Abstract
As activation of the Ras/Raf/MEK/ERK pathway is a critical component of M-CSF-promoted osteoclast survival, determining specific mechanism by which M-CSF activates this signal transduction pathway is paramount towards advancing treatment of pathological conditions resulting in increased bone turnover. The p21 Activated Kinase PAK1 modulates activation of the Raf/MEK/ERK pathway by either directly activating Raf or priming MEK for activation by Raf. Therefore a role for PAK1 in M-CSF-mediated activation of the MEK/ERK pathway controlling osteoclast survival was assessed. Here we show that PAK1 is activated by M-CSF in a Ras-dependent mechanism that promotes osteoclast survival. Surprisingly, PAK1 did not modulate Raf activation or Raf-mediated MEK activation. M-CSF mediated activation of Raf was required for PAK1 activation and osteoclast survival promoted by PAK1. This survival response was MEK-independent as expression of constitutively active MEK did not rescue osteoclasts from apoptosis induced by blocking PAK1 function. Functionally, PAK1 promoted osteoclast survival by modulating expression of the IAP family member Survivin. M-CSF therefore functions to promote PAK1 activation as a novel MEK-independent Raf target to control Survivin-mediated osteoclast survival.
Keywords: Osteoclast, Apoptosis, MEK, Raf, PAK1, Survivin
Introduction
Bone resorption is mediated by osteoclasts, the multinucleated cells derived from the monocyte/macrophage cell lineage. Since the number of osteoclasts executing bone resorption at sites of remodeling is modulated by the survival of these cells, identifying mechanisms controlling osteoclast apoptosis may provide insight into future therapies to treat conditions of increased bone loss. M-CSF promotes osteoclast survival through activation of the MEK/ERK pathway, although the specific upstream signaling events mediating this response have not been characterized (Gingery et al., 2003; Glantschnig et al., 2003). However, inhibition of upstream signaling required for MEK/ERK activation results in a substantial increase in osteoclast apoptosis over inhibition of MEK/ERK alone (Bradley et al., 2008a; Gingery et al., 2003). These data suggest roles for additional targets outside of the MEK/ERK pathway in mediating osteoclast survival supported by M-CSF.
Raf is a serine/threonine kinase activated by Ras- and PI3K-dependent mechanisms that activates MEK/ERK. While MEK1/2 comprise the sole thoroughly validated targets for Raf, reports have suggested functions for Raf in activation of additional targets outside of the Ras/Raf/MEK/ERK module (Kolch, 2000; Morrison and Cutler, 1997; Schaeffer and Weber, 1999). Raf has been reported to physically interact with and modulate the function of the retinoblastoma (Rb) protein, although evidence of Raf directly regulating Rb has not been demonstrated (Wang et al., 1998). In addition, Ras/Raf-dependent cell cycle regulation has also been attributed to Raf-mediated regulation of Cdc25 (Galaktionov et al., 1995). Two distinct Ras-dependent mechanisms are utilized during Raf activation. Ras directly activates Raf by mediating dimerization of Raf at the plasma membrane (Kolch, 2000). Ras may also act indirectly by inducing PI3K-promoted RafSer338 and RafSer341 phosphorylation, thus inducing Raf activation (Zang et al., 2002; Zang et al., 2001). In contrast to these stimulatory effects, Akt-mediated phosphorylation of RafSer259 inactivates Raf (Chong et al., 2001; Dougherty et al., 2005; Marais et al., 1997; Zimmermann and Moelling, 1999).
The p21 Activated Kinase PAK1 modulates activation of the canonical Raf/MEK/ERK pathway, although its role in M-CSF-mediated signaling has not been characterized. PAK1 activation is induced by phosphorylation at Thr423 (King et al., 2000) and phospho-mimetic mutation at this site renders PAK1 constitutively active. Autophosphorylation of Ser199/204 may also lock PAK1 in the open and active conformation (Lei et al., 2000). PAK1 has several known substrates, including MEK. Phosphorylation of MEK1Ser298 by PAK1 primes MEK for direct activation by Raf (Coles and Shaw, 2002; Eblen et al., 2002). PAK1 also has been reported to directly activate MEK1/2 in a Raf-independent manner by inducing autophosphorylation of MEK1/2Ser217/221 (Park et al., 2007). As an additional target, Raf is also a reported PAK1 substrate. Through a direct physical interaction, PAK1 promotes RafSer338 phosphorylation, thereby facilitating Raf activation (Zang et al., 2002; Zang et al., 2001).
The Inhibitor of Apoptosis Proteins (IAPs) function to block apoptosis induction. The IAP family consists of XIAP, cIAP1, cIAP2, ILP2, MLIAP, NIAP, Bruce and Survivin (Schimmer, 2004). In general the IAPs function to block induction of apoptosis by binding and inhibiting caspase 3, 7 and/or 9 through steric hindrance, thus serving as a final checkpoint during induction of apoptosis (Riedl et al., 2001). Each of these family members contains at least one baculovirus IAP repeat (BIR) domain. In addition, some family members also contain a RING finger motif and/or a caspase activation recruitment domain (CARD) to mediate caspase binding (Schimmer, 2004). While most members directly bind caspases, Survivin lacks the RING domain mediating caspase interaction and therefore may bind caspases through an additional IAP family member such as XIAP (Dohi et al., 2004). Survivin blocks the induction of apoptosis by binding to active caspase 9, a critical modulator of osteoclast apoptosis (Oursler et al., 2005; Wall et al., 2003). In addition, blocking the phosphorylation of Survivin at Thr34 abolishes its anti-apoptotic function by blocking the association between Survivin and caspase 9 (Wall et al., 2003). Increased expression of Survivin has been associated with activating mutations in Ras, whereas blocking Survivin expression in cancer cell lines decreases viability (Blum et al., 2007; Fukuda and Pelus, 2004; Sarthy et al., 2007; Sommer et al., 2007; Sommer et al., 2003).
While M-CSF-mediated Raf/MEK/ERK activation promotes osteoclast survival, potential MEK-independent Raf targets induced by M-CSF promoting osteoclast survival remain unexplored. Here we document a novel role for PAK1 as a MEK-independent Raf target and show that PAK1 promotes osteoclast survival by modulating expression of the IAP Survivin. These data therefore identify PAK1 as a novel Raf target independent of MEK/ERK activation and show that Survivin is a downstream effector of PAK1 controlling osteoclast survival.
Materials and Methods
Osteoclast Differentiation
Mouse marrow osteoclast precursors were obtained from female BalB/c mice (Taconic, Germantown, NY) as previously described (Sells Galvin et al., 1999). Long bones of the hind limbs were obtained after sacrifice and the marrow was flushed out with sterile PBS. Marrow aspirates were plated at a density of 2.9×107 in 100 mm dishes in alpha MEM supplemented with 10% FBS (Hyclone, Logan, UT) and 25 ng/ml M-CSF (R and D Systems, Minneapolis, MN) for 24 hours. Non-adherent cells were collected and plated in alpha MEM, 10% FBS at a density of 2×105 cells/cm2 and supplemented with 25 ng/ml M-CSF and 100 ng/ml RANKL. Cells were fed after three days in culture with alpha MEM containing 10% FBS, 25 ng/ml M-CSF and 100 ng/ml RANKL and either fixed in 1% paraformaldehyde or treated as described. For apoptosis and osteoclast cell number measurements, six replicate coverslips were plated and analyzed for each treatment. Each experiment was repeated three times, with representative data reported.
Western Blotting
Osteoclasts were treated as above and either harvested immediately for Western blotting or serum starved for one hour and cultured with M-CSF for the indicated time period. For use of signaling chemical inhibitors, osteoclasts were serum starved in each respective inhibitor or vehicle control for one hour prior to treatment. The farnesyl transferase inhibitor, FPT inhibitor III (Calbiochem, San Diego, CA), was used at a concentration of 100 μM to inhibit Ras activation. Cells were then rinsed with PBS and harvested for Western blotting by scraping into SDS sample buffer lacking β-mercaptoethanol and bromphenol blue. To insure that equal cell protein was analyzed, total protein concentrations were determined using the BioRad DC assay (BioRad). Following protein quantification, 1 μl β-mercaptoethanol and bromphenol blue were added to each sample and 60 μg of total protein was subjected to SDS-PAGE. Western blotting was carried out using antibodies directed against total and phospho-MEK1/2Ser217/Ser221 and phospho-MEK1Ser298, total and phospho-ERK, phospho-PAK1Thr423, total PAK1/3, phospho-RafSer338, (Cell Signaling Technology, Beverly, MA) Survivin (Santa Cruz Biotechnology, Santa Cruz, CA) and tubulin (E7, Hybridoma Bank, Iowa State, IA) at a 1:1000 dilution and corresponding secondary antibodies (Cell Signaling Technology) at a 1:10,000 dilution with chemiluminescent detection using the Pierce femto reagent (Pierce, Rockford, IL). Blots were stripped and reprobed using a Western Blot Recycling Kit (Alpha Diagnostics, San Antonio, TX). Each experiment was repeated three times, with representative data shown.
Apoptosis Detection
Fixed osteoclasts were stained for 60 minutes with Hoechst 33258 diluted to 5 μg/ml in PBS with 0.01% Tween 20. The cells were then Tartrate-Resistant Acid Phosphatase (TRAP) stained as previously described (Gingery et al., 2003) and examined using fluorescent microscopy for apoptotic osteoclasts displaying condensed nuclei.
Adenoviral Infections
Adenoviruses for caPAK1 and dnPAK1 were a gift from B. Gerthoffer. The dnRaf adenovirus was received from L. F. Parada whereas the caMEK adenovirus was from S. Tanaka and dnSurvivin was received from D. C. Altieri. Each virus was expanded and titered according to standard procedures. Mature osteoclasts (day 4 when formation of multinucleated cells was observed) were infected with each indicated virus at an MOI of 100 for 18 hours prior to experimental procedures.
Real-Time RT-PCR
Osteoclasts were differentiated as above and serum starved for one hour in alpha MEM. Following serum starvation, osteoclasts were harvested immediately for total RNA or cultured with 25 ng/ml M-CSF for the indicated period, rinsed with PBS and harvested for total RNA. Total RNA was harvested in TRIzol reagent (Invitrogen, Carlsbad, CA) and phenol/chloroform extracted from which 1 μg of RNA was reverse transcribed using oligo dT primers. The resulting cDNA samples were used in real time RT-PCR analysis of Survivin (forward primer: 5′-ctgatttggcccagtgtttt−3′, reverse primer: 5′-gtctcctttgcctggaatga-3′), and tubulin (forward primer: 5′-ctgctcatcagcaagatcagag-3′, reverse primer: 5′-gcattatagggctccaccacag-3′) expression as outlined in Karst et al. (Karst et al., 2004). Fold changes for each sample compared to time zero were then calculated. Data are the result of three replicate biological samples. Each experiment was performed in triplicate.
Statistical Analysis
Data obtained are the mean +/− Standard Error of the Mean (SEM) (N=6) and are representative of three replicate experiments. The effect of treatment was compared with control values using a student’s t-test to determine significant differences using Microsoft Excel Apple software.
Results
M-CSF Mediates Osteoclast Survival Through PAK1
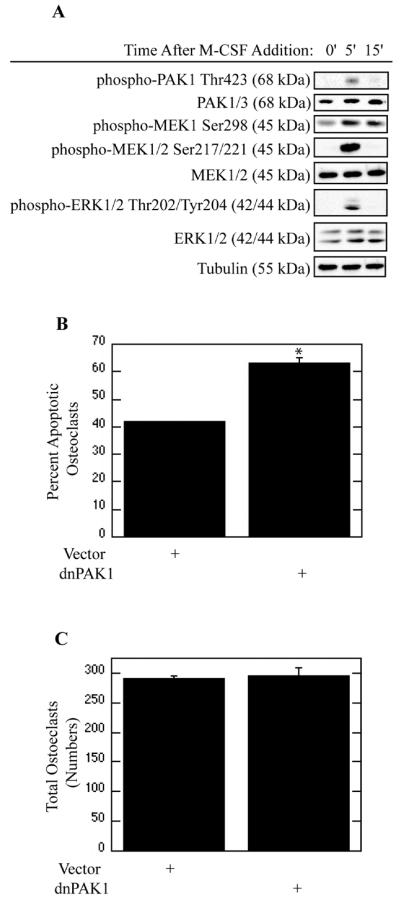
M-CSF induces activation of the MEK/ERK pathway, which is required for promotion of osteoclast survival (Bradley et al., 2008a). To ascertain whether candidate upstream signaling events elicited by M-CSF lead to activation of the MEK/ERK pathway, we first examined if the p21 activated kinase PAK1 was activated in response to M-CSF (Figure 1). Addition of M-CSF rapidly increased phosphorylation of PAK1Thr423 (Figure 1A). In addition, increased phosphorylation of MEK1Ser298, a known PAK1 target, was observed. This increase in PAK1 activity following M-CSF addition was accompanied by an increase in MEK/ERK activation (Figure 1A). As increased PAK1 activity was evident following M-CSF addition, the role of PAK1 in regulation of osteoclast apoptosis was next assayed. Expression of dnPAK1 increased osteoclast apoptosis as compared to vector infection (Figure 1B). Numbers of total osteoclasts associated with each infection were also assessed. No significant change in cell numbers was observed (Figure 1C).
Figure 1. M-CSF Mediates Survival of Mature Osteoclasts through PAK1.
(A) M-CSF Activates PAK1. Day 4 mature osteoclasts were serum starved for one hour and treated with 25 ng/ml M-CSF as indicated. Levels of phospho-PAK1Thr423, total PAK1/3, phospho-MEKSer298, phospho-MEK1/2Ser217/221, total-MEK1/2, phospho-ERK1/2Thr202/Tyr204, total-ERK1/2 and tubulin were assayed by western blotting. (B and C) PAK1 suppresses osteoclast apoptosis. Day 4 mature osteoclasts were infected with the dnPAK1 or vector control adenoviruses for 18 hours. Following infection, osteoclasts were serum starved and treated with M-CSF for eight hours. Shown are (B) Percent Apoptotic Osteoclasts and (C) Total Osteoclasts. *p<0.05 compared to vector infection.
PAK1 is a MEK-Independent Target of the Ras/Raf Pathway
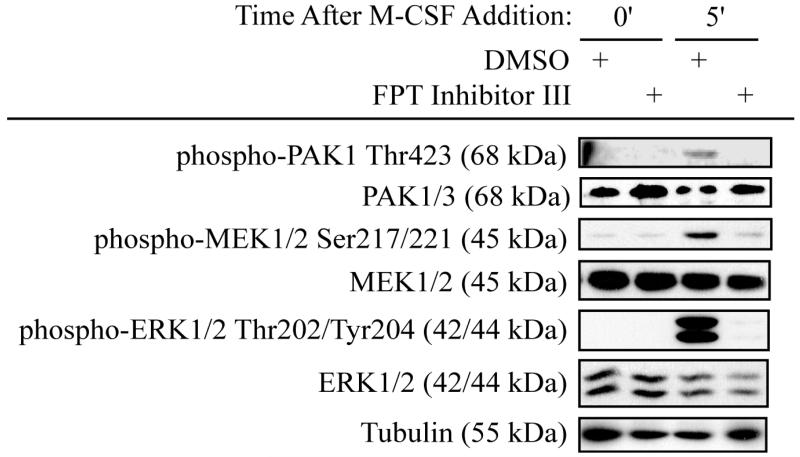
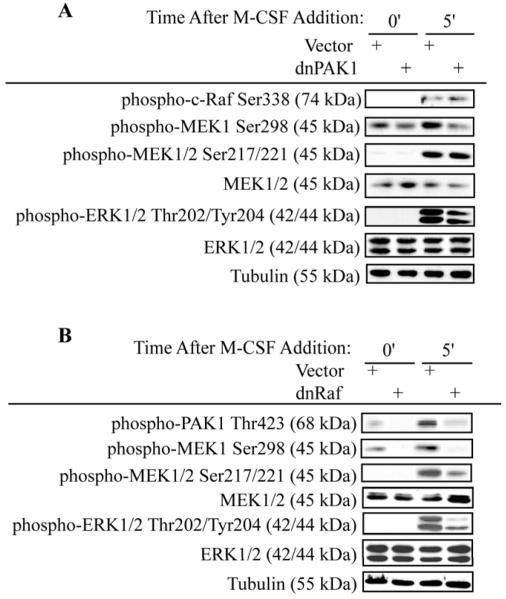
Since Ras is an upstream activator of the MEK/ERK pathway induced by M-CSF, we determined the role of Ras in PAK1 activation in response to M-CSF (Bradley et al., 2008b). Inhibition of Ras through use of a farnesyl transferase inhibitor blocked phosphorylation of PAK1Thr423 in response to M-CSF (Figure 2). A corresponding decrease in MEK/ERK activation was also observed with Ras inhibition (Figure 2). As these data suggested a role for PAK1 as a downstream effector of the Ras/Raf pathway, the function of PAK1 in mediating M-CSF-induced MEK/ERK activation was assayed (Figure 3). Osteoclasts were infected with dnPAK1 or vector control. Following serum starvation, osteoclasts were treated with M-CSF as indicated (Figure 3A and Supplemental Figure 1A). RafSer338 phosphorylation, a known PAK1 target, was unaffected by expression of dnPAK1. However, a decrease in phosphorylation of the PAK1 target MEK1Ser298 was evident with dnPAK1 expression. Given that phosphorylation of MEKSer298 primes MEK for Raf-mediated phosphorylation, we also assayed the effects of blocking PAK1 on MEK/ERK activation (Figure 3A). No significant changes were observed in phosphorylation of MEK1/2Ser217/221, sites required for MEK activation, whereas a slight decrease in ERK activation was observed with dnPAK1 expression (Figure 3A). Because blocking PAK1 did not affect Raf activation and had minimal effects on MEK/ERK activation, we further explored the relationship between Raf and PAK1. While M-CSF induced PAK1Thr423 phosphorylation, this effect was reduced by expression of dnRaf (Figure 3B and Supplemental Figure 1B). A decrease in phosphorylation of the PAK1 target, MEK1Ser298, as well as decreased MEK/ERK activation both at basal levels and in response to M-CSF was observed (Figure 3B).
Figure 2. PAK1 Activation is downstream of Ras.
Day 4 mature osteoclasts were pre-treated with a farnesyl transferase inhibitor to block Ras activation or vehicle control for seven hours. Cells were serum starved and treated with 25 ng/ml M-CSF as indicated in the presence of inhibitor or vehicle control. Western blotting for phospho-PAK1Thr423, total PAK1/3, phospho-MEK1/2Ser217/221, total MEK1/2, phospho-ERKThr202/Tyr204, total ERK1/2 and tubulin was then performed.
Figure 3. PAK1 is a Raf Target.
(A) Blocking PAK1 does not affect Raf activation. Day 4 mature osteoclasts were infected with the dnPAK1 or vector control adenoviruses for 18 hours. Following infection, osteoclasts were serum starved and treated with 25 ng/ml M-CSF as indicated. Levels of phospho-RafSer338, phospho-MEKSer298, phospho-MEK1/2Ser217/221, total-MEK1/2, phospho-ERK1/2Thr202/Tyr204, total-ERK1/2 and tubulin were assayed by western blotting. (B) Blocking Raf abolishes PAK1 activation. Day 4 mature osteoclasts were infected with dnRaf or vector control adenoviruses for 18 hours. Following infection, osteoclasts were serum starved and treated with M-CSF as indicated. Western blotting for phospho-PAK1Thr423, phospho-MEK1Ser298, phospho-MEK1/2Ser217/221, total MEK1/2, phospho-ERKThr202/Tyr204, total ERK1/2 and tubulin was then performed.
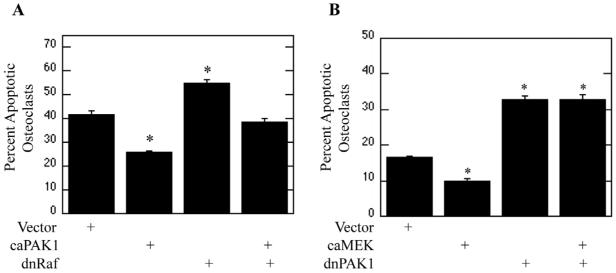
Since inhibition of Raf blocked activation of PAK1, the ability of PAK1 to repress osteoclast apoptosis downstream of Raf was evaluated (Figure 4). Osteoclasts were infected with vector control, caPAK1, dnRaf, or dnRaf and caPAK adenoviruses. Following infection, osteoclasts were serum-starved and treated with M-CSF. Expression of caPAK1 suppressed osteoclast apoptosis, whereas expression of dnRaf promoted osteoclast apoptosis compared to vector control (Figure 4A). Interestingly, expression of caPAK1 diminished dnRaf-induced osteoclast apoptosis (Figure 4A). The observed decrease in the percentage of apoptotic osteoclasts in cells expressing caPAK1 was due to a decreased number of apoptotic osteoclasts combined with an increase in total osteoclasts (Supplemental Figure 2).
Figure 4. PAK1 is a MEK-Independent Raf Target.
(A) PAK1 functions downstream of Raf. Day 4 mature osteoclasts were infected with vector, caPAK1, dnRaf, or caPAK1 and dnRaf adenoviruses for 18 hours. Following infection, osteoclasts were serum starved and treated with 25 ng/ml M-CSF for eight hours. Shown are Percent Apoptotic Osteoclasts. (B) PAK1 and MEK have separate functions. Day 4 mature osteoclasts were infected with vector, caMEK, dnPAK1, or caMEK and dnPAK1 adenoviruses for eighteen hours. Following infection, osteoclasts were serum starved and treated with M-CSF for eight hours. Shown are Percent Apoptotic Osteoclasts. *p<0.05 compared to vector infection.
As PAK1 functioned downstream of Raf and contributes to MEK/ERK activation, roles for PAK1 downstream of the MEK/ERK pathway were next explored. To examine this alternative, the ability of caMEK to suppress dnPAK1-induced osteoclast apoptosis was assayed. Osteoclasts were infected with vector control, caMEK, dnPAK1, or caMEK and dnPAK1 adenoviruses. Following infection, osteoclasts were serum starved and treated with M-CSF. As expected, expression of caMEK repressed osteoclast apoptosis whereas expression of dnPAK1 promoted osteoclast apoptosis (Figure 4B). Interestingly, expression of caMEK was unable to alleviate the effects of dnPAK1 expression (Figure 4B). The observed decrease in the percentage of apoptotic osteoclasts in cells expressing caMEK was due to a decreased number of apoptotic osteoclasts combined with a slight increase in the total number of osteoclasts (Supplemental Figure 2).
Survivin is a Downstream Target of PAK1 and Mediates Osteoclast Survival
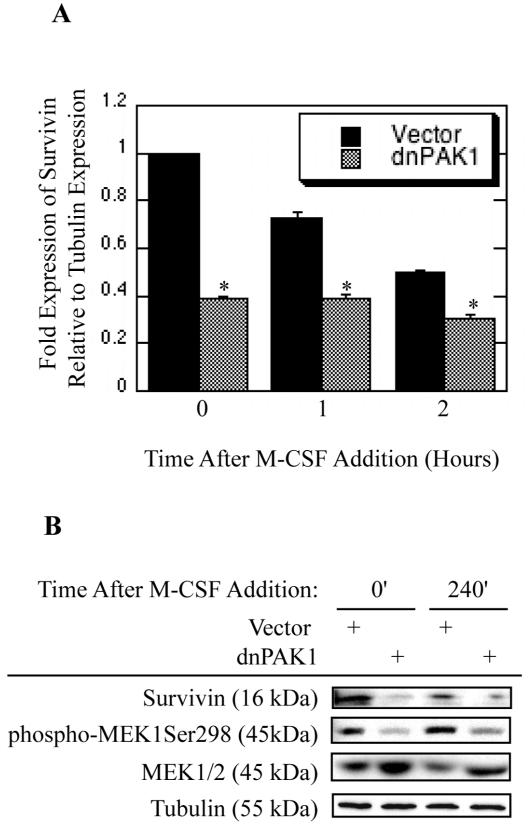
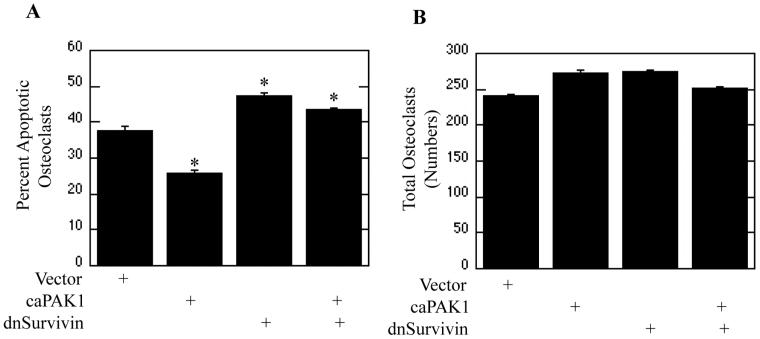
To determine downstream targets of PAK1 mediating the repression of osteoclast apoptosis, Bcl2 and IAP family member expression was evaluated as potential PAK1 targets. Osteoclasts were infected with dnPAK1 or vector control. Following infection osteoclasts were treated with M-CSF as indicated and assayed for transcript expression via real-time RT-PCR. Expression of Survivin, an IAP family member, was reduced by dnPAK1 expression (Figure 5A). We also observed progressive decreases in Survivin expression during culture, indicating that other signaling inputs outside of those elicited by M-CSF may be required to sustain full expression of Survivin (Figure 5A). However, at all time points examined dnPAK1 expression further reduced Survivin expression over vector expression. In addition to transcript expression, the expression of Survivin protein dependent on PAK1 function was also assayed. Declining levels of Survivin protein in vector-infected osteoclasts were observed with increased culture, again supporting a role for other factors in sustaining Survivin expression and osteoclast survival (Figure 5B). Expression of dnPAK1 reduced expression of Survivin protein as compared to vector control while concomitantly decreasing phosphorylation of the known PAK1 target MEK1Ser298 (Figure 5B). Expression of dnPAK1 did not affect expression of Mcl1, Bcl-xl, Bclw, Bim or Bax when compared to vector control (data not shown). The role for Survivin as a downstream target of PAK1 in suppression of osteoclast apoptosis was then examined (Figure 6). Osteoclasts were infected with vector control, caPAK1, dnSurvivin, or dnSurvivin and caPAK1 adenoviruses. Following infection, osteoclasts were serum-starved and cultured with M-CSF. Expression of caPAK1 decreased the percentage of apoptotic osteoclasts, whereas expression of dnSurvivin promoted osteoclast apoptosis (Figure 6A). When used in combination, expression of dnSurvivin abolished the repression of osteoclast apoptosis conferred by caPAK1 expression (Figure 6A). Numbers of total osteoclasts were also determined. No overall difference in the number of osteoclasts was observed with dnSurvivin or caPAK1 alone or in combination as compared to vector control (Figure 6B).
Figure 5. The IAP Survivin is a PAK1 Downstream Effector Controlling Osteoclast Survival.
(A) Blocking PAK1 decreases transcript levels of Survivin. Day 4 mature osteoclasts were infected with the dnPAK1 or vector control adenoviruses for eighteen hours. Following infection, osteoclasts were serum starved and treated with M-CSF as indicated and assayed for Survivin transcript production using real-time PCR. Values are representative of three replicate samples. *p<0.05 compared to vector infection. (B) Blocking PAK1 decreases Survivin expression. Day 4 mature osteoclasts were infected with the dnPAK1 or vector control adenoviruses for eighteen hours. Following infection, osteoclasts were serum starved and treated with M-CSF as indicated. Western blotting for Survivin, phospho-MEKSer298, total MEK1/2 and tubulin was then performed.
Figure 6. Survivin is a PAK1 Downstream Effector.
(A and B) Day 4 mature osteoclasts were infected with vector control, caPAK1, dnSurvivin, or caPAK1 and dnSurvivin adenoviruses for eighteen hours. Following infection, osteoclasts were serum starved and treated with M-CSF for eight hours. Shown are (A) Percent Apoptotic Osteoclasts and (B) Total Osteoclasts. *p<0.05 compared to vector infection.
Discussion
M-CSF promotes survival of cells within the monocyte/macrophage cell lineage, including bone-resorbing osteoclasts. To determine the mechanism by which M-CSF blocks osteoclast apoptosis, the signal transduction pathways induced by M-CSF were first evaluated. Since M-CSF activates the MEK/ERK pathway, other known regulators of MEK/ERK activation were assayed. M-CSF induced Ras-dependent PAK1 activation as demonstrated by an increase in PAK1Thr423 phosphorylation and increased phosphorylation of the PAK1 target MEK1Ser298. Functionally, blocking PAK1 through adenoviral-mediated expression of dnPAK1 increased osteoclast apoptosis. In addition, PAK1 also modulated MEK phosphorylation as blocking PAK1 decreased phosphorylation of the known PAK1 target MEK1Ser298, a site reported to either prime MEK for phosphorylation by Raf or induce autoactivation at Ser217/221 (Coles and Shaw, 2002; Park et al., 2007). This decrease in MEK1Ser298 phosphorylation was not accompanied with a decrease in MEK activating site phosphorylation at Ser217/221 as previously reported (Coles and Shaw, 2002; Park et al., 2007). These data also suggested that although the known PAK1 target site of MEK was blocked by dnPAK1 expression, PAK1 was not involved in modulating M-CSF-induced MEK activation in osteoclasts. In accord, blocking PAK1 did not abolish ERK activating site phosphorylation, but rather attenuated ERK activation in response to M-CSF. This slight decrease in ERK activation could be due to decreased MEKSer298 phosphorylation acting independently of Ser217/221 phosphorylation to regulate MEK activation, as expression of dnPAK1 decreased phosphorylation of MEK1Ser298 and ERK independently of MEKSer217/221 phosphorylation. Since dnPAK1 significantly decreased M-CSF-mediated osteoclast survival while having modest effects on MEK/ERK activation, PAK1 may have functions outside of MEK/ERK in promotion of M-CSF-mediated osteoclast survival. Therefore, we focused studies on this possibility.
To further clarify the role of PAK1 within M-CSF-mediated osteoclast survival as a component of the Ras/Raf pathway, we explored the relationship between Raf and PAK1 activation. Raf is a known PAK1 target through direct physical interaction, which facilitates phosphorylation of RafSer338. In this study, blocking PAK1 function did not affect RafSer338 phosphorylation. This result, coupled with the inability of dnPAK1 to effectively block MEK/ERK activation, as well as the known physical interaction between PAK1 and Raf, implied a potential function for PAK1 as a Raf target. We therefore also assayed for the effects of blocking Raf on activation of PAK1 as well as MEK/ERK in response to M-CSF. As expected, M-CSF activated the MEK/ERK pathway and blocking Raf abolished this activation. As PAK1 reportedly acts upstream of Raf, we unexpectedly observed that blocking Raf also decreased PAK1 activation as evidenced both by a decrease in activating site phosphorylation of PAK1Thr423, as well as decreased PAK1 activity measured by decreased MEK1Ser298 phosphorylation. As further evidence for a function of PAK1 activation downstream of Raf, caPAK1 expression reduced osteoclast apoptosis induced by expression of dnRaf. Further support for a MEK-independent function for PAK1 downstream of Raf is the observation that expression of constitutively active MEK was not able to abolish osteoclast apoptosis promoted by dnPAK1 expression. We have previously shown that M-CSF induces MEK activation (Bradley et al., 2008a). However, the data presented here suggest that PAK1 mediated phosphorylation of MEK1Ser298 does not influence M-CSF-meditated MEK activation, suggesting two parallel Raf-dependent pathways repressing osteoclast apoptosis. These data provide evidence for a role of PAK1 downstream of Raf, illustrating a Raf effector that has not been identified prior to this study and collectively demonstrate a novel function of PAK1 as a MEK-independent Raf target.
To determine how PAK1 functioned to suppress osteoclast apoptosis mediated by M-CSF, expression of apoptosis repressors was assayed. While blocking PAK1 did not affect expression of the tested Bcl2 family members including Mcl1, Bclxl, Bclw, Bim or Bax (data not shown), dnPAK1 expression reduced expression of the IAP Survivin. Survivin has been previously characterized as playing a role in controlling apoptosis and cell cycle progression by regulating the G2/M transition and blocking caspase 9 activity and is induced in response to M-CSF in cultured macrophages (Altieri, 2003; Blanc-Brude et al., 2007). This report is the first documented Survivin expression and function in post-mitotic cells. Survivin was expressed in non-cycling osteoclasts in a PAK1-dependent manner and expression of dnSurvivin, which is unable to bind caspase 9, promoted osteoclast apoptosis. Additional evidence for a function for Survivin downstream of PAK1 was the ability of dnSurvivin to block osteoclast survival promoted by expression of caPAK1. Oncogenic Ras has been demonstrated to enhance expression of Survivin in cycling cells (Sommer et al., 2007; Sommer et al., 2003). Since M-CSF is a known activator of both Ras and PI3K, blocking PAK1 expression through inhibition of Ras provided additional evidence for PAK1 as a functional mediator of M-CSF-induced signaling. Given that PAK1 activation is abolished through blocking Ras activation, the role of Survivin as a PAK1 downstream effector was further established. With increasing culture time following serum starvation, overall levels of Survivin in vector infected cells decreased. This observation may be due to a lack of other mitogeic signals, such as NF-κB activation, in the absence of serum and RANKL (Wu et al., 2003). Thus RANKL and M-CSF signaling, via NF-κB and Raf/PAK1 respectively, may act in concert to promote expression of Survivin, thereby suppressing osteoclast apoptosis.
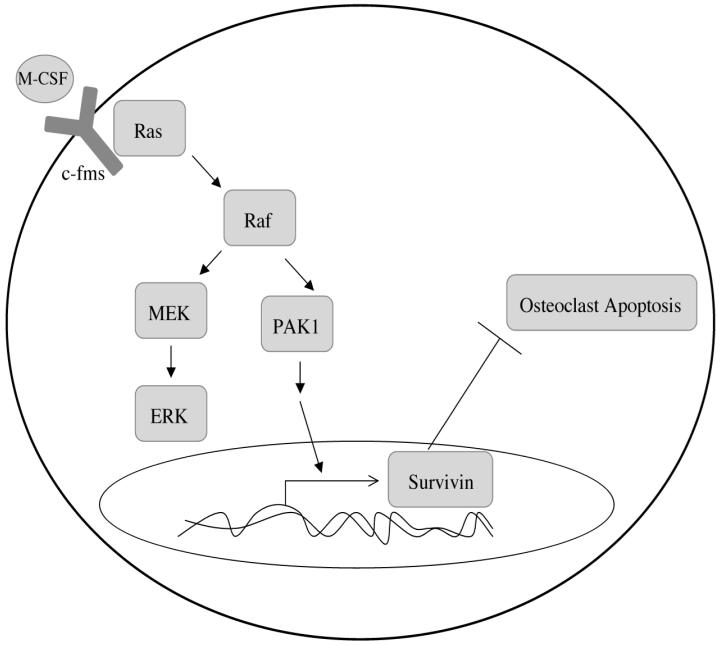
Overall these data illustrate a novel role for PAK1 as a Raf effector in a MEK-independent manner (Figure 7). We have previously shown that inhibition of MEK increases osteoclast apoptosis by 12.5%, whereas inhibition of Raf increases osteoclast apoptosis by 50%, suggesting additional Raf targets outside of MEK (Bradley et al., 2008a; Bradley et al., 2008b). The data presented here may help to explain the increased effectiveness of pharmacological agents blocking Raf function over those blocking MEK function in governing osteoclast apoptosis. In addition, a role for PAK1 in M-CSF-mediated signaling controlling osteoclast survival was also established. Lastly, this study identifies a novel downstream target of PAK1 signaling, Survivin, in the suppression of osteoclast apoptosis mediated by M-CSF, and points to a potential combined effect of M-CSF and RANKL signaling in promoting osteoclast survival through inducing Survivin expression. As Survivin is under study as a target for potential anti-cancer therapy, this IAP family member may also be an attractive target for the treatment of osteoclast-mediated bone resorption, especially in the case of tumor-induced osteolysis (Altieri, 2003).
Figure 7. Model of Raf-independent PAK1 signaling mediating M-CSF promoted osteoclast survival.
Ras-mediated Raf activation in response to M-CSF may promote activation of both MEK/ERK and PAK1 as two independent pathways. M-CSF induced PAK1 activation then acts to promote osteoclast survival through expression of the IAP Survivin.
Supplementary Material
Supplemental Figure 1. PAK1 is a Raf Target. (S1) Blocking PAK1 does not affect Raf activation. Day 4 mature osteoclasts were infected with the dnPAK1 or vector control adenoviruses for 18 hours. Following infection, osteoclasts were serum starved and treated with 25 ng/ml M-CSF as indicated. Levels of phospho-RafSer338, phospho-MEKSer298, phospho-MEK1/2Ser217/221 and phospho-ERK1/2Thr202/Tyr204 were assayed by western blotting. Shown are signal strengths relative to vector infection obtained by scanning each film and quantifying each band using NIH Image software. Each value is the result of three separate experiments. *p<0.05 (S2) Blocking Raf abolishes PAK1 activation. Day 4 mature osteoclasts were infected with dnRaf or vector control adenoviruses for 18 hours. Following infection, osteoclasts were serum starved and treated with M-CSF as indicated. Western blotting for phospho-PAK1Thr423, phospho-MEK1Ser298, phospho-MEK1/2Ser217/221, and phospho-ERKThr202/Tyr204 was then performed. Shown are signal strengths relative to vector infection obtained by scanning each film and quantifying each band using NIH Image software. Each value is the result of three separate experiments. *p<0.05
Supplemental Figure 2. PAK1 is a MEK-Independent Raf Target. (S1 and S2) PAK1 functions downstream of Raf. Day 4 mature osteoclasts were infected with vector, caPAK1, dnRaf, or caPAK1 and dnRaf adenoviruses for 18 hours. Following infection, osteoclasts were serum starved and treated with 25 ng/ml M-CSF for eight hours. Shown are (S1) Apoptotic Osteoclasts and (S2) Total Osteoclasts. (S3 and S4) PAK1 and MEK have separate functions. Day 4 mature osteoclasts were infected with vector, caMEK, dnPAK1, or caMEK and dnPAK1 adenoviruses for eighteen hours. Following infection, osteoclasts were serum starved and treated with M-CSF for eight hours. Shown are (S3) Apoptotic Osteoclasts and (S4) Total Osteoclasts. *p<0.05 compared to vector infection, #p<0.06 compared to vector infection.
Acknowledgments
Contract grant sponsor: Dr. Merry Jo Oursler; Contract grant number: DE14680
References
- Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3(1):46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- Blanc-Brude OP, Teissier E, Castier Y, Leseche G, Bijnens AP, Daemen M, Staels B, Mallat Z, Tedgui A. IAP survivin regulates atherosclerotic macrophage survival. Arterioscler Thromb Vasc Biol. 2007;27(4):901–907. doi: 10.1161/01.ATV.0000258794.57872.3f. [DOI] [PubMed] [Google Scholar]
- Blum R, Elkon R, Yaari S, Zundelevich A, Jacob-Hirsch J, Rechavi G, Shamir R, Kloog Y. Gene expression signature of human cancer cell lines treated with the ras inhibitor salirasib (S-farnesylthiosalicylic acid) Cancer Res. 2007;67(7):3320–3328. doi: 10.1158/0008-5472.CAN-06-4287. [DOI] [PubMed] [Google Scholar]
- Bradley EW, Ruan MM, Oursler MJ. Novel pro-survival functions of the kruppel-like transcription factor EGR2 in promotion of M-CSF-mediated osteoclast survival downstream of the MEK/ERK pathway. J Biol Chem. 2008a doi: 10.1074/jbc.M709500200. [DOI] [PubMed] [Google Scholar]
- Bradley EW, Ruan MM, Vrable A, Oursler MJ. Pathway crosstalk between Ras/Raf and PI3K in promotion of M-CSF-induced MEK/ERK-mediated osteoclast survival. J Cell Biochem. 2008b doi: 10.1002/jcb.21719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong H, Lee J, Guan KL. Positive and negative regulation of Raf kinase activity and function by phosphorylation. Embo J. 2001;20(14):3716–3727. doi: 10.1093/emboj/20.14.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles LC, Shaw PE. PAK1 primes MEK1 for phosphorylation by Raf-1 kinase during cross-cascade activation of the ERK pathway. Oncogene. 2002;21(14):2236–2244. doi: 10.1038/sj.onc.1205302. [DOI] [PubMed] [Google Scholar]
- Dohi T, Okada K, Xia F, Wilford CE, Samuel T, Welsh K, Marusawa H, Zou H, Armstrong R, Matsuzawa S, Salvesen GS, Reed JC, Altieri DC. An IAP-IAP complex inhibits apoptosis. J Biol Chem. 2004;279(33):34087–34090. doi: 10.1074/jbc.C400236200. [DOI] [PubMed] [Google Scholar]
- Dougherty MK, Muller J, Ritt DA, Zhou M, Zhou XZ, Copeland TD, Conrads TP, Veenstra TD, Lu KP, Morrison DK. Regulation of Raf-1 by direct feedback phosphorylation. Mol Cell. 2005;17(2):215–224. doi: 10.1016/j.molcel.2004.11.055. [DOI] [PubMed] [Google Scholar]
- Eblen ST, Slack JK, Weber MJ, Catling AD. Rac-PAK signaling stimulates extracellular signal-regulated kinase (ERK) activation by regulating formation of MEK1-ERK complexes. Mol Cell Biol. 2002;22(17):6023–6033. doi: 10.1128/MCB.22.17.6023-6033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda S, Pelus LM. Activated H-Ras regulates hematopoietic cell survival by modulating Survivin. Biochem Biophys Res Commun. 2004;323(2):636–644. doi: 10.1016/j.bbrc.2004.08.149. [DOI] [PubMed] [Google Scholar]
- Galaktionov K, Jessus C, Beach D. Raf1 interaction with Cdc25 phosphatase ties mitogenic signal transduction to cell cycle activation. Genes Dev. 1995;9(9):1046–1058. doi: 10.1101/gad.9.9.1046. [DOI] [PubMed] [Google Scholar]
- Gingery A, Bradley E, Shaw A, Oursler MJ. Phosphatidylinositol 3-kinase coordinately activates the MEK/ERK and AKT/NFkappaB pathways to maintain osteoclast survival. J Cell Biochem. 2003;89(1):165–179. doi: 10.1002/jcb.10503. [DOI] [PubMed] [Google Scholar]
- Glantschnig H, Fisher JE, Wesolowski G, Rodan GA, Reszka AA. M-CSF, TNFalpha and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ. 2003;10(10):1165–1177. doi: 10.1038/sj.cdd.4401285. [DOI] [PubMed] [Google Scholar]
- Karst M, Gorny G, Galvin RJ, Oursler MJ. Roles of stromal cell RANKL, OPG, and M-CSF expression in biphasic TGF-beta regulation of osteoclast differentiation. J Cell Physiol. 2004;200(1):99–106. doi: 10.1002/jcp.20036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CC, Gardiner EM, Zenke FT, Bohl BP, Newton AC, Hemmings BA, Bokoch GM. p21-activated kinase (PAK1) is phosphorylated and activated by 3-phosphoinositide-dependent kinase-1 (PDK1) J Biol Chem. 2000;275(52):41201–41209. doi: 10.1074/jbc.M006553200. [DOI] [PubMed] [Google Scholar]
- Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351(Pt 2):289–305. [PMC free article] [PubMed] [Google Scholar]
- Lei M, Lu W, Meng W, Parrini MC, Eck MJ, Mayer BJ, Harrison SC. Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell. 2000;102(3):387–397. doi: 10.1016/s0092-8674(00)00043-x. [DOI] [PubMed] [Google Scholar]
- Marais R, Light Y, Paterson HF, Mason CS, Marshall CJ. Differential regulation of Raf-1, A-Raf, and B-Raf by oncogenic ras and tyrosine kinases. J Biol Chem. 1997;272(7):4378–4383. doi: 10.1074/jbc.272.7.4378. [DOI] [PubMed] [Google Scholar]
- Morrison DK, Cutler RE. The complexity of Raf-1 regulation. Curr Opin Cell Biol. 1997;9(2):174–179. doi: 10.1016/s0955-0674(97)80060-9. [DOI] [PubMed] [Google Scholar]
- Oursler MJ, Bradley EW, Elfering SL, Giulivi C. Native, not nitrated, cytochrome c and mitochondria-derived hydrogen peroxide drive osteoclast apoptosis. Am J Physiol Cell Physiol. 2005;288(1):C156–168. doi: 10.1152/ajpcell.00092.2004. [DOI] [PubMed] [Google Scholar]
- Park ER, Eblen ST, Catling AD. MEK1 activation by PAK: A novel mechanism. Cell Signal. 2007;19(7):1488–1496. doi: 10.1016/j.cellsig.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl SJ, Renatus M, Schwarzenbacher R, Zhou Q, Sun C, Fesik SW, Liddington RC, Salvesen GS. Structural basis for the inhibition of caspase-3 by XIAP. Cell. 2001;104(5):791–800. doi: 10.1016/s0092-8674(01)00274-4. [DOI] [PubMed] [Google Scholar]
- Sarthy AV, Morgan-Lappe SE, Zakula D, Vernetti L, Schurdak M, Packer JC, Anderson MG, Shirasawa S, Sasazuki T, Fesik SW. Survivin depletion preferentially reduces the survival of activated K-Ras-transformed cells. Mol Cancer Ther. 2007;6(1):269–276. doi: 10.1158/1535-7163.MCT-06-0560. [DOI] [PubMed] [Google Scholar]
- Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19(4):2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmer AD. Inhibitor of apoptosis proteins: translating basic knowledge into clinical practice. Cancer Res. 2004;64(20):7183–7190. doi: 10.1158/0008-5472.CAN-04-1918. [DOI] [PubMed] [Google Scholar]
- Sells Galvin RJ, Gatlin CL, Horn JW, Fuson TR. TGF-beta enhances osteoclast differentiation in hematopoietic cell cultures stimulated with RANKL and M-CSF. Biochem Biophys Res Commun. 1999;265(1):233–239. doi: 10.1006/bbrc.1999.1632. [DOI] [PubMed] [Google Scholar]
- Sommer KW, Rodgarkia-Dara CJ, Schreiner C, Holzmann K, Krupitza G, Cerni C. Oncogenic c-H-ras deregulates survivin expression: an improvement for survival. FEBS Lett. 2007;581(25):4921–4926. doi: 10.1016/j.febslet.2007.09.023. [DOI] [PubMed] [Google Scholar]
- Sommer KW, Schamberger CJ, Schmidt GE, Sasgary S, Cerni C. Inhibitor of apoptosis protein (IAP) survivin is upregulated by oncogenic c-H-Ras. Oncogene. 2003;22(27):4266–4280. doi: 10.1038/sj.onc.1206509. [DOI] [PubMed] [Google Scholar]
- Wall NR, O’Connor DS, Plescia J, Pommier Y, Altieri DC. Suppression of survivin phosphorylation on Thr34 by flavopiridol enhances tumor cell apoptosis. Cancer Res. 2003;63(1):230–235. [PubMed] [Google Scholar]
- Wang S, Ghosh RN, Chellappan SP. Raf-1 physically interacts with Rb and regulates its function: a link between mitogenic signaling and cell cycle regulation. Mol Cell Biol. 1998;18(12):7487–7498. doi: 10.1128/mcb.18.12.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WS, Xu ZX, Hittelman WN, Salomoni P, Pandolfi PP, Chang KS. Promyelocytic leukemia protein sensitizes tumor necrosis factor alpha-induced apoptosis by inhibiting the NF-kappaB survival pathway. J Biol Chem. 2003;278(14):12294–12304. doi: 10.1074/jbc.M211849200. [DOI] [PubMed] [Google Scholar]
- Zang M, Hayne C, Luo Z. Interaction between active Pak1 and Raf-1 is necessary for phosphorylation and activation of Raf-1. J Biol Chem. 2002;277(6):4395–4405. doi: 10.1074/jbc.M110000200. [DOI] [PubMed] [Google Scholar]
- Zang M, Waelde CA, Xiang X, Rana A, Wen R, Luo Z. Microtubule integrity regulates Pak leading to Ras-independent activation of Raf-1. insights into mechanisms of Raf-1 activation. J Biol Chem. 2001;276(27):25157–25165. doi: 10.1074/jbc.M100152200. [DOI] [PubMed] [Google Scholar]
- Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B) Science. 1999;286(5445):1741–1744. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. PAK1 is a Raf Target. (S1) Blocking PAK1 does not affect Raf activation. Day 4 mature osteoclasts were infected with the dnPAK1 or vector control adenoviruses for 18 hours. Following infection, osteoclasts were serum starved and treated with 25 ng/ml M-CSF as indicated. Levels of phospho-RafSer338, phospho-MEKSer298, phospho-MEK1/2Ser217/221 and phospho-ERK1/2Thr202/Tyr204 were assayed by western blotting. Shown are signal strengths relative to vector infection obtained by scanning each film and quantifying each band using NIH Image software. Each value is the result of three separate experiments. *p<0.05 (S2) Blocking Raf abolishes PAK1 activation. Day 4 mature osteoclasts were infected with dnRaf or vector control adenoviruses for 18 hours. Following infection, osteoclasts were serum starved and treated with M-CSF as indicated. Western blotting for phospho-PAK1Thr423, phospho-MEK1Ser298, phospho-MEK1/2Ser217/221, and phospho-ERKThr202/Tyr204 was then performed. Shown are signal strengths relative to vector infection obtained by scanning each film and quantifying each band using NIH Image software. Each value is the result of three separate experiments. *p<0.05
Supplemental Figure 2. PAK1 is a MEK-Independent Raf Target. (S1 and S2) PAK1 functions downstream of Raf. Day 4 mature osteoclasts were infected with vector, caPAK1, dnRaf, or caPAK1 and dnRaf adenoviruses for 18 hours. Following infection, osteoclasts were serum starved and treated with 25 ng/ml M-CSF for eight hours. Shown are (S1) Apoptotic Osteoclasts and (S2) Total Osteoclasts. (S3 and S4) PAK1 and MEK have separate functions. Day 4 mature osteoclasts were infected with vector, caMEK, dnPAK1, or caMEK and dnPAK1 adenoviruses for eighteen hours. Following infection, osteoclasts were serum starved and treated with M-CSF for eight hours. Shown are (S3) Apoptotic Osteoclasts and (S4) Total Osteoclasts. *p<0.05 compared to vector infection, #p<0.06 compared to vector infection.