Abstract
AIM
To evaluate serum concentrations of angiogenesis-related cytokines in proliferative diabetic retinopathy (PDR) before and after vitrectomy.
METHODS
Serum samples were collected from 30 PDR patients with varying severity before and after vitrectomy. Serum concentrations of vascular endothelial growth factor (VEGF), pigment epithelium-derived factor (PEDF), interleukin-8 (IL-8) and interferon-inducible protein-10 (IP-10) were determined by enzyme-linked immunosorbent assays (ELISA).
RESULTS
Serum concentrations of VEGF, PEDF, IL-8 and IP-10 were significantly higher in PDR patients than that in controls, respectively (P<0.05). VEGF concentration decreased significantly in postoperative samples than that in preoperative samples (P<0.05). The concentrations of PEDF, IL-8 and IP-10 did not exhibit significant changes after vitrectomy.
CONCLUSION
Elevated cytokines levels in serum may be diagnostically useful in PDR. Angiogenesis-related cytokines play important roles in the development of PDR, and would instruct the risk assessment of pathogenetic condition in PDR patients.
Keywords: proliferative diabetic retinopathy, cytokine, vitrectomy, enzyme-linked immunosorbent assay, angiogenesis
INTRODUCTION
Systemic microvascular disease is currently the principal pathological change in patients with diabetes mellitus (DM). Diabetic retinopathy (DR), which is a severe complication of DM, is characterized by retinal ischemia, increased vasopermeability, blood retina barrier breakdown and neovasculization in its pathogenesis[1]. Formation of fibrovascular tissue often leads to substantial morbidity and blindness due to vitreous humor and retina hemorrhage, vitreous fibrovascular proliferation and sequent traction retinal detachment[2]. It has been demonstrated that the imbalance between pro- and anti-angiogenesis factors contributed to neovasculization in proliferative diabetic retinopathy (PDR).
Cytokines play quite vital roles in maintaining the normal physiological function of retina. Although the pathogenesis of PDR is still not well understood, the involvements of cytokines, chemokines, inflammatory cells, and angiogenic factors are known to be implicated in the development and progression of PDR. Moreover, some genetic factors may be involved in the pathogenesis of PDR[3],[4]. The genetic factors could affect the serum levels of angiogenic factors such as VEGF, intercellular adhesion molecule-1 (ICAM-1) and basic fibroblast growth factor (bFGF)[3]-[5]. Recently, vitreous and serum cytokines levels have been studied in patients with PDR. Various cytokines, vascular endothelial growth factor (VEGF) and interleukin-8 (IL-8) promote neovascular growth, while pigment epithelium-derived factor (PEDF) and interferon-inducible protein-10 (IP-10) exhibit preventive effects against retinal neovascularization. Studies have confirmed that intravitreous injection of bevacizumab, an anti-VEGF antibody, could prevent neovascular growth and diminish intraoperative bleeding in PDR patients. The benefits from bevacizumab would be associated with the inhibition of VEGF expression. PEDF is rich in vitreous and retina[6],[7]. It is regulated by oxygen with a modality different from VEGF and down-regulates VEGF expression. Inflammatory cytokines, including IL-8 and IP-10, have been recognized as potent chemo-attractant activators of neutrophils and T lymphocytes[8]. Both of them have participated in the pathogenesis of PDR.
To elucidate the potential value of angiogenesis-related cytokines and chemokines including VEGF, PEDF, IL-8 and IP-10, we collected serum samples of PDR patients before and after vitrectomy, and detected the cytokines concentrations by using ELISA. Herein, we aim to investigate the roles of angiogenesis-related cytokines in the pathophysiology of PDR progression.
SUBJECTS AND METHODS
Subjects
General information
The study is a prospective, interventional, case control study. It was approved by the Institutional Ethics Committee of the Central Hospital of Wuhan. Informed consent was taken from every patient. Thirty DM patients with PDR were selected as experimental group. Thirty cases of age-matched healthy individuals were selected as control group. Patients were classified according to the presence or absence of DR and degree of severity using the final scale of the ETDRS Classification.
Ocular exclusion criteria were intraocular surgery, photocoagulation, uveitis and ocular trauma. For patients with photocoagulation done before, there was at least 3 months post laser time period for inclusion. Systemic exclusion criteria included ischemic cerebrovascular disorders, ischemic cardiovascular disorder, hepatic and renal dysfunction. Careful routine examination including visual acuity, intraocular pressure, slit lamp biomicroscopy and funduscopy were used to establish the diagnosis. Preoperative, intraoperative, and postoperative fundus findings were recorded for each subject. The severity of diabetic retinopathy was assessed by standardized color fundus photography and fluorescein angiography. During the vitrectomy, a standard three-port pars plana vitrectomy (PPV) was performed. Endolaser photocoagulation was performed, and when neovascular tissues were present, they were cut and removed.
Methods
Sample collection
For controls, serum specimens were collected on admission. For PDR patients, serum specimens were collected before and 3 days after vitrectomy. All the samples were obtained in a sterile tube, placed immediately on ice, centrifuged at 2 000r/min for 5 minutes to separate the cell contents, and then rapidly frozen at -80°C until processed. All serum specimens were collected in the morning hours after an overnight fast.
Sample measurement
Serum samples were thawed at room temperature and used for cytokine assays. For VEGF assays, serum samples were diluted 1:2 in a volume of 100µL. For PEDF assays, serum samples were diluted 1:10 in a volume of 100µL. The levels of VEGF, PEDF, IL-8 and IP-10 were measured by enzyme-linked immunosorbent assay (ELISA) using the Quantikine assay kit (R&D systems). The detection steps were referred to the test procedures of the kit. The optical density (ABS) of the final reaction plate was detected by a microplate reader at 450nm wave length. The cytokine concentrations (pg/mL) of the samples were derived according to the standard calibration curve of various cytokines.
Statistics Analysis
All the statistical analyses were done by SPSS version 10.0. Data were expressed as the mean±SD or median and range. Comparisons of groups were analyzed by independent samples t-test. Comparisons of data before and after vitrectomy were analyzed by dependent samples t-test. Statistical significance was defined as P<0.05.
RESULTS
Clinical Characteristics of PDR Patients
Thirty patients with PDR were included in the study (30 eyes), of which male 18 eyes and female 12 eyes. The mean age of the diabetic patients was 52 years (38-73 years). The mean level of HbA1c was 8.0% (5.8%-9.9%), and the duration of diabetes mellitus was 12 years (Table 1).
Table1. Clinical characteristics of PDR patients.
| Control group | PDR group | |
| Cases/eyes | 30/30 | 30/30 |
| Male/female | 16/14 | 18/12 |
| Age(years) | 40-75 | 38-73 |
Cytokine Profiles in PDR Patients
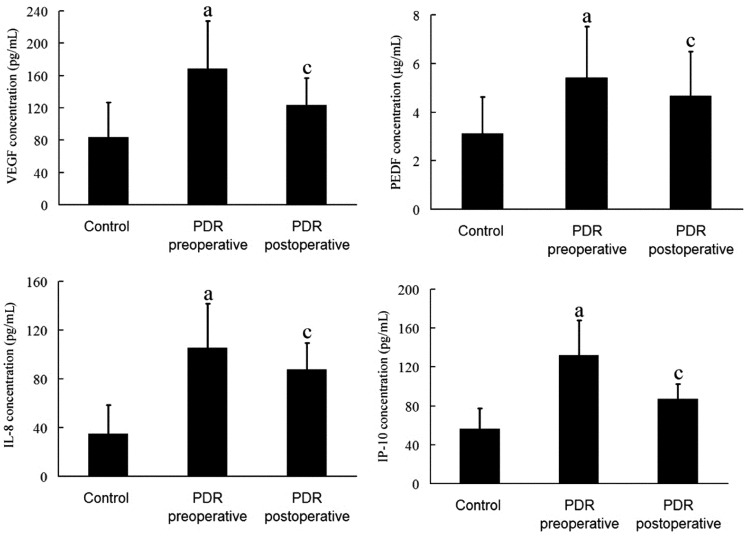
All the serum concentration of various cytokines was shown in Figure 1. Serum concentration of VEGF was 168.45±59.23pg/mL in proliferative diabetic retinopathy, much greater than in controls (83.34±43.27pg/mL, P<0.05). The concentrations of PEDF, IL-8 and IP-10 in PDR group were also greater than that in controls (5.43±2.08 vs 3.12±1.51µg/mL, 105.54±36.22 vs 35.17±23.31pg/mL, 132.12±35.78 vs 56.32±21.14pg/mL, P<0.05). The serum VEGF concentration decreased significantly in postoperative specimens than that in preoperative specimens (P<0.05), while the concentrations of PEDF, IL-8 and IP-10 did not exhibit significant difference.
Figure 1. Cytokines concentrations in the serum of the control and PDR group.
aP<0.05 vs control; cP<0.05 vs PDR preoperative.
DISSCUSION
In the current study, we measured several angiogenesis-related cytokines in the serum of PDR patients. Analysis of data via ELISA identified elevated serum concentrations of VEGF, PEDF, IL-8 and IP-10 in patients with PDR. It was also found that the VEGF level in the serum decreased remarkably after vitrectomy.
Type 2 diabetes is considered to be a kind of chronic inflammatory disease. It can lead to severe microvascular complications, such as proliferative diabetic retinopathy and diabetic optic neuropathy. Studies showed that cytokine-mediated inflammatory response could induce insulin resistance and pancreatic B-cell apoptosis, which would contribute to the development of type 2 diabetes and the related complications.
VEGF is a multifunctional cytokine which increases microvascular permeability and directly stimulates endothelial cell growth and angiogenesis. In the pathogenesis of diabetic retinopathy, VEGF is the principal cytokine to promote angiogenesis, while PEDF acts as a main antagonistic component. VEGF can also stimulate the breakdown of the blood retina barrier, indirectly promote the progression of DR. In some researches regarding various cytokines, it has been demonstrated that hypoxia could induce VEGF expression in vitro and in vivo, and mediate ischemia-induced retinal neovascularization[9]. Moreover, it has been confirmed that the levels of VEGF in vitreous and serum of diabetic patients are much higher than those in health controls[10].
PEDF, which belongs to serine protease inhibitor gene superfamily, exhibits a variety of biological activity. In diabetic complications, PEDF exerts effects of neovasculization inhibition, anti-inflammation, antioxidation, neurotrophy and neuroprotection[11],[12]. PEDF was first purified from human retinal pigment epithelial cells, and was maintained high levels in retinal matrix. As the primary angiogenesis inhibitor, PEDF could restrain the growth and migration of retinal vascular endothelial cell, and contribute to the angiogenic homeostasis in ocular tissues. It can also inhibit retinal neovasculization induced by hypoxia, and promote retinal reparation against mechanical, light- and hypoxia-induced injuries. Also, PEDF could inhibit the proliferation and migration of endothelia cells induced by VEGF in vitro. It has been reported that VEGF could upregulate PEDF expression via VEGFR-1. In the present study, the results showed that serum VEGF and PEDF levels increased in PDR patients, which were in accordance with the previous studies. We infer that the elevation of serum PEDF levels in PDR correlated significantly with the changes of VEGF. The increase of PEDF might be due to the compensatory response to inhibit the increased VEGF.
It has been verified that vitrectomy relieves the retinal hypoxia in ischemic areas. Successful vitrectomy can inhibit the progression of retinal neovascularization in diabetic retinopathy[13]. The photocoagulation is associated with reduced neovascular activity. It may act on the outer retina including retinal pigment epithelium, and initiate some cascades to inhibit retinal neovascularization. Spranger et al[14],[15] demonstrated that the VEGF concentrations in the vitreous of PDR patients decreased after retinal photocoagulation, while PEDF increased after the photocoagulation therapy. We speculate that vitrectomy can remove the vitreous and proliferative membranes and reduce the oxygen consumption of the retina. The retinal laser photocoagulation to the avascular area and new blood vessels can also destroy the photoreceptors; reduce the oxygen consumption of the outer retina. Both of vitrectomy and photocoagulation reduce VEGF production in these areas and decreases new vessel formation. However, we could not detect significant concentration changes of PEDF after vitrectomy, probably owing to the insufficient effects of vitrectomy and retinal photocoagulation to the cytokine levels in serum. Considering the clues mentioned above, we supposed that the imbalance of pro- and anti-angiogenesis cytokines would contribute to the retinal neovasculization. At present, the anti-VEGF therapy towards diabetic retinopathy is provided with certain theoretical evidence. However, we inferred that the therapeutic effect of anti-VEGF only on diabetic retinopathy would be insufficient, because other cytokines except VEGF, especially some chemokines, also participate in the pathogenesis of PDR.
Chemokines belong to a superfamily of cytokines. They are micromolecular cell products which orchestrate the recruitment of leukocytes into the sites of inflammation. Recently, it was demonstrated that chemokines play pivotal roles in mediating angiogenesis and fibrosis[16]. IL-8 is a cytokine mainly produced by monocytes/macrophages, and is involved in the pathogenesis of various autoimmune diseases. Retinal vascular endothelial cells secrete IL-8 in the course of retinal vascularization[17]. Furthermore, retinal glial cells reactively secrete IL-8 in response to hypoxia[18]. It has been demonstrated that IL-8 increased in the hypoxic microenvironment of the diabetic retinopathy, and that IL-8 could mediate angiogenesis via both VEGF dependent and independent mechanisms[19],[20]. In the vitreous of patients with PDR, increased levels of IL-8 have been found. Moreover, elevated vitreous level of IL-8 may be in association with large retinal vessel obliteration and related to ischemia[21]. In this study, the results showed that IL-8 increased in PDR patients. We supposed that elevated IL-8 level could be a marker of ischemic inflammatory reaction. Anti-IL-8 therapy would inhibit retinal neovasculization in PDR.
IP-10 is a kind of chemokine to promote the activation of T-helper cells and the migration of lymphocytes, which are signs of T cell response. Several studies reported that IP-10 is a potent inhibitor of angiogenesis and may have an inhibitory effect on fibrosis[22]. In vitro and in vivo studies have confirmed that VEGF could induce IP-10 expression in the pathogenesis of PDR[23]. The increased expression of IP-10 induced by VEGF would contribute to the main pro-inflammatory mechanism in the immune responses of PDR. A previous report documented elevated IP-10 levels in vitreous humor samples from patients with PDR[24]. Our study demonstrated increased serum levels of inflammatory cytokines IL-8 and IP-10 in PDR patients. However, the results in this study showed no significant difference of the serum IL-8 and IP-10 levels after vitreoretinal surgery. But it seemed undeniable that IL-8 and IP-10 participated in the inflammatory and immunological processes of PDR.
In conclusion, the serum levels of angiogenesis-related cytokines (VEGF, PEDF, IL-8 and IP-10) increased in PDR patients. Furthermore, the serum VEGF levels decreased greatly after vitrectomy. The angiogenesis-related cytokines may act as key regulators in the pathogenesis of PDR and provide potential tools for risk assessment in PDR patients. However, it still needs further studies to explore the effects and underlying relationships of various cytokines in diabetic retinopathy.
REFERENCES
- 1.Kim SJ, Kim S, Park J, Lee HK, Park KS, Yu HG, Kim Y. Differential expression of vitreous proteins in proliferative diabetic retinopathy. Curr Eye Res. 2006;31(3):231–240. doi: 10.1080/02713680600557030. [DOI] [PubMed] [Google Scholar]
- 2.Kohno R, Hata Y, Mochizuki Y, Arita R, Kawahara S, Kita T, Miyazaki M, Hisatomi T, Ikeda Y, Aiello LP, Ishibashi T. Histopathology of neovascular tissue from eyes With proliferative diabetic retinopathy after intravitreal bevacizumab injection. Am J Ophthalmol. 2010;150(2):223–229. doi: 10.1016/j.ajo.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 3.Petrovic MG, Osredkar J, Saraga-Babić M, Petrovic D. K469E polymorphism of the intracellular adhesion molecule 1 gene is associated with proliferative diabetic retinopathy in Caucasians with type 2 diabetes. Clin Experiment Ophthalmol. 2008;36(5):468–472. doi: 10.1111/j.1442-9071.2008.01785.x. [DOI] [PubMed] [Google Scholar]
- 4.Petrovic MG, Krkovic M, Osredkar J, Hawlina M, Petrovic D. Polymorphisms in the promoter region of the basic fibroblast growth factor gene and proliferative diabetic retinopathy in Caucasians with type 2 diabetes. Clin Experiment Ophthalmol. 2008;36(2):168–172. doi: 10.1111/j.1442-9071.2007.01647.x. [DOI] [PubMed] [Google Scholar]
- 5.Petrovic D, Verhovec R, Globocnik Petrovic M, Osredkar J, Peterlin B. Association of vascular endothelial growth factor gene polymorphism with myocardial infarction in patients with type 2 diabetes. Cardiology. 2007;107(4):291–295. doi: 10.1159/000099064. [DOI] [PubMed] [Google Scholar]
- 6.Forooghian F, Kertes PJ, Eng KT, Agrón E, Chew EY. Alterations in the intraocular cytokine milieu after intravitreal bevacizumab. Invest Ophthalmol Vis Sci. 2010;1(5):2388–2392. doi: 10.1167/iovs.09-4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Geest RJ, Lesnik-Oberstein SY, Tan HS, Mura M, Goldschmeding R, Van Noorden CJ, Klaassen I, Schlingemann RO. A shift in the balance of vascular endothelial growth factor and connective tissue growth factor by bevacizumab causes the angiofibrotic switch in proliferative diabetic retinopathy. Br J Ophthalmol. 2012;96(4):587–590. doi: 10.1136/bjophthalmol-2011-301005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elner SG, Elner VM, Jaffe GJ, Stuart A, Kunkel SL, Strieter RM. Cytokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Curr Eye Res. 1995;14(11):1045–1053. doi: 10.3109/02713689508998529. [DOI] [PubMed] [Google Scholar]
- 9.Lu M, Kuroki M, Amano S, Tolentino M, Keough K, Kim I, Bucala R, Adamis AP. Advanced glycation end products increase retinal vascular endothelial growth factor expression. J Clin Invest. 1998;101(6):1219–1224. doi: 10.1172/JCI1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrovič MG, Korošec P, Košnik M, Osredkar J, Hawlina M, Peterlin B, Petrovič D. Local and genetic determinants of vascular endothelial growth factor expression in advanced proliferative diabetic retinopathy. Mol Vis. 2008;14:1382–1387. [PMC free article] [PubMed] [Google Scholar]
- 11.Jenkins AJ, Zhang SX, Rowley KG, Karschimkus CS, Nelson CL, Chung JS, O'Neal DN, Januszewski AS, Croft KD, Mori TA, Dragicevic G, Harper CA, Best JD, Lyons TJ, Ma JX. Increased serum pigment epithelium-derived factor is associated with microvascular complications, vascular stiffness and inflammation in Type 1 diabetes. Diabet Med. 2007;24(12):1345–1351. doi: 10.1111/j.1464-5491.2007.02281.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhang SX, Wang JJ, Gao G, Shao C, Mott R, Ma JX. Pigment epithelium-derived factor (PEDF) is an endogenous anti-inflammatory factor. FASEB J. 2006;20(2):323–325. doi: 10.1096/fj.05-4313fje. [DOI] [PubMed] [Google Scholar]
- 13.Laatikainen L, Summanen P. Long-term visual results of vitreous surgery in diabetic eye disease. Acta Ophthalmol (Copenh) 1989;67(1):21–29. doi: 10.1111/j.1755-3768.1989.tb00718.x. [DOI] [PubMed] [Google Scholar]
- 14.Spranger J, Hammes H-P, Preissner KT, Schatz H, Pfeiffer AF. Release of the angiogenesis inhibitor angiostatin in patients with proliferative diabetic retinopathy: association with retinal photocoagulation. Diabetologia. 2000;43(11):1404–1407. doi: 10.1007/s001250051546. [DOI] [PubMed] [Google Scholar]
- 15.Spranger J, Osterhoff M, Reimann M, Möhlig M, Ristow M, Francis MK, Cristofalo V, Hammes HP, Smith G, Boulton M, Pfeiffer AF. Loss of the antiangiogenic pigment epithelium-derived factor in patients with angiogenic eye disease. Diabetes. 2001;50(12):2641–2645. doi: 10.2337/diabetes.50.12.2641. [DOI] [PubMed] [Google Scholar]
- 16.Agostini C, Gurrieri C. Chemokine/cytokine cocktail in idiopathic pulmonary fibrosis. Proc Am Thorac Soc. 2006;3(4):357–363. doi: 10.1513/pats.200601-010TK. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida A, Yoshida S, Hata Y, Khalil AK, Ishibashi T, Inomata H. The role of NF-kappaB in retinal neovascularization in the rat. Possible involvement of cytokine-induced neutrophil chemoattractant (CINC), a member of the interleukin-8 family. J Histochem Cytochem. 1998;46(4):429–436. doi: 10.1177/002215549804600402. [DOI] [PubMed] [Google Scholar]
- 18.Karakurum M, Shreeniwas R, Chen J, Pinsky D, Yan SD, Anderson M, Sunouchi K, Major J, Hamilton T, Kuwabara K. Hypoxic induction of interleukin-8 gene expression in human endothelial cells. J Clin Invest. 1994;93(4):1564–1570. doi: 10.1172/JCI117135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li M, Zhang Y, Feurino LW, Wang H, Fisher WE, Brunicardi FC, Chen C, Yao Q. Interleukin-8 increases vascular endothelial growth factor and neuropilin expression and stimulates ERK activation in human pancreatic cancer. Cancer Sci. 2008;99(4):733–737. doi: 10.1111/j.1349-7006.2008.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizukami Y, Jo WS, Duerr EM, Gala M, Li J, Zhang X, Zimmer MA, Iliopoulos O, Zukerberg LR, Kohgo Y, Lynch MP, Rueda BR, Chung DC. Induction of interleukin-8 preserves the angiogenic response in HIF-1alpha-deficient colon cancer cells. Nat Med. 2005;11(9):992–997. doi: 10.1038/nm1294. [DOI] [PubMed] [Google Scholar]
- 21.Petrovic MG, Korosec P, Kosnik M, Hawlina M. Vitreous levels of interleukin-8 in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2007;143(1):175–176. doi: 10.1016/j.ajo.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 22.Keane MP, Belperio JA, Arenberg DA, Burdick MD, Zu ZJ, Xue YY, Strieter RM. IFN-gamma-inducible protein-10 attenuates bleomycin-induced pulmonary fibrosis via inhibition of angiogenesis. J Immunol. 1999;163(10):5686–5692. [PubMed] [Google Scholar]
- 23.Boulday G, Haskova Z, Reinders ME, Pal S, Briscoe DM. Vascular endothelial growth factor induced signaling pathways in endothelial cells that mediate overexpression of the chemokines IFN gamma- inducible protein of 10KDa in vitro and in vivo. J Immunol. 2006;176(5):3098–3107. doi: 10.4049/jimmunol.176.5.3098. [DOI] [PubMed] [Google Scholar]
- 24.Elner SG, Strieter R, Bian ZM, Kunkel S, Mokhtarzaden L, Johnson M, Lukacs N, Elner VM. Interferon-induced protein 10 and interleukin 8. C-X-C chemokines present in proliferative diabetic retinopathy. Arch Ophthalmol. 1998;116(12):1597–1601. doi: 10.1001/archopht.116.12.1597. [DOI] [PubMed] [Google Scholar]