Abstract

This report describes development of a series of novel bivalent molecules with a pharmacophore derived from the D2/D3 agonist 5-OH-DPAT. The spacer length in the bivalent compounds had a pronounced influence on affinity for D2 receptors. A 23-fold increase of D2 affinity was observed at a spacer length of 9 or 10 (compounds 11d and 14b) as compared to monovalent 5-OH-DPAT (Ki; 2.5 and 2.0 vs 59 nM for 11d and 14b vs 5-OH-DPAT, respectively). The functional potency of 11d and 14b indicated a 24- and 94-fold increase in potency at the D2 receptor as compared to 5-OH-DPAT (EC50; 1.7 and 0.44 vs 41 nM for 11d and 14b vs 5-OH-DPAT, respectively). These are the most potent bivalent agonists for the D2 receptor known to date. This synergism is consonant with cooperative interaction at the two orthosteric binding sites in the homodimeric receptor.
Keywords: bivalent ligands, D2/D3 dopamine receptors, cooperative gain, affinity, potency, synergism
G protein-coupled receptors (GPCRs) make up one of the largest and diverse family of mammalian proteins. Consequently, GPCRs are targeted most frequently in therapeutics development for numerous diseases.1,2 The prototypical GPCR structure consists of seven trans membrane domains as the central core, connected by extracellular and intracellular loops. The traditional concept of participation of a monomeric receptor in the signal transduction process has been increasingly challenged by evidence for the existence of homo- and heterodimeric receptors.3 A number of GPCRs have been shown to form dimers, which include opioid receptors,4 adrenergic receptors,5 and muscarinic receptors.6 Dopamine receptors are also G protein-coupled and act via modulation of adenylase cyclase activity. Both dopamine D2 and D3 receptors can exist as homodimers in cell lines expressing these receptors.7,8 Furthermore, the dopamine D2 receptor has been shown to form a dimer in human and rat brain tissues.9 Recently, dopamine D1 and D2 receptors also have been shown to form heterodimers.10
Bivalent ligands for GPCRs have been reported going back more than a decade.4,11,12 Bivalent ligands typically consist of two pharmacophoric moieties connected by a spacer with an appropriate length, which then allows the two “heads” to interact either with two orthosteric binding sites of receptors on adjacent protomers of a dimer or with another relevant, allosteric site at the same receptor. Bivalents are classified as either homo- or heterobivalent ligands depending on the nature of pharmacophores.13 Such simultaneous cooperative interaction results in increased affinity theoretically equivalent to the product of the binding contribution of both putative sites.14,15 This can also give rise to higher functional potency, greater selectivity, and better therapeutic agents.16 Bivalent ligands with varying linker lengths for dopamine receptors and other GPCR have been reported.17−21
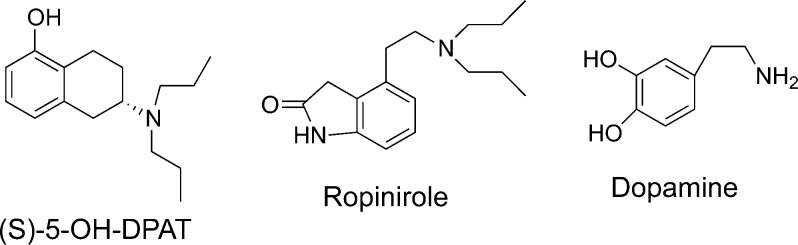
In our quest to probe dimeric forms of either D2 or D3 receptors in cells expressing these cloned receptors, we have designed a prototypical structure of bivalent molecules by linking the well-known dopamine D2/D3 agonist molecule 5-hydroxy-2-(dipropylamino)tetralin (5-OH-DPAT) via a methylene spacer of varying length (Figure 1). Site-directed mutagenesis has demonstrated that the amino tetralin moiety interacts with an agonist binding domain involving TM-3 and TM-5 for activation of either D2 or D3 receptors.22,23 In our own study, we have demonstrated the critical importance of basic nitrogen and hydroxyl functionalities in TM3 and TM5 for binding activity of 5-OH-DPAT.24 Suitable potent bivalent agonists or antagonists for dopamine receptors might find potential therapeutic use for treatment of neuropsychiatric and neurodegenerative diseases like Parkinson's disease.
Figure 1.
Molecular structures of known dopamine D2 and D3 agonists.
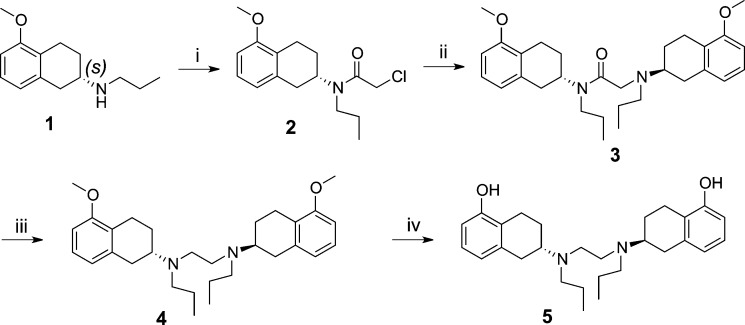
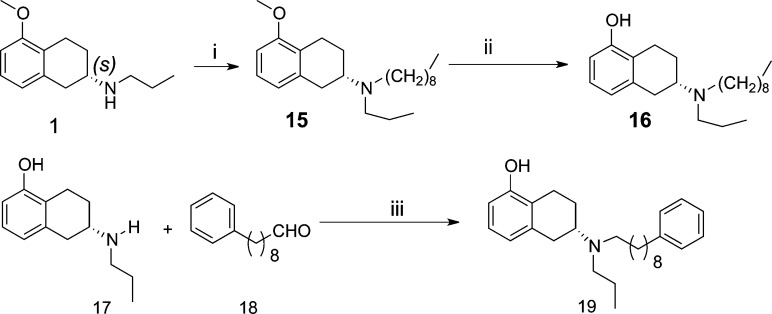
Scheme 1 describes synthesis of target compound 5. Optically active (−)-5-methoxy aminotetralin25 was acylated with chloroacetyl chloride under basic conditions to produce intermediate 2. Further N-alkylation of 1 with intermediate 2 in presence of a base provided 3, which upon reduction with lithium aluminum hydride (LAH) produced 4. Finally, demethylation in presence of boron tribromide at −40 °C produced the final bivalent target compound 5.
Scheme 1.
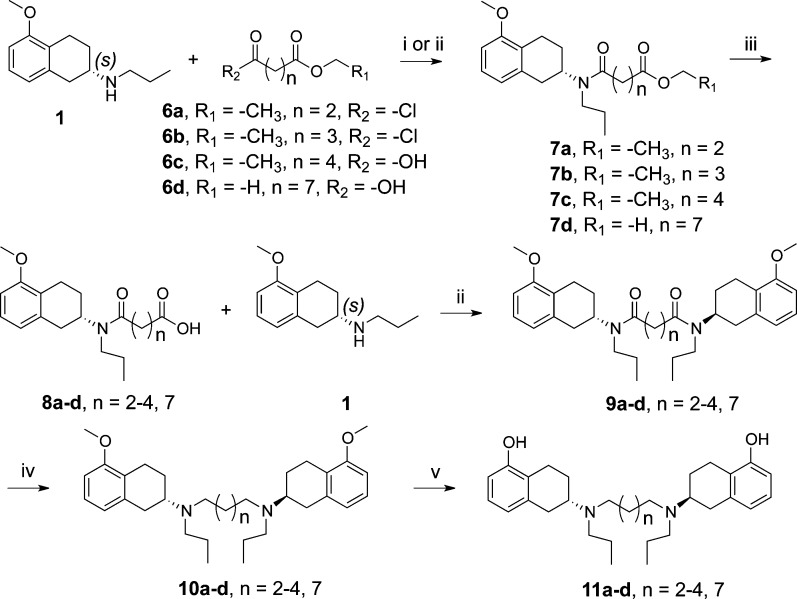
Similarly, Scheme 2 describes synthesis of compounds 11a–d. Compound 1 was treated with either appropriate acid chloride or acid to yield intermediates 7a–d. Hydrolysis of ester by lithium hydroxide produced acid intermediate 8a–d. Acid coupling with amine 1 under standard 1-[3-(dimethylamino)propyl]-3-ethylcarbodiimide-hydrochloride (EDCI) coupling conditions in the presence of hydroxybenzotriazole (HOBt) produced amide intermediate 9a–d. Reduction of amide followed by demethylation yielded the final compounds 11a–d.
Scheme 2.
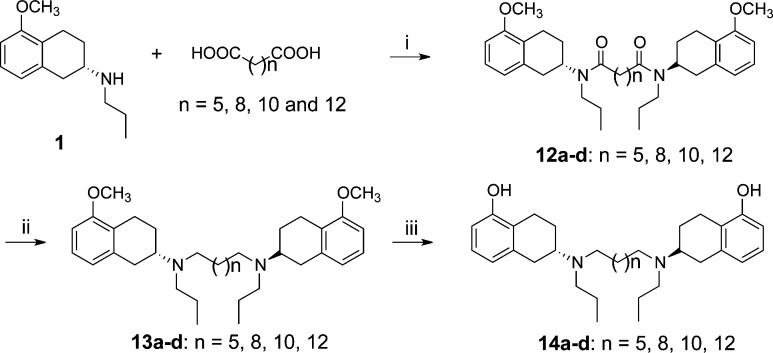
Scheme 3 describes synthesis of compounds 14a–d. Two molecules of amine 1 were coupled with the appropriate diacid to produce intermediate diamides 12a–d, which on reduction with LAH produced amines 13a–d. The final targets 14a–d were synthesized after demethylation with boron tribromide. Scheme 4 describes the synthesis of compounds 16 and 19. In both cases, straightforward synthesis approaches were adopted.
Scheme 3.
Scheme 4.
In our design of this series of bivalent ligands, we incorporated methylene units to build the spacer separating the two pharmacophoric head groups, with a spacer length varying from 2 to 14 methylene units (Table 1). We included the agonist 5-OH-DPAT as the parent compound for comparison purposes. The four-linker 11a exhibited similar affinity at the D2 receptor as 5-OH-DPAT (Ki; 70 vs 59 nM for 11a vs 5-OH-DPAT, respectively) (Table 1). Moving from a 4-linker to a 5-linker did not affect the affinity of 11b significantly as compared to 11a. Interestingly, switching from the 5-linker (11b) to the 6-linker (11c) induced a significant, 12-fold increase, consonant with a cooperative interaction resulting in synergistic enhancement of affinity at D2. The synthesis of 7-, 9- and 10-linkers produced 14a, 11d, and 14b, which exhibited further improvement in affinity, with 9- and 10-linkers producing the highest affinity for D2 receptors. A further increase of the spacer length to 12 methylene units (14c) decreased the affinity to a level that was comparable to compound 5 with a linker length of 2. Upon further increase of the spacer length to 14 (14d), the affinity dropped to that of 5-OH-DPAT (see also Figure 2). At the D3 receptor, less dramatic changes occurred upon varying the linker length, and the 4-linker compound 11a started the bivalent compounds of this spacer length at an affinity lower (higher Ki) than the parent compound 5-OHDPAT (Table 1 and Figure 2). Increasing the length from four linkers up gradually (but modestly) increased D3 affinity, with the 6-linker compound displaying the first significant affinity enhancement, just as observed for D2 affinity. The highest D3 affinity was seen with a linker length of nine methylene units [11d, Ki (D3); 0.91 nM], whereas further elongation (14b, 14c, and 14d) resulted in a lowering of affinity. In comparison, in the case of the D2 receptor, 11d along with 14b with a 10-linker spacer, produced the highest affinity [Ki (D2); 2.0–2.5 nM; statistically the two compounds were indistinguishable at D2]. Compounds 11d and 14b were selected for further functional analysis (see below).
Table 1. Inhibition Constants for Competing for [3H]Spiperone Binding to Cloned rD2L and rD3 Receptors Expressed in HEK Cellsa.
|
Ki (nM) |
||||
|---|---|---|---|---|
| D-compound | HEK-rD2 | Hill slopes HEK-rD2 | HEK-rD3 | D2/D3 |
| parent | ||||
| 5-OH-DPAT | 59 ± 11 | 0.83 ± 0.07b | 1.4 ± 0.3 | 43 |
| bivalent (spacer length) | ||||
| 5 (2) | 16 ± 2 | 1.1 ± 0.1 | 1.1 ± 0.3 | 14 |
| 11a (4) | 70 ± 22 | 0.56 ± 0.04 | 4.0 ± 1.0 | 17 |
| 11b (5) | 75 ± 19 | 0.72 ± 0.06 | 3.6 ± 0.6 | 21 |
| 11c (6) | 5.9 ± 0.2 | 1.1 ± 0.01 | 2.2 ± 0.4 | 2.7 |
| 14a (7) | 3.9 ± 0.7 | 1.5 ± 0.2 | 1.1 ± 0.4 | 3.6 |
| 11d (9) | 2.5 ± 0.5 | 1.3 ± 0.1 | 0.9 ± 0.3 | 2.7 |
| 14b (10) | 2.0 ± 0.4 | 1.4 ± 0.1 | 1.8 ± 0.6 | 1.1 |
| 14c (12) | 11 ± 2 | 1.8 ± 0.2 | 10 ± 3 | 1.0 |
| 14d (14) | 55 ± 11 | 1.4 ± 0.2 | 31 ± 5 | 1.7 |
| monovalent | ||||
| 16 | 69 (4) ± 9 | 7.3 ± 1.1 | 9.4 | |
| 19 | 140 ± 30 | 23 ± 4 | 6.1 | |
Results are means ± SEMs for 3–7 experiments each performed in triplicate.
Average of conglomerate of 27 separate experiments (including data sets collected previously under the same conditions).
Figure 2.
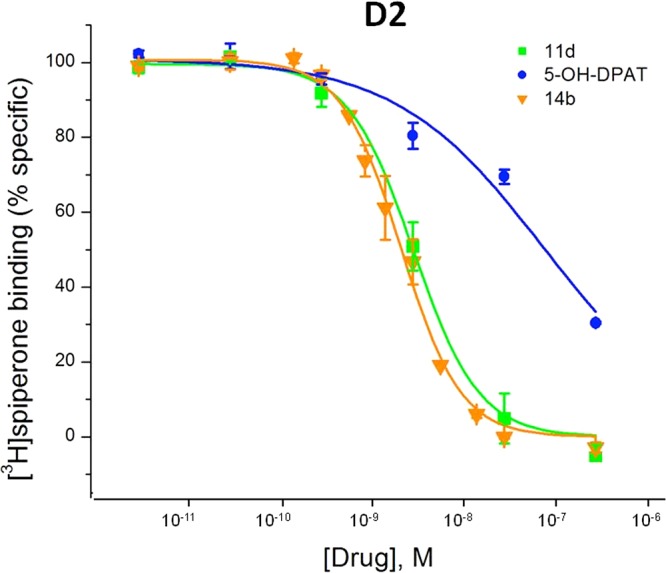
Interaction of 11d, 14b, and 5-OH-DPAT with rD2 receptors expressed in HEK-293 cells. Isotherms were obtained in competition experiments with [3H]spiperone as described in the experimental section (see the Supporting Information). The Hill slopes for the curve 11d (nH = 1.31) and 14b (nH = 1.43) were higher than monovalent 5-OH-DPAT (nH = 0.83), indicating a possible cooperative binding interaction with the D2 receptor dimer. Each point is the average ± SEM (vertical bar) of 4–6 independent experiments assayed in triplicate. For Ki values for each curve, see Table 1. The averages shown for 5-OH-DPAT are from a subset of six experiments. The x-axis concentrations are expressed as Ki taking into account the conversion from IC50 as indicated in the methods (see the Supporting Information).
The gain in affinity for 11d and 14b at D2 (and to a lesser extent at D3) is consonant with a cooperative effect of simultaneous interaction of the two heads with two distal binding sites. As pointed out by Kuhhorn et al.,17 binding of bivalent ligands to two sites will induce cooperativity with binding of the first head facilitating binding of the second head due to being placed in the vicinity of the second binding site. This binding cooperativity can be reflected in increased Hill numbers (as compared with unity for binding of monovalent neutral antagonists). Thus, neutral antagonist binding to D2 receptors displays Hill numbers up to 2.0 in the case of the bivalent compounds in the study of Kuhhorn et al.17 However, McRobb et al.19 report no increases in Hill numbers over unity for clozapine propylamine bivalent antagonists at D2 receptors, indicating that this is a ligand-dependent phenomenon. Full monovalent agonists, recognizing high-affinity D2 states, generally display Hill numbers below unity (0.5–0.7).17 Our compounds are full agonists (Table 2) and display increases in Hill number as a function of bivalent binding to D2 (Table 1). Thus, 11a, the 4-linker compound, has a Hill less than unity as does 5-OH-DPAT, and with increasing linker length, the Hill numbers go up in the 1.3–1.8 range. The two bivalent compounds with the highest D2 potency, 11d and 14b, had Hill numbers of 1.3 and 1.4, with clearly steeper binding inhibition curves as compared with 5-OH-DPAT with an average Hill of 0.8. Therefore, our Hill numbers strongly indicate cooperative binding of our lead bivalent compounds.
Table 2. Stimulation of [35S]GTPgS Binding to hD2 and hD3 Receptors Expressed in CHO Cellsa.
| hCHO-D2 |
hCHO-D3 |
||||
|---|---|---|---|---|---|
| compd | EC50 (nM)b [35S]GTPγS | % Emax | EC50 (nM)b [35S]GTPγS | % Emax | D2/D3 |
| dopamine | 240 ± 40 | 100 | 5.8 ± 1.3 | 100 | 42 |
| ropiniroleb | 300 ± 10 | 74 ± 0.9 | 10 ± 2 | 67 ± 8 | 30 |
| 5-OH-DPAT | 41 ± 6 | 80 ± 4 | 0.63 ± 0.08 | 75 ± 4 | 65 |
| 11d | 1.7 ± 0.1 | 100 ± 3 | 0.53 ± 0.04 | 92 ± 1 | 3.2 |
| 14b | 0.44 ± 0.09 | 97 ± 4 | 0.94 ± 0.28 | 74 ± 5 | 0.46 |
EC50 values (nM) are means ± SEMs for 3–6 experiments each performed in triplicate.
Data from ref (27).
To assess any contribution of a nine methylene spacer, by itself, to affinity for D2/D3 receptors, monovalent 16 with a nine methylene spacer length was synthesized and monitored for D2/D3 receptor binding. It is apparent that 16 exhibited similar affinity for D2 as 5-OH-DPAT (Ki; 59 vs 68.8 nM for 5-OH-DPAT vs 16, respectively) and exhibited lower affinity for D3 as compared to 5-OH-DPAT. In our effort to further evaluate any contribution of hydrophobic or related effect in production of high affinity in 11d and 14b, nine-linker 19 with a terminal phenyl group was designed and synthesized. The binding data indicate much weaker activity of 19 at D2 (Ki = 141) and D3 receptors (23 nM). This clearly indicates that the methylene spacer units did not contribute toward the enhanced affinity of 11d and 14b. It furthermore indicates that enhanced interaction of lead bivalent compounds does not result from enhanced hydrophobic or π-stacking interaction.
Following binding analysis, selected lead 11d and 14b were subjected to the [35S]GTPγS (guanosine 5′-[g-thio]triphosphate) functional assay for D2 and D3 receptors and compared with the full agonists dopamine, ropinirole, and 5-OH-DPAT (Table 2). The assays were carried out with the cloned human D2 and D3 receptors expressed in Chinese hamster ovary (CHO) cells. Dopamine and ropinirole, as observed by previously, were more potent in activating D3 than D2. The results also indicated that 11d and 14b potently stimulate both D2 and D3 receptors. In this regard, 11d and 14b exhibited more than 24- and 94-fold potency as compared to its monovalent counterpart 5-OH-DPAT in activating the D2 receptor (EC50 values of 1.7 and 0.44 nM, respectively, vs 41 nM for 5-OH-DPAT, Table 2 and Figure 2), gains similar to or exceeding those observed for binding affinity. The 94-fold increase for 14b indicates remarkable synergistic enhancement of potency for this compound for the D2 receptor. The linker length of our lead bivalent compounds is ∼13.5 Å (between the two pharmacophoric N-atoms) or ∼22.6 Å (between the two pharmacophoric −OH groups), which is different from the bivalent compounds reported by others. In this regard, there are a number of different linker lengths for bivalent interactions that have been reported in the literature. The antagonist/antagonist bivalent ligands reported by Gmeiner et al. for interaction at the D2 receptor were based on aryl piperazine and aminoindane moieties.17,18 In a recent report by McRobb et al. on a bivalent antagonist, clozapine-like compound at the D2 receptor, a different, shorter linker length was reported.19 In addition, for agonist interaction at other GPCRs, a shorter linker length was also reported for optimal interaction.21 At this point, it is not known what dominant interaction forces might play a role in initiating the binding interaction of these varying bivalent ligands with the D2 homodimer.
In the case of the D3 receptor, there was not the same gain for bivalent interaction as observed for D2 (Table 2). The present bivalent ligands have higher receptor affinities for D3 receptor to begin with (as compared with their corresponding D2 affinities and potencies, Tables 1 and 2); therefore, it may be more difficult to show additional gains in potency through bivalent interactions. D3 receptors also couple to G protein less effectively than D2 receptors,26 and we speculate that this underlies the generally higher Hill numbers observed for D3 binding (data not shown).
In summary, we have designed a novel series of bivalent ligands for interaction with dopamine D2 and D3 receptors. The results demonstrate an effect of chain length on affinity and potency of our bivalent molecules for their interaction with dopamine receptors, primarily of the D2 subtype (see Figure 2). At the critical chain length of six methylene groups in 11c, a significant increase in affinity for D2 receptors took place as compared to spacer length of five methylene groups in 11b. As mentioned earlier, such a 12-fold increase in affinity might indicate initiation of synergistic cooperative binding. Further optimization led to bivalent 11d and 14b with enhanced affinity at both D2 and D3 receptors. Such interaction was further substantiated by results on the functional activity of 11d and 14b, which indicated a significant 26–94-fold enhancement of D2 potency as compared to monovalent 5-OH-DPAT. These bivalent compounds, 14b in particular, are the most potent bivalent D2 agonists known to date. The significant enhancement in D2 potency is in agreement with interaction at the two orthosteric binding sites at two different D2 protomers in the homodimeric receptor.
Acknowledgments
We are grateful to Dr. K. Neve, Oregon Health and Science University (Portland, OR), for D2L and D3 expressing HEK cells. We are also grateful to Dr. J. Shine, Garvan Institute for Medical Research (Sydney, Australia), for D2L expressing CHO cells.
Glossary
Abbreviations
- GTPγS
guanosine 5′-[g-thio]triphosphate
- 5-OH-DPAT
5-hydroxy-2-(dipropylamino)tetralin
- CHO
Chinese hamster ovary
- HEK
human embryonic kidney
Supporting Information Available
Elemental analysis data for all final targets. This material is available free of charge via the Internet at http://pubs.acs.org.
This work is supported by the National Institute of Neurological Disorders and Stroke/National Institute of Health (NS047198, A.K.D.).
The authors declare no competing financial interest.
Author Present Address
∥ CSIR-NEIST, Jorhat, Assam, 785006, India.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Overington J. P.; Al-Lazikani B.; Hopkins A. L. How many drug targets are there?. Nat Rev. Drug Discovery 2006, 5, 993–996. [DOI] [PubMed] [Google Scholar]
- Lagerstrom M. C.; Schioth H. B. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug Discovery 2008, 7, 339–357. [DOI] [PubMed] [Google Scholar]
- Park P. S.; Filipek S.; Wells J. W.; Palczewski K. Oligomerization of G protein-coupled receptors: Past, present, and future. Biochemistry 2004, 43, 15643–15656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portoghese P. S. From models to molecules: opioid receptor dimers, bivalent ligands, and selective opioid receptor probes. J. Med. Chem. 2001, 44, 2259–2269. [DOI] [PubMed] [Google Scholar]
- Hebert T. E.; Moffett S.; Morello J. P.; Loisel T. P.; Bichet D. G.; Barret C.; Bouvier M. A peptide derived from a beta2-adrenergic receptor transmembrane domain inhibits both receptor dimerization and activation. J. Biol. Chem. 1996, 271, 16384–16392. [DOI] [PubMed] [Google Scholar]
- Hern J. A.; Baig A. H.; Mashanov G. I.; Birdsall B.; Corriea J. E.; Lazareno S.; Molloy J. E.; Birdsall N. J. Formation and dissociation of M1 muscarinic receptor dimers seen by total internal reflection fluorescence imaging of single molecules. Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 2693–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. P.; O'Dowd B. F.; Ng G. Y.; Varghese G.; Akil H.; Mansour A.; Nguyen T.; George S. R. Inhibition of cell surface expression by mutant receptors demonstrates that D2 dopamine receptors exist as oligomers in the cell. Mol. Pharmacol. 2000, 58, 120–128. [DOI] [PubMed] [Google Scholar]
- Nimchinsky E. A.; Hof P. R.; Janssen W. G.; Morrison J. H.; Schmauss C. Expression of dopamine D3 receptor dimers and tetramers in brain and in transfected cells. J. Biol. Chem. 1997, 272, 29229–29237. [DOI] [PubMed] [Google Scholar]
- Zawarynski P.; Tallerico T.; Seeman P.; Lee S. P.; O'Dowd B. F.; George S. R. Dopamine D2 receptor dimers in human and rat brain. FEBS Lett. 1998, 441, 383–386. [DOI] [PubMed] [Google Scholar]
- Rashid A. J.; So C. H.; Kong M. M.; Furtak T.; El-Ghundi M.; Cheng R.; O'Dowd B. F.; George S. R. D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 654–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portoghese P. S.; Larson D. L.; Sayre L. M.; Yim C. B.; Ronsisvalle G.; Tam S. W.; Takemori A. E. Opioid agonist and antagonist bivalent ligands. The relationship between spacer length and selectivity at multiple opioid receptors. J. Med. Chem. 1986, 29, 1855–1861. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Gilliam A.; Maitra R.; Damaj M. I.; Tajuba J. M.; Seltzman H. H.; Thomas B. F. Synthesis and biological evaluation of bivalent ligands for the cannabinoid 1 receptor. J. Med. Chem. 2010, 53, 7048–7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan R. G.; Sharma S. K.; Xie Z.; Daniels D. J.; Portoghese P. S. A bivalent ligand (KDN-21) reveals spinal delta and kappa opioid receptors are organized as heterodimers that give rise to delta(1) and kappa(2) phenotypes. Selective targeting of delta-kappa heterodimers. J. Med. Chem. 2004, 47, 2969–2972. [DOI] [PubMed] [Google Scholar]
- Portoghese P. S. Bivalent ligands and the message-address concept in the design of selective opioid receptor antagonists. Trends Pharmacol. Sci. 1989, 10, 230–235. [DOI] [PubMed] [Google Scholar]
- Kramer R. H.; Karpen J. W. Spanning binding sites on allosteric proteins with polymer-linked ligand dimers. Nature 1998, 395, 710–713. [DOI] [PubMed] [Google Scholar]
- Morphy R.; Kay C.; Rankovic Z. From magic bullets to designed multiple ligands. Drug Discovery Today 2004, 9, 641–651. [DOI] [PubMed] [Google Scholar]
- Kuhhorn J.; Hubner H.; Gmeiner P. Bivalent dopamine D2 receptor ligands: synthesis and binding properties. J. Med. Chem. 2011, 54, 4896–4903. [DOI] [PubMed] [Google Scholar]
- Kuhhorn J.; Gotz A.; Hubner H.; Thompson D.; Whistler J.; Gmeiner P. Development of a bivalent dopamine D(2) receptor agonist. J. Med. Chem. 2011, 54, 7911–7919. [DOI] [PubMed] [Google Scholar]
- McRobb F. M.; Crosby I. T.; Yuriev E.; Lane J. R.; Capuano B. Homobivalent ligands of the atypical antipsychotic clozapine: Design, synthesis, and pharmacological evaluation. J. Med. Chem. 2012, 55, 1622–1634. [DOI] [PubMed] [Google Scholar]
- Huber D.; Lober S.; Hubner H.; Gmeiner P. Bivalent molecular probes for dopamine D2-like receptors. Bioorg. Med. Chem. 2012, 20, 455–466. [DOI] [PubMed] [Google Scholar]
- Birnkammer T.; Spickenreither A.; Brunskole I.; Lopuch M.; Kagermeier N.; Bernhardt G.; Dove S.; Seifert R.; Elz S.; Buschauer A. The bivalent ligand approach leads to highly potent and selective acylguanidine-type histamine H(2) receptor agonists. J. Med. Chem. 2012, 55, 1147–1160. [DOI] [PubMed] [Google Scholar]
- Cox B. A.; Henningsen R. A.; Spanoyannis A.; Neve R. L.; Neve K. A. Contributions of Conserved Serine Residues to the Interactions of Ligands with Dopamine D2 Receptors. J. Neurochem. 1992, 59, 627–635. [DOI] [PubMed] [Google Scholar]
- Mansour A.; Meng F.; Meador-Woodruff J. H.; Taylor L. P.; Civelli O.; Akil H. Site-directed mutagenesis of the human dopamine D2 receptor. Eur. J. Pharmacol. 1992, 227, 205–214. [DOI] [PubMed] [Google Scholar]
- Kortagere S.; Cheng S. Y.; Antonio T.; Zhen J.; Reith M. E.; Dutta A. K. Interaction of novel hybrid compounds with the D3 dopamine receptor: Site-directed mutagenesis and homology modeling studies. Biochem. Pharmacol. 2011, 81, 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S.; Zhang S.; Fernandez F.; Ghosh B.; Zhen J.; Kuzhikandathil E.; Reith M. E.; Dutta A. K. Further structure-activity relationships study of hybrid 7-{[2-(4-phenylpiperazin-1-yl)ethyl]propylamino}-5,6,7,8-tetrahydronaphthalen-2-o l analogues: Identification of a high-affinity D3-preferring agonist with potent in vivo activity with long duration of action. J. Med. Chem. 2008, 51, 101–117. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A.; Cussac D.; Audinot V.; Pasteau V.; Gavaudan S.; Millan M. J. G protein activation by human dopamine D3 receptors in high-expressing Chinese hamster ovary cells: A guanosine-5′-O-(3-[35S]thio)- triphosphate binding and antibody study. Mol. Pharmacol. 1999, 55, 564–574. [PubMed] [Google Scholar]
- Ghosh B.; Antonio T.; Reith M. E. A.; Dutta A. K. Discovery of 4-(4-(2-((5-Hydroxy-1,2,3,4-tetrahydronaphthalen-2-yl)(propyl)amino)ethyl)piperazin-1-yl)quinolin-8-ol and Its Analogues as Highly Potent Dopamine D2/D3 Agonists and as Iron Chelator: In Vivo Activity Indicates Potential Application in Symptomatic and Neuroprotective Therapy for Parkinson's Disease. J. Med. Chem. 2010, 53, 2114–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.