Abstract
AIM
To investigate the complications of intravitreal triamcinolone acetonide (IVTA) for the treatment of macular edema, and to determine the risk factors for intraocular pressure (IOP) elevation.
METHODS
Charts of patients with macular edema secondary to branch retinal vein occlusion (BRVO), diabetic retinopathy and uveitis who had received IVTA injections were reviewed to document its complications. IOP elevation was defined as a pressure of ≥24mmHg at some point during follow-up. Multivariate logistic regression analysis was performed to characterize baseline risk factors for this elevation.
RESULTS
The study included 111 eyes of 65 female and 46 male patients with a mean follow-up of (11.6±5.1) months. Of the 111 eyes, 52 (46.8%) had macular edema secondary to BRVO, 44 (39.6%) had clinically significant diabetic macular edema (CSDME) and 15 (13.5%) had non-infectious uveitis with macular edema. IOP was recorded ≥ 24mmHg in 38 eyes (34.2%) during the follow-up. Higher baseline IOP (P=0.022), younger age (P=0.003), and male gender (P=0.014) were significant risk factors for IOP elevation after IVTA injection. Eyes with prior vitrectomy were less likely to have IOP elevation (P=0.054). Two eyes (5.2% of eyes with increased IOP) underwent trabeculectomy, and 9 eyes (16.3% of the phakic eyes) necessitated cataract surgery. Other complications included branch vein occlusion (1.8%), sterile endophthalmitis (0.9%) and pseudohypopyon (0.9%).
CONCLUSION
IVTA has side effects with IOP elevation and cataract formation being the two most common. A subset of patients is more prone to developing increased IOP following IVTA, namely, younger male patients with higher baseline IOP.
Keywords: intravitreal triamcinolone, complications, risk factors, intraocular pressure
INTRODUCTION
Intravitreal triamcinolone acetonide (IVTA) has been and is still being used for the management of a variety of ophthalmic conditions including choroidal neovascularization[1]-[3], macular edema secondary to retinal vein occlusions[4],[5], clinically significant diabetic macular edema (CSDME)[6]-[8] and noninfectious uveitic macular edema[9]-[11]. The safety of IVTA has been shown in animal studies and human trials[11]-[16]; nevertheless, it can be associated with several complications. Potential risks of IVTA injection include intraocular hemorrhage, endophthalmitis, retinal tear/detachment and corticosteroid-related complications such as elevated intraocular pressure (IOP) and lens opacifications.
The most commonly reported side effect of IVTA is transient IOP elevation and several studies have evaluated the risk factors for increased IOP after IVTA[17],[18]. Identifying the risk factors for the development of such complications may enable better management for patients. Although conflicting results have been reported, some previous studies have suggested that some variables including young age and high baseline IOP were the risk factors for elevated IOP after IVTA[17],[18]. However, an optimal balance between risk and benefit profile has yet to be completely determined and a literature search did not reveal any studies to determine risk factors for elevated IOP after IVTA in the Turkish population.
In this study, the aim was to audit the complications following IVTA and investigate risk factors significantly associated with post-injection IOP elevation in a cohort of patients with macular edema secondary to branch retinal vein occlusion (BRVO), diabetes and uveitis.
SUBJECTS and Methods
Subjects
This study was conducted according to the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of SB Diskapi Yildirim Beyazit Research and Training Hospital. Informed consent for IVTA injection was obtained from all participants.
In this retrospective chart review, consecutive patients who had received one or more IVTA between August 2006 to December 2010 for the management of macular edema secondary to BRVO, diabetic retinopathy and uveitis were included. Only the patients who were regularly followed for more than 3 months after the IVTA injection were included in the study. In patients who had bilateral IVTA injections, only one eye was chosen randomly.
Patients with ocular hypertension, glaucoma, or glaucoma suspects were excluded from the study. In patients who were phakic at baseline, lenticular status was documented using a four-point grading system modified from the Lens Opacities Classification System II[19]: grade 1, mild; grade 2, moderate; grade 3, pronounced; and grade, 4 severe cataract. Eyes having lens opacities of grade 3 and more at baseline and receiving posterior subtenon triamcinolone acetonide injections in the prior 3 months were also excluded.
Methods
All injections were performed using topical anesthetic (Proparakain HCl) drops under sterile conditions. A dose of 40mg/mL of triamcinolone acetonide (Kenacort-A; 40mg/mL, Bristol-Myers Squibb Co., Princeton, NJ) was injected into the vitreous cavity using a 27-gauge needle 3mm to 3.5mm posterior to the limbus in the inferotemporal quadrant. At the end of the procedure, patients were supplied with levofloxacin drops and requested to use them 4 times a day for 3 days. Patency of the central retinal artery, injection site, visual acuity and IOP were routinely examined after the injection. Eyes receiving multiple injections were given injections at a minimum interval of 3 months. Re-treatment was based on clinical or OCT-based evidence of persistent macular edema or deterioration in visual acuity. As this was a retrospective study, the choice of re-treatment was at the discretion of the physician.
Patients had a complete eye examination including best-corrected visual acuity (BCVA), Goldman applanation tonometry, anterior segment assessment, and dilated fundus examination. Patients were followed for the development of complications associated with the treatment and were usually reevaluated 1 day, 1 week and 1 month after the injection, followed by reexaminations at approximately 3 months intervals, unless presence of any adverse event necessitated more frequent examination.
Patient demographic and clinical information were obtained from the clinical chart and any complications including cataract progression, IOP elevation, vitreous hemorrhage and endophthalmitis were also noted. Cataract progression was defined as any change in lens opacification grade or increase in the grade of a posterior subcapsular cataract (PSC) during the follow-up period.
An association was sought between post-injection IOP elevation, defined as an IOP ≥ 24mmHg[18], and several clinical parameters including patients' baseline IOP, age, gender, primary disease (BRVO vs CSDME vs uveitis) integrity of the posterior capsule (intact vs open), any previous posterior sub-Tenon's injection of triamcinolone acetonide or not, lens status (phakic vs pseudophakic and aphakic), history of vitrectomy (vitrectomised and nonvitrectomised eyes), and number of IVTA injections (single vs multiple). Since the distribution of IOP might be skewed to the right in some situations such as aging, history of diabetes, systolic blood pressure, obesity and female gender[20]-[22], we used a cut-off point of 24mmHg as previously defined in the literature[18]. Also, a study by Smithen et al[18] demonstrated a strong correlation between initial pressure and the occurrence of an IOP ≥ 24mmHg during follow-up in nonglaucomatous patients after IVTA treatment. Thus, a pressure elevation 24mmHg or higher might have more serious implications. Elevated IOP and other complications were treated at the discretion of the clinician.
Statistical Analysis
Statistical analysis was conducted using the SAS software 9.0 (SAS, Cary, NC, USA). Multivariate logistic regression models and t-test were used to analyze the data. The odds ratio and 95% confidence intervals were calculated. A P value below 0.05 was considered statistically significant.
Results
Of 138 eyes, 111 eyes of 111 patients (65 females and 46 males) that met the inclusion criteria were studied. Thirteen eyes were excluded due to inadequate follow-up and 11 eyes were excluded due to the missing data in their charts. One eye having lens opacities of grade 3 and two glaucomatous eyes were also excluded. Of the 111 eyes, 52 (46.8%) had BRVO, 44 (39.6%) had CSDME and 15 (13.5%) had uveitis. The mean age was (62.4±12.8) years (24-83 years). The mean age for male patients was (59.1±13.8) years (24-75 years) and was (65.5±11.8) years (37-83 years) for female patients. Fifty-five eyes (49.5%) were phakic, 54 (48.6%) were pseudophakic and 2 (1.8%) were aphakic. Twenty-four eyes (21.6%) had sub-Tenon's triamcinolone injections at least three months before receiving IVTA injection. Thirty-two eyes (28.8%) had two IVTA injections; 4 eyes (3.6%) had three and 1 eye (0.9%) had four IVTA injections during the follow-up. The mean follow-up was (11.6±5.1) months (3-29 months) with no significant difference between males and females. Eighty-seven patients (78.3%) were followed-up for at least 6 months after the first injection.
Intraocular Pressure
The mean baseline IOP was (15.1±3.1)mmHg (5-20mmHg). Of the 111 patients, 38(34.2%) experienced an IOP value of 24mmHg or higher anytime during the follow-up period; among them, 22(57.9%) were male and 16 (42.1%) were female. The mean highest IOP during the follow-up period for all patients was (22.8±7.0) mmHg (14-52mmHg); (7.7±7.0)mmHg higher than the baseline (2-34mmHg); more specifically, it was (21.9±6.3)mmHg for female patients (15-49) and (24.2±7.8)mmHg for male patients (14-52mmHg). The first IOP elevation occurred at a median of 10 weeks (1-44 weeks). The average IOP rose by 7.6%, 26.1%, 39.6%, 18.4%, 13.8%, 6.8% and 5.3% from baseline at 1 week, 4, 12, 24, 36, 48 and 60 weeks after the injection, respectively. IOP elevation peaked at a median of 19 weeks (1-118).
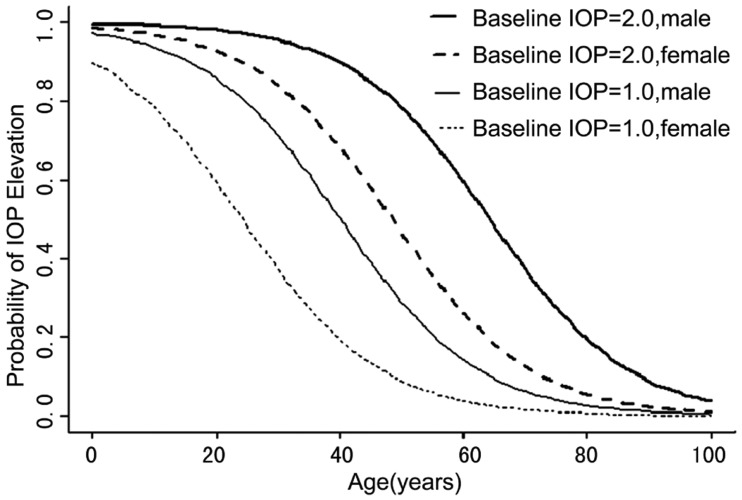
Multivariate logistic regression of IOP elevation with patient characteristics indicates that significant risk factors include higher baseline IOP (P=0.022), younger age (P=0.003), and male gender (P=0.014) (Table 1). The odds ratio of having IOP elevation for a male patient versus a female patient with the same age, baseline IOP and other clinical parameters is 4.02 (95% confidence interval [CI], 1.36-10.75). Younger patients exhibited IOP elevation more than older patients, with an odds ratio (OR) of 0.62 (95% CI, 0.42 -1.02; P=0.003). Further subgroup analysis showed that the mean age of male patients who had IOP elevation was (54.5±12.5) years (24-68 years), 13.8 years younger than those male patients who did not experience IOP elevation (P=0.001, t-test). In contrast, the mean age of female patients who experienced IOP elevation was similar to the ones that did not have IOP elevation [(64.4±10.9 years, (37-76 years) vs (66.8±11.4) years (44-83 years), respectively; P=0.19)]. The mean baseline IOP for patients who experienced IOP elevation was (16.4±3.37)mmHg (5-20mmHg), and was (14.6±2.89) mmHg (7-20mmHg) for patients who did not. Patients exhibited IOP elevation had significantly higher baseline IOP than the other patients, with an OR of 1.42 (95% CI, 1.01-2.29; P=0.022). The impact of baseline IOP, age and gender became even more obvious once the predictive probability of post IVTA IOP elevation was plotted (Figure 1).
Table 1. Multivariate regression analysis assessing the risk factors of intraocular pressure elevation after intravitreal triamcinolone acetonide injection.
| Variables | n | IOP≥24 mmHg n(%) | IOP<24 mmHg n(%) | OR (95% CI) | P |
| Mean age ±SD (yrs) | 111 | 61.0±13.1 | 72.4±10.6 | 0.62 (0.42-1.02) | 0.003 |
| Gender | |||||
| Male | 46 | 22(47.8) | 24(52.2) | 4.02 (1.36-10.75) | 0.014 |
| Female | 65 | 16(24.6) | 49(75.3) | ||
| Mean baseline IOP ±SD (mmHg) | 111 | 16.4±3.4 | 14.6±2.9 | 1.42 (1.01-2.29) | 0.022 |
| Lens status | |||||
| Phakic | 55 | 21(38.2) | 34(61.8) | 1.13 (0.37-3.50) | 0.159 |
| Pseudophakic and aphakic | 56 | 17(30.4) | 39(69.6) | ||
| Posterior Capsule | |||||
| Open | 17 | 5(29.4) | 12(70.6) | 0.59(0.12-2.91) | 0.311 |
| Intact | 94 | 33(35.1) | 61(64.9) | ||
| Multiple IVTA injection | |||||
| Yes | 37 | 15(40.5) | 22(59.5) | 1.16(0.37-3.50) | 0.627 |
| No | 74 | 23(31.1) | 51(68.9) | ||
| Prior sub-Tenon's injection | |||||
| Yes | 24 | 12(50) | 12(50) | 1.57 (0.26- 2.02) | 0.654 |
| No | 87 | 26(29.9) | 61(71.1) | ||
| Retinal diseases | 0,98 | ||||
| BRVO | 52 | 12(23.1) | 40(76.9) | 1.12 (0.11-8.43) | 0.95 |
| DME | 44 | 20(45.5) | 24(54.5) | 1.26 (0.28-4.85) | 0.84 |
| Uveitis | 15 | 6(40) | 9(60) | 1.13 (0.36-4.91) | 0.87 |
| Prior Vitrectomy | |||||
| Yes | 11 | 3(27.3) | 8(73.7) | 0.27(0.12-2.45) | 0.054 |
| No | 100 | 35(35) | 65(65) |
IOP=intraocular pressure; SD=standard deviation; a=years; BRVO=branch retinal vein occlusion; DME= diabetic macular edema; OD=Odds Ratio, CI=Confidence Interval.
n(%)
Figure 1. Predicted probability of IOP elevation as influenced by baseline IOP (5mmHg above or below mean baseline IOP 15mmHg), age (0-100 years), and gender (male vs female) was calculated based on regression coefficients estimated from multivariate logistic regression.
After correcting for other clinical parameters, multivariate analysis did not show any additional risk of post-injection IOP elevation in different disease groups (12/52 in BRVO, 20/44 in CSDME and 6/15 in uveitis, P=0.98, Table 1). Similarly, no association was found between the post-IVTA IOP elevation and status of the lens, integrity of posterior capsule, prior posterior sub-Tenon's capsule triamcinolone acetonide injection and frequency of IVTA injections (Table 1). However, eyes with prior vitrectomy were less likely to have IOP elevation (P=0.057).
IOP was controlled with medical treatment in 27 out of 38 (71.1%) eyes; however, required trabeculectomy in 2 (5.2% of eyes with increased IOP). In 9 eyes (23.7%), treatment was not initiated since the IOP increase had been mild. The mean duration of antiglaucoma treatment was (5.3±3.6) months.
Lens Changes
Nine of the 55 phakic eyes had mild or moderate lenticular opacities in the beginning of the study. The development or progression of lens opacities was occurred in 21 (38.2%) eyes during the follow-up. Of the 21 eyes showing progression of lenticular opacities, 9 had CSDME, 8 had BRVO and 4 had uveitis. Progression of lens opacities was statistically independent of preinjection diagnosis (P > 0.05). The mean time interval between the IVTA injection and the diagnosis of progression of lens opacities was 17.2 months with a range of 3-24 months. Posterior subcapsular opacities were the most common (10 eyes, 47.6%), followed by nuclear (6 eyes, 28.6%), cortical (4 eyes, 19.1%), and combined posterior subcapsular and nuclear (1 eye, 4.7%) opacities. Nine of these opacities necessitated (16.3% of the phakic eyes) cataract surgery at the end of the follow-up period. Age of the patients that developed lenticular opacities (63 ± 10.8 years, range 24-74 years) was not different than the ones who did not [(61.7 ± 12.2 years (29-72), P=0.76]. Also, no association was found between IOP elevation and the lenticular changes (P=0.49). Nine of seventeen (53%) phakic eyes that had multiple IVTA injections developed lens opacities.
Other Complications
One eye (0.9%) with BRVO developed sterile endophthalmitis characterized by some sign of mild intraocular inflammation on the slit lamp examination within 6 days after IVTA injection. The hypopyon was not observed and the patient did not complain of eye pain. The eye was followed up closely, and inflammatory reaction both in the anterior chamber and vitreous spontaneously disappeared 3 weeks after the injection. In one aphakic eye (0.9%) triamcinolone acetonide leaked into the anterior chamber and deposited in the anterior chamber as pseudohypopyon one day after IVTA. These white crystalline deposits suspended in the aqueous disappeared spontaneously without an increase in IOP. Branch vein occlusion developed in 2 eyes (1.8%) having CSDME 6 months after IVTA injection. Both of these eyes did not have a documented IOP rise. No other complications such as endophthalmitis or vitreous hemorrhage have been observed.
Discussion
It is important to identify the patients at risk for IOP elevation after IVTA treatment as IVTA is still being used for the management of a variety of ophthalmic disorders. Our series confirmed the previous reports that one of the most common complications of IVTA injection is steroid-induced IOP elevation[13],[23]; 34% of the eyes experienced IOP ≥24 mmHg. Depending on the dosage of injected IVTA, follow-up period and the definition of elevated IOP, the incidence of IOP increase after IVTA may vary in the literature, but can be as high as 83.3%[11].
We found that higher baseline IOP and younger age had a significant effect on IOP increase after IVTA. Baseline IOP had significant influence on the risk of IOP elevation. Another study also confirmed the role of baseline IOP; an eye with a baseline IOP 1mmHg higher than the group mean had an increased chance of an elevated IOP[24]. In addition, other researchers reported that patients with a higher baseline IOP tended to experience more frequent pressure elevation in nonglaucomatous eyes[18],[23]. The finding that younger age was associated with IOP rise after IVTA, is consistent with previous studies[10],[17]. However, other researchers failed to show association between IOP elevation after IVTA and younger age[18],[23]. This discrepancy between the studies may be due the differences in the patient age distribution. Indeed, Vasconcelos-Santos et al[23] recently suggested that at least for patients older than 40 years of age, age may not be an important risk factor for IOP elevation after IVTA.
We also found that male gender were at greater risk for the development of an IOP elevation. Males were nearly four times more likely to experience an IOP elevation after IVTA. Similarly, Rhee et al[24] reported that women were less likely to experience an IOP elevation at a rate nearly three and a half times that of their male counterparts at every follow up time point in the group of eyes that received second IVTA injections. However, other studies failed to show associations between IOP elevation and gender[17],[23]. The fact that male patients are more prone to developing IOP elevation needs further investigation and may be explained by gender-related differences in such as the number and function of the steroid receptors or metabolism.
The results confirmed previous reports that failed to show an association between IOP elevation and lens status, integrity of posterior capsule, prior posterior sub-Tenon's capsule triamcinolone injections, frequency of IVTA injections and primary ocular pathology[10],[17],[18]. However, a strong correlation between preexisting uveitis and IOP elevation after IVTA injection was reported by Galor et al[25]. This conflicting result may be related to our small sample size which may have limited our ability to demonstrate the association between preexisting uveitis and IOP elevation. Although not statistically significant, IOP elevation after IVTA was observed more frequently in nonvitrectomised eyes than vitrectomised eyes in the present study. Similarly, another published study by Vasconcelos-Santos et al[23] showed lower peak IOP in the vitrectomised group than in the nonvitrectomised group. One possible explanation of decreased incidence of IOP rise in vitrectomised eyes might be faster clearance of triamcinolone acetonide from vitreous in vitrectomised eyes than in the nonvitrectomised eyes[15],[26].
Although the incidence of increased IOP is high after IVTA, in general, most of our patients with elevated IOP after IVTA had only a mild to moderate increase and could be managed with topical glaucoma medications. In the present study, only 2 eyes underwent trabeculectomy resulting in lowering the IOP to normal levels in both eyes. Although the mechanism of post-IVTA IOP increase is not completely understood, the only histopathological specimen obtained during a trabeculectomy showed necrotic changes without deposition of triamcinolone acetonide[9]. The elevation in IOP after steroid treatment is thought to be an increased resistance to aqueous outflow due to microstructral changes at the trabecular meshwork[27].
In addition to elevated IOP, cataract is one of the two most common side effects of intravitreal triamcinolone[28],[29]. In the present study, cataract formation or progression was the second most common complication after IVTA injection and a considerable proportion of eyes (38%) showed progression in lens opacity. The most common type of lens opacity among these eyes was posterior subcapsular opacity (48%) followed by nuclear (28%), cortical (19%) and combined posterior subcapsular and nuclear (5%) opacities. Our results are in accordance with previously published reports that showed an increased rate of PSC formation after IVTA[28],[30]. Among the various subtypes of lens opacification, the rate of PSC formation was found highest after IVTA in a study by Thopmson[30]. The author observed the development and progression of primarily PSC in almost 50% of eyes by one year after IVTA (4mg) injection. Gillies et al[13] observed PSC in 8.9% of the eyes 12 months and 24.2% of eyes 24 months after IVTA injection. Challa et al[31] found progression of nuclear sclerosis cataract in 6 (23%) of 26 eyes at 8 to 12 months after a single injection of IVTA. The increase in the degree of cataract and cataract surgery is significantly correlated with the duration of the follow up[30],[32]. A recent Diabetic Retinopathy Clinical Research Network (DRCR.net) study of people with diabetic macular edema also showed an increase in the rate of cataract during follow-up[8]. In this multicenter, randomized clinical trial, about 55% of phakic eyes treated with IVTA combined with focal/grid laser treatment underwent cataract surgery over the 2 years of follow-up, compared to 15% of phakic eyes that underwent cataract surgery over the one year follow-up[8].
The result of the present study is in contrast with a study by Gillies and colleagues[28], who found an association between steroid-induced elevated IOP and steroid-induced cataract. Gillies et al[28] reported that progression of posterior subcapsular and cortical cataract was significantly higher among eyes experiencing an elevation of IOP after IVTA. However, our study suggests the lack of an association between IOP elevation and cataractous changes. Similarly, no association between progression of PSC and elevated IOP was reported in paper by Thompson[30] and occurrence of PSC progression was observed in many eyes without significant increases in IOP. Reasons for the discrepancy between the studies may be due to the amount of triamcinolone acetonide injected into the vitreous or differences between the study populations.
Sterile endophthalmitis is a rare complication of IVTA injection and is defined as true intraocular inflammation which is not attributed to an infectious process following injection of IVTA[33]. Although the etiology of sterile endophthalmitis is unclear, contamination of the triamcinalone vial with endotoxins or toxic effect of the preservatives present in the vial has been postulated as possible causes[34],[35]. The reported incidence of sterile endophthalmitis following IVTA ranges from 0.1% to 1.6%. Similarly, the rate of steril endophthalmitis of this study was 0.9%.
Pseudohypopyon is also another uncommon complication of IVTA injection and is thought to represent a dispersion of triamcinolone crystals in the anterior chamber. It occurred in one aphakic eye in our study. The pseudohypopyon resolved without intervention within a week. In a retrospective study by Moshfeghi and coworkers[36], the rate of pseudohypopyon was 0.8%. Pseudohypopyon appear to be more common in pseudophakic eyes and usually resolve spontaneously within days or weeks[35]-[38].
IVTA has been and is still being used in the management of retinal venous occlusive diseases. Therefore, our finding of branch vein occlusion in two eyes with CSDME that had no documented IOP increase should be looked into carefully. Certainly, we may not exclude the possibility that patients might have experienced an undocumented IOP rise that might have triggered the vein occlusion. The occurrence of the retinal vein occlusions after IVTA injections has been reported previously[8]. Gillies et al[13] also reported an eye (1/75) with hemiretinal vein occlusion 2 months after IVTA injection.
The limitations of this study are inherited due to its retrospective, non-randomized study design and sample size. The retrospective design with a relatively irregular follow-up may actually have led to an underestimation of the incidence of IOP elevations as the patients might have experienced an undocumented IOP rise. However, scarce studies are available to characterize risk factors that posed increased risk for elevated IOP following intravitreal triamcinolone injection.
In conclusion, despite the limitations of the study, the most common adverse effects associated with IVTA injection were elevation of intraocular pressure and progression of cataract. Despite rare, the IOP elevation may be persistent and need filtrating surgery. Our results suggested that three clinical characteristics, which were high baseline IOP, male gender and younger age were an important risk factor for development of significant IOP elevation after IVTA injection. Further subgroup analysis also showed that probability of post IVTA IOP elevation after IVTA injection increased namely in young male patients with higher baseline IOP. To best of our knowledge, this analysis has not been previously reported. However, further prospective studies with long-term follow-up are needed to confirm these findings. We believe being aware of the risk factors associated with IOP elevation after IVTA injection help ophthalmologists to permit better individualization of the risk–benefit ratio for this treatment.
REFERENCES
- 1.Danis RP, Ciulla TA, Pratt LM, Anliker W. Intravitreal triamcinolone acetonide in exudative age-related macular degeneration. Retina. 2000;20(3):244–250. [PubMed] [Google Scholar]
- 2.Gillies MC, Simpson JM, Luo W, Penfold P, Hunyor AB, Chua W, Mitchell P, Billson F. A randomized clinical trial of a single dose of intravitreal triamcinolone acetonide for neovascular age-related macular degeneration: one-year results. Arch Ophthalmol. 2003;121(5):667–673. doi: 10.1001/archopht.121.5.667. [DOI] [PubMed] [Google Scholar]
- 3.Spaide RF, Sorenson J, Maranan L. Photodynamic therapy with verteporfin combined with intravitreal injection of triamcinolone acetonide for choroidal neovascularization. Ophthalmology. 2005;112(2):301–304. doi: 10.1016/j.ophtha.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Scott IU, Ip MS, VanVeldhuisen PC, Oden NL, Blodi BA, Fisher M, Chan CK, Gonzalez VH, Singerman LJ, Tolentino M, SCORE Study Research Group A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular Edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6. Arch Ophthalmol. 2009;127(9):1115–1128. doi: 10.1001/archophthalmol.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding X, Li J, Hu X, Yu S, Pan J, Tang S. Prospective study of intravitreal triamcinolone acetonide versus bevacizumab for macular edema secondary to central retinal vein occlusion. Retina. 2011;31(5):838–845. doi: 10.1097/IAE.0b013e3181f4420d. [DOI] [PubMed] [Google Scholar]
- 6.Martidis A, Duker JS, Greenberg PB, Rogers AH, Puliafito CA, Reichel E, Baumal C. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002;109(5):920–927. doi: 10.1016/s0161-6420(02)00975-2. [DOI] [PubMed] [Google Scholar]
- 7.Massin P, Audren F, Haouchine B, Erginay A, Bergmann JF, Benosman R, Caulin C, Gaudric A. Intravitreal triamcinolone acetonide for diabetic diffuse macular edema: preliminary results of a prospective controlled trial. Ophthalmology. 2004;111(2):218–224; discussion 224–225. doi: 10.1016/j.ophtha.2003.05.037. [DOI] [PubMed] [Google Scholar]
- 8.Diabetic Retinopathy Clinical Research Network. Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, Ferris FL, 3rd, Friedman SM, Glassman AR, Miller KM, Scott IU, Stockdale CR, Sun JK. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064–1077 e35. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antcliff RJ, Spalton DJ, Stanford MR, Graham EM, ffytche TJ, Marshall J. Intravitreal triamcinolone for uveitic cystoid macular edema: an optical coherence tomography study. Ophthalmology. 2001;108(4):765–772. doi: 10.1016/s0161-6420(00)00658-8. [DOI] [PubMed] [Google Scholar]
- 10.Kok H, Lau C, Maycock N, McCluskey P, Lightman S. Outcome of intravitreal triamcinolone in uveitis. Ophthalmology. 2005;112(11):1916 e1–1917. doi: 10.1016/j.ophtha.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Young S, Larkin G, Branley M, Lightman S. Safety and efficacy of intravitreal triamcinolone for cystoid macular oedema in uveitis. Clin Experiment Ophthalmol. 2001;29(1):2–6. doi: 10.1046/j.1442-9071.2001.00360.x. [DOI] [PubMed] [Google Scholar]
- 12.Dierks D, Lei B, Zhang K, Hainsworth DP. Electroretinographic effects of an intravitreal injection of triamcinolone in rabbit retina. Arch Ophthalmol. 2005;123(11):1563–1569. doi: 10.1001/archopht.123.11.1563. [DOI] [PubMed] [Google Scholar]
- 13.Gillies MC, Simpson JM, Billson FA, Luo W, Penfold P, Chua W, Mitchell P, Zhu M, Hunyor AB. Safety of an intravitreal injection of triamcinolone: results from a randomized clinical trial. Arch Ophthalmol. 2004;122(3):336–340. doi: 10.1001/archopht.122.3.336. [DOI] [PubMed] [Google Scholar]
- 14.McCuen BW. The lack of toxicity of intravitreally administered triamcinolone acetonide. Am J Ophthalmol. 1981;91(6):785–788. doi: 10.1016/0002-9394(81)90013-1. [DOI] [PubMed] [Google Scholar]
- 15.Schindler RH, Chandler D, Thresher R, Machemer R. The clearance of intravitreal triamcinolone acetonide. Am J Ophthalmol. 1982;93(4):415–417. doi: 10.1016/0002-9394(82)90130-1. [DOI] [PubMed] [Google Scholar]
- 16.Scholes GN, O'Brien WJ, Abrams GW, Kubicek MF. Clearance of triamcinolone from vitreous. Arch Ophthalmol. 1985;103(10):1567–1569. doi: 10.1001/archopht.1985.01050100143037. [DOI] [PubMed] [Google Scholar]
- 17.Jonas JB, Degenring RF, Kreissig I, Akkoyun I, Kamppeter BA. Intraocular pressure elevation after intravitreal triamcinolone acetonide injection. Ophthalmology. 2005;112(4):593–598. doi: 10.1016/j.ophtha.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 18.Smithen LM, Ober MD, Maranan L, Spaide RF. Intravitreal triamcinolone acetonide and intraocular pressure. Am J Ophthalmol. 2004;138(5):740–743. doi: 10.1016/j.ajo.2004.06.067. [DOI] [PubMed] [Google Scholar]
- 19.Chylack LT, Jr, Leske MC, McCarthy D, Khu P, Kashiwagi T, Sperduto R. Lens opacities classification system II (LOCS II) Arch Ophthalmol. 1989;107(7):991–997. doi: 10.1001/archopht.1989.01070020053028. [DOI] [PubMed] [Google Scholar]
- 20.Hashemi H, Kashi AH, Fotouhi A, Mohammad K. Distribution of intraocular pressure in healthy Iranian individuals: the Tehran Eye Study. Br J Ophthalmol. 2005;89(6):652–657. doi: 10.1136/bjo.2004.058057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein BE, Klein R, Linton KL. Intraocular pressure in an American community. The Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1992;33(7):2224–2228. [PubMed] [Google Scholar]
- 22.Wu SY, Leske MC. Associations with intraocular pressure in the Barbados Eye Study. Arch Ophthalmol. 1997;115(12):1572–1576. doi: 10.1001/archopht.1997.01100160742012. [DOI] [PubMed] [Google Scholar]
- 23.Vasconcelos-Santos DV, Nehemy PG, Schachat AP, Nehemy MB. Secondary ocular hypertension after intravitreal injection of 4 mg of triamcinolone acetonide: incidence and risk factors. Retina. 2008;28(4):573–580. doi: 10.1097/IAE.0b013e31816079e8. [DOI] [PubMed] [Google Scholar]
- 24.Rhee DJ, Peck RE, Belmont J, Martidis A, Liu M, Chang J, Fontanarosa J, Moster MR. Intraocular pressure alterations following intravitreal triamcinolone acetonide. Br J Ophthalmol. 2006;90(8):999–1003. doi: 10.1136/bjo.2006.090340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galor A, Margolis R, Brasil OM, Perez VL, Kaiser PK, Sears JE, Lowder CY, Smith SD. Adverse events after intravitreal triamcinolone in patients with and without uveitis. Ophthalmology. 2007;114(10):1912–1918. doi: 10.1016/j.ophtha.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 26.Chin HS, Park TS, Moon YS, Oh JH. Difference in clearance of intravitreal triamcinolone acetonide between vitrectomized and nonvitrectomized eyes. Retina. 2005;25(5):556–560. doi: 10.1097/00006982-200507000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Clark AF, Brotchie D, Read AT, Hellberg P, English-Wright S, Pang IH, Ethier CR, Grierson I. Dexamethasone alters F-actin architecture and promotes cross-linked actin network formation in human trabecular meshwork tissue. Cell Motil Cytoskeleton. 2005;60(2):83–95. doi: 10.1002/cm.20049. [DOI] [PubMed] [Google Scholar]
- 28.Gillies MC, Kuzniarz M, Craig J, Ball M, Luo W, Simpson JM. Intravitreal triamcinolone-induced elevated intraocular pressure is associated with the development of posterior subcapsular cataract. Ophthalmology. 2005;112(1):139–143. doi: 10.1016/j.ophtha.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 29.Cekic O, Chang S, Tseng JJ, Akar Y, Barile GR, Schiff WM. Cataract progression after intravitreal triamcinolone injection. Am J Ophthalmol. 2005;139(6):993–998. doi: 10.1016/j.ajo.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 30.Thompson JT. Cataract formation and other complications of intravitreal triamcinolone for macular edema. Am J Ophthalmol. 2006;141(4):629–637. doi: 10.1016/j.ajo.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 31.Challa JK, Gillies MC, Penfold PL, Gyory JF, Hunyor AB, Billson FA. Exudative macular degeneration and intravitreal triamcinolone: 18 month follow up. Aust N Z J Ophthalmol. 1998;26(4):277–281. doi: 10.1111/j.1442-9071.1998.tb01330.x. [DOI] [PubMed] [Google Scholar]
- 32.Jonas JB, Kreissig I, Hugger P, Sauder G, Panda-Jonas S, Degenring R. Intravitreal triamcinolone acetonide for exudative age related macular degeneration. Br J Ophthalmol. 2003;87(4):462–468. doi: 10.1136/bjo.87.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jager RD, Aiello LP, Patel SC, Cunningham ET., Jr Risks of intravitreous injection: a comprehensive review. Retina. 2004;24(5):676–698. doi: 10.1097/00006982-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Roth DB, Chieh J, Spirn MJ, Green SN, Yarian DL, Chaudhry NA. Noninfectious endophthalmitis associated with intravitreal triamcinolone injection. Arch Ophthalmol. 2003;121(9):1279–1282. doi: 10.1001/archopht.121.9.1279. [DOI] [PubMed] [Google Scholar]
- 35.Nelson ML, Tennant MT, Sivalingam A, Regillo CD, Belmont JB, Martidis A. Infectious and presumed noninfectious endophthalmitis after intravitreal triamcinolone acetonide injection. Retina. 2003;23(5):686–6891. doi: 10.1097/00006982-200310000-00014. [DOI] [PubMed] [Google Scholar]
- 36.Moshfeghi AA, Scott IU, Flynn HW, Jr, Puliafito CA. Pseudohypopyon after intravitreal triamcinolone acetonide injection for cystoid macular edema. Am J Ophthalmol. 2004;138(3):489–492. doi: 10.1016/j.ajo.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 37.Amato JE, Lee DH, Santos BA, Akduman L. Steroid hypopyon following intravitreal triamcinolone acetonide injection in a pseudophakic patient. Ocul Immunol Inflamm. 2005;13(2–3):245–247. doi: 10.1080/09273940590928562. [DOI] [PubMed] [Google Scholar]
- 38.Ozkiris A, Erkilic K. Complications of intravitreal injection of triamcinolone acetonide. Can J Ophthalmol. 2005;40(1):63–68. doi: 10.1016/S0008-4182(05)80119-X. [DOI] [PubMed] [Google Scholar]