Abstract
AIM
To examine the relationship between angiogenesis and lymphangigenesis in recurrent pterygia.
METHODS
Tissues from 34 excised recurrent pterygia (including 12 Grade 1, 10 Grade 2, and 12 Grade 3) were involved in the study and tissues from 7 nasal epibulbar conjunctivae segments were used as controls. Sections from each pterygium were immunostained with CD31 and LYVE-1 monoclonal antibodies to evaluate lymphatic microvessel density (LMVD) and blood microvessel density (BMVD), and the relationship between LMVD and BMVD in the pterygium was examined.
RESULTS
There was a large number of CD31(+)LYVE-1(−) blood vessels but only a few CD31(+)LYVE-1(+) lymphatic vessels in grades 1 and 2 pterygium. However, lymphatic vessels were dramatically increased in grade 3 pterygium. LMVD correlated closely with BMVD in all pterygia, including grades 1, 2 and 3 peterygium patients (all P values <0.01). Although both the density of blood and lymphatic vessels increased in recurrent pterygia, lymphatic vessels developed much faster than blood vessels, especially in grade 3 pterygia.
CONCLUSION
There is a significant but not parallel relationship between angiogenesis and lymphangiogenesis in recurrent pterygium. The outgrowth of blood and lymphatic vessels provide evidence that immunological mechanism may play a role in the development and recurrence of pterygium.
Keywords: angiogenesis, lymphangiogenesis, recurrent pterygium
INTRODUCTION
Pterygium is an ocular surface disease of humans attributed to chronic ultraviolet-B (UV-B) exposure. Clinically, the condition involves invasive centripetal growth with associated inflammation and neovascularisation[1]. Currently, surgery is the only method for treating pterygia. However, despite the various surgical procedures that have been described for pterygium excision, recurrence remains a significant problem that seems to be even more likely in cases of recurrent pterygium[2],[3]. Moreover, compared to the pathogenesis of primary pterygia, little is known about that in recurrent pterygia.
More recently, we examined the density of lymphatic vessels in pterygia and discovered lymphangiogenesis occurred and developed[4]. In addition, we also found the lymphangiogenesis was associated closely with the degree of pterygia, which meant the more lymphatic vessels existed, the more severity of pterygia was. Whereas the blood vessels provide a route of entry for immune effector cells (e.g., CD4+ alloreactive T lymphocytes and memory T lymphocytes), lymphangiogenesis enables the exit of antigenic material, for example, antigen-presenting cells (APCs), from the ocular surface to the regional lymph node[5]-[7]. In our previous studies, corneal lymphangiogenesis was found to be in parallel with hemangiogenesis[8]-[10]. Therefore, a question on whether such a significant relationship was also present in recurrent pterygia we felt warranted further investigation. If such is the case, it will provide evidence that immunological mechanism play a role in the development and recurrence of pterygium.
In the current study, we focus on the relationship between angiogenesis and lymphangiogenesis in recurrent pterygium. Findings from the study may potentially broaden our understanding of immune mechanisms that can be instrumental in the recurrence of pterygia.
SUBJECTS AND METHODS
Subjects
A total of 34 patients with recurrent pterygia (15 males and 19 females, average age, 53.2 ± 17.3 years) were included in the study at the Department of Ophthalmology, the third Affiliated Hospital of Sun Yat-sen University from January 2006 to June 2010. The average time of pterygial resection was (5.1 ± 2.7) years following the final pterygial surgeries. Clinical evaluations on pterygia were performed according to the grading systems described by Awdeh[11]. Briefly, pterygia were pre-operatively graded on a scale of 1-3. Grade 1 denoted a pterygium in which episcleral vessels underlying the body of the pterygium were unobscured and with mild clinical signs of inflammation (conjunctival congestion and edem). Grade 2 was defined as vessels partially visible with moderate inflammation, and Grade 3 as vessels wholly obscured with severe signs of inflammation (conjunctival congestion, edem, relative thickness of the fibrovascular lesion, and general eye redness). Of the 34 subjects, a Grade 1 pterygium was found in 12 (38.6%) patients, a grade 2 was found in 10 (31.8%) patients, and a grade 3 was found in 12 (29.6%) patients (Table 1). Seven nasal epibulbar conjunctival segments, excised during cataract surgery near the limbus, were used as control tissues. All patients and controls were informed of the experimental nature of this procedure. The clinical trial protocol and informed consent form were approved by the Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University.
Table 1. Composition of recurrent pterygia.
| Pterygium patients | Grade 1 | Grade 2 | Grade 3 | Controls |
| Number of patients | 12 | 10 | 12 | 7 |
| Age (a) | 52.0±8.05 | 55.1±3.43 | 43.3±6.51a,b,c | 50.5±3.02 |
| Female/Male | 6/6 | 6/4 | 7/5 | 4/3 |
aP<0.05 vs controls;bP<0.05 vs Grade 1;cP<0.05 vs Grade 2.
Methods
Immunohistochemistry
After being fixed in 10% neutral formalin for 24 hours, embedded in paraffin, serially sectioned in 4µm in thickness, and rehydrated with graded ethanol-water mixtures, excised conjunctivae segments were washed with distilled water. Endogeneous peroxidase activity was blocked after being incubated with 30mL/L hydrogen peroxidase for 20 minutes. For antigen retrieval, tissue sections were then autoclaved at 121°C in 10mmol/L citrate buffer (pH 6.0) for 10 minutes. Then the sections were allowed to cool at room temperature for 30 minutes. Subsequently, sections were incubated for 3 hours with mouse anti human LYVE-1 monoclonal antibody (R&D systems, MN) or mouse anti human CD31 (R&D systems, MN), respectively, and biotin marked rabbit anti mouse immunoglobulin as the secondary antibody. Strept avidin biotin complex (SABC)-peroxidase was used as the immune check system. The slides were visualized for peroxidase activity with diaminobenzidine (DAB) and counterstained with hematoxylin. Negative controls involved substitution of the primary antibody with PBS at the same concentration.
Lymphatic microvessel density and blood microvessel density
Lymphatic microvessel density (LMVD) and blood microvessel density (BMVD) of human excised tissues were evaluated independently by two observers without prior knowledge of the experimental details and tests were repeated once. The CD31(+)LYVE-1(−) vessels of sections were identified as blood vessels, whereas the CD31(+)LYVE-1(+) vessels were recognized as lymphatic vessels. Each sample was excised into 40 sections. Then sections were analyzed using standard light microscopy (Nikon, Eclipse 200). Under a 50× magnification (1.56mm2), the five most lymphvascularized areas were identified and the number of immunostained lymphatic vessels were counted. The LMVD for each case was expressed as the mean value (total number of vessels in 200 microscopic fields /200). Similarly, to calculate BMVD, all blood vessels in 200 fields of the 40 slices were summed and divided by 200.
Statistical Analysis
Analysis of the significance of differences between the two groups was performed using paired Student's t-test (SPSS 12.0 statistical software; SPSS, Inc., Chicago, IL, USA). Pearson's analysis was used to analyze the correlation between LMD and BMD. The values were presented as the mean ± SD. All reported P-values were 2-tailed, and statistical significance was defined at α = 0.05 level.
RESULTS
HE and Immunohistochemical Staining
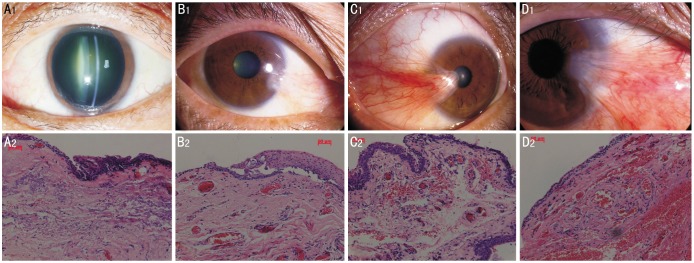
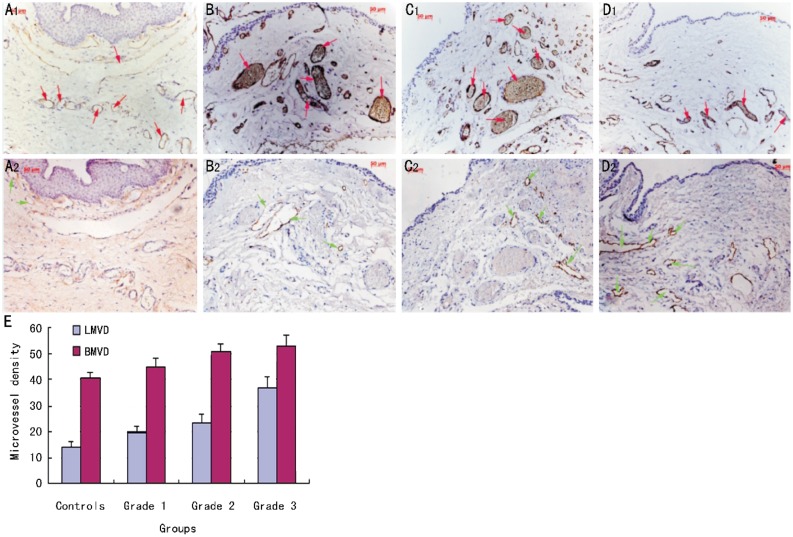
By HE Staining, we found there were some blood vessels and few inflammatory cells in the stroma of controls. Blood vessels mildly increased in grade 1 pterygia. Compared to grade 1 pterygia, more blood vessels and inflammatory cells emerged in the grade 2 pterygia. In grade 3 pterygia, blood vessels dramatically increased in the stroma. Meanwhile, there were a lot of inflammatory cells and red cells infiltrating into the stroma, suggesting the inflammation was serious (Figure 1). Immunohistochemistry was performed on LYVE-1 and CD31 serial sections of human recurrent pterygium tissue. Because CD31 stains blood and lymphatic vessels, and LYVE-1 stains the lymphatic endothelium[12],[13], we could identify and distinguish corneal blood and lymphatic vessels in histologic sections simultaneously. Compared with blood vessels, lymphatic vessels had a relative larger lumen and did not contain erythrocytes. Our data showed that there was a large number of CD31(+)LYVE-1(−) blood vessels but few CD31(+)LYVE-1(+) lymphatic vessels in the conjunctiva of controls. CD31(+)LYVE-1(+) lymphatic vessels were mildly increased in Grade 1 and 2 pterygia but were dramatically increased in Grade 3 pterygia (Figure 2).
Figure 1. HE staining for the conjunctiva of control and petrygia.
There were some blood vessels and few inflammatory cells in the stroma of control conjuctiva. Blood vessels moderately increased in Grade 1 and Grade 2 pterygia, but dramatically increased in Grade 3 pterygia. (A: controls; B: Grade 1 pterygia; C: Grade 2 pterygium; D: Grade 3 pterygia. Magnification for HE staining ×200).
Figure 2. Immunohistochemical staining for blood and lymphatic vessels in petrygia.
There was a number of CD31(+)LYVE-1(−) blood vessels but only few CD31(+)LYVE-1(+) lymphatic vessels in controls. Lymphatic vessels were increased gradually in Grade 1 and 2 pterygia but were dramatically increased in Grade 3 pterygia. (A: controls; B: Grade 1 pterygia; C: Grade 2 pterygium; D: Grade 3 pterygia.;upper panel correspond to CD31 and lower panels to LYVE-1 staining; E: BMVD and LMVD in each group; Red arrows: blood vessels; Green arrows: lymphatic vessels. Magnification for immunohistochemistry ×200).
Relationship Between Hemangiogenesis and Lymphangiogenesis in Recurrent Pterygium
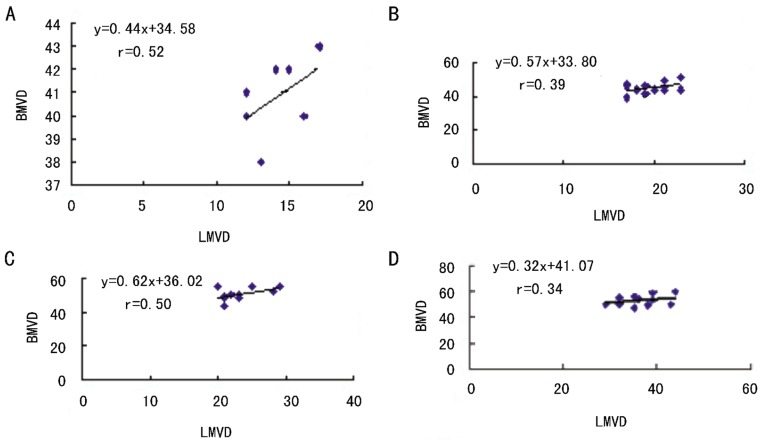
There was a significant relationship between blood and lymphatic vessels in the conjunctiva of controls. To elucidate the relationship between hemangiogenesis and lymphangiogenesis in recurrent pterygium, we divided the patients into three groups according to the degree of pterygia and examied BMVD and LMVD respectively. Our data showed that BMVD was associated closely with LMVD in all three groups (all P values<0.01, Figure 3), suggesting that there was a significant relationship between hemangiogenesis and lymphangigenesis in recurrent pterygia. Subsequently, we compared with increasing rates of BMVD and LMVD in each group. We found that although both the density of blood and lymphatic vessels increased in recurrent pterygia, lymphatic vessels developed much faster than blood vessels, especially in Grade 3 pterygia. LMVD in Grade 3 pterygia was nearly twice as much as that in controls, while the increasing rate of BMVD in Grade 3 pterygia was less than 25% in comparison with that in controls (Table 2). This suggested that the outgrowth of lymphatic vessels (lymphangiogenesis) was not parallel with hamangiogenesis and might play a more important role in seriously recurrent pteygia.
Figure 3. The relationship between LMVD and BMVD in recurrent pterygia.
LMVD correlated closely with BMVD in the conjunctiva of controls and all pterygia, including grades 1, 2 and 3 peterygium patients.
Table 2. BMVD and LMVD in recurrent pterygia.
| Patients | BMVD | LMVD |
| Controls | 40.9±1.68 | 14.1±1.95 |
| Grade 1 | 45.0±3.16a | 19.7±2.19a |
| Grade 2 | 50.5±3.87a,b | 23.3±3.09a,b |
| Grade 3 | 52.8±4.11a,b | 36.7±4.44b,c |
aP<0.05 vs controls; bP <0.05 vs Grade 1 cP<0.05 vs Grade 2.
DISCUSSION
Although current evidence is not definitive, immunologic mechanisms likely contribute to the development of pterygia. Pterygia samples have been shown to have increased levels of cell signaling and adhesion molecules, such as vascular cellular adhesion molecule-1 and intercellular adhesion molecule-1, and aberrant expression of human leucocyte antigen-DR[14],[15]. Other signaling molecules, including E-cadherin and b-catenin, are up-regulated and concentrated in the heads of pterygia[16]. Increased b-catenin has been shown to trigger certain cell cycle proteins and matrix metalloproteinases[17]. An increase in mast cells, lymphocytes, plasma cells, dendritic cells, and CD4+ and CD8+ T cells in pterygia samples has also been documented[14],[15],[18]. Stromal infiltrates in pterygia of T cells, with an increased helper-to-suppressor ratio, and abnormal deposits of immunoglobulins E and I have been described[19],[20]. A subsequent study screened pterygial gene expression compared to normal conjunctival tissue, with a significant increase in macrophage inflammatory protein-4[21]. These immunologic mechanisms have been associated with pterygia, but it is unclear if immunologic mechanisms are involved in pathogenesis or are only secondarily expressed after pterygia formation. The current study discovered that the density of both blood and lymphatic vessels was significantly increased and it suggested that immunological mechanism take part in the development and recurrence of pterygium, since hamangiogenesis and lymphangiogenesis were considered to be two “arms” in immune response and played a key role in ocular immunity[7].
The present study also documented, for the first time to our knowledge, there was a significant relationship between hemangiogenesis and lymphangigenesis in recurrent pterygia. Regarding the relationship between hem- and lymphangiogenesis, evidence from in vivo analysis of rabbit ear transparent chambers points to a sequential in growth of blood vessels first, followed by lymphatic vessels[22]. The same is true for experimental pig wounds and in human skin[23]-[25]. In vascularized human corneas, the degree of corneal lymphangiogenesis was parallel with the degree of hemangiogenesis, which means that in strongly vascularized corneas, there is a significantly higher chance of lymph vessels being present compared with mildly vascularized corneas[26]. Due to invisible features of micro lymphatics, it is difficult for oculists to identify the state of lymphangiogenesis in order to assess the effect of drugs targeting lymphangiogenesis in the treatment of pterygium (if available). But the state of blood vessels can be easily assessed by a slit lamp; we would evaluate the state of lymphangiogenesis according to the state of hemangiogenesis if such a parallel relationship were also present in pterygium. However, in a recent study of ours, we examined LMVD and BMVD in primary pterygium and found the increasing rates of LMVD was much faster than that of BMVD, suggesting that the outgrowth of lymphatic vessels were not parallel with that of blood vessels in primary pterygium[4]. On the basis of it, we focused on the relationship between hemangiogenesis and lymphangiogenesis in recurrent pterygium and discovered a similar change of lymphangiogenesis in comparison with that in primary pterygium. Therefore, the degree of lymphangiogenesis can be informed by the degree of hemangiogenesis neither in primary nor recurrent pterygium.
Interestingly, we also found there was a significant difference in age between Grade 3 pterygia and other groups. In a recent study, Hos et al[27] discovered an age-related change in murine limbal lymphatic vessels and corneal lymphangiogenesis. Lymphatic vascular sprouts and inflammation-induced pathologic corneal lymphangiogenesis decreased with age, which suggested the younger the murine would be, the higher possibility of lymphangiogenesis would have. If such was true, it might partially explain the strong lymphangiogenesis in Grade 3 pterygia patients, who were in the youngest group with the mean age of 43.3 years. In addition to blood vessels, inflammation was proven to be an important factor to induce corneal lymphangiogenesis, as shown in recent experimental and clinical trials[28]. In an earlier study, we found that lymphangiogenesis associated closely with inflammation index in alkali induced inflammatory cornea[29]. The state of ocular inflammation might play a role in the occurrence of lymphangiogenesis in pterygium, since the inflammation was considered to be the common denominator to the postnatal events that overlap with lymphatic vessel growth and was associated closely with the formation and development of pterygia. Meanwhile, the different states of inflammation in Grade 1, 2, and 3 pterygia, to a certain extent, explained a significant but not a parallel relationship in them. Further study awaits to elucidate it.
In summary, present study has revealed the development of blood and lymphatic vessels in recurrent pterygia and has indicated that there was a significant but not parallel relationship between hemangiogenesis and lymphangiogenesis. The development of blood and lymphatic vessels provide evidence that immunological mechanism play a role in the development and recurrence of pterygium. Strategies of immune therapy might be investigated to improve the prognosis of pterygia.
Acknowledgments
We thank Dr. Chao-Yang Li, and Dr. Chuang-Chao Xu for their invaluable technical support.
Footnotes
Foundation items: National Natural Science Foundation of China (No. 81070711); Natural Science Foundation of Guangdong Province, China (No. S2011010006061); Training Program of Young Teachers of Sun Yat-sen University, China (No. 11ykpy42); Program of Low Vision Survey in Guangzhou, China (No A938)
References
- 1.Di Girolamo N, Chui J, Coroneo MT, Wakefield D. Pathogenesis of pterygia: role of cytokines, growth factors, and matrix metalloproteinases. Prog Retin Eye Res. 2004;23(2):195–228. doi: 10.1016/j.preteyeres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Shehadeh-Mashor R, Srinivasan S, Boimer C, Lee K, Tomkins O, Slomovic AR. Management of recurrent pterygium with intraoperative mitomycin C and conjunctival autograft with fibrin glue. Am J Ophthalmol. 2011;152(5):730–732. doi: 10.1016/j.ajo.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 3.Nieuwendaal CP, van der Meulen IJ, Mourits M, Lapid-Gortzak R. Long-term follow-up of pterygium surgery using a conjunctival autograft and Tissucol. Cornea. 2011;30(1):34–36. doi: 10.1097/ICO.0b013e3181dea7f0. [DOI] [PubMed] [Google Scholar]
- 4.Ling S, Liang L, Lin H, Li W, Xu J. Increasing lymphatic microvessel density in primary pterygia. Arch Ophthalmol. 2012;130(6):735–742. doi: 10.1001/archophthalmol.2012.293. [DOI] [PubMed] [Google Scholar]
- 5.Hos D, Regenfuss B, Bock F, Onderka J, Cursiefen C. Blockade of insulin receptor substrate-1 inhibits corneal lymphangiogenesis. Invest Ophthalmol Vis Sci. 2011;52(8):5778–5785. doi: 10.1167/iovs.10-6816. [DOI] [PubMed] [Google Scholar]
- 6.Bachmann B, Taylor RS, Cursiefen C. Corneal neovascularization as a risk factor for graft failure and rejection after keratoplasty: an evidence-based meta-analysis. Ophthalmology. 2010;117(7):1300–1305. doi: 10.1016/j.ophtha.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 7.Cursiefen C, Chen L, Dana MR, Streilein JW. Corneal lymphangiogenesis: evidence, mechanisms, and implications for corneal transplant immunology. Cornea. 2003;22(3):273–281. doi: 10.1097/00003226-200304000-00021. [DOI] [PubMed] [Google Scholar]
- 8.Yan H, Qi C, Ling S, Li W, Liang L. Lymphatic vessels correlate closely with inflammation index in alkali burned cornea. Curr Eye Res. 2010;35(8):685–697. doi: 10.3109/02713681003793136. [DOI] [PubMed] [Google Scholar]
- 9.Ling S, Lin H, Liang L, Xu J, Xu C, Zhao W, Liu Z. Development of new lymphatic vessels in alkali-burned corneas. Acta Ophthalmol. 2009;87(3):315–322. doi: 10.1111/j.1755-3768.2008.01349.x. [DOI] [PubMed] [Google Scholar]
- 10.Ling S, Lin H, Xiang D, Feng G, Zhang X. Clinical and experimental research of corneal lymphangiogenesis after keratoplasty. Ophthalmologica. 2008;222(5):308–316. doi: 10.1159/000144030. [DOI] [PubMed] [Google Scholar]
- 11.Awdeh RM, DeStafeno JJ, Blackmon DM, Cummings TJ, Kim T. The presence of T-lymphocyte subpopulations (CD4 and CD8) in pterygia: evaluation of the inflammatory response. Adv Ther. 2008;25(5):479–487. doi: 10.1007/s12325-008-0056-4. [DOI] [PubMed] [Google Scholar]
- 12.Cao R, Bjorndahl MA, Religa P, Clasper S, Garvin S, Galter D, Meister B, Ikomi F, Tritsaris K, Dissing S, Ohhashi T, Jackson DG, Cao Y. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;6(4):333–345. doi: 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 13.Bjorndahl M, Cao R, Nissen LJ, Clasper S, Johnson LA, Xue Y, Zhou Z, Jackson D, Hansen AJ, Cao Y. Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. Proc Natl Acad Sci USA. 2005;102(43):15593–15598. doi: 10.1073/pnas.0507865102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beden U, Irkec M, Orhan D, Orhan M. The roles of T-lymphocyte subpopulations (CD4 and CD8), intercellular adhesion molecule-1 (ICAM-1), HLA-DR receptor, and mast cells in etiopathogenesis of pterygium. Ocul Immunol Inflamm. 2003;11(2):115–122. doi: 10.1076/ocii.11.2.115.15913. [DOI] [PubMed] [Google Scholar]
- 15.Ioachim-Velogianni E, Tsironi E, Agnantis N, Datseris G, Psilas K. HLA-DR antigen expression in pterygium epithelial cells and lymphocyte subpopulations: an immunohistochemistry study. Ger J Ophthalmol. 1995;4(2):123–129. [PubMed] [Google Scholar]
- 16.Kase S, Osaki M, Sato I, Takahashi S, Nakanishi K, Yoshida K, Ito H, Ohno S. Immunolocalisation of E-cadherin and beta-catenin in human pterygium. Br J Ophthalmol. 2007;91(9):1209–1212. doi: 10.1136/bjo.2007.115709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato N, Shimmura S, Kawakita T, Miyashita H, Ogawa Y, Yoshida S, Higa K, Okano H, Tsubota K. Beta-catenin activation and epithelial-mesenchymal transition in the pathogenesis of pterygium. Invest Ophthalmol Vis Sci. 2007;48(4):1511–1517. doi: 10.1167/iovs.06-1060. [DOI] [PubMed] [Google Scholar]
- 18.Ribatti D, Nico B, Maxia C, Longo V, Murtas D, Mangieri D, Perra MT, De Giorgis M, Piras F, Crivellato E, Sirigu P. Neovascularization and mast cells with tryptase activity increase simultaneously in human pterygium. J Cell Mol Med. 2007;11(3):585–589. doi: 10.1111/j.1582-4934.2007.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinkerton OD, Hokama Y, Shigemura LA. Immunologic basis for the pathogenesis of pterygium. Am J Ophthalmol. 1984;98(2):225–228. doi: 10.1016/0002-9394(87)90358-8. [DOI] [PubMed] [Google Scholar]
- 20.Liu L, Yang D. Immunological studies on the pathogenesis of pterygium. Chin Med Sci J. 1993;8(2):84–88. [PubMed] [Google Scholar]
- 21.John-Aryankalayil M, Dushku N, Jaworski CJ, Cox CA, Schultz G, Smith JA, Ramsey KE, Stephan DA, Freedman KA, Reid TW, Carper DA. Microarray and protein analysis of human pterygium. Mol Vis. 2006;12:55–64. [PubMed] [Google Scholar]
- 22.Clark ER, Clark EL. Observations on the new growth of lymphatic vessels as seen in transparent chambers introduced into rabbit's ear. Am J Anat. 1932;51(1):49–87. [Google Scholar]
- 23.Witmer AN, van Blijswijk BC, Dai J, Hofman P, Partanen TA, Vrensen GF, Schlingemann RO. VEGFR-3 in adult angiogenesis. J Pathol. 2001;195(4):490–497. doi: 10.1002/path.969. [DOI] [PubMed] [Google Scholar]
- 24.Paavonen K, Puolakkainen P, Jussila L, Jahkola T, Alitalo K. Vascular endothelial growth factor receptor-3 in lymphangiogenesis in wound healing. Am J Pathol. 2000;156(5):1499–1504. doi: 10.1016/S0002-9440(10)65021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skobe M, Hamberg LM, Hawighorst T, Schirner M, Wolf GL, Alitalo K, Detmar M. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol. 2001;159(3):893–903. doi: 10.1016/S0002-9440(10)61765-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cursiefen C, Schlötzer-Schrehardt U, Küchle M, Sorokin L, Breiteneder-Geleff S, Alitalo K, Jackson D. Lymphatic vessels in vascularized human corneas: immunohistochemical investigation using LYVE-1 and Podoplanin. Invest Ophthalmol Vis Sci. 2002;43(7):2127–2135. [PubMed] [Google Scholar]
- 27.Hos D, Bachmann B, Bock F, Onderka J, Cursiefen C. Age-related changes in murine limbal lymphatic vessels and corneal lymphangiogenesis. Exp Eye Res. 2008;87(5):427–432. doi: 10.1016/j.exer.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Mouta C, Heroult M. Inflammatory triggers of lymphangiogenesis. Lymphat Res Biol. 2003;1(3):201–218. doi: 10.1089/153968503768330247. [DOI] [PubMed] [Google Scholar]
- 29.Ling SQ, Li WH, Xu JG, Kuang WH, Li CY. The relationship between corneal lymphangiogenesis and inflammation index after corneal alkali injury. Zhonghua Yanke Zazhi. 2010;46(11):1000–1005. [PubMed] [Google Scholar]