Abstract
The most detailed reports of the response of the gastrointestinal system to high dose acute radiation have focused mainly on understanding the histopathology. However, to enable medical countermeasure assessment under the animal rule criteria, it is necessary to have a robust model in which the relationship between radiation dose and intestinal radiation syndrome incidence, timing and severity are established and correlated with histopathology. Although many mortality studies have been published, they have used a variety of mouse strains, ages, radiation sources and husbandry conditions, all of which influence the dose response. Further, it is clear that the level of bone marrow irradiation and supportive care can influence endpoints. In order to create robust baseline data we have generated dose response data in adult male mice, maintained under identical conditions, and exposed to either total or partial-body irradiation. Partial-body irradiation includes both extensive (40%) and minimal (5%) bone marrow sparing models, the latter designed to correlate with an established primate model and allow assessment of effects of any medical countermeasure on all three major radiation syndromes (intestinal, bone marrow and lung) in the surviving mice. Lethal dose (LD30, LD50 and LD70) data are described in the various models, along with the impact of enteric flora and response to supportive care. Correlation with diarrhea severity and histopathology are also described. This data can be used to aid the design of good laboratory practice (GLP) compliant Animal Rule studies that are reflective of the conditions following accidental radiation exposure.
Keywords: modeling, biological factors; radiation damage; radiation dose; mice; X-rays; acute radiation exposure; gastrointestinal
INTRODUCTION
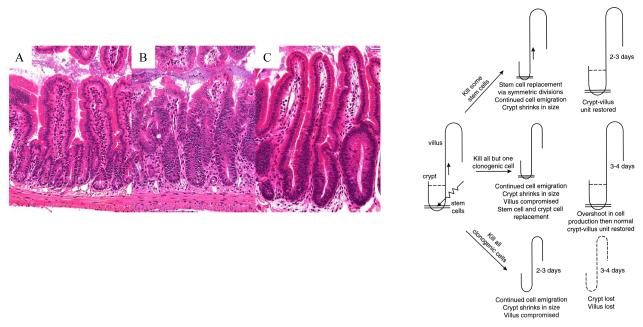
The acute physiological effects of irradiation on the gastrointestinal (GI) system have been repeatedly documented. There is weight loss and diarrhea, leading to dehydration and susceptibility to infection as the intestinal mucosal barrier is interrupted and ultimately becomes ulcerated. The consequences are manifest within a few days of irradiation, due to the interruption of the extremely rapid cell turnover in the normal intestine. Histopathology reveals an increase in crypt cell apoptosis within hours and a subsequent shortening of the crypts and villi (crypt cell output is reduced due to apoptosis and reduced proliferation but the mature differentiated cells continue to migrate upwards and be shed into the intestinal lumen) (Fig. 1) (Potten 1990, Booth and Potten 2001a). This changed morphology is often referred to as ‘villus blunting’, with a resultant impaired functional absorption. If sufficient clonogenic cells towards the crypt base are killed or reproductively sterilized, the crypts themselves are killed, and if sufficient crypts are lost such that the epithelial cell output is insufficient to maintain the barrier, an ulcer may develop (Potten 1995b, Potten et al. 1997). The radiobiological response of the clonogenic cells of the intestine (apoptotic sensitivity, proliferative impairment, tissue death or regeneration) has been extensively characterized by Potten and colleagues, who also demonstrated that prophylactic treatment with certain growth factors such as TGFbeta3, IL-11 or KGF (Kepivance) could protect clonogenic cell number and increase crypt survival following high-dose irradiation, and that this correlated directly with increased animal survival (Potten 1995a, 1996, Potten et al. 1997, Farrell et al. 1998, Booth et al. 2000, Booth and Potten 2001b). However, these studies did not extensively evaluate the effects of post-irradiation mitigators or fully characterize the effects on radiation-induced mortality. The studies also employed high levels of bone marrow protection (head, thorax and forelimbs shielded) in order to focus upon the intestinal response most relevant in an oncology therapy scenario; many patients receive some form of radiation as part of their cancer therapy. Radiation enteritis has been estimated to occur at some point in most patients receiving abdominal or pelvic radiotherapy (Yeoh and Horowitz 1987). Some investigators suggest that the prevalence is dramatically underestimated largely due to lack of clinical recognition (patients not reporting symptoms for fear of compromising tumor treatment). Radiation induced enteritis was first described in 1897 (Walsh 1897), two years after the discovery of X-rays by Roentgen, yet over one hundred years later there are still no effective treatments.
Fig. 1.
Radiation kills the crypt clonogenic cells. As successive clonogenic cells are killed, or reproductively sterilized, the cellular output onto the villi is reduced. Impaired cell production with continued upward cell migration leads to gradual villus and crypt shrinkage (blunting) resulting in reduced nutrient absorption and impaired barrier function. If no clonogenic cells remain in a crypt it will die and if sufficient crypts are killed ulcers will form. However, if one or more clonogenic cells remain the crypt will regenerate (B) and restore the blunted villi, ultimately regenerating the villus, with a slight ‘overshoot’ in cell production (C) before homeostasis and normal levels resume. A control crypt and villus is shown in (A). Adapted from Booth and Potten 2001.
Although these types of studies are sufficient to provide supporting data for investigational new drug (IND) filings with the U.S. Food and Drug Administration (FDA) for oncology treatment applications, they are not suitable for evaluation of radiation mitigators that may be used in the event of a radiation attack or accidents. In addition to events such as those of 11th September 2001, events like the explosions at Japan’s Fukushima Daiichi nuclear power plant in March of 2011 highlight concerns over accidental radiation exposure (although levels of exposure were approximately 10% of those at Chernobyl) and the need for effective treatments. There are currently no U.S. FDA-approved treatments for the mitigation of the gastrointestinal consequences of accidental radiation exposure.
Potential treatments however, cannot be evaluated in these models designed for testing prophylactic treatments, fractionated irradiation models or models employing abdominal only irradiation (high levels of bone marrow shielding). Unless there has been a very focal radiation exposure there will also be involvement of many other organ systems, and their differential responses to various doses of irradiation will impact the gastrointestinal acute radiation syndrome (GI-ARS) dose response. It is therefore implicit that the characterization of GI-ARS links to the other sub-syndromes, particularly that of the hematopoietic system (H-ARS). The effects of bone marrow ablation by irradiation are slower to manifest than the effects of GI-ARS but it is a much more radiosensitive tissue than the intestine (see Orschell et al. in this issue, who has used irradiation and animal conditions comparable to those reported here). Thus, all radiation doses inducing GI-ARS will have a major impact on the bone marrow, which in turn will affect the levels of intestinal inflammation and ability of the body to manage the infection resulting from bacterial translocation through an impaired intestinal mucosal barrier.
The FDA regulations concerning the approval of new drugs or biological products when human efficacy studies are not ethical or feasible are known as the “Animal Rule”(21 CFR 314.600 for drugs; CFR 601.90 for biologics) (Crawford 2002). These regulations state that the pathophysiological mechanism of radiotoxicity and its prevention or substantial reduction by the product must be well understood and demonstrated in more than one animal species expected to react with a response predictive for humans. Further, the endpoint in such studies must be clearly related to the desired benefit in humans, generally the enhancement of survival or prevention of major morbidity. Finally, there must be supporting pharmacokinetic (pK) and pharmacodynamics (pD) data (FDA 2009).
We have therefore been defining the baseline for effectively evaluating medical countermeasures (MCMs) for GI-ARS by attempting to understand the confounding information in the literature and thereby minimize variability and maximize the efficacy with which a MCM can be evaluated. The published data are difficult to interpret due to reports from a range of disparate studies utilizing different strains, sexes and ages of mice, different husbandry conditions, radiation sources, levels of supportive care and bone marrow irradiation. Finally, although morbidity is a crucial endpoint for the Animal Rule, the euthanasia criteria are rarely stated and will have undoubtedly become more stringent in most countries in recent years.
The data presented have been generated from a series of highly-controlled studies using the same mouse strain, age, sex, supplier, husbandry conditions, and irradiation source. Furthermore, all mice were irradiated at the same time of day to eliminate any intestinal circadian variation, which has previously been shown to affect radiosensitivity (Potten et al. 1977, Hendry 1979, Qiu et al. 1994, Shukla et al. 2010). This has therefore allowed full comparison of the responses with differing levels of supportive care and bone marrow survival.
MATERIALS AND METHODS
Mice
All procedures were certified according to the UK Animal (Scientific Procedures) Act 1986. Male C57BL/6 and CBA/Ca mice, aged eight to ten weeks were purchased from Harlan UK and allowed to acclimatize for two weeks prior to irradiation. C57BL/6 have been the standard mouse strain used in these studies and those investigating H-ARS presented by Orschell (this issue). The CBA/Ca strain have also recently been evaluated in these models since Vujaskovic, this issue, has shown strain differences in the presentation of delayed lung injury. By using the same strains of mouse to investigate each radiation sub-syndrome (gastrointestinal, haematological and lung) it may then be possible to link the observations.
Husbandry
All mice were held in individually ventilated cages (IVCs) in a specific pathogen free (SPF) barrier unit. A twelve hour light:dark cycle was maintained with lights being turned on at approximately 0700 hours and off at approximately 1900 hours. There was a constant room temperature of 21+ or − 2°C and a mean relative humidity of 55% + or - 10%. The animals received 2018 extruded rodent diet (Harlan) and sterile acidified water (ad libitum) from time of arrival and throughout the study. Animals were identified by ear punches in cages labeled with the appropriate information necessary to identify the study, dose, animal number and treatment groups.
Supportive Care
Other supportive care was administered as indicated in the results. Ciprofloxacin and levofloxacin (both 0.67g L−1), the same doses as used by Orschell et al. in this issue) were administered in the drinking water from day four post irradiation unless indicated otherwise and were also used to wet the food and generate mash, supplied within the cage to allow for easier access by mice potentially weakened from radiation exposure. Unless otherwise stated, antibiotic administration was started on day four as this was the time when the absolute neutrophil count (ANC) dropped below 500 μl−1 (Orschell et al. in this issue). The use of antibiotic supportive care also links the mouse and nonhuman primate models, antibiotics being used in the primate H-ARS and GI-ARS models (Farese et al. in this issue, MacVittie et al. in this issue).
Irradiation
Animals were irradiated at 15:00 + or - one hour (20 animals per radiation dose per study). Irradiation was performed using a Pantak HF320 X-ray set (Agfa NDT Ltd, Reading, UK), operated at 300kV, 10mA. The X-ray tube has additional filtration to give a radiation quality of 2.3mm Copper half-value layer (HVL). Mice were restrained in a compartmentalized Perspex (plexiglass) jig, positioned at a distance of 700mm below the focus of the X-ray tube. Each rectangular box is divided to provide twelve ventilated restraints, each holding one animal. Thus, twelve mice were irradiated simultaneously. Dosimetry was checked every two months during which time it has remained within + or - 1% of the original value. QA and control procedures were performed prior to and during each irradiation to confirm dose and energy output remain within range.
Animals received either total-body irradiation (TBI) or partial-body irradiation (PBI). Animals receiving partial-body irradiation were anaesthetized intraperitoneally with Ketamine (Fort Dodge) and Rompun (Bayer) to allow immobilization with the restraint and accurate lead shielding of the appropriate area. Animals receiving PBI had either the head, forelimbs and thorax shielded by placement of a lead sheet over this region of the animal (estimated to protect approximately 40% of the bone barrow, PBI BM40) or the hind limbs fibula, tibia and feet shielded using lead tubes (estimated to protect approximately 5% of the bone barrow, PBI BM5) (Boggs 1984; Taketa et al. 1970). Irradiation was delivered at a dose rate of 70.0 (PBI BM40) or 79.5 cGy min−1 (TBI, PBI BM5).
Health Status Monitoring
All animals were weighed and their well-being inspected daily from the initiation of treatment to the end of the study. Any animal demonstrating more than 15% weight loss was considered unwell and humanely euthanized if the weight loss was sustained at greater than 20% for 24hrs and mice also demonstrated signs of a moribund state (withdrawn behavior, reduced body temperature as judged by feeling cool to touch, lack of grooming and dehydration as judged by a persistent skin tent on pinching). During the peak periods of diarrhea incidence and general decline in well-being, animals were inspected several times during each 24 hour period.
The primary endpoint, and key to all FDA studies, was animal mortality. A secondary endpoint in these same studies was the diarrhea severity. Diarrhea scores were recorded as 0, 1, 2 or 3 where 0 is normal stool consistency, 1 is loose stools, 2 is overt diarrhea with peri-anal soilage and 3 is severe liquid faeces and possibly bloody diarrhea with substantial tail soilage. These observations typically begin on days four to five post irradiation. Stool consistency was measured daily, increasing to twice daily from day 4-10 when diarrhea is prevalent.
Statistics
Data was collected on survival and diarrhea severity. Statistical analyses were performed using SAS version 9.3 and S+ 8.1. A variety of analyses were performed (not all presented in this manuscript) including comparisons of the proportion of mice that survived using Mantel-Haenszel test or an exact logistic regression model and the mean survival times (MST) among decedents using analysis of variance (ANOVA). Kaplan-Meier survival curves were also used to present survival data. Logistic regression curves modeling the estimated probability of death against radiation dose were plotted and lethal dose analysis was done to compute LD30,50,70 values. Probit modes were also generated.
Bacterial Flora
In selected studies animals were evaluated for the levels and type of bacterial flora present. In these mice, following removal of blood via cardiac puncture, organs were removed aseptically, homogenized and inoculated onto a range of standard microbiological media and plates read at 24h and 48h for significant isolates.
Anticoagulated blood was transferred aseptically into Oxoid Signal broth. Blood culture bottles were incubated at 37°C for up to 7 days post inoculation. The blood culture bottles were examined daily for bacterial or fungal growth. At the end of the incubation period a sample was removed from the bottles and plates onto blood agar plates and incubated for 48 hours in anaerobic conditions. For the organs, each was aseptically removed then homogenized in sterile PBS. The homogenate was cultured onto 7% blood agar, chocolate blood agar, CLED agar, Sabouraud dextrose agar. Blood agar and chocolate blood agar were incubated at 37°C in 5%CO2/95% air for 48 hours. CLED agar was incubated at 37°C in air for 24 hours. Sabouraud dextrose agar was incubated at 30°C up 5 days. Another 7% blood agar was incubated in anaerobic conditions for 48 hours. After incubation, plates were read and any growth recorded. Identification of the isolates used a subset of the following tests: Gram stain, oxidase, catalyse, Stapharuex Plus and fermentation of sugars using Biomerieux API strips. A qualitative and semi-quantitative assignment of nature of organism(s) was made.
Intestinal Histopathology
Histopathology studies also provided supporting secondary endpoint data. For crypt survival, following fixed time point euthanasia, the small intestine was removed from each animal and Carnoys fixed. The intestines were ‘bundled’ prior to embedding in order to obtain the ideal orientation of the crypts (Merritt et al. 1992). From each mouse a series of small lengths of intestine, approximately 0.5cm long, were placed within a loop of surgical Micropore tape, and the tape tightened to immobilize the lengths. This allows the alignment of many pieces of fixed intestine alongside each other like a series of logs, so that in every section from a mouse there are several well-orientated cross sections. Each paraffin block generated was then sectioned to provide one slide per block, each slide containing two non-serial sections which were stained with H & E. The number of surviving and regenerating crypts per intestinal circumference was scored and the average per mouse and per group determined. A surviving crypt was defined as one that had ten or more tightly packed strongly H & E stained cells (excluding Paneth cells). Only regions that were orientated correctly and did not contain Peyers patches were scored (Peyers patches influence both the number of crypts in a normal circumference and the ability of a crypt to survive insult). The size of surviving crypts varied, influencing the likelihood of observing a surviving crypt in cross section, so a size correction factor was applied to reduce this error, based on the widths of the crypts (Potten et al. 1981). The corrected number of crypts was calculated according to the following equation:
Whilst this calculation has a minor effect on dose response curves, it can provide extra clarity to data where the rate of crypt regeneration is influencing crypt counts (during effective mitigation).
RESULTS
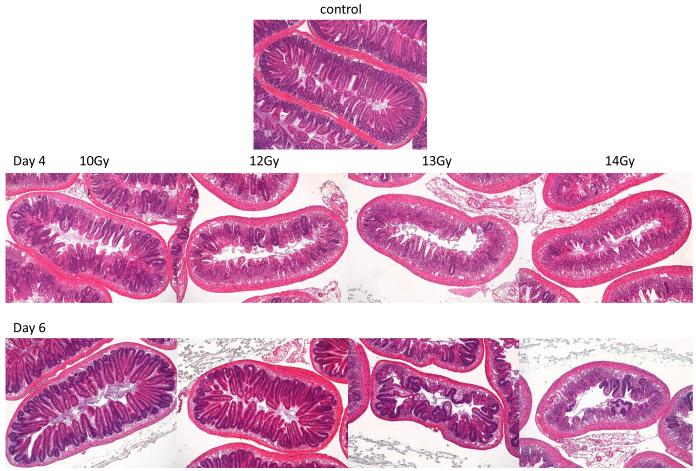
The rapidly proliferating small intestinal epithelium is very radiosensitive. The clonogenic cells in both the large and small intestine have been well characterized (Hendry 1979, Hendry 1993, Potten and Hendry 1983, Potten 1995b, Potten 1998, Potten and Loeffler 1990, Cai et al. 1997a,b) but correlation of the induced pathology with the GI-ARS time course was needed as a baseline for defining the effects of mitigator mechanisms of action. Fig. 2 shows the pathology of the small intestine in adult male C57BL/6 mice four and six days following irradiation. There was a loss of the epithelium within four days after 10 Gy exposure, consistent with published data, due to the transit time of cells to the villus tip and inhibited cell replacement due to the death of the clonogenic cells in many crypts. However, rapid regeneration is triggered and just two days later the epithelium is fully restored, although there is underlying submucosal inflammation. At 12 Gy many more crypts were killed but the epithelium was still restored by day six. However, at higher doses the few surviving crypts on day four produced progeny that migrated to begin the recovery of the barrier, but this was still far from intact on day six.
Fig. 2.
Response of the small intestine to total body irradiation (H&E stained cross sections) A: x5 objective; B: x20 objective. Loss of crypts is visible fours days after 10 Gy, with increasing crypt loss with radiation dose. Two days later the effects of the rapid regeneration can be seen, with crypt numbers being restored after 10 Gy to 12 Gy. At higher doses the progeny of the few surviving crypts are migrating to try and restore the mucosal barrier, although the crypt-villus architecture is still lacking.
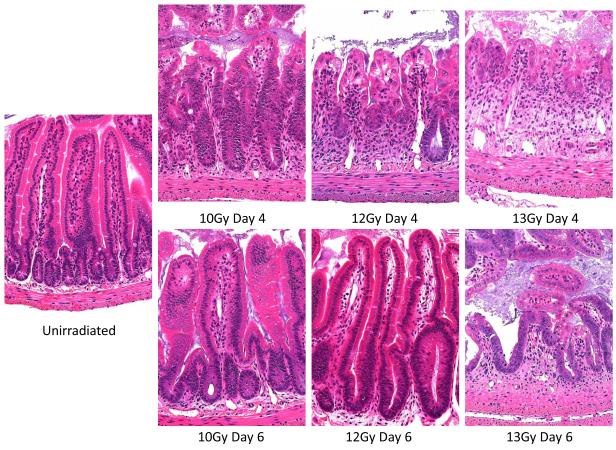
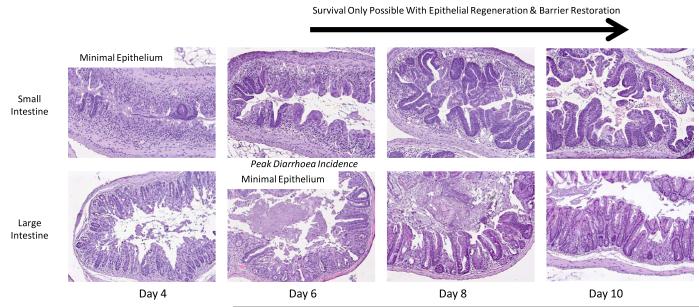
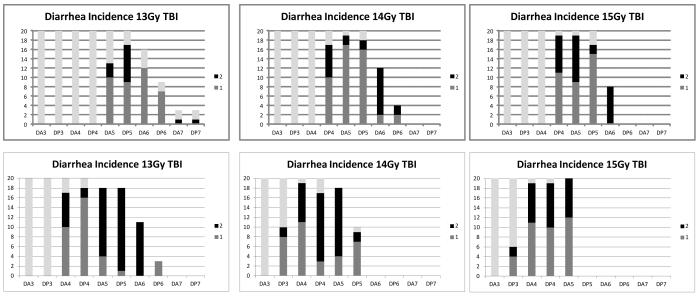
In order to examine the pathology at later time points and the response of the more slowly regenerating large intestine, animals were irradiated with 14 Gy but the head, thorax and forelimbs were lead shielded to protect the bone marrow and allow longer-term survival (Fig. 3). In both regions of the intestine damage was maximal at four to six days post irradiation, with regeneration evident on day eight, but still incomplete on day ten. It can be seen that the small intestinal regeneration is faster than that of the large intestine. Day four to six is coincident with the time of maximal diarrhea incidence (Fig. 6). This data is from C57BL/6 mice but the same data is generated by CBA/Ca mice.
Fig.3.
Response of the small intestine to total body irradiation (H&E stained cross section; x20 objective).
Illustration of the change in histopathology from days 4 to 10 following 14 Gy irradiation (H&E stained cross section; x10 objective). In order to allow survival to the latter time points and illustrate the regenerative capacity of the small and large intestine the bone marrow has been shielded in these studies.
Fig. 6.
Loss of crypts is coincident with the onset of diarrhea, dehydration and mortality. The top plots illustrate the number of C57BL/6 mice per radiation dose (y axis) exhibiting diarrhea of a score 1 (mild) or 2 (severe) from days 3 to 7 (am and pm observations indicated by DA3, DP3, DA4, DP4 etc). The lower plots illustrate the same data in CBA/Ca mice, where the diarrhea occurs slightly earlier than the C57BL/6 mice. Grey bars indicate the number of animals remaining in the study.
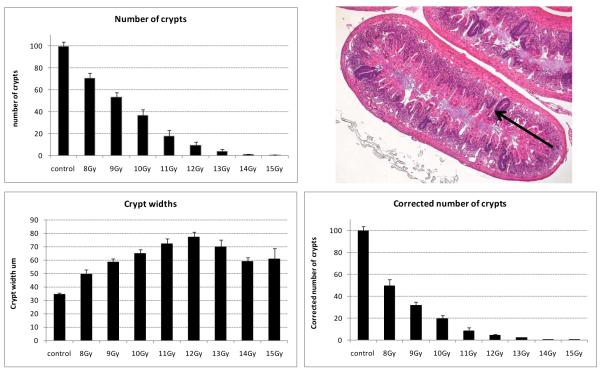
The full dose response of the small intestinal crypt survival post-irradiation was also determined under these irradiation conditions (Fig. 4). Rapid crypt regeneration from surviving clonogenic cells, accompanied by loss of adjacent tissue structure, resulted in the generation of crypts with a dose dependent increase in girth on day four following 8 Gy to 12 Gy. At higher doses, where crypt regeneration is likely to be slower due to the presence of fewer surviving clonogenic cells, there was a slight reduction in girth compared to 10 Gy to 12 Gy. Thus, as has been described previously, in intestinal cross-sections from irradiated animals there is an increased probability of sectioning through a larger surviving crypt than a smaller control crypt, and thus a risk of overestimating the number of surviving crypts (Potten et al. 1981). In assays measuring the efficacy of an agent on crypt survival and regeneration (where increased speed of regeneration hand hence crypt size can influence the probability of being scored) it is therefore necessary to apply the width correction to all measurements of crypt number.
Fig. 4.
There is a radiation dose-dependent crypt kill. The plots illustrate the number of surviving regenerating crypts present in a small intestinal circumference 4 days following total body irradiation of 10 to 12 week old male C57BL/6 mice. Top: The actual number of surviving regenerating crypts present. The arrow in the photograph illustrates an example of a darkly stained surviving, regenerating crypt, derived from at least one clonogenic cell, that is scored in this assay (H&E stained cross section; x5 objective). Bottom: The change in crypt width with radiation dose on day 4 post irradiation and the corrected number of crypts per intestinal circumference once the impact of width is accounted for in the scoring process (see Materials and Methods).
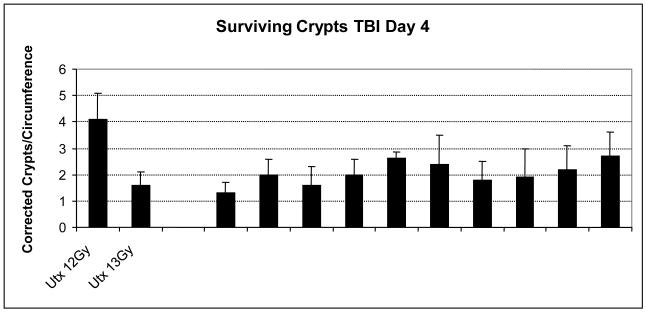
This crypt survival radiosensitivity was very consistent. The number of surviving crypts following 13 Gy TBI from ten totally independent studies performed over three years was remarkably similar (Fig. 5). In each study the mice had received an aqueous vehicle control administration post-irradiation. Thus, although animal morbidity may vary in studies, presumably due to changing environmental conditions, such as the intestinal flora, impacting the steep animal survival curves, the actual level of crypt death remained consistent.
Fig. 5.
Illustration of the consistency of the mean number of surviving crypts following 13 Gy TBI per cross section in saline placebo control groups from a number of different studies over 3 years. In each case the placebo was administered 24h following irradiation, either once or daily. For comparison the number of surviving crypts in untreated irradiated mice following 12 and 13 Gy are also shown.
Further histological endpoints, such as counts of the levels of crypt fission per intestinal cross section, from day six onwards, and the actual proportion of the mucosal surface area that is ulcerated (the length of undulating luminal contour that is devoid of residual mucosa in a cross section) have also been made at a variety of time points following a range of irradiation doses. These can provide further data supporting possible mechanisms of action of mitigators (improved crypt fission and epithelial restitution to restore the barrier) (data not shown).
Animal survival was highly correlative with diarrhea severity following total body irradiation (Fig. 6). At doses of 13 Gy and higher (where there was incomplete epithelial regeneration by day six) diarrhea was first evident on day four, lasting for approximately three days, by which time the animal had either recovered or reached a moribund state and been humanely euthanized. Diarrhea was typically soft or very loose stools, but there was rarely evidence of hemorrhaging. Although survival levels, and hence the relative proportion of animals with diarrhea, may change when the bone marrow is shielded, the time course of the diarrhea remained consistent, and directly correlated with the ulceration seen in the histopathology.
A series of animal dose response curves were performed employing TBI, PBI BM40 and PBI BM5 irradiation. Further, in order to gain insight into the response of different mouse strains, which may be crucial when linking the various radiation induced sub-syndromes where there are strain specific responses, these were performed in both C57BL/6 and CBA/Ca mice (both of the same sex and age and housed and irradiated under identical conditions). The LD30,50,70 data for TBI from a series of pooled studies are shown in Table 1. The increasing morbidity through days six to eight is evident, with the day twenty LD30,50,70 estimates incorporating the later stage hematology sub-syndrome mortalities (consistent with the timing and drop in blood counts observed by Orschell et al, this issue) and other confounding variables due to infection etc. Thus, Probit plots (not shown) illustrate 100% mortality at greater than 8Gy within twenty days. The overlapping 95% confidence intervals reflect the steepness of the dose response curve. The median survival time for decedents was seven days for C57BL/6 mice and six days for the CBA/Ca mice, with deaths generally being + or - two days of the median.
Table 1.
LD30, LD50 and LD70 estimates from C57BL/6 and CBA/Ca mice exposed to total-body irradiation. The lethal dose estimates are derived from mice pooled from several studies (the total number of mice are indicated in parentheses). The predicted lethal dose needed to deliver an LD30, LD50 and LD70 are shown, along with the lower and upper confidence intervals for these figures, for days 6, 7, 8 and 20. The overlap between limits can be seen e.g. the upper confidence limit for the LD30 on day 6 is above the lower confidence limit for the LD50 on the same day.
| C57BL/6 | CBA/Ca | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Day | LD | Dose (Gy) |
Lower 95% CI |
Upper 95% CI |
Group | Day | LD | Dose (Gy) |
Lower 95% CI |
Upper 95% CI |
| TBI (450) | 6 | 30 | 11.1 | 10.8 | 11.4 | TBI (380) | 6 | 30 | 9.7 | 9.4 | 10.0 |
| TBI (450) | 6 | 50 | 11.9 | 11.6 | 12.2 | TBI (380) | 6 | 50 | 10.3 | 10.0 | 10.5 |
| TBI (450) | 6 | 70 | 12.7 | 12.4 | 13.1 | TBI (380) | 6 | 70 | 10.9 | 10.6 | 11.2 |
| TBI (450) | 7 | 30 | 9.1 | 8.7 | 9.4 | TBI (380) | 7 | 30 | 8.7 | 8.4 | 9.0 |
| TBI (450) | 7 | 50 | 10.0 | 9.7 | 10.3 | TBI (380) | 7 | 50 | 9.2 | 9.0 | 9.5 |
| TBI (450) | 7 | 70 | 11.1 | 10.7 | 11.4 | TBI (380) | 7 | 70 | 9.8 | 9.5 | 10.1 |
| TBI (450) | 8 | 30 | 7.9 | 7.5 | 8.3 | TBI (380) | 8 | 30 | 8.1 | 7.8 | 8.3 |
| TBI (450) | 8 | 50 | 8.8 | 8.5 | 9.1 | TBI (380) | 8 | 50 | 8.5 | 8.3 | 8.7 |
| TBI (450) | 8 | 70 | 9.7 | 9.4 | 10.1 | TBI (380) | 8 | 70 | 8.9 | 8.7 | 9.2 |
| TBI (450) | 20 | 30 | 6.3 | 6.1 | 6.5 | TBI (380) | 20 | 30 | 6.5 | 6.2 | 6.7 |
| TBI (450) | 20 | 50 | 6.5 | 6.2 | 6.7 | TBI (380) | 20 | 50 | 6.7 | 6.4 | 6.9 |
| TBI (450) | 20 | 70 | 6.7 | 6.4 | 6.9 | TBI (380) | 20 | 70 | 6.9 | 6.6 | 7.1 |
The same euthanasia criteria were used throughout the reported studies, with animals inspected several times per 24h during days four to ten. It was observed that several animals exposed to TBI, in addition to weight loss, diarrhea and resultant dehydration, also developed a swollen muzzle that, within the next 24h, spread distally to cause a swollen head, with animals becoming moribund during this time. However, this observation was slightly reduced in animals treated post irradiation with ciprofloxacin, which also presumably reduced the levels of enteric flora. The impact of reducing this incidence on the LD30,50,70 estimates is shown in Table 2.
Table 2.
Effect of ciprofloxacin on the LD30, LD50 and LD70 estimates during the GI syndrome time points, from C57BL/6 and CBA/Ca mice exposed to total-body irradiation.
| C57BL/6 | CBA/Ca | ||||||
|---|---|---|---|---|---|---|---|
| Day | LD | Dose (Gy) |
Dose + Cipro (Gy) |
Day | LD | Dose (Gy) |
Dose + Cipro (Gy) |
| 6 | 30 | 11.1 | 11.7 | 6 | 30 | 9.7 | 10.1 |
| 6 | 50 | 11.9 | 12.6 | 6 | 50 | 10.3 | 10.9 |
| 6 | 70 | 12.7 | 13.6 | 6 | 70 | 10.9 | 11.9 |
| 7 | 30 | 9.1 | 8.8 | 7 | 30 | 8.7 | 9.8 |
| 7 | 50 | 10.0 | 10.3 | 7 | 50 | 9.2 | 10.6 |
| 7 | 70 | 11.1 | 12.1 | 7 | 70 | 9.8 | 11.4 |
| 8 | 30 | 7.9 | 7.6 | 8 | 30 | 8.1 | 9.5 |
| 8 | 50 | 8.8 | 8.9 | 8 | 50 | 8.5 | 10.3 |
| 8 | 70 | 9.7 | 10.5 | 8 | 70 | 8.9 | 11.2 |
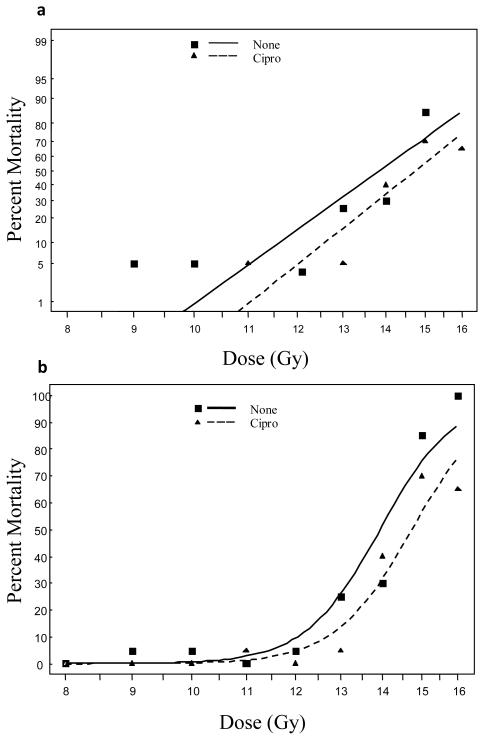
There was a dramatically increased survival if the head, thorax and forelimbs were shielded from irradiation; with day seven LD50 in C57BL/6 mice increasing from 10 Gy in TBI animals to 14 Gy in PBI BM40 animals, and 15.8 Gy if the animals were also treated with ciprofloxacin (Table. 3 and 4). The same data are presented as Probit and Logit plots for the C57BL/6 mice in Figure 7, where the dose shift effect of the ciprofloxacin is clearly illustrated. It was also observed in these mice that the average daily maximum diarrhea in the ciprofloxacin treated mice was marginally lower compared to the unsupported mice (p-value = 0.061). Similarly the global maximum diarrhea grade was also lower (p-value = 0.027). However, although of milder severity, the diarrhea duration in the ciprofloxacin treated mice was generally longer compared to the unsupported mice (p-value = 0.022).
Table 3.
LD30, LD50 and LD70 estimates from C57BL/6 mice exposed to partial-body irradiation (40% bone marrow shielding). This is effectively abdominal only irradiation. The predicted lethal dose needed to deliver an LD30, LD50 and LD70 are shown, along with the lower and upper confidence limits for these figures, for days 6, 7, 8 and 20. This has not yet been performed in CBA/Ca mice.
| Group | Day | LD | Dose (Gy) |
Lower 95% CI |
Upper 5% CI |
|---|---|---|---|---|---|
| PBI BM40 (310) | 6 | 30 | 13.9 | 13.6 | 14.2 |
| PBI BM40 (310) | 6 | 50 | 14.4 | 14.2 | 14.7 |
| PBI BM40 (310) | 6 | 70 | 15.0 | 14.7 | 15.4 |
| PBI BM40 (310) | 7 | 30 | 13.5 | 13.3 | 13.8 |
| PBI BM40 (310) | 7 | 50 | 14.0 | 13.8 | 14.3 |
| PBI BM40 (310) | 7 | 70 | 14.5 | 14.2 | 14.8 |
| PBI BM40 (310) | 8 | 30 | 13.4 | 13.1 | 13.7 |
| PBI BM40 (310) | 8 | 50 | 13.9 | 13.6 | 14.2 |
| PBI BM40 (310) | 8 | 70 | 14.4 | 14.1 | 14.7 |
| PBI BM40 (310) | 20 | 30 | 13.4 | 13.1 | 13.6 |
| PBI BM40 (310) | 20 | 50 | 13.9 | 13.6 | 14.1 |
| PBI BM40 (310) | 20 | 70 | 14.4 | 14.1 | 14.7 |
Table 4.
LD30, LD50 and LD70 estimates from C57BL/6 and CBA/Ca mice exposed to partial- body irradiation (40% bone marrow shielding and receiving post-irradiation antibiotic support (ciprofloxacin). The predicted lethal dose needed to deliver an LD30, LD50 and LD70 are shown, along with the lower and upper confidence limits for these figures, for days 6, 7, 8 and 20.
| C57BL/6 | CBA/Ca | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Day | LD | Dose (Gy) |
Lower 95% CI |
Upper 95% CI |
Group | Day | LD | Dose (Gy) |
Lower 95% CI |
Upper 95% CI |
|
PBI BM40 +
Cipro (240) |
6 | 30 | 15.4 | 15.0 | 15.8 |
PBI BM40 +
Cipro (180) |
6 | 30 | 15.6 | 15.0 | 16.3 |
|
PBI BM40 +
Cipro (240) |
6 | 50 | 16.1 | 15.6 | 16.6 |
PBI BM40 +
Cipro (180) |
6 | 50 | 16.5 | 15.5 | 17.5 |
|
PBI BM40 +
Cipro (240) |
6 | 70 | 16.7 | 16.0 | 17.5 |
PBI BM40 +
Cipro (180) |
6 | 70 | 17.3 | 15.9 | 18.9 |
|
PBI BM40 +
Cipro (240) |
7 | 30 | 15.2 | 14.8 | 15.6 |
PBI BM40 +
Cipro (180) |
7 | 30 | 15.0 | 14.5 | 15.4 |
|
PBI BM40 +
Cipro (240) |
7 | 50 | 15.8 | 15.3 | 16.3 |
PBI BM40 +
Cipro (180) |
7 | 50 | 15.7 | 15.1 | 16.2 |
|
PBI BM40 +
Cipro (240) |
7 | 70 | 16.5 | 15.8 | 17.1 |
PBI BM40 +
Cipro (180) |
7 | 70 | 16.4 | 15.6 | 17.2 |
|
PBI BM40 +
Cipro (240) |
8 | 30 | 14.6 | 14.3 | 15.0 |
PBI BM40 +
Cipro (180) |
8 | 30 | 14.9 | 14.5 | 15.4 |
|
PBI BM40 +
Cipro (240) |
8 | 50 | 15.2 | 14.9 | 15.6 |
PBI BM40 +
Cipro (180) |
8 | 50 | 15.6 | 15.1 | 16.1 |
|
PBI BM40 +
Cipro (240) |
8 | 70 | 15.9 | 15.4 | 16.4 |
PBI BM40 +
Cipro (180) |
8 | 70 | 16.2 | 15.5 | 17.0 |
|
PBI BM40 +
Cipro (240) |
20 | 30 | 14.2 | 13.9 | 14.6 |
PBI BM40 +
Cipro (180) |
20 | 30 | 14.9 | 14.5 | 15.4 |
|
PBI BM40 +
Cipro (240) |
20 | 50 | 14.8 | 14.5 | 15.2 |
PBI BM40 +
Cipro (180) |
20 | 50 | 15.6 | 15.1 | 16.1 |
|
PBI BM40 +
Cipro (240) |
20 | 70 | 15.5 | 15.0 | 15.9 |
PBI BM40 +
Cipro (180) |
20 | 70 | 16.2 | 15.5 | 17.0 |
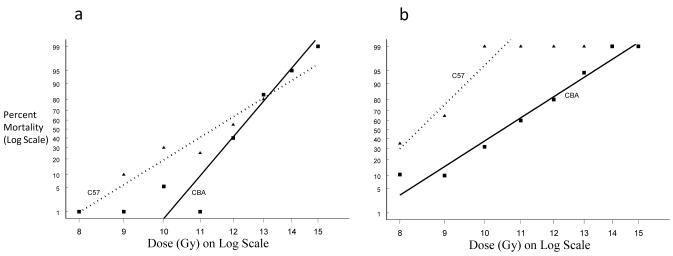
Fig. 7.
a) Dose response curves using a Probit model b) Logit of C57BL/6 survival following PBI BM40. Ciprofloxacin significantly reduced the mortality rate of mice at 20 days (odds ratio = 0.389, p-value = 0.013).
The median survival time of the decedents remained similar to the TBI mice (six days), consistent with GI-ARS timeframe mortality, but with improved survival levels when the bone marrow was protected.
In CBA/Ca mice the LD30,50,70 estimates were also shifted with similar lethal dose estimates (within the confidence limits of the C57BL/6 mice) (data not shown). The PBI BM40 model allows one to concentrate on GI-specific effects, and is highly applicable to evaluating potential GI mucositis therapies for oncology supportive care indications, but is not a reality scenario for radiation exposure as a result of a nuclear accident or terrorist attack. Similarly, it is also unlikely that all persons involved will receive TBI. Survival of 2.5 to 5% of the bone marrow is both a reality model and allows modeling of later stage interacting sub-syndromes. Thus, we have developed a 5% shielding model that is similar to that used in the primate model reported in this issue. The LD30,50,70 estimates in both C57BL/6 and CBA/Ca mice are shown in Table 5 with the Probit plots for the unsupported (no antibiotics) animals in Fig. 8. There was a large dose shift from the TBI lethal dose values during the GI-ARS timescale of days six to eight, with both strains of mice again being remarkably consistent. A similar dose shift was seen in the LD50/15 values in the primates with 5% bone marrow sparing (MacVittie et al. in this issue). Thus, in both species sparing just 5% of the bone marrow is sufficient to influence the mortalities during the early stage GI-ARS time frame. In these mice there was a biphasic pattern of weight loss in the mice exposed to high dose irradiation (greater than 10 Gy); an initial weight loss over the first six days coincident with GI-ARS, followed by a brief recovery, and in the surviving mice a second period of weight loss from days ten to sixteen, coincident with some animals yielding to H-ARS (data not shown). Studies are underway to determine whether this correlates with a change in crypt survival/regeneration. We have compared crypt survival following TBI and PBI BM40 and demonstrated increased crypt survival following bone marrow shielding, supporting a contribution to the effect on animal survival (data not shown since these studies were not performed as a simultaneous head to head comparison).
Table 5.
LD30, LD50 and LD70 estimates from C57BL/6 and CBA/Ca mice exposed to partial- body irradiation (5% bone marrow shielding). The predicted lethal dose needed to deliver an LD30, LD50 and LD70 are shown, along with the lower and upper confidence limits for these figures, for days 6, 7, 8 and 20.
| C57BL/6 | CBA/Ca | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Day | LD | Dose (Gy) |
Lower 95% CI |
Upper 95% CI |
Group | Day | LD | Dose (Gy) |
Lower 95% CI |
Upper 95% CI |
|
PBI BM5 +
(160) |
6 | 30 | 13.6 | 13.1 | 14.2 |
PBI BM5 +
(155) |
6 | 30 | 14.1 | 13.7 | 14.6 |
|
PBI BM5 +
(160) |
6 | 50 | 14.6 | 13.9 | 15.2 |
PBI BM5 +
(155) |
6 | 50 | 14.8 | 14.2 | 15.4 |
|
PBI BM5 +
(160) |
6 | 70 | 15.6 | 14.5 | 16.7 |
PBI BM5 +
(155) |
6 | 70 | 15.5 | 14.7 | 16.4 |
|
PBI BM5 +
(160) |
7 | 30 | 11.7 | 11.1 | 12.2 |
PBI BM5 +
(155) |
7 | 30 | 12.5 | 12.1 | 12.9 |
|
PBI BM5 +
(160) |
7 | 50 | 12.7 | 12.2 | 13.3 |
PBI BM5 +
(155) |
7 | 50 | 13.0 | 12.7 | 13.4 |
|
PBI BM5 +
(160) |
7 | 70 | 13.9 | 13.1 | 14.7 |
PBI BM5 +
(155) |
7 | 70 | 13.6 | 13.2 | 14.1 |
|
PBI BM5 +
(160) |
8 | 30 | 10.6 | 10.2 | 11.1 |
PBI BM5 +
(155) |
8 | 30 | 11.8 | 11.5 | 12.2 |
|
PBI BM5 +
(160) |
8 | 50 | 11.5 | 11.0 | 11.9 |
PBI BM5 +
(155) |
8 | 50 | 12.2 | 11.9 | 12.6 |
|
PBI BM5 +
(160) |
8 | 70 | 12.3 | 11.8 | 12.9 |
PBI BM5 +
(155) |
8 | 70 | 12.7 | 12.3 | 13.0 |
|
PBI BM5 +
(160) |
20 | 30 | 8.0 | 7.6 | 8.4 |
PBI BM5 +
(155) |
20 | 30 | 9.8 | 9.4 | 10.3 |
|
PBI BM5 +
(160) |
20 | 50 | 8.4 | 8.1 | 8.8 |
PBI BM5 +
(155) |
20 | 50 | 10.5 | 10.1 | 11.0 |
|
PBI BM5 +
(160) |
20 | 70 | 8.8 | 8.5 | 9.2 |
PBI BM5 +
(155) |
20 | 70 | 11.3 | 10.8 | 11.8 |
Fig. 8.
Dose response curves using Probit models C57BL/6 and CBA/Ca survival following PBI BM5 a) day 8 and b) day 20. There was a large difference in the incidence of the swollen muzzle syndrome in the two strains of mice, with associated mortality being higher in the C57BL/6 mice.
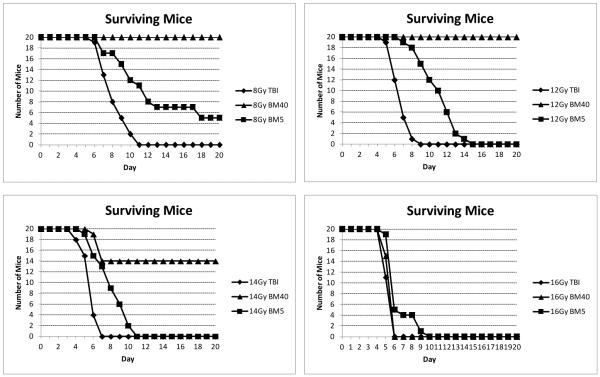
Comparison of the time course of all cause mortalities in the different radiation models is illustrated in Fig. 9. At 8 Gy [insufficient to cause GI-ARS but results in a hematological sub-syndrome dose (H-ARS)] there were no deaths when 40% of the bone marrow was shielded. However, following TBI there was 100% mortality by day eleven. Protection of just 5% of the bone marrow increased the survival time and reduced the level of animal deaths within twenty days. Increasing the dose to 12 Gy (a dose that is lethal to the bone marrow but not the GI) caused 100% mortality in both the TBI and 5% shielding model, but no deaths in the 40% shielding model; however, there was an increased survival time with the 5% model compared to TBI. When doses were increased to the GI-ARS-inducing levels of 14 Gy to 16 Gy, mortality was increased, and became absolute in all models at 16 Gy.
Fig. 9.
Examples of animal survival in three independent studies in C57BL/6 mice receiving post-irradiation ciprofloxacin. The plots directly compare the survival time course in TBI, PBI BM5 and PBI BM40. These study data contributed towards the much larger data set used to generate the lethal dose tables.
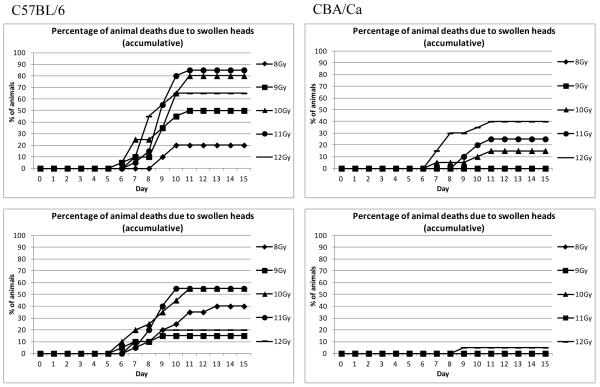
In both the TBI and PBI BM5 models there were a number of animals becoming moribund between six and twelve days post irradiation, even at the radiation doses below 12 Gy (i.e. at times and doses when the intestinal epithelial barrier appeared to be restored in H & E sections). However, this was not entirely due to H-ARS since many of these animals developed swollen muzzles, which rapidly developed into a swollen head, prior to necessary euthanasia. This was more prevalent in the C57BL/6 mice than the CBA/Ca mice, and was almost completely inhibited by ciprofloxacin in the CBA/Ca mice (but not the C57BL/6 mice) suggesting a bacterial cause (Fig. 10, Table 6).
Fig. 10.
The incidence of notable swollen muzzles in a single study. Two strains of mice were exposed to PBI BM5 irradiation (doses that cause H-ARS but minimal GI-ARS). Top: Unsupported animals, Bottom: Animals receiving ciprofloxacin from day 4 post irradiation. The incidence was clearly lower in the CBA/Ca mice and dramatically reduced by ciprofloxacin.
Table 6.
LD30, LD50 and LD70 estimates from C57BL/6 and CBA/Ca mice exposed to partial- body irradiation (5% bone marrow shielding and receiving post-irradiation antibiotic support (ciprofloxacin).The predicted lethal dose needed to deliver an LD30, LD50 and LD70 are shown, along with the lower and upper confidence limits for these figures, for days 6, 7, 8 and 20.
| C57BL/6 | CBA/Ca | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Day | LD | Dose (Gy) |
Lower 95% CI |
Upper 95% CI |
Group | Day | LD | Dose (Gy) |
Lower 95% CI |
Upper 95% CI |
|
PBI BM5 + Cipro (180) |
6 | 30 | 13.9 | 13.2 | 14.7 |
PBI BM5 + Cipro (258) |
6 | 30 | 13.2 | 12.7 | 13.7 |
|
PBI BM5 + Cipro (180) |
6 | 50 | 15.5 | 14.4 | 16.5 |
PBI BM5 + Cipro (258) |
6 | 50 | 14.4 | 13.8 | 15.0 |
|
PBI BM5 + Cipro (180) |
6 | 70 | 17.2 | 15.6 | 19.0 |
PBI BM5 + Cipro (258) |
6 | 70 | 15.6 | 14.7 | 16.5 |
|
PBI BM5 + Cipro (180) |
7 | 30 | 12.6 | 11.8 | 13.6 |
PBI BM5 + Cipro (258) |
7 | 30 | 13.2 | 12.7 | 13.7 |
|
PBI BM5 + Cipro (180) |
7 | 50 | 14.9 | 13.6 | 16.3 |
PBI BM5 + Cipro (258) |
7 | 50 | 14.4 | 13.8 | 15.0 |
|
PBI BM5 + Cipro (180) |
7 | 70 | 17.5 | 15.3 | 20.1 |
PBI BM5 + Cipro (258) |
7 | 70 | 15.6 | 14.7 | 16.5 |
|
PBI BM5 + Cipro (180) |
8 | 30 | 11.6 | 10.6 | 12.6 |
PBI BM5 + Cipro (258) |
8 | 30 | 13.1 | 12.6 | 13.5 |
|
PBI BM5 + Cipro (180) |
8 | 50 | 14.1 | 12.9 | 15.4 |
PBI BM5 + Cipro (258) |
8 | 50 | 14.1 | 13.6 | 14.7 |
|
PBI BM5 + Cipro (180) |
8 | 70 | 17.2 | 14.8 | 20.0 |
PBI BM5 + Cipro (258) |
8 | 70 | 15.3 | 14.5 | 16.1 |
|
PBI BM5 + Cipro (180) |
20 | 30 | 6.8 | 5.8 | 8.0 |
PBI BM5 + Cipro (258) |
20 | 30 | 10.9 | 10.5 | 11.4 |
|
PBI BM5 + Cipro (180) |
20 | 50 | 7.5 | 6.7 | 8.4 |
PBI BM5 + Cipro (258) |
20 | 50 | 12.0 | 11.6 | 12.5 |
|
PBI BM5 + Cipro (180) |
20 | 70 | 8.2 | 7.6 | 8.9 |
PBI BM5 + Cipro (258) |
20 | 70 | 13.3 | 12.7 | 13.9 |
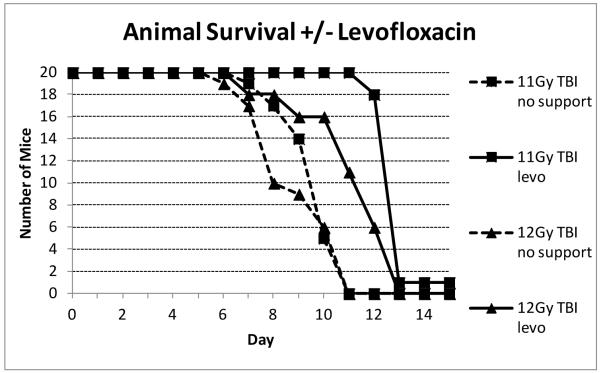
This was therefore further investigated with the use of a broader spectrum gram positive antibiotic, levofloxacin. In the first study mice were exposed to TBI radiation doses that would be lethal for the bone marrow but not the GI tract (Fig. 11). It was found that addition of levofloxacin dramatically increased the survival time of the decedents by three to four days until mice eventually died in the second week, presumably due to H-ARS. After PBI BM5, where survival times were extended due to the 5% bone marrow sparing, this mitigation was only evident at the higher dose of 13 Gy (data not shown).
Fig. 11.
Effect of levofloxacin on C57BL/6 animal survival. Groups of twenty C57BL/6 adult male mice per group were irradiated with TBI. All mice received acid water but the addition of levofloxacin in the water/wetted chow was added from day 4 post irradiation. Mice were checked twice daily and euthanized when moribund or on day 15 post irradiation. The number of surviving mice per time point is plotted.
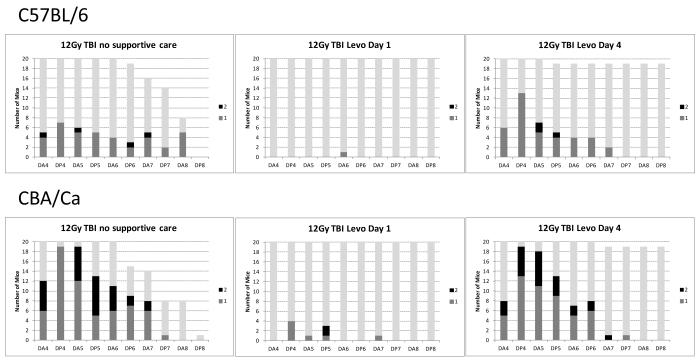
The response to levofloxacin was then compared in both C57BL/6 mice and CBA/Ca mice, with the antibiotic administered from different times post irradiation (from day one, before ulceration is evident, and from day four). The levels of survival and impact on diarrhea severity over the first eight days were dramatic and consistent in both strains of mice (Fig. 12). The data indicates that levofloxacin increased animal survival time in both strains of mice, and again even when administration was delayed until four days post irradiation. Administration from day one almost completely blocked diarrhea induced by 12 Gy. However, a delay in treatment until day four was too late to stop the diarrhea. In all cases there was 100% mortality by day fourteen but the survival time was again extended by the levofloxacin.
Fig. 12.
Effect of levofloxacin administered from either day 1 or day 4 post irradiation. levofloxacin prevented mortalities during the first 8 days post irradiation and also prevented diarrhea if administered from day 1 (+24h), but not day 4 post irradiation. This observation was consistent in two strains of adult (10-12 week) male mice. Many animals in the group receiving no antibiotic developed swollen muzzles and had faecal associated enterobacteriacae (E.coli and K.pneumoniae) faecal streptococci (S.faecalis and S.faecium) or P.aeruginosa in more than one organ. These samples also had a positive blood culture containing the same organisms, confirming the leaking of gut flora in to the blood. The infection and incidence of swollen muzzles was eliminated by levofloxacin.
In additional animals, scheduled euthanasia was performed on days six to ten for microbial analysis. The presence of a swollen muzzle was also recorded. All samples were analyzed in a blinded manner - to both mouse strain and treatment, which was subsequently unblinded. When significant bacteria were present there were large quantities of Enterobacteriacae (E. coli) that possibly originated in the gut; in addition Streptococcus faecalis and Streptococcus faecium either individually (also probably originating from the gut) or as mixtures of organisms were present in more than one organ. These samples also had positive blood cultures containing the same organisms as those present in the organs indicating general sepsis (the organs were probably ‘seeded’ from the blood). This is indicative of translocation of microbes from the gut into the blood allowing organisms to be transported to other organs. There was a striking correlation of bacterial growth inhibition and levofloxacin treatment. Most animals with heavy bacterial loads also demonstrated the swollen muzzle syndrome, although this was not absolute, presumably because the increased infection preceded the development of the syndrome. The data therefore confirmed the leakage of gut flora into the blood post irradiation, followed by systemic distribution to the organs, including the brain. This therefore caused septicemia and meningitis, which could be prevented by the levofloxacin. Studies using the PBI BM40 model did not demonstrate swollen muzzle syndrome, presumably at least in part because the maintenance of 40% of the bone marrow maintained sufficient immune function. In the PBI BM5 model (where less bone marrow is protected but the muzzle itself is irradiated) the incidence of swollen muzzles was reduced/delayed but not abrogated compared to TBI. In the model described by Kirsch et al. 2010 in which both the head and forelimbs were shielded (equivalent to protecting 25% of the bone marrow according to Taketa et al. 1962) no swollen muzzles were observed but there was in increased animal survival compared to TBI.
Thus, the involvement of the mouse flora, (i.e. husbandry conditions, and antibiotic support in addition to acidified water) plays a crucial role in the timecourse of animal mortalities involving high levels of bone marrow toxicity. This, of course, may be in addition to irradiation compromization of the upper GI/respiratory and oral mucosa although studies performed in-house employing doses of 20-30 Gy irradiation in a single exposure to the muzzle (the remainder of the animal being lead shielded) do not cause this syndrome indicating disruption of this mucosa alone is not sufficient to cause the response.
DISCUSSION
There are no FDA-approved MCMs to mitigate the lethal effects of GI-ARS. Although there have been guidance documents relating to studies necessary to achieve such licensure (FDA 2009), to date this has not been rigorously tested due to the lack of countermeasures available and progressing to pivotal studies. The primary endpoint is clearly enhancement of survival but the baseline upon which such a mitigator might be evaluated (the level of associated supportive care, bone marrow sparing etc) has not been decided. The impact of these factors needs to be defined in order to design an evaluation trial that is both well understood and suitably represents a human irradiation ‘reality’ situation.
Data to help define these baseline conditions are presented here. Mouse strain differences in radiosensitivity and survival have been observed for over 50 years (Grahn and Hamilton 1956; Hanson et al. 1987). These findings may represent innate differences, particularly in the immune response, but also the enteric flora population. Indeed Ciorba recently manipulated radiosenstivity using Lactobacilli (Ciorba et al. 2011) and others have shown that changes in the bacterial population can impact radiosensitivity and animal survival (Duran-Struuck et al. 2008).
By restricting our studies to two inbred strains and performing all studies in the same husbandry conditions (including individually ventilated rather than open top cages), we have attempted to restrict the levels of variation and generate a more robust model. For the same reasons we have restricted studies to males (where the impact of the estrus cycle does not complicate responses) and used adult mice aged ten to twelve weeks. This latter decision is crucial since many published studies describing potential mitigators have used much younger mice (often five to eight weeks of age) and induced GI-ARS by lower doses of irradiation than we have identified. This is directly related to the difference in the radiosensitivity of the crypt clonogenic population in young mice. These juvenile animals are still growing quite rapidly and are consequently increasing the length of their intestine. As a result the stem and overall clonogenic cell population is also expanding as more crypts are constantly generated by ongoing crypt fission. The factors that regulate these processes are not known but are likely to have both similarities and differences to the regeneration induced by irradiation.
The human population is, however, clearly far more diverse and so understanding the reasons for variations in responses remains to be more fully investigated. The differential effects of mitigators on outbred mice of either sex, and pediatric, juvenile and aged populations are highly relevant, and must therefore also be pursued.
Further, the stem cells of the crypt are known to have a circadian rhythm which again influences their radiosensitivity (Potten et al. 1977, Hendry 1975, Qiu et al. 1994). We have therefore performed all the irradiations in these studies at the same time of day, in order to reduce variability.
By restricting the study variables (strain, sex, age, husbandry, irradiation source) there is now a directly comparable database from which countermeasure studies can be designed. Further, since the conditions are as closely related as possible to those of Orschell et al. (in this issue), the GI-ARS and H-ARS databases from these mouse studies are compatible.
The primary aim of these studies was to define the dose response and time course of GI-ARS in the mice. Given that the main symptom of GI-ARS is diarrhea it was noted that, irrespective of radiation dose or mouse strain, this symptom began 3.5 to 5 days post irradiation. The severity rather than the timing of the onset of diarrhea was dose dependent. This is consistent with the time taken for a cell generated from the clonogenic cells towards the crypt base to transit up the crypt and villus (Kaur and Potten 1986), and hence the time taken for that epithelium to be lost and an ulcer generated if that exponential cell generation is interrupted by irradiation sterilization of the clonogens. There was a direct correlation with the timing of the diarrhea and evidence of sustained loss of mucosal integrity.
The level of clonogenic death on day four (measured by the reciprocal of small intestinal crypt loss, crypt survival and regeneration) is a directly quantifiable measure of acute GI damage, and is dose dependent (Withers and Elkind 1970, Potten and Hendry 1985, Potten and Hendry 1995, Hendry at al. 1992, Hendry 1993, Roberts and Potten 1994, Withers et al. 1993). Following 10 Gy, although only approximately 20% of the original crypt number survive and regenerate, this number is sufficient to rapidly restore the mucosa via proliferation, crypt fission and cell migration. At 11 Gy this is reduced to less than 10%, and less than 5% at 12 Gy. When too few crypts remain to restore the barrier, mortalities become high. Effects of treatments on surviving crypt number can therefore be measured, and MCM induced improvements directly correlated to an equivalent radiation dose, to generate a dose modification factor (DMF).
Exactly the same crypt measurements are possible in the large intestine. The kinetics of this tissue are slightly slower, with crypt survival best evaluated on day five post irradiation rather than day four (Cai et al. 1997a,b).
Subsequent analysis of small intestinal crypt levels at later times (days six to eight), alongside measures of crypt fission (Cairnie and Millen 1975) and epithelial restitution, allow quantification of the speed of epithelial regeneration and comparisons of mechanisms of action in treatments versus placebo. Tissues taken at such timepoints can also be taken for expression analysis to substantiate the hypothesized mechanism of action, in addition to correlating with systemic cytokine effects etc.
These histopathology observations were then correlated with the LD30,50,70 parameters, which were defined with 95% confidence intervals. The LD estimates are heavily dependent upon the level of bone marrow sparing, with differing levels in the TBI, PBI BM40 and PBI BM5 models.
In each case the LD was defined by the day (six, seven, eight and twenty) due to the rapidly progressive mortality during the early timepoints. The day twenty LD estimates also include deaths due to the later stage H-ARS sub-syndrome in all but the PBI BM40 model. The dose shift in the PBI BM5 model compared to TBI presumably reflects the rapid response of the residual bone marrow and the influence of remotely produced endogenous mitigating factors or cells. This is consistent with reports of GI-ARS or intestinal mucositis induced by radiotherapy mitigation by bone marrow derived cells or growth factors (Terry and Travis 1989; Takaba et al. 2010, Saha et al. 2011, Gao et al. 2012). This influence may be reduced by lower levels of bone marrow sparing such as 2.5%. The advantage of these low level bone marrow sparing models is that the response of the surviving animals (particularly after effective mitigation) can then be assessed for later syndrome effects (H-ARS, lung or upper GI abnormalities). It is therefore a reality, multi-syndrome model. In contrast, the PBI BM40 model allows sub-syndrome separation by protecting the bone marrow and preventing H-ARS.
Evidence of the effects of the combination of head irradiation, enteric flora and immune tolerance in each strain of mouse was clear from the appearance of septicemia in animals exposed to TBI. C57BL/6 mice were more susceptible than the CBA/Ca mice. Thus, in the C57BL/6 mice even a low level or short-term interruption of the mucosal barrier induced by low doses of irradiation (below the GI-ARS dose but at lethal bone marrow doses) was sufficient to allow enteric bacterial translocation through a leaky epithelium and induce septicemia, which these highly immunocompromized animals could not tolerate. Hematological readouts (complete blood counts) were not measured at these key time points in these studies and so the strain responses cannot be compared. However, it is clear that this response is likely to be responsible for the variable levels of survival seen in many in-house and published studies, despite the relative consistency of the crypt measurements. The baseline lethal doses will undoubtedly drift with changes in the enteric flora and immune status of the mice.
The dramatic effect of the levofloxacin and lesser effects of ciprofloxacin were consistent with the importance of controlling these enteric flora, and the fact that others have observed that germ-free animals have improved survival after irradiation (Crawford and Gordon 2005). When levofloxacin was included in the water, the incidence of obvious septicemia as presented by the swollen muzzles and heads was averted, and animal survival dramatically improved. If given 24h following irradiation, diarrhea and resulting dehydration was also averted, but not if given 96h post irradiation. Given that antibiotics should be widely available following a nuclear incident, it is prudent to assume that any useful mitigator must be effective in the presence of such antibiotic supportive care. Further, other higher species such as the non-human primate and dog models routinely administer antibiotics as a standard of care. Thus, for consistency between models antibiotic use is recommended, either with TBI or a model employing minimal bone marrow sparing.
Although not reported in this paper, several studies were also undertaken to evaluate the beneficial effect of rehydration on animal survival (injectable or oral water or Ringers solution) since this has also been a beneficial means of supportive care in rodents (Taketa 1962). All these studies employed maximal bone marrow sparing (PBI BM40) and so will not contribute to baseline medical countermeasure evaluation conditions. However, it was clear that at doses of 14 Gy there were beneficial survival effects. Unfortunately, in traumatized mice the stress of additional handling and dosing (especially repeated oral gavages) countered the beneficial effect of the saline itself, such that at 15 Gy or later timepoints the treatments were counterproductive. This is consistent with H-ARS studies (Orschell et al. this issue) when it was observed that daily PBS injections increased animal mortality, presumably due to the handling stresses. However, it is clear that the maintenance of fluid support itself is beneficial, and hence any countermeasure evaluation should take into account rehydration and handling effects (the former can also cause apparent increases in diarrhea levels compared to dehydrated mice).
Current studies are underway to redefine the dose response (LD30,50,70) timelines for mice supported with levofloxacin in TBI and minimal bone marrow sparing models, and quantify the diarrhea severity and histology timelines in these reality models. In particular, direct correlations of the levels of crypt survival with the different levels of bone marrow shielding +/- levofloxacin are underway.
CONCLUSION
These data define the dose response of GI-ARS in mice, along with the time course of the histopathology and resultant main physiological symptom, diarrhea. Studies have been performed multiple times in two strains of mice with various levels of bone marrow sparing and antibiotic supportive care generating validated models with lethal dose values and 95% confidence intervals. This, along with future estimates combining TBI or minimal bone marrow sparing with levofloxacin will therefore provide a baseline for evaluation of mitigators of GI-ARS as described by the FDA Animal Rule.
Acknowledgments
This work supported by NIAID contracts HHSN266200500043C and HHSN272201000046C.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Boggs DR. The total marrow mass of the mouse: a simplified method of measurement. Am J Hematol. 1984;16(3):277–286. doi: 10.1002/ajh.2830160309. [DOI] [PubMed] [Google Scholar]
- Booth C, Potten CS. The Intestine as a Model for Studying Stem Cell Behaviour. In: Teicher BA, editor. Tumor Models in Cancer Research. Humana Press; Totawa, New Jersey: 2001a. pp. 337–357. [Google Scholar]
- Booth D, Potten CS. Protection against mucosal injury by growth factors and cytokines. J Natl Cancer Inst Monogr. 2001b;29:16–20. doi: 10.1093/oxfordjournals.jncimonographs.a003433. [DOI] [PubMed] [Google Scholar]
- Booth D, Haley JD, Bruskin AM, Potten CS. Transforming growth factor beta 3 protects murine small intestinal crypt stem cells and animal survival after irradiation, possibly by reducing stem cell cycling. Int J Cancer. 2000;86(1):53–59. doi: 10.1002/(sici)1097-0215(20000401)86:1<53::aid-ijc8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Cai WB, Roberts SA, Potten CS. The number of clonogenic cells in crypts in three regions of murine large intestine. Int J Radiat Biol. 1997a;71(5):573–579. doi: 10.1080/095530097143905. [DOI] [PubMed] [Google Scholar]
- Cai WB, Roberts SA, Bowley E, Hendry JH, Potten CS. Differential survival of murine small and large intestinal crypts following ionizing radiation. Int J Radiat Biol. 1997b;71(2):145–155. doi: 10.1080/095530097144265. [DOI] [PubMed] [Google Scholar]
- Cairnie AB, Millen BH. Fission of crypts in the small intestine of the irradiated mouse. Cell Tissue Kinet. 1975;8(2):189–196. doi: 10.1111/j.1365-2184.1975.tb01219.x. [DOI] [PubMed] [Google Scholar]
- Ciorba MA, Riehl TE, Rao MS, Moon C, Ee X, Nava GM, Walker MR, Marinshaw JM, Stappenbeck TS, Stenson WF. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut. 2011 doi: 10.1136/gutjnl-2011-300367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford LM. New drug and biological drug products; Evidence needed to demonstrate effectiveness of new drugs when human efficacy studies are not ethical or feasible. Federal Register. 2002;67:37988–37998. 21 CFR parts 314 and 601, FDA, HHS; ACTION: Final Rule. [PubMed] [Google Scholar]
- Crawford PA, Gordon JI. Microbial regulation of intestinal radiosensitivity. Proc. Natl. Acad. Sci. USA. 2005;102(37):13254–13259. doi: 10.1073/pnas.0504830102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Struuck R, Hartigan A, Clouthier SG, Dyson MC, Lowler K, Gatza E, Tawara I, Toubai T, Weisiger E, Hugunin K, Reddy P, Wilkinson JE. Differential susceptibility of C57BL/6NCr and B6.Cg-Ptprca mice to commensal bacteria after whole-body irradiation in translational bone marrow transplantation studies. J Transl Med. 2008;6:10–20. doi: 10.1186/1479-5876-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell CL, Bready JV, Rex KL, Chen JN, DiPalma CR, Whitcomb KL, Yin S, Hill DC, Wiemann B, Starnes CO, Havill AM, Lu ZN, Aukerman SL, Pierce GF, Thomason A, Potten CS, Ulich TR, Lacey DL. Keratinocyte growth factor protects mice from chemotherapy and radiation induced gastrointestinal injury and mortality. Cancer Res. 1998;58(5):933–939. [PubMed] [Google Scholar]
- FDA C. CBER Guidance for Industry: Animal Models-Essential Elements to Address Efficacy Under the Animal Rule. 2009:1–19. [Google Scholar]
- Gao Z, Zhang Q, Han Y, Cheng X, Lu Y, Fan L, Wu Z. Mesenchymal stromal cell-conditioned medium prevents radiation-induced small intestine injury in mice. Cytotherapy. 2012;14(3):267–273. doi: 10.3109/14653249.2011.616194. [DOI] [PubMed] [Google Scholar]
- Grahn D, Hamilton KF. Genetic variation in the acute lethal response of four inbred mouse strains to whole body x-irradiation. Radiation Res. 1956;4:189–198. doi: 10.1093/genetics/42.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson WR, Fry RJ, Sallese AR, Frischer H, Ahmad T, Ainsworth EJ. Comparison of intestine and bone marrow radiosensitivity of the BALB/c and the C57BL/6 mouse strains and their B6CF1 offspring. Radiat Res. 1987;110(3):340–352. [PubMed] [Google Scholar]
- Hendry JH. Diurnal variations in radiosensitivity of mouse intestine. Br J Radiol. 1975;48(568):312–314. doi: 10.1259/0007-1285-48-568-312. [DOI] [PubMed] [Google Scholar]
- Hendry JH. A new derivation, from split dose data, of the complete survival curve for clonogenic normal cells in vivo. Radiat Res. 1979;78(3):404–14. [PubMed] [Google Scholar]
- Hendry JH. Quantitation of intestinal clonogens by regeneration following cytotoxicity. Semin. Dev. Biol. 1993;4:303–312. [Google Scholar]
- Hendry JH, Roberts SA, Potten CS. The clonogen content of murine intestinal crypts: dependence on radiation dose used in its determination. Radiat Res. 1992;132(1):115–119. [PubMed] [Google Scholar]
- Kaur P, Potten CS. Cell migration velocities in the crypts of the small intestine after cytotoxic insult are not dependent on mitotic activity. Cell Tissue Kinet. 1986;19(6):601–610. doi: 10.1111/j.1365-2184.1986.tb00761.x. [DOI] [PubMed] [Google Scholar]
- Kirsch DG, Santiago PM, di Tomasso E, Sullivan JM, Hou W-S, Dayton T, Jeffords LB, Sodha P, Mercer K, Cohen R, Takeuchi O, Korsmeyer SJ, Bronson R, Kim CF, Haigis KM, Jain R, Jacks T. p53 Controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science. 2010;327(5965):593–596. doi: 10.1126/science.1166202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt AJ, Jones LS, Potten CS. In: Techniques in Apoptosis. Cotter TG, Martin SJ, editors. Portland Press; London: 1992. pp. 269–300. [Google Scholar]
- Potten CS. A comprehensive study of the radiobiological response of the murine (BDF1) small intestine. Int J Radiat Biol. 1990;58(6):925–973. doi: 10.1080/09553009014552281. [DOI] [PubMed] [Google Scholar]
- Potten CS. Interleukin-11 protects the clonogenic stem cells in murine small-intestinal crypts from impairment of their reproductive capacity by radiation. Int J Cancer. 1995a;62(3):356–361. doi: 10.1002/ijc.2910620321. [DOI] [PubMed] [Google Scholar]
- Potten CS. Structure, function and proliferative organisation of the mammalian gut. In: Potten CS, Hendry JH, editors. Radiation and Gut. Elsevier Science; Amsterdam The Netherlands: 1995b. pp. 1–31. [Google Scholar]
- Potten CS. Protection of the small intestinal clonogenic stem cells from radiation induced damage by pretreatment with interleukin 11 also increases murine survival time. Stem Cells. 1996;14(4):452–459. doi: 10.1002/stem.140452. [DOI] [PubMed] [Google Scholar]
- Potten CS. Stem cells in gastrointestinal epithelium: numbers, characteristics and death. Philos Trans R Soc Lond B Biol Sci. 1998;353(1370):821–830. doi: 10.1098/rstb.1998.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS, Hendry JH. Stem cells in murine small intestine. In: Potten CS, editor. Identification and Characterisation of Stem Cells. Churchill Livingstone; Edinburgh: 1983. pp. 155–199. [Google Scholar]
- Potten CS, Hendry JH. The micro colony assay in mouse small intestine. In: Potten CS, Hendry JH, editors. Cell Clones: Manual of Mammalian Cell Techniques. Churchill Livingstone; Edinburgh: 1985. pp. 50–60. [Google Scholar]
- Potten CS, Hendry JH. Clonal Regeneration Studies. In: Potten CS, Hendry JH, editors. Radiation and Gut. Elsevier Science; The Netherlands: 1995. pp. 45–59. [Google Scholar]
- Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110(4):1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- Potten CS, Booth C, Pritchard DM. The intestinal epithelial stem cell: the mucosal governor. Int J Exp Pathol. 1997a;78(4):219–243. doi: 10.1046/j.1365-2613.1997.280362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS, Booth D, Haley JD. Pretreatment with transforming growth factor beta-3 protects small intestinal stem cells against radiation damage in vivo. Br J Cancer. 1997b;75(10):1454–1459. doi: 10.1038/bjc.1997.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS, Al-Barwari SE, Hume WJ, Searle J. Circadian rhythms of presumptive stem cells in three different epithelia of the mouse. Cell Tissue Kinet. 1977;10(6):557–568. doi: 10.1111/j.1365-2184.1977.tb00312.x. [DOI] [PubMed] [Google Scholar]
- Potten CS, Rezvani M, Hendry JH, Moore JV, Major D. Correction of intestinal microcolony counts for variation in size. Int J Radiat Biol Relat Stud Phys Chem Med. 1981;40(3):321–326. doi: 10.1080/09553008114551251. [DOI] [PubMed] [Google Scholar]
- Qiu JM, Roberts SA, Potten CS. Cell migration in the small and large bowel of mice shows a strong circadian rhythm. Epithelial Cell Biol. 1994;3(4):137–148. [PubMed] [Google Scholar]
- Roberts SA, Potten CS. Clonogen content of intestinal crypts: its deduction using a microcolony assay on whole mount preparations and its dependence on radiation dose. Int J Radiat Biol. 1994;65(4):477–481. doi: 10.1080/09553009414550551. [DOI] [PubMed] [Google Scholar]
- Saha S, Bhanja P, Kabarriti R, Liu L, Alfieri AA, Guha C. Bone marrow stromal cell transplantation mitigates radiation-induced gastrointestinal syndrome in mice. PLoS One. 2011;6(9):e24072. doi: 10.1371/journal.pone.0024072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla P, Gupta D, Bisht SS, Pant MC, Bhatt ML, Gupta R, Srivastava K, Gupta S, Dhawan A, Mishra D, Negi MP. Circadian variation in radiation-induced intestinal mucositis in patients with cervical carcinoma. Cancer. 2010;116(8):2031–2035. doi: 10.1002/cncr.24867. [DOI] [PubMed] [Google Scholar]
- Takaba J, Mishima Y, Hatake K, Kasahara T. Role of bone marrow-derived monocytes/macrophages in the repair of mucosal damage caused by irradiation and/or anticancer drugs in colitis model. Mediators Inflamm. 2010:634145. doi: 10.1155/2010/634145. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketa ST. Water-electrolyte and antibiotic therapy against acute (3 to 5 day) intestinal radiation death in the rat. Radiation Res. 1962;16:312–326. [PubMed] [Google Scholar]
- Taketa ST, Carsten AL, Cohn SH, Atkins HL, Bond VP. Active bone marrow distribution in the monkey. Life Sci. 1970;9(3):169–174. doi: 10.1016/0024-3205(70)90310-3. [DOI] [PubMed] [Google Scholar]
- Terry NH, Travis EL. The influence of bone marrow depletion on intestinal radiation damage. Int J Radiat Oncol Biol Phys. 1989;17(3):569–573. doi: 10.1016/0360-3016(89)90108-9. [DOI] [PubMed] [Google Scholar]
- Withers HR, Elkind M. Microcolony survival assay for cells of mouse intestinal mucosa exposed to radiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;17(3):261–267. doi: 10.1080/09553007014550291. [DOI] [PubMed] [Google Scholar]
- Withers HR, Mason KA, Taylor JMG. The number of clonogenic cells in a mouse jejunal crypt. Radiother Oncol. 1993;26(3):238–243. doi: 10.1016/0167-8140(93)90265-a. [DOI] [PubMed] [Google Scholar]
- Walsh D. Deep tissue traumatism from roentgen ray exposure. Br Med J. 1897;2:272–273. doi: 10.1136/bmj.2.1909.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh EK, Horowitz M. Radiation enteritis. Surg Gynecol Obstet. 1987;165(4):373–379. [PubMed] [Google Scholar]