Abstract
AIM
To investigate the effect of simulated dynamic intraocular pressure (SDIOP) during uncomplicated phacoemulsification on postoperative macular and peripapillary retinal nerve fiber layer (RNFL) thickness.
METHODS
Macular and RNFL thicknesses in one eye of patients (n=30) undergoing uncomplicated phacoemulsification were measured by optical coherence tomography preoperatively and 1 week postoperatively. The best-corrected visual acuity, SDIOP, irrigation time (IT), effective phacoemulsification time, entire surgical duration, blood pressure, and heart rate were recorded.
RESULTS
The mean SDIOP and IT was (74.9 ± 27.4)cmH2O and (178.4 ± 21.6) seconds respectively. We divided our patients into two groups based upon IT with greater than 90cmH2O (P>90IT). In Group A (n=14), the P>90IT was greater than the mean P>90IT, and in Group B (n=16), the P>90IT was less than the mean P>90IT. For all patients there was a significant increase in macular thickness one week after cataract surgery (P=0.001). While the RNFL thickness tended to increase, the change was not significant. The postoperative macular thickness of Group A, (277.8 ± 13.7)µm, was significantly thicker than that of Group B, (267.9 ± 15.0)µm (P=0.004). The postoperative peripapillary RNFL thickness of Group A, (96.8 ± 10.8) µm, was not significantly different from Group B. For Group A, the change in macular thickness was positively correlated with P>90IT (R2=0.524, P=0.02). There was no statistical difference in postoperative visual acuity between Groups A and B.
CONCLUSION
After uncomplicated phacoemulsification, increased macular thickness is associated with the IT under high SDIOP. The effect of high SDIOP is limited to the sub-clinical level.
Keywords: intraocular pressure, retina, optical coherence tomography, phacoemulsification
INTRODUCTION
Phacoemulsification with endocapsular intraocular lens (IOL) implantation has become the preferred surgical procedure among cataract surgeons. With the advancement of technology, many surgeons prefer to use maximum vacuum to complete the surgery rapidly. However, high vacuums carry the potential risk of anterior chamber instability. To maintain anterior chamber stability during surgery, more infusion is provided, resulting in transient elevations of intraocular pressure (IOP)[1]. Several studies have evaluated the ocular damage caused by temporary IOP elevation[2]-[4]. Findl et al[3] reported that a 20mmHg increase in IOP for 5 minutes caused reduced blood flow to the optic nerve, retina, and choroid in healthy subjects. The association between nonarteritic anterior ischemic optic neuropathy (NAION) and cataract surgery has also been documented[5]-[8]. These reports documented the possible adverse effects of transient IOP fluctuations on retinal and optic nerve function and visual acuity recovery. However, the relationship between intra-operative spikes of dynamic IOP and the postoperative physiological state of retina remains unclear.
During the early postoperative period, subtle visual acuity loss can easily be overlooked; thus, some anatomic retinal changes can go undetected. Optical coherence tomography (OCT) has been established as a noninvasive, noncontact diagnostic tool that objectively quantifies retinal thickness variations with a precision of 10µm[9],[10]. Some increase in retinal thickness, as detected by OCT, tends to occur after uneventful cataract surgery[11]-[14].
To the best of our knowledge, no study has been conducted in vivo to investigate the effect of changes in dynamic IOP during phacoemulsification on postoperative changes in retinal thickness. Thus, we investigated whether or not a subclinical increase in retinal thickness in the early postoperative course was associated with the level and duration of irrigation during uncomplicated phacoemulsification with endocapsular IOL implantation.
SUBJECTS AND METHODS
Subjects
This prospective study was comprised of a consecutive series of cataract patients scheduled for phacoemulsification and foldable IOL implantation. Patients were eligible only if the media opacity due to the cataract was moderate and OCT examination could be performed. Patients were excluded if the cataract was so dense that an OCT examination was impossible or unreliable. Other exclusion criteria included media opacification for reasons other than cataract, retinal pathologies, age-related macular degeneration, glaucoma, uveitis, amblyopia, diabetes mellitus, or other systemic diseases that could affect the eyes. Informed consent was obtained from each patient before the operation, and the study protocol was approved by the Institutional Review Board of Wenzhou Medical College. The study complied with the Declaration of Helsinki.
Methods
Surgical technique
Mydriasis was induced 30 minutes before surgery with eye drops containing 0.5% tropicamide and 0.5% phenylephrine (Mydrin-P, Santen, Osaka, Japan). All procedures were performed using topical anesthesia (Alcaine, Alcon Laboratories, Ft. Worth, TX, USA) by one experienced surgeon (YEZ) using the Alcon Infiniti Vision System (Alcon Laboratories, Inc.) at the Wenzhou Eye Hospital from December 2008 to May 2009. There were no surgical complications.
A 3.0-mm clear corneal incision and a 1.0-mm side puncture were made firstly. After introducting of viscoelastic material (Medical Hyaluronan Gel, Bausch & Lomb Freda, Shandong, China) into the anterior chamber, a 5.5mm capsulorhexis was made, followed by hydrodissection and phacoemulsification with the phaco-chop technique using the Ozil Torsional Handpiece (Alcon Laboratories, Inc.). Following aspiration of the cortical mass, the anterior and posterior capsular surfaces were polished, and a single-piece foldable IOL (Acrysof Single-Piece IOL, Alcon Laboratories Inc.) was implanted. Once the viscoelastic material was removed, the clear cornea wound was closed with hydration.
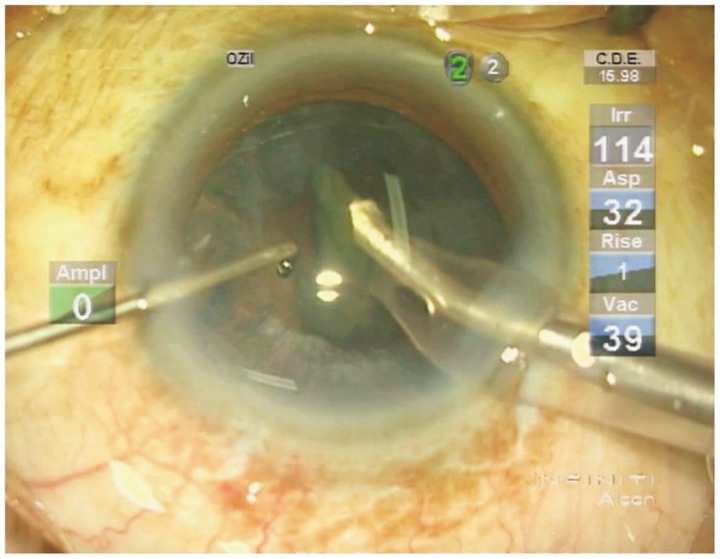
The irrigation column height was set at 120cm above the anterior chamber, and the maximum vacuum was set at 500mmHg during the nucleus removal, cortical cleanup, and viscoelastic removal stages. The irrigation column height was lowered to 80cm during the capsular polishing stage, and the maximum vacuum was set at 150mmHg during anterior capsular polishing and 25mmHg during posterior capsular polishing. The intraoperative effective phacoemulsification time (EPT) and energy of the phacomachine were documented. The whole surgical procedure as well as dynamic parameters during phacoemulsification were recorded in video format by the Alcon Infiniti Vision System (Figure 1) and exported for further analysis. Blood pressure and heart rate were measured three times each by electrocardiogram (ECG) monitor. Systolic pressure (SP) and diastolic pressure (DP) were recorded separately and mean arterial pressure (MAP) was calculated as follows: MAP = 1/3(SP) + 2/3(DP).
Figure 1. Video display during phacoemulsification. The whole surgical procedure, as well as the dynamic parameters during phacoemulsification, was recorded by the video module. The dynamic irrigation pressure (Irr) as displayed in the upper right portion of the screen was defined as the simulated dynamic intraocular pressure (SDIOP).
Postoperatively, antibiotic drops (Cravit, Santen, Osaka, Japan) five times per day and corticosteroid drops (Predforte, Allergan, Irvine, CA, USA) five times per day regimen was started. After the postoperative 1st week, the antibiotic drops were stopped and corticosteroid drops were tapered to end in 4 weeks.
Measurements
All patients underwent a complete ophthalmologic examination preoperatively, including refraction, best-corrected visual acuity (BCVA), IOP, slit-lamp examination, dilated fundus examination, and biometric measurements with the IOL-Master (Zeiss-Meditec, Jena, Germany). An OCT scan was performed with the OCT3 (Stratus OCT, Carl Zeiss Ophthalmic Systems, Inc., Humphrey Division, Dublin, CA, USA) to obtain cross-sectional images of the macula and peripapillary retinal nerve fiber layer (RNFL) through the dilated pupils.
Each OCT measurement was performed in the morning between 9:00 and 10:00 a.m. by the same examiner, minimizing changes due to diurnal and inter-examiner variations. To center the scan, the patient was instructed to stare at an internal fixation point, and the operator identified retinal landmarks. The standard fast macular thickness scan protocol was selected to obtain six consecutive scans centered on the fovea, equally spaced 30° apart. To determine foveal thickness, the OCT images were analyzed with the Stratus OCT-software macular thickness protocol (Version 4.04, Carl Zeiss Meditec). The mean foveal thickness was defined as the average thickness at the intersection of the 6 radial scans of the central sector with a 600µm diameter. The fast RNFL scans were used to measure and calculate the overall and quadrant RNFL thicknesses. The scans were assessed by the software to ascertain proper detection of RNFL boundaries. The thickness of the RNFL was determined by automated computer algorithm that identified the anterior and posterior margins. The analysis algorithm averaged the measurements around the circular scan to obtain the mean RNFL thickness of the 4 quadrants. All of the OCT maps were checked for artifacts. In cases where the retinal surfaces were not found by the built-in algorithm, the measurements were repeated until good quality measurements were achieved. The average of three qualified scans was used to calculate the mean retinal thickness.
Examinations one week after surgery including refraction, BCVA, IOP, slit-lamp examination, and OCT scan. BCVA results were converted into LogMAR for statistical analysis.
Video Analysis
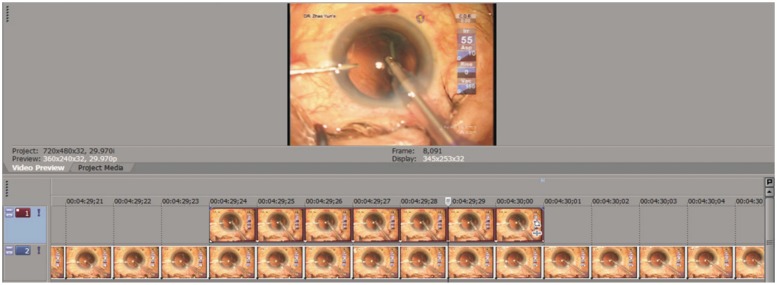
Video for each surgery was exported and analyzed (Figure 2) with video editing software (Vegas Pro 9.0, Sony Creative Software, Inc., Middleton, WI, USA). In the video, irrigation pressure (Irr) was displayed in real-time (Figure 1). We defined the irrigation pressure as simulated dynamic intraocular pressure (SDIOP). During the operation, the instantaneous SDIOP change was displayed frame-by-frame on the time line and analyzed with the Vegas Pro software. The duration of each frame was 0.3 second. All frames were categorized into two levels of pressure depending on the value of SDIOP. For Level 1 the SDIOP was less than 90cm H2O, and for Level 2, SDIOP was equal to or greater than 90cmH2O. The cumulative duration of each level was then calculated (Figure 2).
Figure 2. Analysis interface of the video editing software. The video was deconstructed into frames as shown on the timelines. Depending on the value of irrigation pressure (SDIOP), all frames were categorized into two levels: Level 1 (top, SDIOP < 90cm H2O), and Level 2 (bottom, SDIOP ≥ 90cm H2O). The duration of each frame was 0.3 second. The cumulative duration of each level was then calculated.
Statistical Analysis
Sample size calculation, descriptive statistics, including means ± standard deviations, and statistical analysis were performed with two standard software programs (BIAS 4.0, Ackermann, Frankfurt, Germany; SPSS 11.0 for Windows XP, SPSS Inc., Chicago, IL, USA). The sample size was calculated for a significance level of 0.05 and a power of 0.8. Paired t-tests were used to compare retinal thicknesses of each individual eye before and after phacoemulsification. Linear regression analysis and Pearson's correlation analysis were carried out to analyze the correlation between the change in OCT-measured retinal thickness and SDIOP, EPT, phaco energy, and biometric data. Statistical significance was assessed at the 5% level.
RESULTS
All of the patients were Chinese, and one eye of each (n=30; 17 right and 13 left eyes; 14 males, 16 females; age 71.5±8.4 years; age range: 67-89 years) was included for analysis. The duration of surgery was (7.5±3.1) minutes (Table 1). The EPT was (67.3±24.5) seconds, and the phaco energy was (23.3±11.5)%. The SDIOP was (74.9±27.4)cmH2O (range: 56.5-91.7cmH2O). The instantaneous SDIOP varied from 50cmH2O to 130cmH2O. For each case, we defined the cumulative irrigation time with SDIOP over 90cmH2O as P>90IT. The total phaco irrigation time (TPIT) was (178.4±21.6) seconds (range: 149.5-214.9 seconds). The ratio of P>90IT/TPIT was 0.74±0.033 (range: 0.69 to 0.81). The mean P>90IT (MP>90IT) for all patients was (132.7±15.1) seconds (range: 91.4-153.6 seconds). We then divided our patients into two groups. Group A consisted of 14 patients for whom the individual P>90IT was greater than the MP>90IT, and Group B consisted of 16 patients for whom the individual P>90IT was less than the MP>90IT. The patient demographics and operation data of these two groups were compared with ANOVA and Pearson Chi-Square tests (Table 1), and there were no significant differences between them.
Table 1. Comparison of patient demographics and intra-operative data between groups.
| Demographics and operation data | All eyes (n=30) | Group A (n=14) | Group B (n=16) | P |
| Age (a) | 71.5±8.4 | 73.0±10.2 | 70.2±7.9 | 0.65 |
| Female (n) | 16 | 7 | 9 | 10.15 |
| Duration of surgery (minutes) | 7.5±3.1 | 7.3±2.4 | 7.7±3.2 | 0.24 |
| EPT (seconds) | 67.3±24.5 | 64.3±26.3 | 68.8±25.1 | 0.08 |
| Phaco energy(%) | 23.3±11.5% | 25.3±13.3% | 22.3±10.9% | 0.10 |
| SP (mmHg) | 139.38 ± 13.0 | 142.22 ± 14.4 | 137.59 ± 12.8 | 0.15 |
| DP (mmHg)Heart rate (BPM) | 74.3±14.475.5±18.2 | 73.5±13.677.1±15.8 | 75.0±15.874.1±18.4 | 0.120.07 |
1Statistical analysis with Pearson Chi-Square, Continuity Correction (SPSS 11.0); EPT: effective phaco time, SP: Systolic blood pressure, DP: Diastolic blood pressure, BPM: beats per minute
There was no statistical difference in the pre-surgical macular thickness between Group A and B patients. However, collectively and for each group, there was a significant increase in macular thickness one week after surgery (Table 2). The postoperative macular thickness of Group A, (277.8±13.7)µm, was significantly greater than that of Group B, (267.9±15.0)µm (P<0.004). For all patients, the pre-surgical peripapillary RNFL thickness was 90.7±10.3, and it was increased slightly, but not significantly, one week after surgery. The postoperative peripapillary RNFL thickness of Group A, (96.8±10.8)µm, was thicker than that of Group B, (91.9±8.5)µm, but the difference was not significant (Table 2).
Table 2. Preoperative and postoperative macular thickness and peripapillary RNFL thickness.
| All eyes (n=30) | 1Group A (n=14) | 2Group B (n=16) | |
| Macular thickness (µm) | |||
| Preoperative | 252.7±14.3 | 253.2±15.6 | 250.3±12.9; 3P=0.560 |
| Postoperative (1 week) | 272.2±16.5; 4P=0.001 | 277.8±13.7; 4P=0.001 | 267.9±15.0; 3P=0.004, 4P=0.002 |
| Peripapillary RNFL thickness (µm) | |||
| Preoperative | 90.7±10.3 | 92.3±9.9 | 89.3±11.5; 3P=0.095 |
| Postoperative (1 week) | 94.2±9.5; 4P=0.072 | 96.8±10.8; 4P=0.060 | 91.9±8.5; 3P=0.053; 4P=0.820 |
1P>90IT > MP>90IT; 2P>90IT < MP>90IT; 3Group A vs group B; 4Preoperative vs postoperative.
For all patients, stepwise multivariate linear regression showed that TPIT and the ratio of P>90IT/TPIT each contributed significantly to the increase in macular thickness (P=0.019 and 0.035 respectively). SDIOP, EPT, phaco energy, IOP, axial length, anterior chamber depth, DP, MAP, heart rate, gender, and age were not significantly correlated with the increase (all P>0.05).
For Group A, the change in macular thickness was positively correlated with P>90IT (ΔThickness= -69.70+0.62 P>90IT, R2=0.524, P=0.02). For Group B, there were no statistically significant correlations between the change in macular thickness and IOP, DP, MAP, heart rate, P>90IT, or other surgical parameters.
For all patients, pre-operative visual acuity (LogMAR) was 0.43±0.21, and it increased significantly (P=0.001) to 0.11±0.15 one week after surgery. There was no statistical difference in postoperative visual acuity between Groups A and B (P=0.156).
DISCUSSION
During a modern phacoemulsification procedure, high vacuum along with high irrigation pressure, achieved by increased irrigation bottle height or forced infusion, are commonly used. However, increased irrigation pressure may lead to significantly elevated intraocular pressure[1]. The good agreement between the measured static and theoretical IOPs based on the bottle height above the patient's eye level has been proved by studies both in vitro and in vivo[2],[15]. The Alcon Infiniti Vision System has a Fluidic Management System (FMS) with an irrigation pressure sensor that measures the dynamic irrigation pressure. The FMS is connected to the anterior chamber via a tube. In accordance with the principle of fluid dynamics, the dynamic change of IOP is determined to a large extent by the irrigation pressure. The most accurate measurement of intra-operative IOP is achieved by inserting a pressure transducer into the anterior chamber as described by previous studies[16],[17]. However, it would be unethical to incorporate this invasive method during phacoemulsification. Therefore to facilitate understanding and discussion, we defined the dynamic irrigation pressure as displayed by the FMS as the SDIOP.
The intraocular pressure fluctuated during the surgery and high pressures were transient and intermittent. During the nucleus emulsification and cortex aspiration processes, we found that the SDIOP was over 90 cmH2O (68.4mmHg) for (132.7±15.1) seconds, accounting for 74.4% of the total perfusion time period. The maximum SDIOP measured in this study was 130cmH2O (95.6mmHg), which is much higher than the pressure of central retinal artery (60mmHg)[2]. These data suggest that the retinal artery may experience almost no perfusion most of the time during the phacoemulsification procedure. Obviously, this may cause transient ocular ischemia. In addition, we monitored the blood pressure and heart rate of all patients during the surgery. The diastolic pressure was much lower than systolic pressure, thus the period of non-perfusion was more likely to occur during diastole. This has been confirmed by previous studies regarding the effect of changes in blood pressure on perfusion pressure and intraocular pressure[18]-[20]. Such intraocular pressure may explain the occurrence of acute optic nerve ischemia after cataract surgery[5]-[8]. While in most cases visual acuity is restored, there is the possibility of subclinical retinal changes during intra-operative spikes of intraocular pressure.
OCT is a noninvasive, noncontact imaging method with a precision of 10µm that is an ideal tool for measuring retinal thickness[9],[10]. In contrast to fluorescein angiography, OCT is capable of quantifying retinal thickness changes and giving a pseudohistological insight into retinal structure. Although fluorescein angiography can detect retinal leakage, OCT allows quantification of macular thickness and mapping of the retinal damage. Furthermore, there are reports that visual acuity may be correlated more closely to retinal thickness than to the amount of fluorescein extravasation[21]. Using OCT imagery, we found that cataract patients had a significant increase in macular thickness one week after uncomplicated phacoemulsification. This is consistent with other studies[11]-[14],[22]. We also measured the peripapillary RNFL thickness and found a slight, but not statistically significant, increase in RNFL thickness. In contrast, El-Ashry et al[14] found that the increase in RNFL thickness was significant. The difference between our results and theirs could be attributed to the size, age, and/or ethnicity of the study population, surgical or imaging methodologies, or other factors.
The most important reason for the decline in post-cataract surgery visual acuity is the development of cystoid macular edema (CME), which has an incidence as high as 2%[23]. Hyperfluorescence in angiography is associated with increased retinal thickness and possibly disturbed retinal architecture. Even though the rate of positive angiographic findings with the modern phacoemulsification can range from 9% to 19%[24],[25], the increased macular thickness after cataract surgery remains mostly at the subclinical level and does not affect visual acuity[26],[27]. The etiology of the postoperative retinal changes is not yet to be identified. The three most commonly cited factors for the mechanism of postoperative macular edema development are mechanical traction due to vitreoretinal adhesion, increased prostaglandin production due to anterior segment ischemia, and prostaglandin production secondary to free radical release in the postoperative period with more light exposure to retina[26]. Von et al[27] studied the influence of risk factors on the postoperative course of foveal thickness. They evaluated phacoemulsification time and energy, age, anterior chamber depth, axial length, and preoperative BCVA for differences between operated eyes with and without foveal thickening. None of these considerations turned out to be significant. In present study, we found a positive correlation between the increase in macular thickness and the irrigation time in the sub-group of patients undergoing longer irrigation time with high SDIOP (Group A). However, the relationship was not significant in the other sub-group (Group B) with shorter irrigation time. We propose that the effect of high SDIOP spikes on postoperative macular thickness may be temporally cumulative. Retinal ischemia and macular edema tend to occur in cases with prolonged low arterial perfusion due to high intra-operative IOP.
Slight change in peripapillary RNFL thickness occurred after uneventful cataract surgery, especially in the sub-group with longer duration of irrigation under high SDIOP. Various studies have speculated that autoregulatory responses in ocular arteries occur within certain limits. Geijer and Bill[28] found that high elevations in IOP reduced the perfusion pressures and caused marked reductions in optic nerve blood flow in monkeys. Riva et al[29] suggested that the recovery of optic nerve head blood flow to baseline after acute changes in ocular perfusion pressure was affected by the duration of IOP elevation[29]-[31]. Garhöfer et al[32] reported that a short-term increase of IOP up to 43mmHg does not alter retina or optic nerve head regulation. However, large fluctuations in IOP overwhelm the ocular autoregulatory capacity and lead to compromised posterior segment blood flow[30],[29],[33]. We believe that transiently increased IOP could be one important factor that contributes to the changes in peripapillary RNFL thickness. While we did not find that the increase in RNFL thickness was statistically significant, others did[14]. The clinical significance of these changes remains to be determined, therefore further studies on importance of transient IOP increases during cataract surgery and the clinical sequelae are warranted.
It is the common clinical experience that most eyes do well after cataract surgery. Previous studies have also suggested that cataract extraction increases the incidence of NAION[7],[34],[35], which may result from the transient elevations of IOP that occur immediately after cataract surgery[5],[6]. McCulley et al[5],[7] found that it occurs with an average onset of approximately 35 days, ranging widely from hours to nearly five months postoperatively. Cataract extraction and closed vitrectomy may also lead to retinal and choroidal ischemic infarctions[36]. In present study, all patients had healthy optic nerves according to the preoperative assessment, and the postoperative changes seen by OCT were limited to the sub-clinical level. Studies suggest that cataract patients with compromised optic nerves, such as occur in glaucoma and atrophic optic nerve are more prone to have optic nerve damage during phacoemulsification[7],[34]. Nguyen et al[34] evaluated seven patients with nonarteritic anterior and posterior ischemic optic neuropathy after cataract extraction and found that all of them had vascular risk factors. A history of NAION in the fellow eye has been suggested as an additional risk factor for the incidence of NAION after cataract extraction[37].
In present study, the mean BCVA increased significantly in the postoperative period and was not affected by the change in retina thickness. On one hand, the transient effect on retinal function and structure of high IOP during cataract surgery might be reversible and limited to the sub-clinical level in healthy subjects. On the other hand, visual acuity determination with the LogMAR visual acuity chart might not be sensitive enough to detect the sub-clinical changes in visual function. This is a potential limitation of the present study. Thus, more precise visual function assessments such as contrast sensitivity or visual field[38] tests should be applied in the future studies. Furthermore, the relationship between the transient intra-operative reduced ocular blood flow and long-term effect of visual function remain to be determined.
In conclusion, significant increase in macular thickness as measured by OCT was associated with the irrigation time under high SDIOP after uncomplicated phacoemulsification. The adverse effects of high SDIOP on post-operative visual function remain unclear.
Footnotes
Foundation item: Supported by Research Grants from Zhejiang Provincial Nature Science Foundation, China (No.Y12H120008); Innovation Guiding Program of the Eye Hospital, Wenzhou Medical College, China (No. YNCX201008)
REFERENCES
- 1.Khng C, Packer M, Fine IH, Hoffman RS, Moreira FB. Intraocular pressure during phacoemulsification. J Cataract Refract Surg. 2006;32(2):301–308. doi: 10.1016/j.jcrs.2005.08.062. [DOI] [PubMed] [Google Scholar]
- 2.Zhao H, Ai Y, Niu C, Guan W, Li X, Qin L. Research on influences of transient high IOP during LASIK on retinal functions and ultrastructure. J Ophthalmol. 2009;2009:230528. doi: 10.1155/2009/230528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Findl O, Strenn K, Wolzt M. Effects of changes in intraocular pressure on human ocular haemodynamics. Curr Eye Res. 1997;16(10):1024–1029. doi: 10.1076/ceyr.16.10.1024.9024. [DOI] [PubMed] [Google Scholar]
- 4.Quigley HA, McKinnon SJ, Zack DJ. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest Ophthalmol Vis Sci. 2000;41(11):3460–3466. [PubMed] [Google Scholar]
- 5.McCulley TJ, Lam BL, Feuer WJ. Nonarteritic anterior ischemic optic neuropathy and surgery of the anterior segment: temporal relationship analysis. Am J Ophthalmol. 2003;136(6):1171–1172. doi: 10.1016/s0002-9394(03)00676-7. [DOI] [PubMed] [Google Scholar]
- 6.McCulley TJ, Lam BL, Feuer WJ. A comparison of risk factors for postoperative and spontaneous nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol. 2005;25(1):22–24. doi: 10.1097/00041327-200503000-00006. [DOI] [PubMed] [Google Scholar]
- 7.McCulley TJ, Lam BL, Feuer WJ. Incidence of nonarteritic anterior ischemic optic neuropathy associated with cataract extraction. Ophthalmology. 2001;108(7):1275–1278. doi: 10.1016/s0161-6420(01)00631-5. [DOI] [PubMed] [Google Scholar]
- 8.Fontes BM, Jung LS, Soriano ES, Chicani CF. Nonarteritic anterior ischemic optic neuropathy after uneventful phacoemulsification: case report. Arq Bras Oftalmol. 2007;70(3):544–546. doi: 10.1590/s0004-27492007000300029. [DOI] [PubMed] [Google Scholar]
- 9.Paunescu LA, Schuman JS, Price LL. Reproducibility of nerve fiber thickness, macular thickness, and optic nerve head measurements using Stratus OCT. Invest Ophthalmol Vis Sci. 2004;45(6):1716–1724. doi: 10.1167/iovs.03-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung CK, Chan WM, Yung WH. Comparison of macular and peripapillary measurements for the detection of glaucoma: an optical coherence tomography study. Ophthalmology. 2005;112(3):391–400. doi: 10.1016/j.ophtha.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Ching HY, Wong AC, Wong CC, Woo DC, Chan CW. Cystoid macular oedema and changes in retinal thickness after phacoemulsification with optical coherence tomography. Eye (Lond) 2006;20(3):297–303. doi: 10.1038/sj.eye.6701864. [DOI] [PubMed] [Google Scholar]
- 12.Cagini C, Fiore T, Iaccheri B, Piccinelli F, Ricci MA, Fruttini D. Macular thickness measured by optical coherence tomography in a healthy population before and after uncomplicated cataract phacoemulsification surgery. Curr Eye Res. 2009;34(12):1036–1041. doi: 10.3109/02713680903288937. [DOI] [PubMed] [Google Scholar]
- 13.Sourdille P, Santiago PY. Optical coherence tomography of macular thickness after cataract surgery. J Cataract Refract Surg. 1999;25(2):256–261. doi: 10.1016/s0886-3350(99)80136-9. [DOI] [PubMed] [Google Scholar]
- 14.El-Ashry M, Appaswamy S, Deokule S, Pagliarini S. The effect of phacoemulsification cataract surgery on the measurement of retinal nerve fiber layer thickness using optical coherence tomography. Curr Eye Res. 2006;31(5):409–413. doi: 10.1080/02713680600646882. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y, Li X, Tao A, Guan W, Li X, Qin L. Intraocular pressure and calculated diastolic ocular perfusion pressure during three simulated steps of phacoemulsification in vivo. Invest Ophthalmol Vis Sci. 2009;50(6):2927–2931. doi: 10.1167/iovs.08-2996. [DOI] [PubMed] [Google Scholar]
- 16.Grinbaum A, Blumenthal M, Assia E. Comparison of intraocular pressure profiles during cataract surgery by phacoemulsification and extracapsular cataract extraction. Ophthalmic Surg Lasers Imaging. 2003;34(3):182–186. [PubMed] [Google Scholar]
- 17.Feltgen N, Leifert D, Funk J. Correlation between central corneal thickness, applanation tonometry, and direct intracameral IOP readings. Br J Ophthalmol. 2001;85(1):85–87. doi: 10.1136/bjo.85.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein BEK, Klein R, Knudtson MD. Intraocular pressure and systemic blood pressure: longitudinal perspective: the Beaver Dam Eye Study. Br J Ophthalmol. 2005;89(3):284–287. doi: 10.1136/bjo.2004.048710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karabatakis VE, Natsis KI, Chatzibalis TE. Correlating intraocular pressure, blood pressure, and heart rate changes after jogging. Eur J Ophthalmol. 2004;14(2):117–122. doi: 10.1177/112067210401400206. [DOI] [PubMed] [Google Scholar]
- 20.Perlman JI, Delany CM, Sothern RB. Relationships between 24h observations in intraocular pressure vs blood pressure, heart rate, nitric oxide and age in the medical chronobiology aging project. Clin Ter. 2007;158(1):31–47. [PubMed] [Google Scholar]
- 21.Nussenblatt RB, Kaufman SC, Palestine AG, Davis MD, Ferris FL., 3rd Macular thickening and visual acuity. Measurement in patients with cystoid macular edema. Ophthalmology. 1987;94(9):1134–1139. doi: 10.1016/s0161-6420(87)33314-7. [DOI] [PubMed] [Google Scholar]
- 22.Degenring RF, Vey S, Kamppeter B, Budde WM, Jonas JB, Sauder G. Effect of uncomplicated phacoemulsification on the central retina in diabetic and non-diabetic subjects. Graefes Arch Clin Exp Ophthalmol. 2007;245(1):18–23. doi: 10.1007/s00417-006-0377-4. [DOI] [PubMed] [Google Scholar]
- 23.Stark WJ, Jr, Maumenee AE, Fagadau W. Cystoid macular edema in pseudophakia. Surv Ophthalmol. 1984;28(Suppl):442–451. doi: 10.1016/0039-6257(84)90226-1. [DOI] [PubMed] [Google Scholar]
- 24.Mentes J, Erakgun T, Afrashi F, Kerci G. Incidence of cystoid macular edema after uncomplicated phacoemulsification. Ophthalmologica. 2003;217(6):408–412. doi: 10.1159/000073070. [DOI] [PubMed] [Google Scholar]
- 25.Ursell PG, Spalton DJ, Whitcup SM, Nussenblatt RB. Cystoid macular edema after phacoemulsification: relationship to blood-aqueous barrier damage and visual acuity. J Cataract Refract Surg. 1999;25(11):1492–1497. doi: 10.1016/s0886-3350(99)00196-0. [DOI] [PubMed] [Google Scholar]
- 26.Perente I, Utine CA, Ozturker C. Evaluation of macular changes after uncomplicated phacoemulsification surgery by optical coherence tomography. Curr Eye Res. 2007;32(3):241–247. doi: 10.1080/02713680601160610. [DOI] [PubMed] [Google Scholar]
- 27.Von Jagow B, Ohrloff C, Kohnen T. Macular thickness after uneventful cataract surgery determined by optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2007;245(12):1765–1771. doi: 10.1007/s00417-007-0605-6. [DOI] [PubMed] [Google Scholar]
- 28.Geijer C, Bill A. Effects of raised intraocular pressure on retinal, prelaminar, laminar, and retrolaminar optic nerve blood flow in monkeys. Invest Ophthalmol Vis Sci. 1979;18(10):1030–1042. [PubMed] [Google Scholar]
- 29.Riva CE, Hero M, Titze P, Petrig B. Autoregulation of human optic nerve head blood flow in response to acute changes in ocular perfusion pressure. Graefes Arch Clin Exp Ophthalmol. 1997;235(10):618–626. doi: 10.1007/BF00946937. [DOI] [PubMed] [Google Scholar]
- 30.Fuchsjager-Mayrl G, Wally B, Georgopoulos M. Ocular blood flow and systemic blood pressure in patients with primary open-angle glaucoma and ocular hypertension. Invest Ophthalmol Vis Sci. 2004;45(3):834–839. doi: 10.1167/iovs.03-0461. [DOI] [PubMed] [Google Scholar]
- 31.Kaeser P, Orgul S, Zawinka C, Reinhard G, Flammer J. Influence of change in body position on choroidal blood flow in normal subjects. Br J Ophthalmol. 2005;89(10):1302–1305. doi: 10.1136/bjo.2005.067884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garhöfer G, Resch H, Weigert G, Lung S, Simader C, Schmetterer L. Short-term increase of intraocular pressure does not alter the response of retinal and optic nerve head blood flow to flicker stimulation. Invest Ophthalmol Vis Sci. 2005;46(5):1721–1725. doi: 10.1167/iovs.04-1347. [DOI] [PubMed] [Google Scholar]
- 33.Moorhead LC, Gardner TW, Lambert HM. Dynamic intraocular pressure measurements during vitrectomy. Arch Ophthalmol. 2005;123(11):1514–1523. doi: 10.1001/archopht.123.11.1514. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen LT, Taravella MJ, Pelak VS. Determining whether delayed nonarteritic ischemic optic neuropathy associated with cataract extraction is a true entity. J Cataract Refract Surg. 2006;32(12):2105–2109. doi: 10.1016/j.jcrs.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 35.Lam BL, Jabaly-Habib H, Al-Sheikh N. Risk of non-arteritic anterior ischaemic optic neuropathy (NAION) after cataract extraction in the fellow eye of patients with prior unilateral NAION. Br J Ophthalmol. 2007;91(5):585–587. doi: 10.1136/bjo.2006.0107276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gass JD, Parrish R. Outer retinal ischemic infarction--a newly recognized complication of cataract extraction and closed vitrectomy. Part 1. A case report. Ophthalmology. 1982;89(12):1467–1471. doi: 10.1016/s0161-6420(82)34615-1. [DOI] [PubMed] [Google Scholar]
- 37.Serrano LA, Behrens MM, Carroll FD. Postcataract extraction ischemic optic neuropathy. Arch Ophthalmol. 1982;100(7):1177–1178. doi: 10.1001/archopht.1982.01030040155030. [DOI] [PubMed] [Google Scholar]
- 38.Trible JR, Anderson DR. Factors associated with intraocular pressure-induced acute visual field depression. Arch Ophthalmol. 1997;115(12):1523–1527. doi: 10.1001/archopht.1997.01100160693005. [DOI] [PubMed] [Google Scholar]