Abstract
In recent years, the broad application of optical coherence tomography and vitrectomy, combined with research efforts in maculopathy in high myopia have provided many achievements, such as the new classification of myopic traction maculopathy (MTM). Here, we review the latest developments in the diagnosis and treatment of MTM, including its conception, clinical characteristics, pathogenesis, clinical stages, and the options for surgical treatment.
Keywords: myopic traction maculopathy, optical coherence tomography, high myopia, vitrectomy
INTRODUCTION
The pathological significance of high myopia is not due to ametropia but rather the degree of retinopathy. Retinopathy in high myopia is closely related to the lengthening of the ocular axis and occurs preferentially at the posterior pole and the periphery. Retinopathy at the posterior pole in high myopia, and maculopathy in particular, is a major cause of legal blindness in the world. While much research has been undertaken on maculopathy in high myopia, the historic lack of common diagnostic tools has halted significant gains in our understanding of this condition. However, the combination of the recent achievements and the broad application of optical coherence tomography (OCT) and vitrectomy has resulted in the conception and classification of myopic traction maculopathy (MTM).
In 1999, Takano and Kishi[1] identified foveal retinoschisis and retinal detachment without retinal holes in severely myopic eyes by OCT. Others also described similar pathological features of high myopia maculopathy[2]. In 2004, Panozzo and Mercanti[3] retrospectively reviewed their medical records and OCT findings for 125 eyes with high myopia. They found epiretinal traction in 58 eyes (46.4%) and retinal damage in 43 eyes (34.4%), of which 36 had epiretinal traction (83.7%). They were the first to use the term ‘myopic traction maculopathy’ to refer to these pathologies of the posterior pole in high myopia, such as macular retinoschisis, shallow retinal detachment without retinal holes, lamellar macular holes, and macular holes with or without retinal detachment. Distinguishing these conditions from epiretinal traction syndromes found in eyes without myopia was an important advancement because their distinct features have significantly different clinical effects on retinal tissue and visual function.
In 2007, Panozzo and Mercanti[4] suggested that the term ‘retinoschisis’ used in the literature for high myopic maculopathy was not appropriate as the terms inner or outer retinal schisis suggested a complete separation between the retinal layers that resulted in the irreversible and total loss of retinal function. Since all of the previously published case series of foveal retinoschisis reported good visual improvement without fixed scotoma, this retinal damage is not a schisis but rather due to retinal swelling with the accumulation of fluid. Smiddy et al[5] supported this notion and suggested that myopic maculoschisis, foveal schisis, and vitreoschisis in high myopia should all fall under the family of MTM.
CLINICAL FEATURES
MTM is more prevalent in populations with high myopia, particularly in females[4]. Patients usually complain of visual decrease and/or metamorphopsia[3], but some harbour no subjective symptoms[6]. Although there are no specific features in the early stages of MTM that can be identified by routine examinations[1], OCT can be used to detect retinoschisis, shallow retinal detachment without retinal holes, lamellar macular holes, and other clinical characteristics[4]. Macular holes with or without retinal detachment can only be discovered by an ophthalmoscope at the later stages.
The OCT image features of macular retinoschisis include the significant thickening of the retina at the position of the schisis and the delamination of the neuroretina by a low echo cavum that is separated by an erect columnar microstructure. Macular retinoschisis can be divided into two types: inner and outer retinal schisis. When outer retinal schisis occurs, the neurepithelium layer is divided into two layers. There is a thin layer on the outside and a thick layer on the inside, with the low echo cavum located in the outer part of the neurepithelium layer (Figure 1A). When inner retinal schisis occurs, the neurepithelium layer is also divided into two layers. There is a thin layer on the outside and a thick layer on the inside, with the low echo cavum located in the inner part of the neurepithelium layer (Figure 1B)[2]. The main OCT findings that differentiate macular retinoschisis from retinal detachment are a columnar microstructure and the smooth inner walls of the low echo cavum (Figures 1, 2)[1]. In high myopia, macular retinoschisis is always accompanied by foveal detachment, lamellar macular holes, macular epiretinal membrane, or vitreoretinal traction. In a study of 29 eyes with high myopia and macular retinoschisis, 10 eyes also had foveal detachment (34.5%), 8 had macular epiretinal membrane (27.6%), 5 had vitreoretinal traction, 7 had lamellar macular holes (24.1%), and 4 had two or more of the above lesions[7].
Figure 1. OCT image of high myopia.
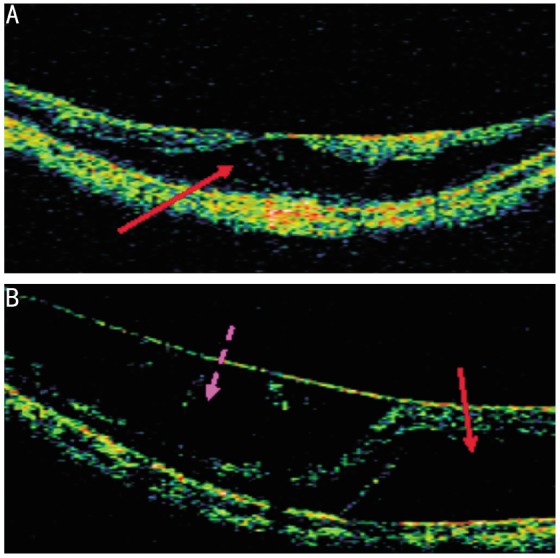
A: outer retinal schisis (Patient 1, female, 65 years old, left eye). The solid arrow shows outer retinal schisis and the inner wall of the low echo cavum is not smooth; B: inner macular retinoschisis combined with shallow macular detachment (Patient 2, female, 57 years old, right eye). The dotted arrow shows inner macular retinoschisis, and there is a connective columnar microstructure in the low echo cavum. The solid arrow shows shallow macular detachment, and the low echo cavum is smooth.
Figure 2. OCT image of a high myopia macular hole accompanied by shallow retinal detachment.
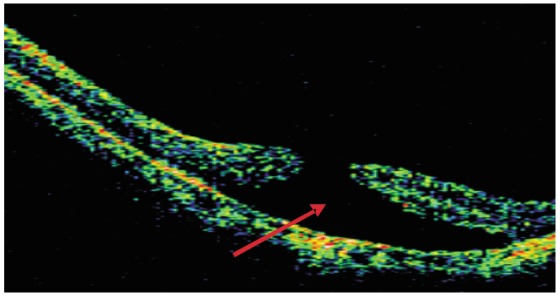
(Patient 3, female, 46 years old, right eye). The solid arrow shows shallow retinal detachment, and the low echo cavum is smooth.
OCT of eyes with shallow macular detachment accompanied by macular retinoschisis display two walls of limited shallow macular detachment forming the shape of the Chinese character eight (八). At the top is a thin neurepithelium layer and at the bottom is a smooth but strong reflection pigment epithelial layer (Figure 3).
Figure 3. OCT image of high myopia inner macular retinoschisis combined with shallow foveal detachment.
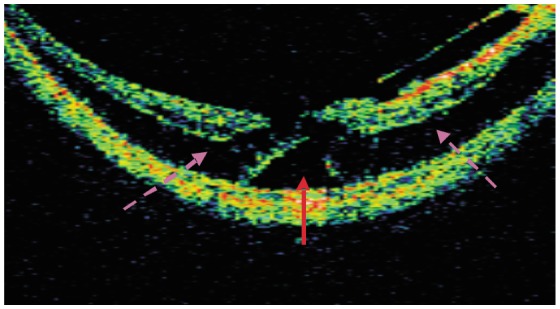
(Patient 4, female, 60 years old, left eye). The dotted arrow shows inner macular retinoschisis. The solid arrow shows shallow foveal detachment.
PATHOGENESIS
The pathogenesis of MTM is complicated[8] and may develop due to several factors relating to the retina itself: preretinal factors, and subretinal factors. The factors relating to the retina itself include: 1) thickening[9] and/or stiffening[10] of the internal limiting membrane (ILM), which can prevent the retina from adapting to high myopia posterior scleral staphyloma that produce centripetal traction and tangential traction to the retina; 2) choroidal atrophy, which can lead to the shortage in blood supply in the retina that reduces adhesion between the nerve layers; and 3) the retinal pigment epithelium and choroid may succumb to atrophy, thus reducing the adhesion between the retinal pigment epithelium and neuroretina[11]. Preretinal factors include: 1) incomplete posterior vitreous detachment[12] or adhesion[13] between the remaining vitreous and the retina, which can produce centripetal traction and tangential traction to the retina; 2) the Bruch membrane, which can be ruptured, and the pigment epithelium, which can migrate to the front of the retina in high myopia forming an epiretinal membrane whose shrinkage produces tangential traction to the retina[14],[15]; and 3) small retinal vessels that can cause traction on the retina[16]. One subretinal factor is the formation of posterior scleral staphyloma from the extension of the axis of the eye[17], which produces centrifugal traction on the retina. While some researchers postulate that vitreous traction contributes the most toward MTM pathogenesis[2],[15],[18],[19], others hold the opinion that the extension of the eye axis and the formation of staphyloma may also be major contributing factors[5],[20].
Panozzo and Mercanti[4] reported that women constituted to 87.5% of MTM patients, suggesting that MTM may be linked to hormonal differences[4]. Others have shown that high myopia is a polygenic inherited disease. Seven pairs of related genes have been identified, with six located on autosomal chromosomes and one on the X chromosome (Xq28)[21]. The higher incidence of MTM in women indicates that it may be a sex-linked genetic disease, but further research is needed to confirm this hypothesis.
DEVELOPMENT
Panozzo and Mercanti[4] suggested that high myopia macular holes and macular holes accompanied by retinal detachment occur in the advanced stages of MTM while macular retinoschisis, shallow retinal detachment without holes, and lamellar macular holes occur in the early stages of MTM. In 1999, Tokano and Kishi speculated that the formation of high myopia macular holes is the result of macular retinoschisis or shallow retinal detachment without holes. This hypothesis has been confirmed by several other groups[3],[5],[7],[22]-[24]. Although Panozzo and Mercanti[4] did not observe the developmental process of MTM, they did study some patients with bilateral high myopia eyes who had stable macular retinoschisis in one eye and a macular hole with posterior retinal detachment in the other eye.
The development of early MTM into macular holes may be related to the following factors: if the central fovea itself is thin[23], the existence of posterior scleral staphyloma[25], preretinal traction (macular epiretinal membrane, incomplete posterior vitreous detachment, and the remaining vitreous)[2],[3],[7],[23],[24], MTM that is accompanied by central foveal detachment[7], and the degree of stiffness of the internal limiting membrane[10]. Gaucher et al[7] discovered that myopic foveal retinoschisis may not affect vision for several years, but it is more likely to occur if preretinal structures have formed. Therefore, early MTM can be divided into two types: affected vision and unaffected vision. Patients with affected vision may seek medical attention earlier than those with unaffected vision, thus increasing their chances of preventing the illness from reaching a more advanced stage.
Retinal detachment occurs more readily after the formation of high myopia macular holes compared to idiopathic macular holes because of vitreous retinal traction, low adhesion between the pigment epithelial layer and neuroepithelial layer, posterior scleral staphyloma, and obvious liquefaction of the high myopia vitreous[3].
SURGICAL TREATMENT
It is currently unknown as to the stage when MTM patients benefit the most from surgical intervention. Some researchers have suggested that surgery should be performed in any case of MTM with sight threatening or are at risk of sight threatening[4],[26]. However, others have suggested that patients in the early stages should be observed for at least 6 months[27] because of the possibility of spontaneous remission[12].
Along with the factors that influence the pathogenesis of MTM, the factors that affect the vitreoretinal traction interface are also deemed important[17]. Since Kelly and Wendel[28] first applied vitrectomy to treat idiopathic macular holes in 1991, it has gradually become a standard surgical procedure. Vitrectomy is widely used in the treatment of MTM. However, joint internal limiting membrane peeling (ILMP) remains a controversial outcome. The ILM is an important factor in the pathogenesis of MTM, and ILMP can reduce retinal traction[29] to allow for anatomical repositioning[10],[30]-[32]. It can also stimulate the proliferation of the pigment epithelial layer, which is helpful for closing macular holes[33]. Futagami et al[34] reported a case of postoperative relief after ILMP in a patient with recurrent MTM. The patient had undergone vitrectomy three years previously, but not joint ILMP. Their report supports the importance of ILMP. However, the retina in high myopia is very thin and ILMP can easily cause macular holes. The curative effect of ILMP may not only encompass the peeling of the ILM but may also include the simultaneous peeling of collagen fibers and the cell components of the remaining vitreous, all of which are the main factors leading to MTM[13],[35].
In MTM, a thin retina, abnormal vitreoretinal adhesions, and stiff ILM may contribute to the occurrence and development of high myopia MTM. Therefore, it is particularly important that the remaining vitreous cortex and ILM develop effectively. Triamcinolone acetonide (TA) can affect the development of both the remaining vitreous cortex and ILM. TA is a long-term corticosteroid, which has been effectively applied in ophthalmic treatment for nearly 60 years. TA has been used via intraocular injection since 1974[36]. In 2000, Perman et al[37] described a marked vitreous cortex and auxiliary vitreous body separation after applying TA during vitrectomy. In 2004, Kimura et al[38] applied TA to assist ILMP. Triamcinolone Acetonide-assisted Internal Limiting Membrane Peeling (TA-ILMP) helps to reduce the inflammatory response after surgery, lowering the risk of proliferative vitreoretinopathy. Therefore, it can prevent the need for subsequent operations[39]. Takeuchi et al[40] and Hikichi et al[41] found that TA remaining at the macular holes has no influence on anatomical repositioning or visual acuity. Moreover, in a multi-center prospective study, Yamakiri et al[42] found that TA-ILMP reduced the incidence of retinal breaks and detachment during the operation, and also caused no adverse reactions.
In addition to vitrectomy in combination with gas injection or silicone oil injection, traditional operations for high myopia macular holes accompanied with retinal detachment include such procedures as macular buckling, scleral shortening, and vitrectomy combined with a laser to close macular holes. Chen et al[43] compared the retina anatomical reattachment rates of 6 different procedures: vitrectomy joint long-term gas injection, scleral buckling joint vitrectomy combined with epiretinal membrane peeling and long-term gas injection, vitrectomy joint epiretinal membrane peeling combined with long-term gas injection, scleral buckling joint vitrectomy combined with long-term gas injection, and scleral buckling joint vitrectomy combined with silicone oil injection. They recorded postoperative retinal reattachment rates of 12.5%, 40.0%, 42.8%, 50.0%, 57.1%, and 75.0%, respectively, suggesting that the more aggressive treatment in silicone oil tamponade may have to be performed in high myopic eyes that have posterior staphyloma with marked chorioretinal atrophy. When the efficacy of posterior scleral reinforcement was compared to vitrectomy for treating high myopia macular holes and retinal detachment, the one-time retinal reattachment rate for posterior scleral reinforcement (30 eyes) was 93.3%, and 100% after an additional operation. However, the one-time retinal reattachment rate for vitrectomy (28 eyes) was 50%, and 86% after an additional operation. Visual acuity was also significantly better after posterior scleral reinforcement compared to vitrectomy (P < 0.005)[44].
While there are a variety of surgical procedures for MTM, there is no standard method of treatment. Recent advancements in vitreous drug injections used for vitreous liquefaction or posterior vitreous detachment make this another viable choice for MTM treatment[45].
OUTLOOK
With the emergence of OCT, our understanding of the clinical characteristics, pathogenesis, and lesion development in myopic traction maculopathy has gradually improved. However, more long-term and in-depth studies are required. The main method for treating MTM involves surgical intervention, but there are a variety of procedures available and no standard has been agreed upon. In addition, the optimal timing for performing surgery on MTM patients remains unclear, and must be addressed by comparative studies using larger sample sizes. Injection of drugs into the vitreous used for vitreous liquefaction or posterior vitreous detachment is also a new avenue for the treatment of MTM.
REFERENCES
- 1.Takano M, Kishi S. Foveal retinoschisis and retinal detachment in severely myopic eyes with posterior staphyloma. Am J Ophthalmol. 1999;128(4):472–476. doi: 10.1016/s0002-9394(99)00186-5. [DOI] [PubMed] [Google Scholar]
- 2.Benhamou N, Massin P, Haouchine B, Erginay A, Gaudric A. Macular retinoschisis in highly myopic eyes. Am J Ophthalmol. 2002;133(6):794–800. doi: 10.1016/s0002-9394(02)01394-6. [DOI] [PubMed] [Google Scholar]
- 3.Panozzo G, Mercanti A. Optical coherence tomography findings in myopic traction maculopathy. Arch Ophthalmol. 2004;122(10):1455–1460. doi: 10.1001/archopht.122.10.1455. [DOI] [PubMed] [Google Scholar]
- 4.Panozzo G, Mercanti A. Vitrectomy for myopic traction maculopathy. Arch Ophthalmol. 2007;125(6):767–772. doi: 10.1001/archopht.125.6.767. [DOI] [PubMed] [Google Scholar]
- 5.Smiddy WE, Kim SS, Lujan BJ, Gregori G. Myopic traction maculopathy: spectral domain optical coherence tomographic imaging and a hypothesized mechanism. Ophthalmic Surg Lasers Imaging. 2009;40(2):169–173. doi: 10.3928/15428877-20090301-21. [DOI] [PubMed] [Google Scholar]
- 6.Baba T, Ohno-Matsui K, Futagami S, Yoshida T, Yasuzumi K, Kojima A, Tokoro T, Mochizuki M. Prevalence and characteristics of foveal retinal detachment without macular hole in high myopia. Am J Ophthalmol. 2003;135(3):338–342. doi: 10.1016/s0002-9394(02)01937-2. [DOI] [PubMed] [Google Scholar]
- 7.Gaucher D, Haouchine B, Tadayoni R, Massin P, Erginay A, Benhamou N, Gaudric A. Long-term follow-up of high myopic foveoschisis: natural course and surgical outcome. Am J Ophthalmol. 2007;143(3):455–462. doi: 10.1016/j.ajo.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 8.Vanderbeek BL, Johnson MW. The diversity of traction mechanisms in myopic traction maculopathy. Am J Ophthalmol. 2012;153(1):93–102. doi: 10.1016/j.ajo.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Bando H, Ikuno Y, Choi JS, Tano Y, Yamanaka I, Ishibashi T. Ultrastructure of internal limiting membrane in myopic foveoschisis. Am J Ophthalmol. 2005;139(1):197–199. doi: 10.1016/j.ajo.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 10.Kuhn F. Internal limiting membrane removal for macular detachment in highly myopic eyes. Am J Ophthalmol. 2003;135(4):547–549. doi: 10.1016/s0002-9394(02)02057-3. [DOI] [PubMed] [Google Scholar]
- 11.Cho H, Choi A, Kang SW. Effect of internal limiting membrane removal in treatment of retinal detachment caused by myopic macular hole. Korean J Ophthalmol. 2004;18(2):141–147. doi: 10.3341/kjo.2004.18.2.141. [DOI] [PubMed] [Google Scholar]
- 12.Polito A, Lanzetta P, Del Borrello M, Bandello F. Spontaneous resolution of a shallow detachment of the macula in a highly myopic eye. Am J Ophthalmol. 2003;135(4):546–547. doi: 10.1016/s0002-9394(02)02080-9. [DOI] [PubMed] [Google Scholar]
- 13.Spaide RF, Fisher Y. Removal of adherent cortical vitreous plaques without removing the internal limiting membrane in the repair of macular detachments in highly myopic eyes. Retina. 2005;25(3):290–295. doi: 10.1097/00006982-200504000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Stirpe M, Michels RG. Retinal detachment in highly myopic eyes due to macular holes and epiretinal traction. Retina. 1990;10(2):113–114. doi: 10.1097/00006982-199004000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Ishida S, Yamazaki K, Shinoda K, Kawashima S, Oguchi Y. Macular hole retinal detachment in highly myopic eyes: ultrastructure of surgically removed epiretinal membrane and clinicopathologic correlation. Retina. 2000;20(2):176–183. [PubMed] [Google Scholar]
- 16.Ikuno Y, Gomi F, Tano Y. Potent retinal arteriolar traction as a possible cause of myopic foveoschisis. Am J Ophthalmol. 2005;139(3):462–467. doi: 10.1016/j.ajo.2004.09.078. [DOI] [PubMed] [Google Scholar]
- 17.Wu PC, Chen YJ, Chen YH, Chen CH, Shin SJ, Tsai CL, Kuo HK. Factors associated with foveoschisis and foveal detachment without macular hole in high myopia. Eye (Lond) 2009;23(2):356–361. doi: 10.1038/sj.eye.6703038. [DOI] [PubMed] [Google Scholar]
- 18.Oshima Y, Ikuno Y, Motokura M, Nakae K, Tano Y. Complete epiretinal membrane separation in highly myopic eyes with retinal detachment resulting from a macular hole. Am J Ophthalmol. 1998;126(5):669–676. doi: 10.1016/s0002-9394(98)00180-9. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi H, Kishi S. Vitreous surgery for highly myopic eyes with foveal detachment and retinoschisis. Ophthalmology. 2003;110(9):1702–1707. doi: 10.1016/S0161-6420(03)00714-0. [DOI] [PubMed] [Google Scholar]
- 20.Robichaud JL, Besada E, Basler L, Frauens BJ. Spectral domain optical coherence tomography of myopic traction maculopathy. Ophthalmic Surg Lasers Imaging. 2011;82(10):607–173. doi: 10.1016/j.optm.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Soubrane G. Choroidal neovascularization in pathologic myopia: recent developments in diagnosis and treatment. Surv Ophthalmol. 2008;53(2):121–138. doi: 10.1016/j.survophthal.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Ikuno Y, Tano Y. Early macular holes with retinoschisis in highly myopic eyes. Am J Ophthalmol. 2003;136(4):741–800. doi: 10.1016/s0002-9394(03)00319-2. [DOI] [PubMed] [Google Scholar]
- 23.Shimada N, Ohno-Matsui K, Baba T, Futagami S, Tokoro T, Mochizuki M. Natural course of macular retinoschisis in highly myopic eyes without macular hole or retinal detachment. Am J Ophthalmol. 2006;142(3):497–500. doi: 10.1016/j.ajo.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 24.Matsumura N, Ikuno Y, Tano Y. Posterior vitreous detachment and macular hole formation in myopic foveoschisis. Am J Ophthalmol. 2004;138(6):1071–1073. doi: 10.1016/j.ajo.2004.06.064. [DOI] [PubMed] [Google Scholar]
- 25.Oie Y, Ikuno Y, Fujikado T, Tano Y. Relation of posterior staphyloma in highly myopic eyes with macular hole and retinal detachment. Jpn J Ophthalmol. 2005;49(6):530–532. doi: 10.1007/s10384-005-0249-1. [DOI] [PubMed] [Google Scholar]
- 26.Muller B, Joussen AM. Myopic traction maculopathy - vitreoretinal traction syndrome in high myopic eyes and posterior staphyloma. Klin Monbl Augenheilkd. 2011;228(9):771–779. doi: 10.1055/s-0031-1281714. [DOI] [PubMed] [Google Scholar]
- 27.Ratiglia R, Osnaghi S, Bindella A, Pirondini C. Posterior traction retinal detachment in highly myopic eyes: clinical features and surgical outcome as evaluated by optical coherence tomography. Retina. 2005;25(4):473–478. doi: 10.1097/00006982-200506000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol. 1991;109(5):654–659. doi: 10.1001/archopht.1991.01080050068031. [DOI] [PubMed] [Google Scholar]
- 29.Konidaris V, Androudi S, Brazitikos P. Myopic traction maculopathy: study with spectral domain optical coherence tomography and review of the literature. Hippokratia. 2009;13(2):110–113. [PMC free article] [PubMed] [Google Scholar]
- 30.Kadonosono K, Yazama F, Itoh N, Uchio E, Nakamura S, Akura J, Sawada H, Ohno S. Treatment of retinal detachment resulting from myopic macular hole with internal limiting membrane removal. Am J Ophthalmol. 2001;131(2):203–207. doi: 10.1016/s0002-9394(00)00728-5. [DOI] [PubMed] [Google Scholar]
- 31.Kanda S, Uemura A, Sakamoto Y, Kita H. Vitrectomy with internal limiting membrane peeling for macular retinoschisis and retinal detachment without macular hole in highly myopic eyes. Am J Ophthalmol. 2003;136(1):177–180. doi: 10.1016/s0002-9394(03)00243-5. [DOI] [PubMed] [Google Scholar]
- 32.Ikuno Y, Sayanagi K, Ohji M, Kamei M, Gomi F, Harino S, Fujikado T, Tano Y. Vitrectomy and internal limiting membrane peeling for myopic foveoschisis. Am J Ophthalmol. 2004;137(4):719–724. doi: 10.1016/j.ajo.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 33.Wu Y, Zhao PQ, Jiang CH. Evaluation of vitrectomy for macular hole with retinal detachment in high myopia by optical coherence tomography. Zhongguo Shiyong Yanke Zazhi. 2004;22(8):613–616. [Google Scholar]
- 34.Futagami S, Inoue M, Hirakata A. Removal of internal limiting membrane for recurrent myopic traction maculopathy. Clin Experiment Ophthalmol. 2008;36(8):782–785. doi: 10.1111/j.1442-9071.2008.01887.x. [DOI] [PubMed] [Google Scholar]
- 35.Kwok AK, Lai TY, Yip WW. Vitrectomy and gas tamponade without internal limiting membrane peeling for myopic foveoschisis. Br J Ophthalmol. 2005;89(9):1180–1183. doi: 10.1136/bjo.2005.069427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jermak CM, Dellacroce JT, Heffez J, Peyman GA. Triamcinolone acetonide in ocular therapeutics. Surv Ophthalmol. 2007;52(5):503–522. doi: 10.1016/j.survophthal.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Peyman GA, Cheema R, Conway MD, Fang T. Triamcinolone acetonide as an aid to visualization of the vitreous and the posterior hyaloid during pars plana vitrectomy. Retina. 2000;20(5):554–555. doi: 10.1097/00006982-200005000-00024. [DOI] [PubMed] [Google Scholar]
- 38.Kimura H, Kuroda S, Nagata M. Triamcinolone acetonide-assisted peeling of the internal limiting membrane. Am J Ophthalmol. 2004;137(1):172–173. doi: 10.1016/s0002-9394(03)00782-7. [DOI] [PubMed] [Google Scholar]
- 39.Enaida H, Hata Y, Ueno A, Nakamura T, Hisatomi T, Miyazaki M, Fujisawa K, Sakamoto T, Ishibashi T. Possible benefits of triamcinolone-assisted pars plana vitrectomy for retinal diseases. Retina. 2003;23(6):764–770. doi: 10.1097/00006982-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Takeuchi M, Katagiri Y, Usui M. Residual triamcinolone acetonide in the macular hole after vitrectomy. Am J Ophthalmol. 2003;136(6):1174–1176. doi: 10.1016/s0002-9394(03)00674-3. [DOI] [PubMed] [Google Scholar]
- 41.Hikichi T, Furukawa Y, Ohtsuka H, Higuchi M, Matsushita T, Ariga H, Kosaka S, Matsushita R. Improvement of visual acuity one-year after vitreous surgery in eyes with residual triamcinolone acetonide at the macular hole. Am J Ophthalmol. 2008;145(2):267–272. doi: 10.1016/j.ajo.2007.09.035. [DOI] [PubMed] [Google Scholar]
- 42.Yamakiri K, Sakamoto T, Noda Y, Nakahara M, Ogino N, Kubota T, Yokoyama M, Furukawa M, Sonoda Y, Yamada T, Doi N, Enaida H, Hata Y, Ishibashi T. Reduced incidence of intraoperative complications in a multicenter controlled clinical trial of triamcinolone in vitrectomy. Ophthalmology. 2007;114(2):289–296. doi: 10.1016/j.ophtha.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 43.Chen YP, Chen TL, Yang KR, Lee WH, Kuo YH, Chao AN, Wu WC, Chen KJ, Lai CC. Treatment of retinal detachment resulting from posterior staphyloma-associated macular hole in highly myopic eyes. Retina. 2006;26(1):25–31. doi: 10.1097/00006982-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Ando F, Ohba N, Touura K, Hirose H. Anatomical and visual outcomes after episcleral macular buckling compared with those after pars plana vitrectomy for retinal detachment caused by macular hole in highly myopic eyes. Retina. 2007;27(1):37–44. doi: 10.1097/01.iae.0000256660.48993.9e. [DOI] [PubMed] [Google Scholar]
- 45.Schneider EW, Johnson MW. Emerging nonsurgical methods for the treatment of vitreomacular adhesion: a review. Clin Ophthalmol. 2011;5:1151–1165. doi: 10.2147/OPTH.S14840. [DOI] [PMC free article] [PubMed] [Google Scholar]


