Abstract
AIM
To determine whether the PI3K/AKT/mTOR pathway is activated in proliferative vitreoretinopathy (PVR) in homo-sapiens.
METHODS
The retina of controls and patients with PVR were collected and their levels of PI3K, phospho-AKT, phospho-mTOR, phospho-p70S6k and phospho-4EBP-1 were determined by Western blot. The cultured human retinal pigment epithelial cell line D407 was treated with a specific mTOR inhibitor, rapamycin (RAPA) or a PI3K inhibitor, LY294002, of various concentrations and durations. Cell morphology was observed by phase contrast microscopy and the proliferation and apoptosis of treated cells were determined by MTT assay and flow cytometry.
RESULTS
Levels of PI3K, phospho-AKT, phospho-mTOR, phospho-P70S6K and phospho-4EBP1 was increased in the retina in PVR (P<0.05). In D407 cells, both RAPA and LY294002 significantly inhibited cell proliferation and cell cycle progression, and promoted apoptosis (P <0.05); morphologically, the cells became smaller. Both RAPA and LY294002 reduced levels of phospho-AKT, phospho-mTOR, phospho-p70S6k and phospho-4EBP1 expression (P <0.05). RAPA, but not LY294002, had no significant effect on PI3K expression.
CONCLUSION
PI3K/AKT/mTOR signaling pathway is highly activated in the retinal pigment epithelial cells of PVR. The inhibitors of PI3K/AKT/mTOR signaling pathway, RAPA and LY294002, could inhibited the PI3K/AKT/mTOR signaling pathway by reducing the levels of phosphorylation of mTOR pathway components.
Keywords: human retinal pigment epithelial cell, proliferative vitreoretinopathy, PI3K/AKT/mTOR signal pathway
INTRODUCTION
Proliferative vitreoretinopathy (PVR) is a disease process in which ectopic sheets of cells develop in the vitreous and/or on the inner or outer surface of the retina after rhegmatogenous retinal detachment. Improvements in surgical technology have resulted in a high rate of treatment success, although multiple operations are often needed because of re-proliferation of the vitreous or epiretinal membranes[1].
The retinal pigment epithelium (RPE) is a monolayer of differentiated cells located between the neural retina and the choroidal vasculature. In early PVR stages, the attachment of RPE cells to the retinal surfaces followed by cytoplasmic spreading and cellular migration along these surfaces. Later stages of PVR pathogenesis are characterized by cellular proliferation and membrane formation with subsequent cell-mediated membrane contraction and tractional re-detachment of the retina[2]. Recently, the roles of RPE cells are highly concerned in the development of PVR. Experimental studies (including electron microscopy, light microscopy, and immunohistochemistry) have found that, in PVR, RPE cells not only lose their non-proliferative characteristics, but differentiate into macrophages and similar fibroblast-like cells. Normal adult RPE cells almost never proliferate, although various factors can stimulate their proliferation, with loss of normal contact inhibition. The morphology of the RPE cells is also altered; macrophage-like movements and smooth muscle cell-like contractions occur, and, like inflammatory cells, the RPE cells produce inflammatory chemokines. These RPE cell transformations play an important role in the development of PVR. However, the molecular mechanism of their proliferation is unclear and thus there is a lack of effective treatment for PVR[2],[3].
Although numerous attempts have been made to reduce the incidence of PVR and subsequent membrane formation, no effective pharmacologic treatment has been found. Various antiproliferative agents have been tested as adjunctive treatments for PVR, including 5-fluorouracil[3], daunorubicin[4], retinoids[5], 20-benzoyloxycinnamaldehyde (BCA)[6] and alkylphosphocholines[2],[7]. However, data on the clinical efficacy and safety of most of these drugs are limited at present.
Mammalian target of rapamycin (mTOR) is a key intermediary in multiple mitogenic signaling pathways and plays a central role in modulating proliferation and angiogenesis in normal tissues and neoplastic processes[8]. mTOR signaling is essential to intestinal cell migration[9]. PI3K, through AKT, mTOR, p70S6K and key cell cycle proteins such as cyclins, promotes progression through the G1 phase, accelerating the cell cycle[10]. In tumor cell lines, mTOR function can be inhibited by a P13K/AKT pathway-mediated signal that causes cell cycle arrest, inhibiting tumor growth. mTOR may be a target for the development of new anticancer drugs.
Rapamycin (RAPA), an mTOR inhibitor, can strongly inhibits migration of human umbilical vein epithelial cells at doses that do not cause cytotoxicity or apoptosis in vitro[11]. The basic principle is the inhibition of mTOR and its downstream targets 4EBP1/eIF4E, which inhibits the translation of key mRNAs and thereby blocks cell cycle progression from the G1 to the S phase; this results in cell cycle stagnation and apoptosis[8]-[10]. The P13K-specific inhibitor LY294002 inhibits cell growth, but because of its rapid metabolism, short duration of action and toxicity is not used clinically[11]. Little research has been conducted on the role of mTOR in the pathogenesis of PVR, or RPE cell spreading and migration in vitro or in animal experiments.
The purpose of the present study was to assess whether the PI3K/AKT/mTOR pathway is activated in RPE cells in PVR. Specific inhibitors of the PI3K/AKT/mTOR pathway were investigated, with the aim of developing new targets and drugs for the treatment of this refractory eye disease.
MATERIALS AND METHODS
Materials
Human retina
For the control group, 15 retinas were obtained from patients who had suffered ocular trauma. The proliferative membrane groups comprised 30 cases of diabetic retinopathy with PVR (PVR-1) and 30 cases of ocular trauma with PVR (PVR-2). The PVR was standard grade C or above. Samples were obtained by vitreoretinal surgery with an Alcon vitrectomy machine, using a standard pars plana procedure, tweezers and a retinal hook. Strips of epiretinal membrane were transferred to Eppendorf tubes containing TRIzol and stored at -80°C. The use of human tissue material is in accordance with the Declaration of Helsinki on the use of human material for research.
Cell culture and drug treatment
Human RPE D407 cells were purchased from the Central Laboratory of Central South University Xiangya and cultured in DMEM Base with 10% FBS and 10µL/mL penicillin-streptomycin solution. Unless otherwise stated, the cells were maintained at 37°C in a humidified 5% CO2 and 95% air incubator.
RAPA (Sigma, Archri) concentrations were 30ng/mL, 90ng/mL and 180ng/mL. LY294002 (Cell Signaling Inc.) concentrations were 15µmol/L, 30µmol/L and 45µmol/L). Zero wells contained culture medium, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and dimethyl sulfoxide (DMSO); control wells contained cells, culture medium, MTT and DMSO.
Methods
Western blot
After treated with inhibitors, RAPA and LY294002, for 24 hours, the cells were picked out for western blot assay. Tissue and cells were washed twice with ice-cold phosphate buffered saline (PBS), homogenized on ice in 10 volumes (w/v) of lysis buffer containing 20mmol/L Tris-HCl, 1mmol/L EDTA, 50mmol/L NaCl, 50mmol/L NaF, 1mmol/L Na3VO4, 1% Triton-X100 and 1mmol/L PMSF using a homogenizer (DLA×900; Heidoph). The homogenate was centrifuged at 15 000r/min for 30 minutes at 4°C. The supernatant was collected and the protein content determined by BCA assay using a BCA Protein Assay Kit-23227 (Pierce Biotechnology). From each sample preparation, 50µg of total protein was separated by 8% SDS-PAGE and then transferred to PVDF blotting membranes. The total protein extracts were analyzed by immunoblotting with primary antibodies specific for phospho-mTOR (1:400; Cell Signaling), phospho-AKT (1:400; Santa Cruz Biotechnology), PI3K (1:400; Santa Cruz Biotechnology), phospho-p70S6k (1:400; Santa Cruz Biotechnology), phospho-4EBP1 (1:400; Santa Cruz Biotechnology) and β-actin (a housekeeping protein used as a loading control to assure equal amounts of protein in all lanes). After blocking nonspecific binding with 5% BSA in TBS (pH 7.5) containing 0.05% Tween-20 (TBST), primary antibodies were incubated on the membranes overnight at 4°C. Following three washes in TBST, the membranes were incubated for 2 hours at 37°C with secondary antibodies (1:2000, ZDR-5306) labeled with horseradish peroxidase (all from Zhongshan Biotechnology). Immunoreactive strips were identified using a DAB system (DAB Kit-0031; Maixin Biotechnology), as directed by the manufacturer. A BioImaging System (UVP, Upland, CA, USA) was used to capture specific bands, and the optical density (OD) of each band was measured using Image J software. The ratio between the OD of the proteins of interest and β-actin in each sample was calculated as the relative content and expressed graphically.
3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide assay
A tetrazolium dye-reduction (MTT) assay (Sigma-Aldrich) was used to determine cell survival and proliferation. RPE cells were seeded in 96-well plates (Costar, USA) (150µL/well at a density of 3×104/well in DMEM and 10% FCS) and incubated with RAPA (30ng/mL, 90ng/mL or 180ng/mL for 6, 12 or 24 hours) or LY294002 (15µmol/L, 30µmol/L or 45µmol/L for 6, 12 or 24 hours). The medium was then removed, the cells were washed with PBS, and 150µL/well of MTT solution (1.5mL MTT stock, 2mg/mL in PBS, plus 28.5mL DMEM) was added. The cells were then incubated at 37°C for 3 hours. The formazan crystals that formed were dissolved by the addition of DMSO (150L/well). The final concentration of DMSO in the cell culture medium was found to have no antiproliferative effect on RPE cells. Absorption was measured with a scanning multi-well spectrophotometer at 570nm (Molecular Probes, Garching, Germany). Experiments were performed in triplicate and repeated five times. RPE cells at the same passage incubated with PBS served as controls.
Flow cytometry
After 6, 12 and 24 hours in culture, cells from each group were collected and digested with trypsin, centrifuged at 1500r/min for 5 minutes, washed twice with PBS, and fixed with 75% ice-cold ethanol at 4°C overnight. Cells (1×106) were centrifuged at 1500r/min for 5 minutes and the pellets resuspended with 50µg/mL propidium iodide (Sigma) for 45 minutes in the dark before analysis. The percentage of cells in the each of the cell cycle phases was determined using a FACS Calibur Flow Cytometer with CellQuest 3.0 software (BD Biosciences). Experiments were performed in triplicate.
Statistical Analysis
Statistical analysis was performed using SPSS v.11.0 (SPSS Inc., Chicago, IL). All results were expressed as the mean ± standard deviation. To determine the significance of differences, analysis of variance was conducted. The Mann–Whitney U test was performed for nonparametric ordinal data. Differences of P < 0.05 were considered statistically significant.
RESULTS
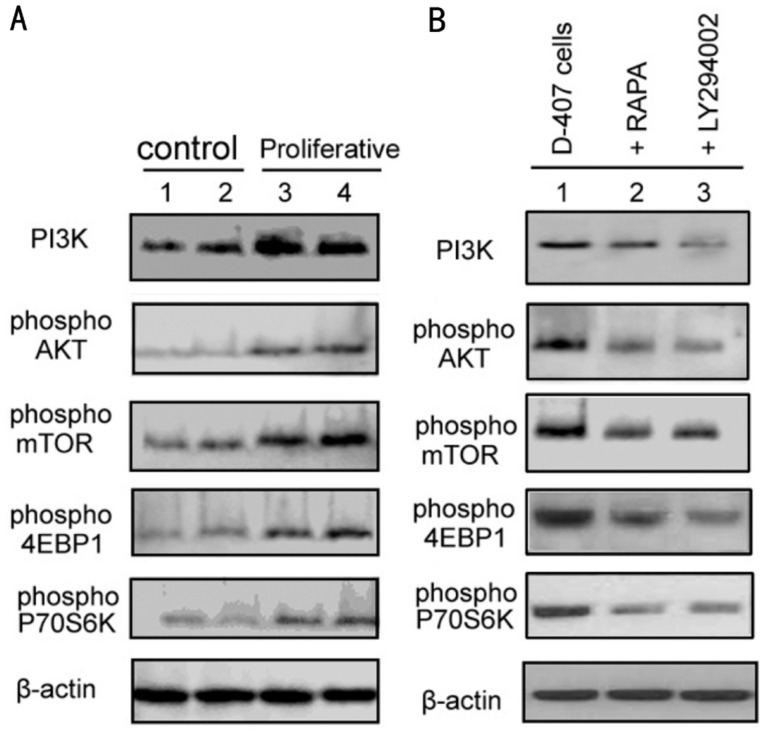
Levels of PI3K, Phospho-AKT, Phospho-mTOR and Phospho-p70s6k Determined by Western Blot as shown in Table 1 and Figure 1A, protein expression of PI3K, levels of phospho-AKT, phospho-mTOR, phospho-4EBP1 and phospho-p70S6K was significantly higher in both PVR-1 and PVR-2 than in control retinal tissues (P<0.05).
Table 1. Western blot to detect the PI3K, phospho-AKT, phospho-mTOR, phospho-p70S6K and phospho-4EBP1 expression.
| Group | PI3K value | p-AKT value | p-mTOR value | p-P70S6K value | p-4EBP1 value |
| Control | 0.30±0.09 | 0.22±0.07 | 0.30±0.04 | 0.15±0.03 | 0.33±0.03 |
| PVR-1 | 1.36±0.05 | 0.73±0.07 | 0.77±0.02 | 0.41±0.04 | 0.72±0.06 |
| PVR-2 | 1.10±0.08 | 0.61±0.06 | 0.68±0.05 | 0.52±0.05 | 0.79±0.10 |
| D-407 | 0.80±0.07 | 0.73±0.06 | 1.05±0.08 | 0.78±0.08 | 1.13±0.04 |
| RAPA | 0.71±0.05 | 0.41±0.04 | 0.41±0.02 | 0.48±0.04 | 0.75±0.08 |
| LY294002 | 0.34±0.04 | 0.30±0.03 | 0.59±0.06 | 0.59±0.05 | 0.59±0.05 |
Figure 1. Western blot was used to detect the PI3K, AKT, mTOR, P70S6K and 4EBP1 expression.
A: 1,2: two cases of control retinal tissue and 3,4: two cases of proliferative membrane tissue; B: 1: D-407 cells, 2: RAPA (90ng/mL, 24 hours), 3: LY294002 (30µmol/L, 24 hours).
Similarly, as we shown in Figure 1B and Table 1, levels of phospho-AKT, phospho-mTOR, phospho-4EBP1 and phospho-p70S6K were significantly decreased in D407 cells treated with LY294002 compared with the control group (P<0.05). Treatment with RAPA only mild suppressed the protein expression of PI3K, without statistical significance. LY294002 treatment significantly inhibited the protein expression of PI3K.
Morphology of RAPA and LY294002 Treated Cells
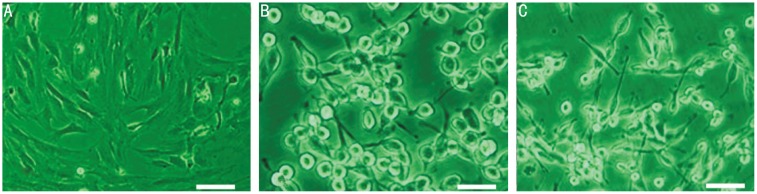
Morphological changes of the RPE cells treated with RAPA (90ng/mL) or LY294002 (30µmol/L) were observed by phase contrast microscopy. It can be seen in Figure 2 that, after treatment with RAPA for 24 hours, the cells were smaller in length, appeared to be floating and showed slow cell proliferation compared with untreated D407 cells. LY294002 treatment for 24 hours also reduced cell length, caused the cells to appear to be floating and slowed their proliferation, compared with untreated cells (Figure 2).
Figure 2. The Change of Rapamycin, LY294002-treated cell morphology.
The morphological changes of RPE cells were observed by phase contrast microscope and Rapamycin(90ng/mL), LY294002(30µmol/L) A: Untreated Human RPE D407 cells; B: Compared with untreated cells, Rapamycin(90ng/mL) treatment (24 hours), the apparent change in cells became smaller length, and appeared floating cells; C: Compared with untreated cells, LY294002 treatment (24 hours), the apparent change in cell morphology, become smaller length, and appeared floating cells.
MTT Assay
An MTT assay was used to determine the growth of cells treated with RAPA for 6, 12 and 24 hours. The OD values, reflecting cell proliferation, differed significantly in RAPA treated cells compared with untreated cells (P<0.05). The higher the concentration of RAPA were applied, the lower the OD and the greater the inhibition of cell proliferation. In addition, at the same concentration of RAPA, the inhibition was greater at 24 hours than at 6 and 12 hours (Table 2).
Table 2. Role of RAPA and LY294002 on the proliferative ability of RPE cells.
| Groups | OD value (Inhibition ratio, %) |
||
| 6 hours | 12 hours | 24 hours | |
| RAPA | |||
| 0 | 0.91±0.06 (0) | 1.13±0.11 (0) | 1.40±0.21 (0) |
| 30ng | 0.94±0.09 (17.00) | 0.83±0.04 (26.40) | 0.69±0.05 (50.71) |
| 90ng | 0.63±0.03 (30.80) | 0.77±0.05 (31.85) | 0.64±0.1 (54.29) |
| 180ng | 0.59±0.03 (35.16) | 0.71±0.04 (37.17) | 0.62±0.13 (55.71) |
| LY294002 | |||
| 0 | 1.02±0.06 (0) | 1.12±0.10 (0) | 1.20±0.14 (0) |
| 15µmol/L | 0.75±0.05 (26.50) | 0.82±0.08 (28.50) | 0.69±0.03 (42.50) |
| 30µmol/L | 0.67±0.03 (34.30) | 0.70±0.06 (37.50) | 0.63±0.11 (47.50) |
| 45µmol/L | 0.50±0.04 (50.76) | 0.64±0.10 (54.10) | 0.62±0.12 (55.70) |
Similar observations were made of cells treated with LY294002 for 6, 12 and 24 hours. Their OD values differed significantly from those of untreated cells (P<0.05). The higher the LY294002 concentration were introduced, the lower the OD and the greater the inhibition of cell proliferation. At the same concentration of LY294002, the inhibition was greater at 24 hours than at 6 and 12 hours (Table 2).
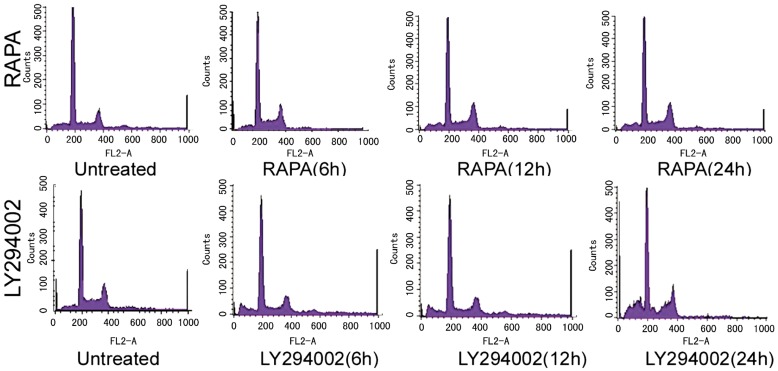
Role of RAPA and LY294002 in the Cell Cycle and Apoptosis of RPE Cells RAPA and LY294002 significantly inhibited cell cycle progression. As shown in Table 3, Table 4 and Figure 3, the number of cells in the G1 phase was significantly increased with RAPA or LY294002 treatment, compared with untreated cells (P<0.05). By blocking cell cycle progression, RAPA and LY294002 inhibited cell growth. RAPA and LY294002 treatment also resulted in significantly reduced numbers of cells in the S phase (P<0.05), further illustrating their inhibition of cell growth.
Table 3. Role of RAPA and LY294002 on cell cycle of RPE cell.
| Groups | Control | 6 hours | 12 hours | 24 hours |
| RAPA | ||||
| G0/G1 phase(%) | 45.03±3.10 | 55.82±2.78 | 66.76±5.98 | 71.96±6.20 |
| S phase (%) | 35.29±2.89 | 21.1±2.13 | 20.9±2.06 | 14.35±1.48 |
| G2/M phase (%) | 19.68±1.12 | 23.04±1.67 | 12.30±1.59 | 13.69±1.35 |
| LY294002 | ||||
| G0/G1 phase(%) | 44.93±2.60 | 60.58±2.78 | 65.16±2.98 | 69.29±5.90 |
| S phase (%) | 35.29±3.49 | 21.56±2.13 | 17.47±1.26 | 15.60±1.48 |
| G2/M phase (%) | 19.68±1.17 | 17.86±1.67 | 17.36±1.59 | 15.11±1.45 |
Table 4. Role of PAPA and LY294002 on apoptosis of RPE cell.
| Time | Control(%) | RAPA(%) | LY294002(%) |
| 6 hours | 6.0±0.55 | 10.42±1.1 | 10.90±0.9 |
| 12 hours | 6.1±0.4 | 16.02±1.45 | 19.29±0.89 |
| 24 hours | 6.2±0.56 | 20.47±1.5 | 15.97±0.72 |
Figure 3. Role of Rapamycin (RAPA), LY294002 on cell cycle, apoptosis of RPE cells.
RAPA significantly induced apoptosis of RPE cells. The proportion of apoptotic cells in the RAPA treatment group (6 hours: 10.42%; 24 hours: 16.02%) was significantly higher than that in the untreated group (6%, P<0.05). A similar effect was observed with LY294002. The proportion of apoptotic cells in the LY294002 treatment group (6 hours: 10.90%; 12 hours: 19.29%; 24 hours: 15.97%) was significantly higher than that in the untreated group. This proportion varied significantly with time, being highest at 12 hours (P<0.05).
Compared with the untreated group, PAPA, LY294002 significantly inhibited the cells in G1 phase, and with the PAPA, LY294002 increased the role of time, stagnation in the number of cells in G1 phase increased, PAPA, LY294002 treatment group compared with untreated cells in G1 phase group were higher than 16.93%, 19.33%, the difference was statistically significant (P<0.05). That PAPA, LY294002 can inhibit cell cycle progression, thereby inhibiting cell growth. On the other hand, PAPA, LY294002 treatment, the synthesis phase in S phase significantly reduced the number of cells (P <0.05), and further that PAPA, LY294002 on cell growth inhibition.
DISCUSSION
Although dramatic improvements have been made in vitreoretinal surgery in the past a few years, proliferative vitreoretinopathy (PVR) is still the common cause of severe visual loss and blindness.
Blinding conditions that can result from retinal detachment are PVR and subretinal fibrosis, which is the cellular membrane on the vitreal or photoreceptor surface of the retina. Though it is frequently not possible to adequately peel sub-retinal membranes, the commonly used treatment is surgical removal. Besides, surgical intervention is often not a permanent solution because recurrence of secondary membranes is common. Ideally, a pharmacologic adjunct could be given at the time of reattachment surgery to prevent membrane formation or, alternatively, at the time of membrane removal to prevent continued growth of the tissue. To date no pharmacologic approaches have proven clinically effective at inhibiting this process.
Phosphorylation of AKT leads to activation of mTOR and then of p70S6K and 4EBPI, whereas phosphorylation of 4EBPI leads to its inactivation and binding with eIF4E. Combined with other translation initiation factors, protein synthesis then begins[5]-[7]. Phosphorylation of p70S6K also promotes protein synthesis. The principal roles of P13K/AKT are: 1) anti-apoptosis, via phosphorylation of the forkhead family transcription factor FKHR, thereby reducing transcription, and through inhibition of BAD phosphorylation activity[11]; 2) regulation of the cell cycle via phosphorylation of P21CIPI and P27KIP1, phosphorylation of the forkhead family transcription factor AFX, reduction of P27KIP transcription, increased cyclin D transcription and increased cyclin D mRNA translation[12]; 3) promotion of angiogenesis via activation of eNOS, HIF-1 (a regulator of VEGF expression) and NF-kB, increasing expression of COX-2[13]; and 4) inhibition of invasion and metastasis via mTOR, inhibition of P70S6K activation, actin filament remodeling and cell motility, and increased MMP-2 mRNA and protein expression[14]. Other signal transduction pathways and gene changes are involved in tumor progression, but the activation of PI3K/AKT is a critical prerequisite[15],[16]. Specific inhibition of LY294002 can prevent P13K phosphorylation, thereby blocking the activation pathway[17],[18].
By Western blot, we found that PI3K, phosphorylation of AKT, mTOR, 4EBP1 and p70S6K occurred in control retinal tissue but was significantly up-regulated in the proliferative membrane, indicating that the PI3K/AKT/mTOR signaling pathway plays a role in cell growth and proliferation. Stimulated by growth factors and nutritional factors, activation by mTOR of downstream target proteins regulates cell growth and metabolism. Two important downstream proteins are p70S6K (ribosomal S6-kinase) and 4EBP1 (eukaryotic initiation factor 4E-binding protein). p70S6k is a key regulator of cell growth, and p70S6K expression levels are increased in proliferative retinas. 4EBP1 is a mRNA5 cap binding protein; eIF4E binds to it and inhibits its activity. On activation of mTOR and phosphorylation of 4EBP1, the eIF4E/4EBP1 combination inhibits protein translation[10]. Our results show that phosphorylation level of 4EBP1 was significantly increased in proliferative membranes compared with control retinas.
To find a target for anti-proliferative drug action, we used human cell line D407 to study the role of the PI3K/AKT/mTOR pathway and its inhibitors in PVR. On treatment with RAPA, the proportion of RPE cells in the G0/G1 phase was increased and those in the S phase were decreased. RAPA-induced apoptosis of RPE cells indicates that RAPA blocks the mTOR/p70S6K signaling pathway, with up-regulated phosphorylation of p70S6K and 4EBP1 expression, arresting the cell cycle in G0/G1.
An MTT assay showed that the higher the concentration of RAPA, the lower the OD, and with increasing concentration of RAPA, the inhibition of cell proliferation increased. While, at the same concentration of RAPA, the inhibition was greater at 24 hours than at 6 and 12 hours. Thus, we concluded that RAPA could significantly inhibit cell proliferation in a dose-dependent manner.
To further elucidate the role of the mTOR/p70S6K signaling pathway, Western blot was used to determine the effect of RAPA on mTOR and its downstream targets. The results showed that RAPA statistically significantly reduced mTOR protein expression. Besides, RAPA treatment also inhibited the phosphorylation of two downstream targets, p70S6K and 4EBP1. This study demonstrated that, in RPE cells with an activated mTOR/p7OS6K signaling pathway, there is a high degree of phosphorylation of p70S6K and 4EBP1, but RAPA can inhibit mTOR activity and decrease the level of phosphorylation of p70S6K and 4EBP1, suggesting that the drug in fact have a effect on phosphorylation. When RPE cells were treated with LY294002, the proportion of cells in the G0/G1 phase was increased and that in the S phase was decreased compared with untreated cells; the difference was statistically significant. LY294002 caused cell cycle arrest in RPE cells by blocking the PI3K/AKT/mTOR signaling pathway, down-regulating phosphorylation of PI3K, AKT, mTOR, p70S6K, and 4EBP1. Compared with untreated RPE cells, the proportion of apoptotic cells in the LY294002 treated group was 13.29% higher. The fact that the rate of apoptosis decreased after 12 hours suggests that the metabolism of LY294002 is rapid and its action of short duration.
In summary, the data presented here suggest that RAPA and LY294002 is effective agents for decreasing proliferation of RPE cells. The data has shown that RPE cell proliferation is associated with the PI3K/AKT/mTOR cell signaling pathway. The finding of two inhibitory targets in present study will provide a foundation for further studies on LY294002 and RAPA, and the treatment of PVR.
Footnotes
Foundation items: Scientific Research Project of Education Department of Liaoning Province, China (No.L2010676); Project of Science and Technology Commission of Shenyang City, China (No. F10-149-9-58); Doctoral Foundation of Ministry of Education of China (No.20102104120027)
References
- 1.Campochiaro PA. Pathogenesis of proliferative vitreoretinopathy. In: Ogden DH, Schachat AP, Murphy RP, Glaser BM, Ryan SJ, editors. Retina. London: C.V. Mosby; 2001. pp. 2221–2227. [Google Scholar]
- 2.Eibl KH, Kook D, Priglinger S, Haritoglou C, Yu A, Kampik A, Welge-Lussen U. Inhibition of human retinal pigment epithelial cell attachment, spreading, and migration by alkylphosphocholines. Invest Ophthalmol Vis Sci. 2006;47(1):364–370. doi: 10.1167/iovs.05-0657. [DOI] [PubMed] [Google Scholar]
- 3.Blankenship GW. Evaluation of a single intravitreal injection of 5- fluorouracil in vitrectomy cases. Graefes Arch Clin Exp Ophthalmol. 1989;227(6):565–568. doi: 10.1007/BF02169453. [DOI] [PubMed] [Google Scholar]
- 4.Khawly JA, Saloupis P, Hatchell DL, Machemer R. Daunorubicin treatment in a refined experimental model of proliferative vitreoretinopathy. Graefes Arch Clin Exp Ophthalmol. 1991;229(5):464–467. doi: 10.1007/BF00166311. [DOI] [PubMed] [Google Scholar]
- 5.Araiz JJ, Refojo MF, Arroyo MH, Leong FL, Albert DM, Tolentino FI. Antiproliferative effect of retinoic acid in intravitreous silicone oil in an animal model of proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1993;34(3):522–530. [PubMed] [Google Scholar]
- 6.Lee JJ, Park JK, Kim YT, Kwon BM, Kang SG, Yoo YD, Yu YS, Chung H. Effect of 2′-benzoyl-oxycinnamaldehyde on RPE cells in vitro and in an experimental proliferative vitreoretinopathy model. Invest Ophthalmol Vis Sci. 2002;43(9):3117–3124. [PubMed] [Google Scholar]
- 7.Eibl KH, Banas B, Schoenfeld CL, May CA, Neubauer AS, Priglinger S, Kampik A, Welge-Lussen U. Alkylphosphocholines inhibit proliferation of human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2003;44(8):3556–3561. doi: 10.1167/iovs.02-1172. [DOI] [PubMed] [Google Scholar]
- 8.Rao RD, Buckner JC, Sarkaria JN. Mammalian target of rapamycin (mTOR) inhibitors as anti-cancer agents. Curr Cancer Drug Targets. 2004;4(8):621–635. doi: 10.2174/1568009043332718. [DOI] [PubMed] [Google Scholar]
- 9.Rhoads JM, Niu X, Odle J, Graves LM. Role of mTOR signaling in intestinal cell migration. Am J Physiol Gastrointest Liver Physiol. 2006;291(3):G510–517. doi: 10.1152/ajpgi.00189.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon YS, Hong HS, Kim JC, Shin JS, Son Y. Inhibitory effect of rapamycin on corneal neovascularization in vitro and in vivo. Invest Ophthalmol Vis Sci. 2005;46(2):454–460. doi: 10.1167/iovs.04-0753. [DOI] [PubMed] [Google Scholar]
- 11.Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369(6483):756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- 12.Browne GJ, Proud CG. Regulation of peptide-chain elongation in mammalian cell. Eur J Biochem. 2002;269(22):5360–5368. doi: 10.1046/j.1432-1033.2002.03290.x. [DOI] [PubMed] [Google Scholar]
- 13.Rowinsky EK. Targeting the molecular target of rapamycin (mTOR) Curr Opin Oncol. 2004;16(6):564–575. doi: 10.1097/01.cco.0000143964.74936.d1. [DOI] [PubMed] [Google Scholar]
- 14.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and rapor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14(14):1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 15.Treins C, Giorgetti-Peraldi S, Murdaca J, Monthouël-Kartmann MN, Van Obberghen E. Regulation of hypoxia-inducible factor (HIF)-1 activity and expression of HIF hydroxylases in response to insulin-like growth factor I. Mol Endocrinol. 2005;19(5):1304–1317. doi: 10.1210/me.2004-0239. [DOI] [PubMed] [Google Scholar]
- 16.Zhang B, Cao H, Rao GN. 15(S)-hydroxyeicosatetraenoic acid induces angiogenesis via activation of P13K-Akt-mTOR-S6K1 signaling. Cancer Res. 2005;65(16):7283–7291. doi: 10.1158/0008-5472.CAN-05-0633. [DOI] [PubMed] [Google Scholar]
- 17.Schalm SS, Fingar DC, Sabatini DM, Blenis J. TOS motif-mediated raptor binding regulates 4E-BP1 multisite phosphorylation and function. Curr Biol. 2003;13(10):797–806. doi: 10.1016/s0960-9822(03)00329-4. [DOI] [PubMed] [Google Scholar]
- 18.Xu G, Zhang W, Bertram P, Zheng XF, McLeod H. Pharmacogenomic profiling of the P13K/PTEN-AKT-mTOR pathway in common human tumors. Int J Oncol. 2004;24(4):893–900. [PubMed] [Google Scholar]