Abstract
AIM
To evaluate the short-term effect of the fixed combination of brinzolamide-timolol on the ocular surface in glaucoma patients.
METHODS
This is a prospective study of 23 eyes of 23 patients with newly diagnosed glaucoma. Schirmer I test, tear break-up time (BUT) measurement, conjunctival impression cytology and central corneal thickness (CCT) measurements were performed in one of the eyes of each patients before and 4 weeks after brinzolamide-timolol fixed combination therapy. All patients were asked to answer the OSDI questionnaire form about the ocular surface symptoms at baseline and at 1 week and 4 weeks follow-up visits.
RESULTS
After brinzolamide-timolol fixed combination theraphy Schirmer I, BUT and CCT values decreased but the only statistically significant decrease was seen in BUT test (P=0.03). OSDI scores increased during the follow-up but this increase was not statistically significant (P=0.22, P=0.42 respectively). Impression cytology findings ranged from 0.78±0.42 to 0.95±0.36 according to the Nelson classification. There was no statistically significant difference between baseline and 4 weeks follow up in impression cytology grades (P=0.15).
CONCLUSION
The results of our study indicate that short-term use of brinzolamide-timolol fixed combination theraphy does not have a profound effect on ocular surface except BUT values.
Keywords: fixed combination antiglaucoma drug, ocular surface, impression cytology
INTRODUCTION
Glaucoma is a leading cause of irreversible blindness throughout the world. Worldwide it has became the second most common cause of bilateral blindness[1]. At the present time, reduction of intraocular pressure (IOP) is the only proven effective therapy for reducing the risk of development or progression of glaucoma[2]. As in other disciplines of medicine, the decision on selection of hypotensive agent must be based on patient's findings. Also potential ocular and systemic side-effects of the treatment with the patient's overall health and life expectancy must be taken into account. Adverse effects of topical hypotensive agents may have undesirable effect on ocular surface. In many studies ocular surface changes with anti-glaucomatous drugs have been investigated[3]-[5]. In addition, in many studies it has been demonstrated that these changes were identified as a risk factor for failure of trabeculectomy[6],[7].
Although sometimes single ocular hypotensive agent is adequate, fixed combinations of topical hypotensive agents are frequently indicated to achieve more IOP reduction[8]. Besides the advantages of more IOP reduction, patient compliance to a one bottle instead of two and elimination of the washout effect are the other benefits of fixed combinations[9]. Also in a recent study Cho et al[10] showed that fixed combination antiglaucoma drugs can reduce the adverse ocular surface changes instead of two concomitant drugs in an experimental animal study. The authors explained the reduction in side effects were due to the small concentration of preservatives in the fixed combination.
A fixed combination of brinzolamide and timolol has recently been developed. Brinzolamide is a topical carbonic anhydrase inhibitor, reduces IOP by decreasing aqueous humour secretion[11]. Timolol is a β adrenergic blocker and reduces IOP by decreasing the aqueous humour formation[12]. In recent studies brinzolamide 1% and timolol 0.5% fixed combination was found to be well tolerated with less ocular discomfort[13],[14].
The purpose of this study was to evaluate the short-term effect of the fixed combination of brinzolamide-timolol on the ocular surface in glaucoma patients. We both evaluated the subjective symptoms via a questionnaire form (Ocular Surface Disease Index) and objective findings by Schirmer's test I, impression cytology and tear break-up time (BUT) test.
SUBJECTS AND METHODS
Subjects
Patients were eligible for participation if they met the following inclusion criteria: 18 years of age or older with open angle glaucoma without any medication previously and had not used any other topical medication for the last 1 month. Patients were required to have an IOP 21mmHg or more in at least 1 eye on 2 eligibility visits without any hypotensive agent.
Exclusion criteria were including known hypersensitivity to any component of the brinzolamide-timolol fixed combination, use of topical artificial tear preparations, any history or evidence of ocular surface disorders, any previous ocular surgery or laser treatment, a history of ocular infection or inflammation within the previous 3 months. Patients with any systemic disease or ocular disease that can affect ocular surface and also female patients with pregnancy and lactation were excluded.
Twenty five patients were enrolled; however 23 patients completed the study. Two patients were excluded because of their hypersensitivity to the medication that they did not know before the treatment. Only one eye of each patient was involved randomly. Patients were instructed to use the drop twice daily at the same time of the day (around 9:00 AM and 9:00 PM). All patients were examined at baseline and at 1week and 4 weeks after the medication beginning at the same time of the day.
This was a prospective, single-masked, single center clinical study. The study was reviewed and approved by institutional ethics committee and was conducted with the Declaration of Helsinki. All participating patients provided written informed consent.
Methods
Procedures
Each patient underwent a full ophthalmic examination, including best-corrected visual acuity, IOP measurement with a Goldmann applanation tonometry, slit-lamp biomicroscopy, gonioscopy, stereoscopic fundus evaluation on the slit lamp using a 90 diopter lens, the Humphrey Field Analyzer (HFA) (Humprey-Zeiss systems, Dublin, CA) Swedish Interactive Threshold Algorithm (SITA) 30-2 test.
All patients were asked to answer the OSDI questionnaire form about the ocular surface symptoms at baseline and at 1 week and 4 weeks follow-up visits. Measurements were performed for all patients at baseline and at 4 week, including biomicroscopic evaluation, Schirmer's 1 test, BUT, central corneal thickness (CCT) and impression cytology after treatment. Ophthalmic examination and glaucoma diagnosis were performed by the same physician (Dr. PF). A masked physician performed the BUT. Two masked pathologists examined the impression cytology specimens.
Subjective Test
Ocular surface disease index
In this study we used the Ocular Surface Disease Index (OSDI) to provide a rapid assessment of Ocular Surface Disease. The 12-item questionnaires in OSDI included ocular complaints and impact on the patient's life within last week. Five levels of symptom were presented and these 5 levels of score rangeed from 0 (none of the time) to 4 (all of the time). The OSDI overall score ranged from 0 to 100 which 100 representing presence of a worse OSD and 0 representing absence of a problem. The total OSDI score was calculated as described previously[15] as follows:
A score of 12 or greater was considered as marker of OSD[16].
Objective Test
Tear function determination test
Schirmer I test:
Schirmer I test with topical anesthesia was performed using Schirmer's paper strip placed in the lateral lower conjunctival sac. The paper strip was removed after 5 minutes and the length of the moistened area was recorded.
Break-up time test:
To measure tear break up time (BUT), a drop of sodium fluorescein dye was instilled in the lower conjunctival sac. The tear film was observed under cobalt blue filtered light. The average interval between the last complete blink and the appearance of first dry spot on the precorneal film was calculated. Mean values of the three repeated measurements were obtained.
Impression cytology
After instillation of a drop of proparacaine 0.5 %, a 4 mm×5mm Whatman nitrocellulose filter paper strip was applied to the inferior bulbar conjunctiva without any pressure. The paper peeled off with a forceps after 5 seconds and immediately transferred into a 95 % ethanol. The specimens were stained with both Papanicolau's modification of Gill's technique and periodic acid-Schiff. The examination was performed according to the Nelson's method[17] under a light microscope. Two observers, similarly blinded, examined the specimens.
Grading system
Grade 0:
The epithelial cells are small and round with eosinophilic-staining cytoplasm. The nucleuses are large, basophilic, and nucleo-cytoplasmic ratio of 1/2. The goblet cells with an intensely PAS-positive cytoplasm are abundant, plump, and oval.
Grade 1:
In Grade 1 the epithelial cells are slightly larger and more polygonal and have eosinophilic-staining cytoplasm. The nucleo-cytoplasmic ratio is 1/3. The goblet cells are decreased in number however; they still maintain their plump oval shape, with an intensely PAS-positive cytoplasm.
Grade 2:
The epithelial cells are larger and polygonal, occasionally multinucleated with variably staining cytoplasm. The goblet cells are markedly decreased in number and are smaller, less intensely PAS-positive with poorly defined cellular borders.
Grade 3:
The epithelial cells are large and polygonal with basophilic-staining cytoplasm. The nuclei are small, pycnotic, and completely absent from many cells. The nucleo-cytoplasmic ratio is larger than 1/6. Goblet cells are completely absent.
Central corneal thickness measurement
Central corneal thickness (CCT) measurements were obtained using Pentacam-Scheimpflug imaging system. The system is based on a 180 degree rotating Scheimpflug camera that takes 12 to 50 single captures. The measurements were repeated 3 times and mean values were obtained. The Pentacam measurements were not affected by corneal deformation from Schirmer I and BUT, since they were performed first.
Statistical Analysis
For statistical analyses SPSS v.15. (SPSS Inc., Chicago, IL, USA) was used. Schirmer I test, BUT, impression cytology grades, CCT values and OSDI scores at baseline and follow up visits were compared using Wilcoxon test. Results are presented as mean ± SD. A P-value less than 0.05 was accepted as being statistically significant.
RESULTS
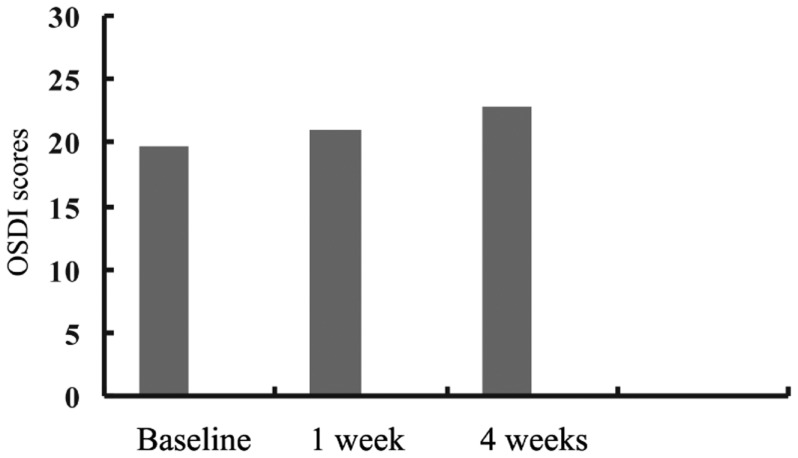
Twenty-three patients with open angle glaucoma 14 women (60.8 %), 9 men (39.2 %) aged 51.5 years (range 20-79 years) were included in the study. Schirmer I test, BUT, CCT values for pre-treatment and post-treatment were shown in Table 1. OSDI scores at baseline and at 1 week and 4 weeks visits were shown in Figure 1.
Table 1. Evaluation of ocular surface values.
| Pre-treatment (min-max) | Post-treatment (min-max) | P | |
| Schirmer I (mm) | 11.82±4.94 | 10.34±3.56 | 0.19 |
| 5.0-25.0 | 4.0-20.0 | ||
| BUT (s) | 12.34±2.7 | 9.86±2.56 | 0.03 |
| 7.0-16.0 | 5.0-15.0 | ||
| CCT (µm) | 541.95±28.03 | 538.13±26.71 | 0.28 |
| 510-594 | 500-581 | ||
| Impession cytology (grade) | 0.78±0.42 | 0.95±0.36 | 0.15 |
| 0-1 | 0-2 |
(x±s)
Figure 1. OSDI scores at baseline and at 1- and 4-week visits.
After brinzolamide-timolol fixed combination therapy, Schirmer I, BUT and CCT values decreased but the only statistically significant decrease was seen in BUT test (P=0.03). OSDI scores increased during the follow up but this increase was not statistically significant (P=0.22, P=0.42 respectively). Impression cytology findings ranged from (0.78±0.42) to (0.95±0.36) according to the Nelson classification. There was no statistically significant difference between baseline and 4 weeks follow up impression cytology grades (P= 0.15).
DISCUSSION
In this study we investigated the effect of brinzolamide-timolol fixed combination therapy on ocular surface. We found no statistically significant difference in OSDI, Schirmer I test, CCT, impression cytology values between pre- and post-treatment. Although there was statistically significant difference between baseline and 4 weeks in BUT values.
Glaucoma is a chronic progressive disease and permanency of the treatment is required for a stable IOP reduction and visual field protection. Poor adherence to treatment is a serious problem for glaucoma patients. Most studies of adherence and persistence with glaucoma medications found that most of the patients discontinued the treatment within the 6 months[18]-[20]. In most of the glaucoma patients, symptoms are mild or absent and treatment is prophylactic. So using eye drops sometimes can be seen meaningless by the patients. Poor adherence is generally related to many factors, and one of them is side effects of the eye drops[21]. Therefore, the effects of the drugs on tear film, conjunctiva and cornea are important issues to be examined. These side effects may occur depending on the active agent or preservative[22]. Brinzolamide-timolol fixed combination contains benzalkonium chloride (BAK). Several studies confirmed the toxic effect of BAK against the ocular surface[23],[24]. However, there is not any study comparing the effects of BAK preserved to BAK free brinzolamide-timolol fixed combination on ocular surface.
The goal of this study was to evaluate the effect of brinzolamide-timolol fixed combination on ocular surface. So we use both objective and subjective methods to strengthen the study. OSDI was used for subjective measurement. OSDI scores were not statistically changed during the follow up from baseline. Although at the baseline visit with the objective markers of ocular surface disease (OSD) such as Schirmer I test and BUT, OSD was not recognized in any patients. The mean OSDI score at baseline was showing OSD with a score of 19.7± 10.8. Similarly to our results, previous studies demonstrated a poor correlation between patient symptoms and objective tests[25],[26]. Possible explanations can be inconsistent clinical signs, the heterogeneity of dry eye disease, and the instability of the tear film in elderly patients[4].
We did not observe any significant difference in Schrimer's 1 and impression cytology grades between baselines and follow up visits. BUT was the only parameter of statistically significant decrease. The time required for onset of the metaplasia has not been demonstrated before, however in a long-term study it has been suggested that it was less than three months[3]. Whereas another study suggested that the time required for onset of the metaplasia is less than one month[27].
The decrease in conjunctival goblet cell density was accepted as the most important parameter in assessing the OSD[28], which results in outside the normal Schirmer and BUT. In a multicenter study with 72 patients switched from dorzolamide/timolol to brinzolamide-timolol combination Rossi et al[29] found significantly improvement in Schirmer and BUT. The improvement in Schirmer and BUT results in patients preference which may improve adherence.
Blurred vision is a problem with brinzolamide-timolol fixed combination because of its suspension form[30]. Despite this problem in a study that was made by Mundorf et al[14] 79.2% of patients preferred brinzolamide-timolol fixed combination. In a recent multicenter study with 14 025 patients Lanzl and Raber[31] demonstrated that 75.9% of the patients preferred brinzolamide-timolol fixed combination over the previous treatment.
Carbonic anhydrase 2 is also expressed in corneal endothelium. So, prescription of a carbonic anhydrase inhibitor (CAI) needs a careful evaluation of corneal endothelial function[19]. The effect of brinzolamide on corneal endothelial cell function has been evaluated by March et al[32]. They observed no meaningful decrease in endothelial cell density or in corneal thickness between treatment groups over the 18 months. CCT was reported as an indicator of corneal endothelial pump and barrier functions[33]. We evaluated the CCT at baseline and 4 weeks visit to assess the effect of brinzolamide-timolol fixed combination on corneal endothelial cell function. We did not find any statistically significant difference in CCT.
In conclusion, we investigated the short-term effect of brinzolamide-timolol fixed combination on corneal surface using objective and subjective methods. We did not observe any significant difference on the parameters except BUT. Long-term studies with a greater number of patients are required to see the effect of Brinzolamide-timolol fixed combination on ocular surface.
REFERENCES
- 1.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80(5):389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The AGIS Investigators The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130(4):429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 3.Turaçli E, Budak K, Kaur A, Mizrak B, Ekinci C. The effects of long-term topical glaucoma medication on conjunctival impression cytology. Int Ophthalmol. 1997;21(1):27–33. doi: 10.1023/a:1005892426045. [DOI] [PubMed] [Google Scholar]
- 4.Skalicky SE, Goldberg I, McCluskey P. Ocular surface disease and quality of life in patients with glaucoma. Am J Ophthalmol. 2012;153(1):1–9. doi: 10.1016/j.ajo.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 5.Broadway DC, Grierson I, O'Brien C, Hitchings RA. Adverse effects of topical antiglaucoma medication. I. The conjunctival cell profile. Arch Ophthalmol. 1994;112(11):1437–1445. doi: 10.1001/archopht.1994.01090230051020. [DOI] [PubMed] [Google Scholar]
- 6.Broadway DC, Grierson I, O'Brien C, Hitchings RA. Adverse effects of topical antiglaucoma medication. II. The outcome of filtration surgery. Arch Ophthalmol. 1994;112(11):1446–1454. doi: 10.1001/archopht.1994.01090230060021. [DOI] [PubMed] [Google Scholar]
- 7.Arici MK, Demircan S, Topalkara A, Güler C, Aker H, Arici DS. Effect of conjunctival structure and inflammatory cell counts on intraocular pressure after trabeculectomy. Ophthalmologica. 1999;213(6):371–375. doi: 10.1159/000027457. [DOI] [PubMed] [Google Scholar]
- 8.Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK, 2nd, Wilson MR, Gordon MO. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 9.Barnebey HS, Orengo-Nania S, Flowers BE, Samples J, Mallick S, Landry TA, Bergamini MV. The safety and efficacy of travoprost 0.004%/timolol 0.5% fixed combination ophthalmic solution. Am J Ophthalmol. 2005;140(1):1–7. doi: 10.1016/j.ajo.2005.02.043. [DOI] [PubMed] [Google Scholar]
- 10.Cho HK, Park MH, Moon JI. Effects of antiglaucoma drugs on the ocular surface in rabbits: a fixed-combination drug versus two concomitant drugs. Jpn J Ophthalmol. 2011;55(6):670–675. doi: 10.1007/s10384-011-0078-3. [DOI] [PubMed] [Google Scholar]
- 11.DeSantis L. Preclinical overview of brinzolamide. Surv Ophthalmol. 2000;44(Suppl 2):119–129. doi: 10.1016/s0039-6257(99)00108-3. [DOI] [PubMed] [Google Scholar]
- 12.Reiss GR, Brubaker RF. The mechanism of betaxolol, a new ocular hypotensive agent. Ophthalmology. 1983;90(11):1369–1372. doi: 10.1016/s0161-6420(83)34380-3. [DOI] [PubMed] [Google Scholar]
- 13.Vold SD, Evans RM, Stewart RH, Walters T, Mallick S. A one-week comfort study of BID-dosed brinzolamide 1%/timolol 0.5% ophthalmic suspension fixed combination compared to BID-dosed dorzolamide 2%/timolol 0.5% ophthalmic solution in patients with open-angle glaucoma or ocular hypertension. J Ocul Pharmacol Ther. 2008;24(6):601–605. doi: 10.1089/jop.2008.0030. [DOI] [PubMed] [Google Scholar]
- 14.Mundorf TK, Rauchman SH, Williams RD, Notivol R, Brinzolamide/Timolol Preference Study Group A patient preference comparison of Azarga (brinzolamide/timolol fixed combination) vs Cosopt (dorzolamide/timolol fixed combination) in patients with open-angle glaucoma or ocular hypertension. Clin Ophthalmol. 2008;2(3):623–628. doi: 10.2147/opth.s4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118(5):615–621. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 16.Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17(5):350–355. doi: 10.1097/IJG.0b013e31815c5f4f. [DOI] [PubMed] [Google Scholar]
- 17.Nelson JD. Impression cytology. Cornea. 1988;7(1):71–81. [PubMed] [Google Scholar]
- 18.Nordstrom BL, Friedman DS, Mozaffari E, Quigley HA, Walker AM. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005;140(4):598–606. doi: 10.1016/j.ajo.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 19.Beckers HJ, Schouten JS, Webers CA, van der Valk R, Hendrikse F. Side effects of commonly used glaucoma medications: comparison of tolerability, chance of discontinuation, and patient satisfaction. Graefes Arch Clin Exp Ophthalmol. 2008;246(10):1485–1490. doi: 10.1007/s00417-008-0875-7. [DOI] [PubMed] [Google Scholar]
- 20.Herndon LW, Brunner TM, Rollins JN. The glaucoma research foundation patient survey: patient understanding of glaucoma and its treatment. Am J Ophthalmol. 2006;141(1 Suppl):22–27. doi: 10.1016/j.ajo.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 21.Patel SC, Spaeth GL. Compliance in patients prescribed eyedrops for glaucoma. Ophthalmic Surg. 1995;26(3):233–236. [PubMed] [Google Scholar]
- 22.Ammar DA, Noecker RJ, Kahook MY. Effects of benzalkonium chloride- and polyquad-preserved combination glaucoma medications on cultured human ocular surface cells. Adv Ther. 2011;28(6):501–510. doi: 10.1007/s12325-011-0029-x. [DOI] [PubMed] [Google Scholar]
- 23.Yee RW, Norcom EG, Zhao XC. Comparison of the relative toxicity of travoprost 0.004% without benzalkonium chloride and latanoprost 0.005% in an immortalized human cornea epithelial cell culture system. Adv Ther. 2006;23(4):511–519. doi: 10.1007/BF02850039. [DOI] [PubMed] [Google Scholar]
- 24.Brasnu E, Brignole-Baudouin F, Riancho L, Guenoun JM, Warnet JM, Baudouin C. In vitro effects of preservative-free tafluprost and preserved latanoprost, travoprost, and bimatoprost in a conjunctival epithelial cell line. Curr Eye Res. 2008;33(4):303–312. doi: 10.1080/02713680801971857. [DOI] [PubMed] [Google Scholar]
- 25.Hay EM, Thomas E, Pal B, Hajeer A, Chambers H, Silman AJ. Weak association between subjective symptoms or and objective testing for dry eyes and dry mouth: results from a population based study. Ann Rheum Dis. 1998;57(1):20–24. doi: 10.1136/ard.57.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schein OD, Tielsch JM, Munõz B, Bandeen-Roche K, West S. Relation between signs and symptoms of dry eye in the elderly. A population-based perspective. Ophthalmology. 1997;104(9):1395–401. doi: 10.1016/s0161-6420(97)30125-0. [DOI] [PubMed] [Google Scholar]
- 27.Herreras JM, Pastor JC, Calonge M, Asensio VM. Ocular surface alteration after long-term treatment with an antiglaucomatous drug. Ophthalmology. 1992;99(7):1082–1088. doi: 10.1016/s0161-6420(92)31847-0. [DOI] [PubMed] [Google Scholar]
- 28.Albietz JM, Bruce AS. The conjunctival epithelium in dry eye subtypes: effect of preserved and non-preserved topical treatments. Curr Eye Res. 2001;22(1):8–18. doi: 10.1076/ceyr.22.1.8.6977. [DOI] [PubMed] [Google Scholar]
- 29.Rossi GC, Pasinetti GM, Sandolo F, Bordin M, Bianchi PE. From dorzolamide 2%/timolol 0.5% to brinzolamide 1%/timolol 0.5% fixed combination: a 6-month, multicenter, open-label tolerability switch study. Expert Opin Pharmacother. 2011;12(16):2425–2431. doi: 10.1517/14656566.2011.589384. [DOI] [PubMed] [Google Scholar]
- 30.Manni G, Denis P, Chew P, Sharpe ED, Orengo-Nania S, Coote MA, Laganovska G, Volksone L, Zeyen T, Filatori I, James J, Aung T. The safety and efficacy of brinzolamide 1%/timolol 0.5% fixed combination versus dorzolamide 2%/timolol 0.5% in patients with open-angle glaucoma or ocular hypertension. J Glaucoma. 2009;18(4):293–300. doi: 10.1097/IJG.0b013e31818fb434. [DOI] [PubMed] [Google Scholar]
- 31.Lanzl I, Raber T. Efficacy and tolerability of the fixed combination of brinzolamide 1% and timolol 0.5% in daily practice. Clin Ophthalmol. 2011;5:291–298. doi: 10.2147/OPTH.S16355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.March WF, Ochsner KI. The long-term safety and efficacy of brinzolamide 1.0% (azopt) in patients with primary open-angle glaucoma or ocular hypertension. The Brinzolamide Long-Term Therapy Study Group. Am J Ophthalmol. 2000;129(2):136–143. doi: 10.1016/s0002-9394(99)00343-8. [DOI] [PubMed] [Google Scholar]
- 33.Lundberg B, Jonsson M, Behndig A. Postoperative corneal swelling correlates strongly to corneal endothelial cell loss after phacoemulsification cataract surgery. Am J Ophthalmol. 2005;139(6):1035–1041. doi: 10.1016/j.ajo.2004.12.080. [DOI] [PubMed] [Google Scholar]