Abstract
AIM
To investigate the role of retinoic acid (RA) and retinaldehyde dehydrogenase-2 (RALDH2) of retina and choroid in the guinea pig lens-induced myopic eyes.
METHODS
Totally 45 guinea pigs, at age of three weeks, were randomly assigned into three groups: the normal control, the lens-induced group and the recovering group. Out of focus was induced by the -6.00D concave lens on the left eye, and lasted for 15 days. All animals underwent biometric measurement (corneal radius of curvature, refraction and axial length). Subsequently, RA content in the retina and RPE/choriod complex was detected by reversed-phase high-performance liquid chromatography. RALDH2 protein in the retina and RPE/choriod complex was evaluated by the immunohistochemical staining and Western blotting.
RESULTS
After wearing -6.00D lens for 15 days, axial length of the lens-induced eye extends and myopia was formed, with RA contents increasing in both the neural retina and RPE/choroid complex. Comparing with the lens-induced group, myopic degree significantly relieved, and its RA contents in both the neural retina and RPE/choroid complex decreased in the recovering group. In the normal control, RALDH2 protein was expressed positively in the retinal nerve fiber layer (RNFL), inner plexiform layer (IPL) and lateral border of outer nuclear layer (ONL). Retinal RALDH2 protein increased in the lens-induced group, and was also positive in the outer plexiform layer (OPL). In the recovering group, retinal RALDH2 protein attenuated the expression in the OPL turns to negative. RALDH2 protein was not expressed in the choroid of any group.
CONCLUSION
RA of retina and chorid participates in the regulation of the lens-induced myopia in guinea pigs, which may be related with retinal RALDH2 protein.
Keywords: retinoic acid, lens-induced myopia, retinaldehyde dehydrogenase-2, guinea pig
INTRODUCTION
Retinoic acid (RA) is a derivant of vitamin A. With the development of the studies on myopic pathogenesis, it has been proved that RA of retina and choroid plays an important role in the formation of myopia. At present, myopic incidence rate in the school age children increases significantly and the age of onset keeps on becoming younger and younger in China[1],[2]. Most of these myopia patients has a correlation with too much work in a short distance, belonging to lens-induced myopia[1]. As the animal model of lens-induced myopia is similar with the human myopia in the formation mechanism[3], we adopt the guinea pig lens-induced myopia to discuss the effects of wearing concave lens on RA content, and its synthesis rate-limiting enzyme-retinaldehyde dehydrogenase-2 (RALDH2) of the retina and choroid to provide the scientific references for the further study on the correlation between RA and the development of myopia.
MATERIALS AND METHODS
Materials
Forty-five clean guinea pigs at age of 3 weeks that each weighing 140-170g were provided by the Animal Department of the Central South University, and randomly divided into 3 groups: the normal control, lens-induced group, recovering group. All animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No.86-23, revised 1986).
The concave lens was -6.00D optical resin lens provided by Hong Kong Optical Lens Co., Ltd. Its diameter was 12mm and the inner arc curvature was 9.61mm. The one-time Murphy dropper was pruned to make it into a frame and the concave lens were agglutinated to the self-made frame (Figure 1). Two small apertures were left at the bottom of the frame for the lens cleaning and air circulation. Three small blades should be kept at the bottom of the frame.
Figure 1. One-time Murphy dropper was pruned to make it into a frame and the concave lens were agglutinated to the self-made frame.
Pentobarbital sodium of 0.3% (30mg/kg) was injected to the abdominal cavity for anaesthesia, and the hairs around the fossa orbitalis were sheared. The self-made frame was sutured and fixed to the soft tissue around the fossa orbitalis of the left eye (Figure 2). The guinea pigs were fed in different cages separately and provided indoor fluorescent light. The cycle for light and dark is 12/12 hours per day. The lens-induced group should wear the concave lens for 15 days. The recovering group should wear the lens for 12 days before removal of the flame and the lens, and then three days of normal visual environment should be offered.
Figure 2. Self-made frame was sutured and fixed to the soft tissue around the fossa orbitalis of the left eye.

According to the scheduled experimental time, the corneal radius and refraction were measured with the photokeratometer and retinoscopy optometry. Type-A ultrasonic was adopted to measure the axial length (accurate to 0.01mm) for three times and the average value was taken.
Methods
The guinea pigs were killed with overdose pentobarbital sodium. The eyeballs were removed and the protomerite tissue was excise and then the vitreous body was put on ice. Three deutomerite calices opticus were picked for each group, and the optic nerve was marked. Neutral formalin buffer solution of 10% was used to make into paraffin block for the RALDH2 immunohistochemistry dyeing. The 12 deutomerite calices opticus left were adopted in each group and were divided along the sagittal plane through the optic nerve into two parts. One part was used for measuring the RA content by the reversed-phase high-performance liquid chromatography and the other part was used for detecting RALDH2 protein.
Neural retina and RPE/choroid complex from the deutomerite calices opticus was separated and weighed. The RA contents in the neural retina and RPE/choroid complex were measured respectively. The atRA standard was purchased from the Sigma-Aldrich (C20H28O2, molecular mass: 300.44). Chromatographic condition was as follows: the Waters uBondapak C18 reversion phase chromatography column (150mm×3.9mm), the mobile phase was V (acetonitrile): V (0.1% glacial acetic acid solution) =86:14, with flow rate of 1.0mL/min, detected wave length of 350nm, column temperature of 25°C, sample size of 20µL. The RA contents (ng) in the neural retina or RPE/choroid complex per mg were calculated.
RALDH2 protein detected by the Western blotting refers to reference[4] in the neural retina and RPE/choroid complex. The first antibody was rabbit anti-RALDH2 polyclonal antibody (Santa Cruz, U.S.A), working concentration 1:500. Target strap was performed grey value analysis by Bandscan 5.0 image analysis software, GAPDH as the internal control; the relative expression of target protein was calculated.
The paraffin section was cut at the thickness of 5µm to conduct the immunohistochemistry dyeing. The SP kit was purchased from Beijing Biosynthesis Biotechnology Co., LTD. The dyeing procedure was conducted according to the instruction in the SP kit. The working concentration of anti-RALDH2 polyclonal antibody was 1:300. The antibody was substitute with double distilled water to conduct negative control.
Statistical Analysis
All the data was demonstrated in mean±SD. The data was processed with SPSS 11.0 statistical package. One-way ANOVA test was adopted to compare the corneal radius, the refraction of eyeballs, axial length, relative expression quantity of RALDH2 protein and the RA content.
RESULTS
Effects of Concave Lens on the Ocular Refractive State
After the -6.00D concave lens was wore for fifteen days, the axial length of the lens-induced eye was extended and myopia was formed (the relative myopic diopter was about 3.6D). Statistical significance was shown in contrast with its self control eye and normal control eye (P<0.01), while there were no obvious changes of corneal radius detected (P>0.05). Compared with the lens-induced group, the myopic degree significantly relieved in the recovering group (P<0.01). There were statistical differences in the diopter, axial length between the normal control and the recovering group (P<0.01,Table 1).
Table 1. Effect of concave lens on ocular refractive state.
| Groups | Eyes | n | Corneal radius ofcurvature (mm) | Refraction (D) | Axial length (mm) |
| Normal control | Left | 15 | 3.53±0.02 | +1.25±0.28 | 7.83±0.05 |
| Right | 15 | 3.53±0.04 | +1.18±0.24 | 7.82±0.06 | |
| Lens-inducation | Left | 15 | 3.53±0.03 | -2.35±0.80d,f | 8.26±0.08d,f |
| Right | 15 | 3.55±0.03 | +1.20±0.30 | 7.85±0.04 | |
| Recovery | Left | 15 | 3.54±0.03 | -0.50±0.25b,d,f | 8.05±0.06b,d,f |
| Right | 15 | 3.53±0.04 | +1.23±0.20 | 7.83±0.05 |
bP<0.01 vs lens-induced group; dP<0.01 vs the left eye; fP<0.01 vs the normal control.
(x±s, n=15)
Effects of Concave Lens on RA Contents in the Neural Retina and RPE/ Choroid Complex
RA content in the neural retina per mg of the left eye from the normal control was about (1.27±0.22)ng, and in the RPE/choroid complex per mg was about (0.25±0.08)ng. RA contents of the neural retina and RPE/choroid complex in the lens-induced group were higher than that in the normal control, and the differences showed statistical significance (Neural retina F=12.384, RPE/choroid F=9.669, P<0.01). In contrast with the lens-induced group, RA contents in both the neural retina and RPE/choroid complex decreased in the recovering group (Neural retina F=7.883, RPE/choroid F=6.585, P<0.01), but its contents was still higher than the normal control (Neural retina F=5.115, RPE/choroid F=3.165, P<0.01), which differences showed statistical significance (Table 2).
Table 2. Effect of concave lens on RALDH2 and RA in the retina and choroids.
| Groups | n | RALDH2 (Ret) | RA (Ret, ng) | RA (RPE/Cho, ng) |
| Normal control | 12 | 0.08±0.02 | 1.27±0.22 | 0.25±0.08 |
| Lens-inducation | 12 | 0.43±0.08a | 2.60±0.30a | 0.63±0.11a |
| Recovery | 12 | 0.26±0.05a,c | 1.74±0.23a,c | 0.36±0.09a,c |
Ret: retina, Cho: choroid. aP<0.05 vs the normal control; cP<0.05 vs the lens-induced group.
(x±s, n=12)
Effects of Concave Lens on RALDH2 Protein in the Retina and the Choroid
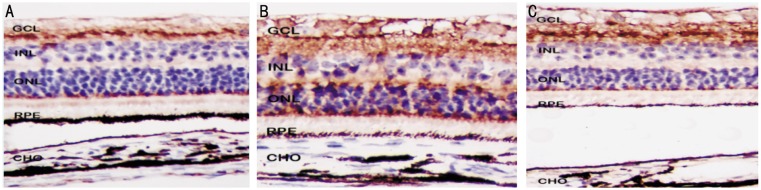
According to the immunohistochemistry dyeing results, RALDH2 protein was expressed positively in retinal nerve fiber layer (RNFL), inner plexiform layer (IPL) and lateral border of the outer nuclear layer (ONL) of the normal control, and the pigmentation was all in the cytolymph with the color being buffy (Figure 3A). RALDH2 protein was not only expressed in retinal these places with enhancement, but also expressed positively in retinal outer plexiform layer (OPL) in the lens-induced group (Figure 3B). In the recovering group, retinal RALDH2 protein attenuated and the expression in OPL turned to negative (Figure 3C). RALDH2 protein was not expressed in the choroid of any group.
Figure 3. A: The positive signal of RALDH2 protein, as brown-yellowish granules, was localized in retinal nerve fiber layer(RNFL), inner plexiform layer(IPL) and out-edge of outer nuclear layer(ONL) in guinea pig. However, no positive signal was found in choroid; B: Compared with the normal control, the increase of retinal RALDH2 protein was found in the lens-induced eyes. The positive signal was localized in the RNFL, IPL, outer plexiform layer(OPL) and out-edge of ONL, and no positive signal was found in choroid; C: Compared with the lens-inducation, the decrease of retinal RALDH2 protein was found in the RNFL, IPL and out-edge of ONL in the recovering group. No positive signal was found in OPL and choroid. SP×600.
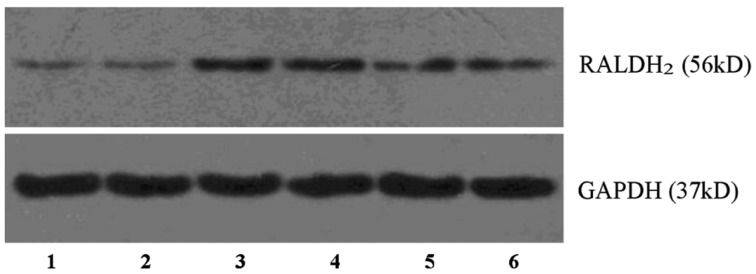
According to the results of Western blotting analysis, RALDH2 protein positive expression can be detected in neural retina of each group, but there was none detected in the RPE/choroid complex. The expression quantity of RALDH2 protein in the neural retina increased in the lens-induced group, and in contrast with the normal control, and its differences showed statistical significance (F=14.706, P<0.01). The expression of RALDH2 protein decreased in the recovering group (F=6.243, P<0.01), but is still higher than the normal control (F=11.576, P<0.01), and the differences showed statistical significance (Table 2, Figure 4).
Figure 4. Western blotting technique detection of RALDH2 protein in the guinea pig neural retina.
1,2: Normal control; 3,4: Lens-inducation; 5,6: Recovery.
DISCUSSION
Since the establishment of the myopic models, the surgeons both at home and abroad had conducted many studies on the correlation between RA and myopia. At present, it is proved that the RA system in the retina and the choroid plays an important role in the formation of the myopia. These studies penetrated most deeply in the studies on the experimental myopia of chickens. It was detected that the RA content increased in the retina and decreased in the choroid of the chickens with form-deprived myopia[5] and lens-induced myopia[6]. It was found that the exogenous RA could interfere with the proliferation of the scleral fibroblasts and chondrocytes of the chickens to affect the development of myopia in vivo and vitro studies[7]. Mertz et al[6] also found that RA had selective effects on the retinal defocusing signal; -15D spherical lens could induce the increase of RA content in the retina and the decrease of RA content in the choroid of the chicken; while +15D spherical lens could induce the decrease of RA content in the retina and increase of RA content in the choroid. Besides, Mertz et al[8] also thought that the choroidal RA might be one of the key factors mediating the transfer of myopic signal from the retina to the sclera, and choroid could directly convert the retinol to RA, which could not only combine with 28D-30D protein to stop it from being washed away by the blood flow but also diffused to the scleral tissue to regulate the scleral remodeling of myopia.
However, the changes of RA in the mammal's experimental myopia were different from the changes of chickens. The RA contents in both the retina and the choroid of myopia increased which had a correlation with the species differences and the eyeball constitutional structure differences[9]-[11]. What's more, its scleral fibroblasts expressed RA receptor and could recognize the biological signal mediated by RA to affect the scleral remodeling procedure by regulating the expression of Fibulin-1 protein[12],[13]. In this research, we found that after the guinea pigs wore the -6.00D concave lens for fifteen days, the axial length of the lens-induced eye extended and myopia was formed, the myopic dimensionality of which was about 3.6D. The RA contents in the retina and the choroid both increased and the RA content in the retina was 4-5 times in the choroid. The above results were consistent with the results of the related researches on the guinea pig myopia conducted by McFadden et al[11]. We also found out that after the guinea pigs from the recovering group wore the -6D lens for ten days and then being offered with 3-day normal visual environment, its myopic degree was significantly lower than in the lens-induced group, and the RA contents in the retina and the choroid both decreased, further proving that RA played an important role in the formation of the lens-induced myopia of guinea pigs. To deeply analyze the reasons for the changes of RA content, we had detected the change of RA synthesis rate-limiting enzyme in the retina and the choroid.
There were mainly three kinds of rate-limiting enzymes participating in the RA synthesis procedure: 1) AHD2: oxidized multiple kinds of aldehydes and had many substrates, and the retinol was one of its main physiological substrates; 2) RALDH2: the key rate-limiting enzyme to catalyze the retinol to convert to RA through dehydrogenation. It selectively took the retinol as the substrate and had higher specificity than AHD2. The activity changes of RALDH2 directly affected the production quantity of RA; 3) V1: only expressed in the retina during the embryo period. So, in this research, we detected the changes of RALDH2 protein in the lens-induced myopia of the guinea pigs. At present, it has been found that RALDH2 is expressed in the retina and the choroid of the chicken, and the expression level in the choroid is much higher than in the retina[14],[15]. The research conducted by Rada et al[16] in 2012 already prove that the high expression of choroidal RALDH2 in the recovering process of the chicken form-deprived myopia was the main cause of the increase of the RA content. However, there exist contradictory conclusions. In the report of the research conducted by Bitzer et al[17] in 2000, RALDH2 mRNA was detected in the retina of the chicken, but not detected in the choroid. As there were different changing trends of RA in the retina and the choroid between the chicken myopia and the guinea pig myopia, and there existed species differences of ocular structure between the two, we could not adopt the correlation between RALDH2 and the chicken myopia for the study of the mammal's myopia. In present research, it has been found that RALDH2 protein was expressed positively in the retina but not expressed in the choroids. After the guinea pigs wore the concave lens for 15 days, retinal RALDH2 protein significantly increased while in the recovering group decreased, the same changing trend with the RA in the retina. According to the immunohistochemistry location analysis, the RALDH2 protein was mainly distributed in the inner layer of the retina (such as RNFL and IPL); in the lens-induced group, the expression of RALDH2 protein increased in the inner layer of the retina, and was also expressed positively in the OPL; in the recovering group, negative expression in the OPL was detected. Besides, we haven't detected the RALDH2 protein in the choroid of the guinea pigs, and its RA content was only 1/4-1/5 of the RA content in the retina. So we presumed that the possible sources of RA may be: 1) directly transferred by the retina; 2) produced through catalysis by other RA synthetases; 3) transferred through the blood flow of the choroid by other parts of the body. All these possibilities call for further researches in the future.
It can be seen that, after the visual environment changes induced by the concave lens being recognized by the retina, retinal RALDH2 protein increases which can induce the increase of RA production, and then the RA content in the choroid increases, finally, participating in the regulation of the guinea pig lens-induced myopia.
Footnotes
Foundation item: National Natural Science Foundation of China (No.81070752)
REFERENCES
- 1.Pi LH, Chen L, Liu Q, Ke N, Fang J, Zhang S, Xiao J, Ye WJ, Xiong Y, Shi H, Zhou XY, Yin ZQ. Prevalence of eye diseases and causes of visual impairment in school-aged children in Western China. J Epidemiol. 2012;22(1):37–44. doi: 10.2188/jea.JE20110063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan DS, Lai C, Lau HH, Cheung EY, Lam DS. Change in vision disorders among Hong Kong preschoolers in 10 years. Clin Experiment Ophthalmol. 2011;39(5):398–403. doi: 10.1111/j.1442-9071.2010.02470.x. [DOI] [PubMed] [Google Scholar]
- 3.Wei X, Zhang JS. Researching advance in pathogenesis of the lens-induced myopia. Int Rev Ophthalmol. 2009;33(5):302–306. [Google Scholar]
- 4.Mao JF, Liu SZ, Qin WJ, Xiang Q. Modulation of TGFβ2 and dopamine by PKC in retinal Müller cells of guinea pig myopic eye. Int J Ophthalmol. 2011;4(4):357–360. doi: 10.3980/j.issn.2222-3959.2011.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seko Y, Shimizu M, Tokoro T. Retinoic acid increases in the retina of the chick with form deprivation myopia. Ophthalmic Res. 1998;30(6):361–367. doi: 10.1159/000055496. [DOI] [PubMed] [Google Scholar]
- 6.Mertz JR, Howlett MHC, McFudden SA, Wallman J. Retinoic acid from both the retina and choroids influences eye growth. Invest Ophthalmol Vis Sci. 1999;40(suppl):4473. [Google Scholar]
- 7.Seko Y, Shimokawa H, Tokoro T. In vivo and in vitro association of retinoic acid with form-deprivation myopia in the chick. Exp Eye Res. 1996;63(4):443–452. doi: 10.1006/exer.1996.0134. [DOI] [PubMed] [Google Scholar]
- 8.Mertz JR, Wallman J. Choroidal retinoic acid synthesis: a possible mediator between refractive error and compensatory eye growth. Exp Eye Res. 2000;70(4):519–527. doi: 10.1006/exer.1999.0813. [DOI] [PubMed] [Google Scholar]
- 9.Huang J, Qu XM, Chu RY. Expressions of cellular retinoic acid binding proteins I and retinoic acid receptor-β in the guinea pig eyes with experimental myopia. Int J Ophthalmol. 2011;4(2):131–136. doi: 10.3980/j.issn.2222-3959.2011.02.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Troilo D, Nickla DL, Mertz JR, Summers Rada JA. Change in the synthesis rates of ocular retinoic acid and scleral glycosaminoglycan during experimentally altered eye growth in marmosets. Invest Ophthalmol Vis Sci. 2006;47(5):1768–1777. doi: 10.1167/iovs.05-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McFadden SA, Howlett MH, Mertz JR. Retinoic acid signals the direction of ocular elongation in the guinea pig eye. Vision Res. 2004;44(7):643–653. doi: 10.1016/j.visres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Yan DS, Zhou XT, Chen XY, Lü F, Wang J, Hu DN, Qu J. Expression of retinoid acid receptors in human scleral fibroblasts and regulation of growth of fibroblasts by retinoic acid. Zhonghua YanKe ZaZhi. 2007;43(8):750–753. [PubMed] [Google Scholar]
- 13.Li C, McFadden SA, Morgan I, Cui D, Hu J, Wan W, Zeng J. All-trans retinoic acid regulates the expression of the extracellular matrix protein fibulin-1 in the guinea pig sclera and human scleral fibroblasts. Mol Vis. 2010;16:689–697. [PMC free article] [PubMed] [Google Scholar]
- 14.Hoover F, Gundersen TE, Ulven SM, Michaille JJ, Blanchet S, Blomhoff R, Glover JC. Quantitative assessment of retinoid signaling pathways in the developing eye and retina of the chicken embryo. J Comp Neurol. 2001;436(3):324–335. [PubMed] [Google Scholar]
- 15.Fischer AJ, Wallman J, Mertz JR, Stell WK. Localization of retinoid binding proteins, retinoid receptors, and retinaldehyde dehydrogenase in the chick eye. J Neurocytol. 1999;28(7):597–609. doi: 10.1023/a:1007071406746. [DOI] [PubMed] [Google Scholar]
- 16.Rada JA, Hollaway LR, Lam W, Li N, Napoli JL. Identification of RALDH2 as a visually regulated retinoic acid synthesizing enzyme in the chick choroid. Invest Ophthalmol Vis Sci. 2012;53(3):1649–1662. doi: 10.1167/iovs.11-8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bitzer M, Feldkaemper M, Schaeffel F. Visually induced changes in components of the retinoic acid system in fundal layers of the chick. Exp Eye Res. 2000;70(1):97–106. doi: 10.1006/exer.1999.0762. [DOI] [PubMed] [Google Scholar]