkylidene rhodanine derivative 2. Aliquots were taken after different time intervals (t), and the residual activity was determined by adding 0.4 mM S2c and 1.2 mM 4,4′-dithiodipyridine. The inset shows a semi-log plot of the same data.
Figure 8 shows the dependence of ki on the S2c substrate concentration in the presence of different inhibitors at fixed inhibitor concentrations. Cephalexin behaves as a competitive inactivator, and from the data a Km value of 0.22 ± 0.04 mM could be calculated for S2c on the basis of the equation ki = ki,0/[1 + (S/Km)], where ki,0 is ki in the absence of substrate. This value is in good agreement with that of Jamin et al. (20). In the presence of compounds 2, 3, and 6, ki values were independent of the substrate concentration, a characteristic of a noncompetitive slow-binding inhibitior with similar ki and ki′ values. vss values were measured with different substrate concentrations in the presence of increasing concentrations of compound 2. The fitting of the data to the equation for vss provided in Materials and Methods (Fig. 9) shows that the results are in agreement with those from a noncompetitive model with similar ki and ki′ values (ki = 11 ± 2 μM; ki′ = 13 ± 6 μM). The inhibition of PBP 2xS by arylalkylidene rhodanine derivatives 2 and 3 was reversible upon dilution, while a slow to very slow reactivation phenomenon was observed with the β-lactams.
FIG. 8.
Influence of S2c substrate concentration on ki value of PBP 2xS in the presence of different inhibitors at fixed concentrations.
FIG. 9.
Inhibition of PBP 2xS by arylalkylidene rhodanine derivative 2 (I) by fitting of the steady-state data. The following values were determined: ki, 11 ± 2 μM; ki′, 13 ± 6 μM.
In conclusion, cephalexin, a β-lactam compound and an active site-directed inactivator of PBPs (11), forms an acyl enzyme with the serine residue in the active site (13). In contrast, arylalkylidene rhodanine derivative 2 behaves as a noncompetitive inhibitor of PBP 2xS and probably does not interact with the active site of this enzyme. Since some of the results might be explained by nonspecific interactions of the hydrophobic molecules with a variety of targets, further experiments are needed to identify the site of interaction and clarify the mechanism of inhibition of PBP 2xS. Furthermore, the inhibition of the other dd-peptidases and PBPs should be studied.
Acknowledgments
We thank M. Nguyen-Distèche for helpful discussions. We thank C. D. Wallace, C. B. Mikesell, and P. M. Koenigs for testing the antibacterial activities and J. L. Brill for determination of cell wall synthesis inhibition. We also acknowledge S. Roychoudhory for valuable input.
This work was supported by Procter & Gamble Pharmaceuticals and grant PAI P5/33 from the Belgian Government.
REFERENCES
- 1.Adam, M., C. Damblon, B. Plaitin, L. Christiaens, and J.-M. Frère. 1990. Chromogenic depsipeptide substrates for β-lactamases and penicillin-senitive dd-peptidases. Biochem. J. 270:525-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adam, M., C. Damblon, M. Jamin, W. Zorzi, V. Dusart, M. Galleni, A. El Kharroubi, G. Piras, B. G. Spratt, W. Keck, J. Coyette, J.-M. Ghuysen, M. Nguyen-Distèche, and J. M. Frère. 1991. Acyltransferase activities of the high-molecular-mass essential penicillin-binding proteins. Biochem. J. 279:601-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adam, M., C. Fraipont, N. Rhazi, M. Nguyen-Distèche, B. Lakaye, J.-M. Frère, B. Devreese, J. van Beeumen, Y. Van Heijenoort, J. van Heijenoort, and J.-M. Ghuysen. 1997. The bimodular G57-V577 polypeptide chain of the class B penicillin-binding protein 3 of Escherichia coli catalyzes peptide bond formation from thiolesters and does not catalyze glycan chain polymerization from lipid II intermediate. J. Bacteriol. 197:6005-6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson, I., A. C. Terwisscha van Scheltinga, and K. Valegard. 2001. Towards new β-lactam antibiotics. Cell. Mol. Life Sci. 58:1897-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baizman, E. R., A. A. Branstrom, C. B. Longley, N. Allanson, M. J. Sofia, D. Gange, and R. C. Goldman. 2000. Antibacterial activity of synthetic analogues based on the disaccharide structure of moenomycin, an inhibitor of bacterial transglycosylase. Microbiology 146:3129-3140. [DOI] [PubMed] [Google Scholar]
- 6.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 7.Broetz, H., G. Bierbaum, P. E. Reynolds, and H.-G. Sahl. 1997. The lantibiotic mersacidin inhibits peptidoglycan biosynthesis at the level of transglycosylation. Eur. J. Biochem. 246:193-199. [DOI] [PubMed] [Google Scholar]
- 8.Chambers, H. F. 2003. Solving staphylococcal resistance to β-lactams. Trends Microbiol. 11:145-148. [DOI] [PubMed] [Google Scholar]
- 9.Copeland, R. A. 1996. Tight binding inhibitors, p. 225-261. In R. A. Copeland (ed.), Enzymes: a practical introduction to structure, mechanism and data analysis. VCH Publishers, Inc., New York, N.Y.
- 10.De Meester, F., B. Joris, G. Reckinger, C. Bellefroid-Bourguignon, and J.-M. Frère. 1987. Automated analysis of enzyme inactivation phenomena. Biochem. Pharmacol. 36:2393-2403. [DOI] [PubMed] [Google Scholar]
- 11.Frère, J.-M., J.-M. Ghuysen, and H. P. Perkins. 1975. Interaction between the exocellular dd-carboxypeptidase-transpeptidase from Streptomyces R61, substrate and β-lactam antibiotics. Eur. J. Biochem. 57:353-359. [DOI] [PubMed] [Google Scholar]
- 12.Frère, J.-M. 1995. Beta-lactamases and bacterial resistance to antibiotics. Mol. Microbiol. 16:385-395. [DOI] [PubMed] [Google Scholar]
- 13.Frère, J.-M., M. Nguyen-Distèche, J. Coyette, and B. Joris. 1992. Mode of action: interaction with the penicillin binding proteins, p. 148-195. In M. Page (ed.), The chemistry of beta-lactams. Chapman and Hall, Glasgow, Scotland.
- 14.Frère, J.-M. 1998. Actinomadura R39 d-Ala-d-Ala carboxypeptidase, p. 439-441. In A. J. Barrett, N. D. Rawlings, and J. F. Woessner (ed.), Handbook of proteolytic enzymes. Academic Press, Inc., New York, N.Y.
- 15.Frère, J. M. 1998. Streptomyces R61 d-Ala-d-Ala carboxypeptidase, p. 427-430. In A. J. Barrett, N. D. Rawlings, and J. F. Woessner (ed.) Handbook of proteolytic enzymes. Academic Press, Inc., New York, N.Y.
- 16.Galleni, M., B. Lakaye, S. Lepage, M. Jamin, I. Thamm, B. Joris, and J.-M. Frère. 1993. A new, highly sensitive method for the detection and quantification of penicillin-binding proteins. Biochem. J. 291:19-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ge, M., Z. Chen, H. R. Onishi, J. Kohler, L. L. Silver, R. Kerns, S. Fukuzawa, C. Thompson, and D. Kahne. 1999. Vancomycin derivatives that inhibit peptidoglycan biosynthesis without binding d-Ala-d-Ala. Science 284:507-510. [DOI] [PubMed] [Google Scholar]
- 18.Granier, B., M. Jamin, M. Adam, M. Galleni, B. Lakaye, W. Zorzi, J. Grandchamps, J.-M. Wilkin, C. Fraipont, B. Joris, C. Duez, M. Nguyen-Distèche, J. Coyette, M. Leyh-Bouille, J. Dusart, L. Christiaens, J.-M. Frère, and J.-M. Ghuysen. 1994. Serine-type d-Ala-d-Ala peptidases and penicillin-binding proteins. Methods Enzymol. 244:249-267. [DOI] [PubMed] [Google Scholar]
- 19.Grant, E. B., D. Guiadeen, E. Z. Baum, B. D. Foleno, H. Jin, D. A. Montenegro, E. A. Nelson, K. Bush, and D. J. Hlasta. 2000. The synthesis and SAR of rhodanines as novel class C β-lactamase inhibitors. Bioorg. Med. Chem. Lett. 10:2179-2182. [DOI] [PubMed] [Google Scholar]
- 20.Jamin, M., C. Damblon, S. Millier, R. Hakenbeck, and J.-M. Frère. 1993. Penicillin-binding protein 2x of Streptococcus pneumoniae: enzymic activities and interactions with β-lactams. Biochem. J. 292:735-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jamin, M., R. Hakenbeck, and J.-M. Frère. 1993. Penicillin binding protein 2x as a major contributor to intrinsic β-lactam resistance of Streptococcus pneumoniae. FEBS Lett. 331:101-104. [DOI] [PubMed] [Google Scholar]
- 22.Laible, G., B. G. Spratt, and R. Hakenbeck. 1991. Interspecies recombinational events during the evolution of altered PBP2x genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Mol. Microbiol. 5:1993-2002. [DOI] [PubMed] [Google Scholar]
- 23.Lakaye, B., C. Damblon, M. Jamin, M. Galleni, S. Lepage, B. Joris, J. Marchand-Brynaert, C. Frydrych, and J.-M. Frère. 1994. Synthesis, purification and kinetic properties of fluorescein-labelled penicillins. Biochem. J. 300:141-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu, W.-P., Y. Sun, M. D. Bauer, S. Paule, P. M. Koenigs, and W. G. Kraft. 1999. Penicillin-binding protein 2a from methicillin-resistant Staphylococcus aureus: kinetic characterization of its interactions with beta-lactams using electrospray mass spectrometry. Biochemistry 38:6537-6546. [DOI] [PubMed] [Google Scholar]
- 25.Lu, W.-P., E. Kincaid, Y. Sun, and M. D. Bauer. 2001. Kinetics of β-lactam interactions with penicillin-susceptible and -resistant penicillin-binding protein 2x proteins from Streptococcus pneumoniae. J. Biol. Chem. 276:31494-31501. [DOI] [PubMed] [Google Scholar]
- 26.Matagne, A., A. Dubus, M. Galleni, and J.-M. Frère. 1999. The β-lactamase cycle: a tale of selective pressure and bacterial ingenuity. Nat. Prod. Rep. 16:1-19. [DOI] [PubMed] [Google Scholar]
- 27.Mouz, N., E. Gordon, A. M. Di Guilmi, I. Petit, Y. Petillot, Y. Dupont, R. Hakenbeck, T. Vernet, and O. Dideberg. 1998. Identification of a structural determinant for resistance to beta-lactam antibiotics in gram-positive bacteria. Proc. Natl. Acad. Sci. USA 95:13403-13406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roychoudhury, S., J. L. Brill, W.-P. Lu, R. E. White, Z. Chen, and T. P. Demuth. 2003. Development of a screening assay to measure the loss of antibacterial activities in the presence of proteins: its use in optimizing compound structure. J. Biomol. Screen. 8:555-558. [DOI] [PubMed] [Google Scholar]
- 29.Terrak, M., T. K. Ghosh, J. van Heijenoort, J. van Beeumen, M. Lampilas, J. Aszodi, J. A. Ayala, J.-M. Ghuysen, and M. Nguyen-Distèche. 1999. The catalytic, glycosyl transferase and acyl transferase modules of the cell wall peptidoglycan-polymerizing penicillin-binding protein 1b of Escherichia coli. Mol. Microbiol. 34:350-364. [DOI] [PubMed] [Google Scholar]
- 30.Vosberg, H.-P., and H. Hoffmann-Berling. 1971. DNA synthesis in nucleotide-permeable Escherichia coli cells. J. Mol. Biol. 58:739-753. [DOI] [PubMed] [Google Scholar]
- 31.Wilkin, J.-M. 1998. Penicillin-binding protein 5, a serine type d-Ala-d-Ala carboxypeptidase. In A. J. Barrett, N. D. Rawlings, and J. F. Woessner (ed.), Handbook of proteolytic enzymes. Academic Press, Inc., New York, N.Y.