Abstract
While M-CSF-mediated MEK/ERK activation promotes osteoclast survival, the signaling pathway by which M-CSF activates MEK/ERK is unresolved. Functions for PI3K, Ras, and Raf have been implicated in support of osteoclast survival, although interaction between these signaling components has not been examined. Therefore, the interplay between PI3K, Ras and Raf in M-CSF-promoted MEK/ERK activation and osteoclast survival was investigated. M-CSF activates Ras to coordinate activation of PI3K and Raf/MEK/ERK, since Ras inhibition decreased PI3K activation and PI3K inhibition did not block M-CSF-mediated Ras activation. As further support for Ras-mediated signaling, constitutively active (ca) Ras promoted MEK/ERK activation and osteoclast survival, which was blocked by inhibition of PI3K or Raf. Moreover, PI3K-selective or Raf-selective caRas were only partially able to promote osteoclast survival when compared to parental caRas. We then examined whether PI3K and Raf function linearly or in parallel downstream of Ras. Expression of caPI3K increased MEK/ERK activation and promoted osteoclast survival downstream of M-CSF, supporting this hypothesis. Blocking Raf did not decrease osteoclast survival and MEK/ERK activation promoted by caPI3K. In addition, PI3K-selective Ras-mediated survival was not blocked by Raf inhibition. Taken together, our data support that Raf signaling is separate from Ras/PI3K signaling and PI3K signaling is separate from Ras/Raf signaling. These data therefore support a role for Ras in coordinate activation of PI3K and Raf acting in parallel to mediate MEK/ERK-promoted osteoclast survival induced by M-CSF.
Keywords: Osteoclast, Apoptosis, MEK, Ras, PI3K, M-CSF
Macrophage-Colony Stimulating Factor (M-CSF) promotes the differentiation and survival of cells within the monocyte/macrophage lineage, including osteoclasts, the multinucleated cells derived for bone resorption [Akiyama et al., 2005; Arai et al., 1999; Fuller et al., 1993; Glantschnig et al., 2003; Takahashi et al., 1988; Udagawa et al., 1990]. Overall increases in osteoclast numbers and activity, when not balanced with corresponding bone deposition, contribute to excessive bone loss seen in conditions such as post-menopausal osteoporosis, Paget’s disease and tumor-induced osteolysis [Rodan and Martin, 2000]. The number of osteoclasts present at sites of bone remodeling depends both on differentiation and survival of osteoclasts [Rodan and Martin, 2000]. As these processes are both supported by M-CSF, identifying the mechanisms by which M-CSF influences osteoclasts will help to target bone loss associated with increased osteoclast numbers.
M-CSF promotes cellular responses through the receptor tyrosine kinase c-fms, which is activated following ligand binding through an autophosphorylation event [Jaworowski et al., 1996]. Once active, c-fms signals through both Ras and PI3K dependant mechanisms to activate the MEK/ERK pathway [Sun et al., 2000; Yang et al., 2006]. Both Ras- and PI3K-dependent osteoclast survival have been reported, however the interplay between these pathways in mediating osteoclast survival has not been addressed [Gingery et al., 2003; Miyazaki et al., 2000]. H-Ras is a small G-protein of the p21 family which, upon farnesylation, is recruited to the plasma membrane and contributes to receptor tyrosine kinasae signaling [Carboni et al., 1995; Cox et al., 1992; Peyssonnaux and Eychene, 2001]. In its active GTP-bound form, Ras promotes activation of downstream signaling components including the Raf/MEK/ERK pathway. A T35S point mutation in Ras induces selective activation of Raf, confirming that Ras mediates Raf activation [Edme et al., 2002; Stoyanov et al., 1995; White et al., 1995]. Once activated, Raf directly phosphorylates and activates MEK1/2 by targeting Ser217 and Ser221 [Alessi et al., 1994].
Activation of Ras can regulate PI3K activity in that a Y40C point mutation in Ras confers selective activation of PI3K [Edme et al., 2002; White et al., 1995]. PI3K is a lipid kinase catalyzing the phosphorylation of PIP2 to PIP3, leading to plasma membrane recruitment of PH-domain containing signaling components. PI3K is composed of two subunits, the p110 subunit, which catalyzes the lipid kinase reaction, and the p85 subunit, which regulates p110 activity. During PI3K activation, the p85 subunit is recruited to phospho-tyrosine residues within the YXXM motif of receptor tyrosine kinases at the plasma membrane leading to activation of the pathway and downstream effectors [Ponzetto et al., 1993].
Ras promotes activation of both Raf and PI3K and PI3K may also act independently of Ras and/or regulate Ras activity. In myeloid progenitors, Ras and PI3K have been reported to individually mediate M-CSF-induced MEK/ERK activation in parallel [Lee and States, 2006]. PI3K can also act upstream of Ras in activation of the MEK/ERK pathway [Wennstrom and Downward, 1999]. In turn, Ras then acts both directly and indirectly to promote Raf activation. Through direct association, Ras may promote dimerization of Raf at the plasma membrane, leading to Raf activation [Kolch, 2000]. Ras may also act indirectly through PI3K to mediate Raf activation by targeted Raf phosphorylation at Ser338 and Ser341 [Zang et al., 2002; Zang et al., 2001], while Akt-mediated phosphorylation of Ser259 inactivates Raf [Chong et al., 2001; Dougherty et al., 2005; Marais et al., 1997; Zimmermann and Moelling, 1999]. As activation of the MEK/ERK pathway is a critical component of M-CSF-promoted osteoclast survival, determining specific mechanism by which M-CSF activates MEK/ERK may contribute to advancing treatment of conditions resulting in increased bone turnover mediated by increased osteoclast numbers.
Given that there are multiple candidate mechanisms for activation of the MEK/ERK pathway by M-CSF, this study focused on characterizing the upstream signaling events involved in M-CSF-mediated activation of the MEK/ERK pathway, which controls osteoclast survival. These data demonstrate that M-CSF-mediated osteoclast survival and MEK/ERK activation are controlled by Ras-coordinated activation of PI3K/MEK/ERK and Raf/MEK/ERK. These data document parallel yet interacting sequence of events induced by M-CSF to promote osteoclast survival.
Materials and Methods
Osteoclast Differentiation
Mouse marrow osteoclast precursors were obtained from female Balb/c mice (Jackson Labs, Bar Harbor, ME) as previously described [Sells Galvin et al., 1999]. Long bones of the hind limbs were obtained after sacrifice and the marrow was flushed out with sterile PBS. Marrow aspirates were plated at a density of 2.9×107 in 100 mm dishes in alpha MEM (αMEM) supplemented with 10% FBS (v/v) (Hyclone, Logan, UT), 1% antimycotic/antibiotic (base media) and supplemented with 25 ng/ml M-CSF (R and D Systems, Minneapolis, MN) for 24 hours. Non-adherent cells were collected and plated in base media at a density of 2×105 cells/cm2 and supplemented with 25 ng/ml M-CSF and 100 ng/ml rRANKL (differentiation media). Cells were fed after three days in culture with differentiation media and allowed to differentiate until formation of mature, multi-nucleated TRAP-positive osteoclasts formed. Mature osteoclasts were then either fixed in 1% (w/v) paraformaldehyde or treated as described.
Western Blotting
Mature osteoclasts were treated as above and either harvested immediately for Western blotting or serum starved for one hour and cultured with 25 ng/ml M-CSF for the indicated time period. For use of signaling chemical inhibitors, mature osteoclasts were serum starved in each respective inhibitor or vehicle control for one hour prior to treatment. The Raf inhibitor GW5074 (Sigma, St. Louis, MO) was used at a concentration of 5 μM and the PI3K inhibitors LY294002 (Cell Signaling, Beverly, MA) and wortmannin (Cell Signaling) were used at concentrations of 50 μM and 10 μM respectively. To inhibit Ras activation, cells were pre-treated with 100 μM farnesyl transferase inhibitor, FPT inhibitor III (Calbiochem, San Diego, CA), for eight hours prior to and during serum-starve and culture with 25 ng/ml M-CSF as detailed in the figure legends. Cells were rinsed with PBS and harvested for Western blotting by scraping into SDS sample buffer lacking β-mercaptoethanol and bromphenol blue. To insure that equal cell protein was analyzed, total protein concentrations were determined using the BioRad DC assay (BioRad). Following protein quantification, 1μl β-mercaptoethanol and bromphenol blue were added to each sample and 60μg of total protein was subjected to SDS-PAGE. Western blotting was carried out using antibodies directed against total and phospho-MEK1/2Ser217/Ser221, total and phospho-ERKThr202/Tyr204, phospho-RafSer338 and phospho-RafSer259, phospho-tyrosine p85 binding motif YXXM, (Cell Signaling) and tubulin (E7, Hybridoma Bank, Iowa State, IA) at a 1:1000 dilution and corresponding secondary antibodies (Cell Signaling) at a 1:10,000 dilution with chemiluminescent detection using the Pierce femto reagent (Pierce, Rockford, IL). Blots were stripped and reprobed using a Western Blot Recycling Kit (Alpha Diagnostics, San Antonio, TX).
Apoptosis Detection
Fixed mature osteoclasts were stained for 60 minutes with Hoechst 33258 diluted to 5 mg/ml in PBS with 0.01% Tween 20. The cells were then Tartrate-Resistant Acid Phosphatase (TRAP) stained as previously described [Gingery et al., 2003] and examined using fluorescent microscopy for apoptotic osteoclasts, as defined by TRAP-positive cells with three or more nuclei, displaying condensed nuclei. Six replicate coverslips were plated and analyzed for each treatment. Each experiment was repeated three times, with representative data reported.
Ras ELISA
Mature osteoclasts were treated as described and Ras activation was assayed using a Ras GTPase Chemi ELISA kit according to manufacture’s instructions (Active Motif, Carlsbad, CA). Briefly, cell lysates were centrifuged and the resulting supernatants were incubated in a microtiter plate coated with a Raf-RBD peptide recognizing GTP-bound Ras. HeLa cell lysates treated with EGF were used as a positive control (Active Motif). Following incubation, each well was washed three times and incubated with primary antibody recognizing H-Ras and a corresponding HRP-linked secondary antibody and incubated with the provided chemiluminescent detection reagent. Signals for each treatment were then read as relative light units using a luminometer.
Ras GTPase Assay
Mature osteoclasts were treated as described and levels of GTP-bound Ras were evaluated using the Pierce EX-detect Ras Activation kit according to the manufacture’s specifications. Osetoclasts were serum starved for one hour and treated with 25 ng/ml M-CSF in the presence of the PI3K inhibitor LY294002 or vehicle control. Cell lysates were collected and assayed for protein concentrations using the BioRad Dc Assay. A 40 μg sample was evaluated for levels of total Ras via western blotting. Following protein quantification, 500 μg of cell lysate was incubated with GST-Raf-RBD in the presence of gel-immobilized resin for one hour to isolate active Ras. After three washes of the resin, GTP-bound Ras was eluted with sample buffer containing beta-mercaptoethanol. Samples were probed for total GTP-bound Ras with each treatment via western blotting.
Adenovirus Infections
Adenoviral constructs for caRas, dnRas and dnRaf were a gift from L. F. Parada. The caPI3K and dnPI3K adenoviruses were obtained from Keqiang Ye. The pathway selective forms of Ras were made from RasV12T35S and RasV12Y40C expression constructs obtain from J. Downward. These expression constructs were used to manufacture corresponding adenoviruses using the Ad-EadyXL system according to manufacture’s specifications (Stratagene). Each virus was expanded and titered according to standard procedures. Mature osteoclasts were infected with each indicated virus at a multiplicity of infection (MOI) of 100 for 18 hours prior to experimental procedures.
Statistical Analysis
Data obtained are the mean +/− standard deviation (N=6) and are representative of three replicate experiments. The effect of treatment was compared with control values using a student’s t-test to asses significant differences using Microsoft Excel Apple software.
Results
M-CSF Activates Components of Ras and PI3K Signal Transduction Pathways to Promote Osteoclast Survival
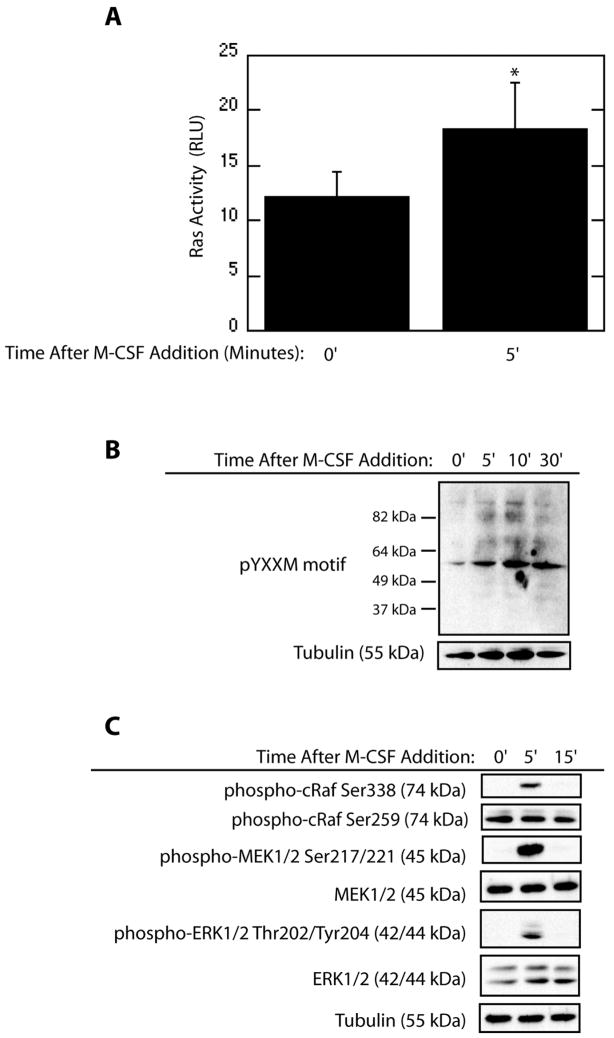
M-CSF transiently activates the MEK/ERK pathway to promote osteoclast survival [Gingery et al., 2003; Glantschnig et al., 2003]. Since Ras is a canonical upstream activator of the MEK/ERK pathway, a Ras activity ELISA was employed to determine if M-CSF increased Ras activation. A significant increase in osteoclast Ras activity was observed five minutes after M-CSF addition (Figure 1A). The level of Ras activation was comparable to levels induced by EGF treatment of HeLa cells (data not shown). PI3K has also been implicated in promotion of osteoclast survival, and acts as an upstream activator of the MEK/ERK pathway. To assay for PI3K activation in response to M-CSF in mature osteoclasts, western blotting for phospho-tyrosine motifs within the YXXM motif was performed. M-CSF caused an increase in phosphorylation of these residues in multiple proteins, supporting increased PI3K activity following M-CSF treatment (Figure 1B). Downstream signal transduction pathway components were also assayed for activation via western blotting. Increased phosphorylation of Raf-Ser338 was evident following M-CSF treatment (Figure 1C) with no concomitant increase in phosphorylation of the inhibitory Raf-Ser259 residue. In addition, activation of MEK/ERK was observed as levels of phospho-MEK1/2 and phospho-ERK1/2 increased following M-CSF addition.
Figure 1.
M-CSF Activates Ras, PI3K as well as Downstream Signaling Components in Mature Osteoclasts. (A) Ras activity is induced by M-CSF. Osteoclasts were serum-starved for 60 minutes and treated with M-CSF for the indicated time. Ras Activation was assayed using an ELISA-based assay for quantification of GTP-bound Ras. (B) PI3K Activity is promoted by M-CSF. Western blotting for phospho-tyrosine residues within the YXXM motif and tubulin was performed following serum-starve and M-CSF treatment as indicated. (C) Downstream effectors of Ras and PI3K are activated by M-CSF. Levels of phospho-MEK1/2Ser217/221, total-MEK1/2, phospho-ERK1/2Thr202/Tyr204, total-ERK1/2, phospho-RafSer338, phospho-RafSer259, and tubulin were assayed by western blotting following treatment with M-CSF.
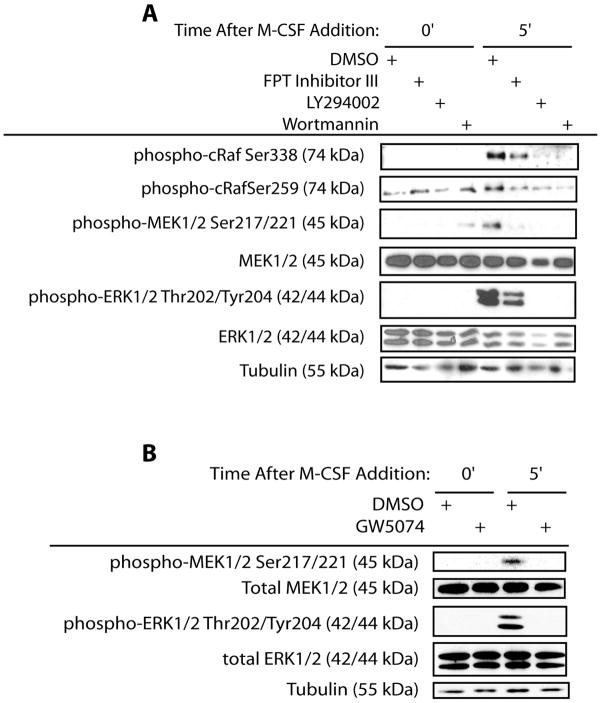
To determine the role of PI3K, Ras, Raf, MEK and ERK in M-CSF-mediated activation of the MEK/ERK pathway, chemical inhibitors or dominant negative (dn) forms for respective components were employed. M-CSF-induced phosphorylation of RafSer338 was decreased by inhibition of either Ras or PI3K (Figure 2A). Inhibitory site phosphorylation of RafSer259 was not affected with Ras or PI3K inhibition prior to M-CSF addition (Figure 2A). While M-CSF did not induce phosphorylation of RafSer259, inhibition of both PI3K and Ras decreased phosphorylation of this site following M-CSF addition (Figure 2A). Moreover, M-CSF-induced MEK/ERK activation was decreased by inhibition of Ras or PI3K (Figure 2A). To assay for the role of Raf in pathway activation, the Raf chemical inhibitor GW5074 was employed. Raf chemical inhibition decreased MEK/ERK activation as assessed by decreased levels of phospho-MEK1/2 and phospho-ERK1/2 (Figure 2B).
Figure 2.
Inhibition of Pathway Constituents Blocks M-CSF-Mediated Pathway Activation. (A) Inhibition of PI3K by LY294002 and Ras activation by FPT Inhibitor III abolishes activation of downstream components. Western blotting for phospho-MEK1/2Ser217/221, total-MEK1/2, phospho-ERK1/2Thr202/Tyr204, total-ERK1/2, phospho-RafSer338, phospho-RafSer259, and tubulin was performed following treatment of mature osteoclasts with M-CSF for the indicated times. (B) Inhibition of Raf by GW5074 blocks pathway activation. Levels phospho-MEK1/2Ser217/221, total-MEK1/2, phospho-ERK1/2Thr202/Tyr204, total-ERK1/2, phospho-RafSer338, and tubulin were assayed by western blotting following treatment with M-CSF in mature osteoclasts.
Roles for these signaling components in promotion of M-CSF-mediated osteoclast survival were examined. Chemical inhibition of PI3K significantly increased the percentage of apoptotic osteoclasts as compared to vehicle control (Figure 3A). Expression of dnRas also significantly increased the percentage of apoptotic osteoclasts as compared to vehicle treatment (Figure 3B). In addition, Raf chemical inhibition significantly increased the percentage of apoptotic osteoclasts as compared to vehicle control (Figure 3C).
Figure 3.
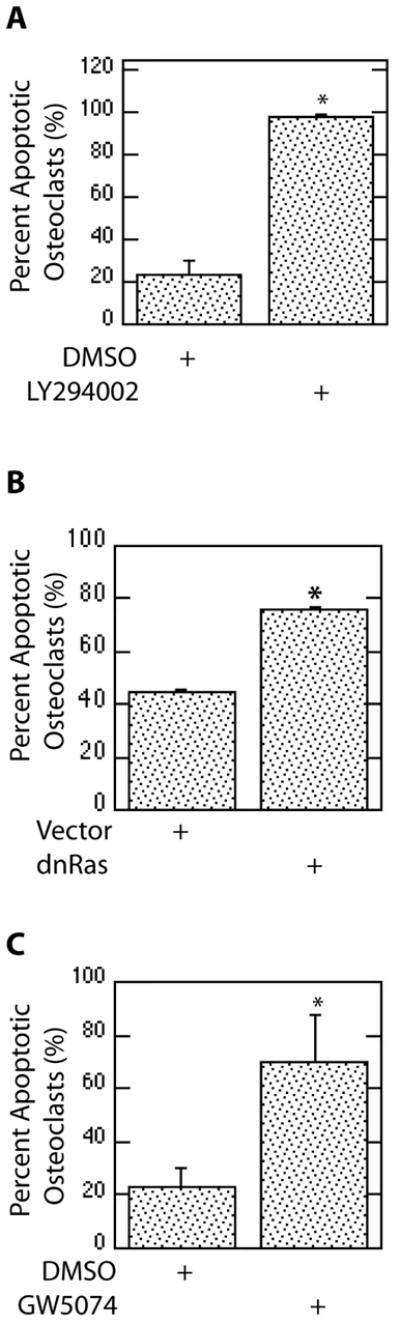
Inhibition of Pathway Constituents Blocks M-CSF-Mediated Osteoclast Survival. (A) Inhibition of PI3K promotes osteoclast apoptosis. Mature osteoclasts were treated with M-CSF in the presence of the PI3K inhibitor LY294002 or DMSO vehicle control and assayed for percent apoptotic multinucleated cells. (B) Inhibition of Ras increases osteoclast apoptosis. Mature osteoclasts infected with a dnRas adenovirus or vector control. Percent apoptotic multinucleated cells were then determined with each treatment. (C) Inhibition of Raf decreases osteoclast survival. Percent apoptotic TRAP-positive multi-nucleated osteoclasts were assayed following treatment with M-CSF in the presence of the Raf inhibitor GW5074 or vehicle control.
MEK/ERK Activation and Osteoclast Survival Involves Ras-Mediated Activation of PI3K and Raf
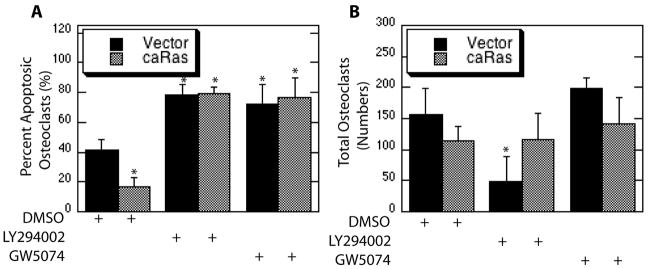
To determine the function of each signaling component downstream of Ras, osteoclasts were infected with a parental caRas or two-pathway specific forms of Ras and subsequently treated with downstream inhibitors. While expression of caRas promoted osteoclast survival as compared to vector infection, this response was blocked through chemical inhibition of either PI3K or Raf (Figure 4A). Significant changes in overall cell numbers were not observed with Raf inhibition in osteoclast expressing either caRas or vector control, whereas a significant decrease in total cell numbers was associated with PI3K inhibition alone (Figure 4B). This decrease in total cell numbers associated with PI3K inhibition was abolished by expression of caRas (Figure 4B). These results suggest roles for Raf and PI3K downstream of Ras in promotion of osteoclast survival.
Figure 4.
Blocking PI3K and Raf Abolishes Survival of Mature Osteoclasts Promoted by Expression of caRas. Mature osteoclasts were infected with the caRas adenovirus or vector control. Following infection osteoclasts were serum starved in the presence of the PI3K inhibitor LY294002, the Raf inhibitor GW5074 or DMSO vehicle control and treated with M-CSF. Shown are (A) Percent Apoptotic Osteoclasts and (B) Total Osteoclasts.
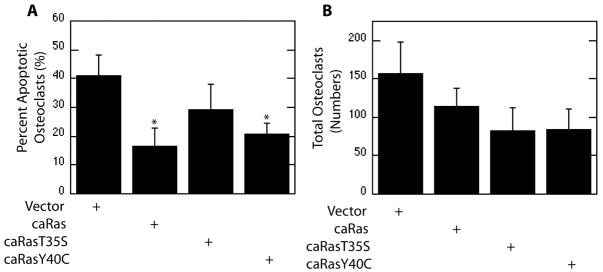
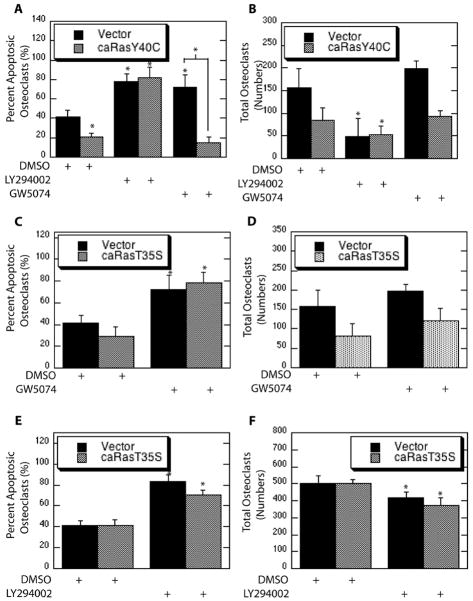
To evaluate the specific roles of each of these signaling components downstream of Ras, point mutants conferring PI3K-selective and Raf-selective caRas (caRasY40C and caRasT35S) were utilized. Expression of parental caRas promoted osteoclast survival as evidenced by decreased apoptosis. A significant decrease in the percentage of apoptotic osteoclasts was not observed with expression of Raf-selective caRas (T35S), whereas expression of PI3K-selective caRas suppressed osteoclast apoptosis (Figure 5A). Although a trend towards decreased total numbers of osteoclasts was evident with expression of both PI3K-selective and Raf-selective caRas as compared to vector infections, changes were not statistically significant (Figure 5B).
Figure 5.
PI3K and Raf Function Downstream of Ras to Promote Osteoclast Survival. Mature osteoclasts were infected with a parental caRas adenovirus, the Raf-selective caRas adenovirus (T35S), the PI3K-selective caRas adenovirus (Y40C), or vector control. Shown are (A) Percent apoptotic osteoclasts and (B) Total Osteoclasts.
To further examine functions of PI3K and Raf downstream of Ras, pathway selective forms of caRas were utilized in combination with inhibition of downstream signaling counterparts. Expression of PI3K-selective caRas decreased osteoclast apoptosis and chemical inhibition of PI3K blocked this effect (Figure 6A). Raf inhibition blocked osteoclast survival promoted by M-CSF and expression of PI3K-selective
Figure 6.
Blocking Raf and PI3K Blocks Selective Ras-Promoted Osteoclast Survival. (A and B) Mature osteoclasts were infected with the PI3K-selecitve caRas adenovirus or vector control. Following infection osteoclasts were serum starved in the presence of the PI3K inhibitor LY294002, the Raf inhibitor GW5074 or DMSO vehicle control and treated with M-CSF. Shown are (A) Percent Apoptotic Osteoclasts and (B) Total Osteoclasts. (C and D) Mature osteoclasts were infected with the Raf-selective caRas adenovirus or vector control. Following infection osteoclasts were serum starved in the presence of the Raf inhibitor GW5074 or DMSO vehicle control and treated with M-CSF. Shown are (C) Percent Apoptotic Osteoclasts and (D) Total Osteoclasts. (E and F) Mature osteoclasts were infected with the Raf-selective caRas adenovirus or vector control. Following infection osteoclasts were serum starved in the presence of the PI3K inhibitor LY294002 or DMSO vehicle control and treated with M-CSF. Shown are (E) Percent Apoptotic Osteoclasts and (F) Total Osteoclasts.
Ras abolished this effect (Figure 6A). Moreover, Raf inhibition did not impact apoptosis repressed by PI3K-selective Ras (Figure 6A). In contrast, Raf inhibition increased apoptosis independent of Raf-selective Ras expression (Figure 6C). No changes in overall total numbers of osteoclasts were observed (Figure 6D). These data again demonstrate roles for PI3K and Raf downstream of Ras in mediating M-CSF promoted osteoclast survival.
Crosstalk from Raf-selective Ras to PI3K in M-CSF-mediated ostoeclast survival was next assayed. Once again, expression of Raf-selective Ras alone did not decrease the percentage of apoptotic osteoclasts whereas PI3K inhibition caused an increase in osteoclast apoptosis (Figure 6E). In contrast, a significant difference was observed between osteoclasts expressing Raf-selective Ras treated with the PI3K inhibitor compared to vehicle control (Figure 6E). PI3K inhibition also significantly reduced numbers of total osteoclasts with either Raf-selective Ras or vector expression (Figure 6F).
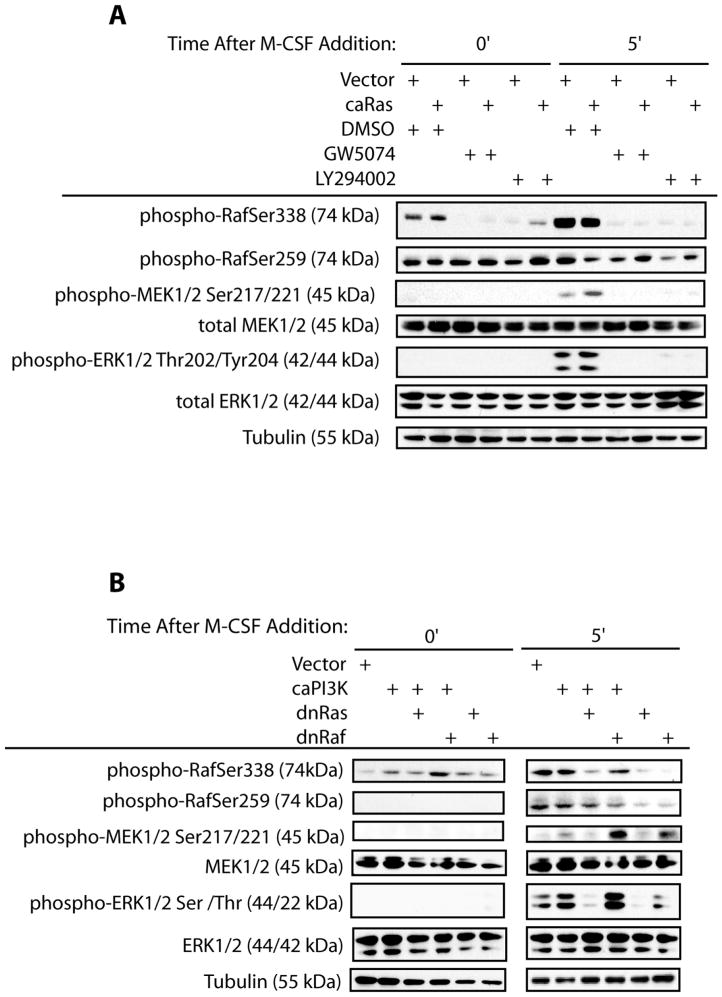
The ability of each downstream Ras effector to mediate activation of the MEK/ERK pathway downstream of M-CSF was next evaluated (Figure 7). To examine this, osteoclasts were infected with parental caRas and treated in combination with downstream signaling inhibitors. We examined phosphorylation at the activating site (Ser338) and the inactivating site (Ser259) of Raf. Expression of caRas did not affect basal phosphorylation of RafSer338 or RafSer259. Raf inhibition diminished phosphorylation at Ser338 in both vector and caRas infected osteoclasts, but did not affect Raf phosphorylation at Ser259. PI3K inhibition abolished phosphorylation at Ser338, but did not affect Raf phosphorylation at Ser259 prior to M-CSF addition. Basal phosphorylation of MEK/ERK was not evident with any treatment. M-CSF-induced pathway activation was next examined. Although RafSer338 phosphorylation was not increased by expression of caRas as compared to vector infection, expression of caRas decreased phospho-RafSer259 levels as compared to vector infection following M-CSF treatment. Phosphorylation of RafSer338 induced by M-CSF was blocked by chemical inhibition of Raf and PI3K in both vector and caRas infected cells (Figure 7A). Chemical inhibition of both Raf and PI3K decreased levels of phospho-RafSer259 in vector-infected cells whereas levels slightly increased in caRas infected cells. Expression of caRas increased MEK1/2 activation induced by M-CSF as compared to vector infection, whereas inhibition of Raf or PI3K blocked both M-CSF-induced and caRas-promoted MEK1/2 activation. M-CSF-induced ERK1/2 activation was likewise increased by expression of caRas as compared to vector control. Inhibition of Raf or PI3K decreased ERK1/2 activation as compared to either caRas or vector control infection following M-CSF treatment. These data again support role for PI3K and Raf downstream of Ras.
Figure 7.
Blocking Ras- and PI3K-Promoted MEK/ERK Activation. (A) Inhibition of downstream signaling components blocks Ras-mediated pathway activation. Osteoclasts were infected with the caRas adenovirus of vector control. Following infection, osteoclasts were serum starved and treated with M-CSF in the presence of the PI3K inhibitor LY294002, the Raf inhibitor GW5074 or DMSO vehicle control. Western blotting for phospho-MEK1/2Ser217/221, total-MEK1/2, phospho-ERK1/2Thr202/Tyr204, total-ERK1/2, phospho-RafSer338, phospho-RafSer259, and tubulin was performed. (B) Inhibition of Raf does not block PI3K-mediated pathway activation. Osteoclasts were infected with the caPI3K, dnRaf, dnRas, caPI3K and dnRaf, caPI3K and dnRas or vector control adenoviruses. Following infection, osteoclasts were serum starved and treated with M-CSF as indicated. Levels of phospho-MEK1/2Ser217/221, total-MEK1/2, phospho-ERK1/2Thr202/Tyr204, total-ERK1/2, phospho-RafSer338, phospho-RafSer259, and tubulin were then assessed via western blotting.
The reciprocal experiment was performed to determine the ability of caPI3K to promote pathway activation in the presence of downstream inhibitors. Expression of caPI3K increased basal but did not enhance Raf phosphorylation above the levels observed with M-CSF treatment (Figure 7B). Co-expression of caPI3K and dnRas abrogated the increase in basal phospho-RafSer338 promoted by caPI3K expression alone. In addition, co-expression of caPI3K and dnRas also decreased M-CSF-induced phosphorylation of RafSer338 (Figure 7B). In contrast, co-expression of caPI3K and dnRaf increased basal levels of phospho-RafSer338 and did not change levels of M-CSF-induced phospho-RafSer338 as compared to caPI3K expression alone or vector control (Figure 7B). Expression of either dnRas or dnRaf did not alter basal phosphorylation of RafSer338 but decreased levels of phospho-RafSer338 induced by M-CSF (Figure 7B). Basal phosphorylation of RafSer259 was not observed with any treatment (Figure 7B). Phospho-RafSer259 increased following M-CSF treatment, which was unaffected by expression of caPI3K, either alone or in combination with dnRas expression (Figure 7B). Phosphorylation of RafSer259 was decreased by co-expression of dnRaf with caPI3K, or by expression of dnRas or dnRaf alone following M-CSF addition (Figure 7B). M-CSF-induced phosphorylation of MEK/ERK was increased by caPI3K expression as compared to vector infection, whereas basal phosphorylation was not affected (Figure 7B). Co-expression of dnRas decreased MEK/ERK activation promoted by caPI3K whereas co-expression of dnRaf increased MEK/ERK activation (Figure 7B). Expression of either dnRas or dnRaf alone decreased MEK/ERK activation (Figure 7B). These data suggest that although PI3K and Raf both function downstream of Ras, PI3K and Raf may function in parallel.
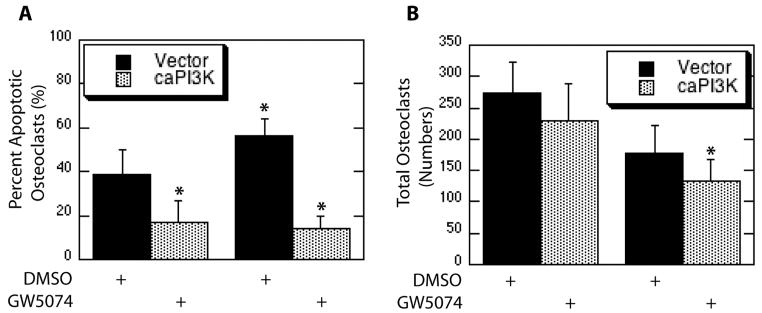
Since Raf inhibition by GW5074 did not abolish pathway activation promoted by expression of caPI3K, the ability of caPI3K to repress osteoclast apoptosis with Raf inhibition was also determined. Expression of caPI3K decreased osteoclast apoptosis (Figure 8A). Raf inhibition increased the percentage of apoptotic osteoclasts and this effect was blocked by expression of caPI3K (Figure 8A). Significant changes in numbers of total osteoclasts were not observed with caPI3K expression or Raf inhibition alone, although decreased numbers of total osteoclasts were observed in caPI3K infected cells combined with Raf inhibition (Figure 8B). These data further solidify the parallel roles for PI3K and Raf.
Figure 8.
Blocking Raf Does Not Abolish PI3K-Promoted Osteoclast Survival. Mature osteoclasts were infected with the Raf-selective caPI3K adenovirus or vector control. Following infection osteoclasts were serum starved in the presence of the Raf inhibitor GW5074 or DMSO vehicle control and treated with M-CSF. Shown are (A) Percent Apoptotic Osteoclasts and (B) Total Osteoclasts.
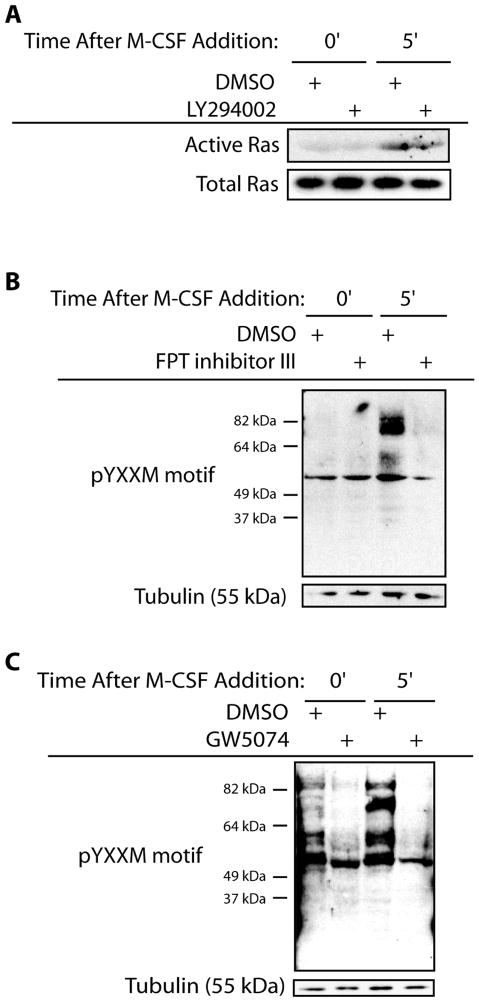
Pathway selective forms of Ras increased osteoclast survival and influenced MEK/ERK activation, supporting that PI3K activity is downstream of Ras. Therefore the ability of Ras to activate PI3K was next evaluated. M-CSF treatment caused increased Ras activation which was not abolished through PI3K inhibition (Figure 9A). Ras inhibition decreased phosphorylation of tyrosine residues within the YXXM motif following M-CSF addition supporting that Ras inhibition decreased PI3K activation (Figure 9B). The ability of Raf to modulate PI3K activation was also determined. Inhibition of Raf by GW5074 decreased levels of phospho-tyrosine residues within the YXXM motif (Figure 9C). Thus, PI3K and Raf function in linear and separate pathways downstream of Ras to mediate osteoclast survival promoted by M-CSF-induced MEK/ERK activation.
Figure 9.
PI3K Functions Downstream of Ras. (A) Inhibition of PI3K does not affect Ras activation. Mature osteoclasts were serum starved and treated with M-CSF in the presence of the PI3K inhibitor LY294002 or vehicle control. Cell lysates were then assayed for levels of GTP-bound Ras through interaction with the Ras-binding domain of Raf. Western blotting for Ras was then performed from the isolated samples. Levels for total Ras from unfractionated samples were also assessed via western blotting. (B) Inhibition of Ras decreases PI3K activation. Mature osteoclasts were pre-treated with a farnesyl transferase inhibitor to block Ras activation or DMSO vehicle control for seven hours. Cells were serum starved and treated with M-CSF as indicated in the presence of inhibitor or DMSO vehicle control. Western blotting for phospho-tyrosine residues within the YXXM motif was then performed along with western blotting for levels of tubulin. (C) Inhibition of Raf reduces PI3K activation. Mature osteoclasts were serum starved and treated with M-CSF as indicated in the presence of the Raf inhibitor GW5074 or DMSO vehicle control. Western blotting for phospho-tyrosine residues within the YXXM motif was then performed along with western blotting for levels of tubulin.
Discussion
M-CSF promotes survival of cells within the monocyte-macrophage lineage including bone-resorbing osteoclasts. As M-CSF acts through activation of the MEK/ERK pathway to induce pro-survival responses, the signal transduction events connecting M-CSF to activation of the MEK/ERK pathway were evaluated. M-CSF activates two signal transduction pathways, the MEK/ERK and the Akt pathways. We have examined the mechanism by which M-CSF activates MEK/ERK to promote osteoclast survival. Blocking Ras, Raf and PI3K blocks M-CSF-induced activation of the MEK/ERK pathway. In addition, inhibition of any of these pathway components abolishes M-CSF-conferred repression of osteoclast apoptosis. Given these data, specific interactions between the Ras/Raf and PI3K pathways in promoting osteoclast survival dependent on MEK/ERK activation induced by M-CSF were evaluated.
Since Ras has been reported to promote activation of PI3K and PI3K also modulates activity of the Ras/Raf pathway, crosstalk between these two signal transduction pathways was examined. We first assessed the functional interaction between Ras and PI3K activation. Blocking Ras blocked PI3K activity, whereas the reciprocal was not evident in that inhibition of PI3K did not affect Ras activation induced by M-CSF. PI3K therefore functions downstream of M-CSF-mediated Ras activation. These data suggested that Ras coordinated downstream signaling components, including PI3K, in response to M-CSF. These data highlight a central role for Ras in coordination of osteoclast survival which is also supported by studies showing that blocking Ras activation reduces osteoclast differentiation and increases apoptosis of pre-osteoclasts [Coxon et al., 2000; Luckman et al., 1998].
Our data demonstrate that PI3K functions downstream of Ras. Although caPI3K expression promoted MEK/ERK activation above basal activation, expression of caPI3K was not able to restore pathway activation blocked through expression of dnRas. This result contradicts a functional role for PI3K downstream of Ras. However, this observation could be due to decreased expression of c-fms via expression of dnRas, thus blocking the effects of M-CSF required for full activation promoted by caPI3K [Hume et al., 1997]. Additionally, this effect could illustrate additional required Ras targets. These possibilities are further strengthened by inability of PI3K to modulate Ras activation.
As further demonstration of coordinate pathway activation by Ras, the ability of caRas to bypass inhibition of downstream signaling components was also assayed. Expression of caRas was not able to restore M-CSF promoted osteoclast survival blocked following inhibition of PI3K or Raf, suggesting that PI3K and Raf function downstream of Ras. In addition, while expression of caRas promoted pathway activation, this effect was blocked through inhibition of PI3K or Raf. Additional support for roles of PI3K and Raf downstream of Ras were indicated by the use of pathway selective forms of Ras. However, Ras-mediated signaling through PI3K repressed osteoclast apoptosis more effectively than Ras-mediated signaling through Raf. These findings may indicate that although Raf plays a role in attenuating osteoclast apoptosis, PI3K may activate additional pro-survival pathways outside of the MEK/ERK pathway, consistent with the pleiotropic function of PI3K.
M-CSF has been reported to promote activation of the MEK/ERK pathway via multiple mechanisms depending on the cell type in question. In NIH3T3 cells expressing a mutant c-fms receptor unable to couple M-CSF to Ras activation, M-CSF still activates downstream components of the PI3K signal transduction pathway [Kelley et al., 1999]. While these results imply a function for PI3K independent of Ras, bone marrow macrophages expressing a mutant c-fms receptor lacking a PI3K-binding site are able to activate MEK/ERK activation none the less in a PI3K-dependent fashion [Lee and States, 2000]. Thus, functions of PI3K downstream of Ras may be required for signal transduction mediated by M-CSF. We find this to be true in osteoclast M-CSF-mediated MEK/ERK activation. In addition, other factors outside of Ras and PI3K are required for M-CSF-mediated activation of the MEK/ERK pathway in that expression of either caRas or caPI3K did not increase MEK/ERK activation in the absence of M-CSF.
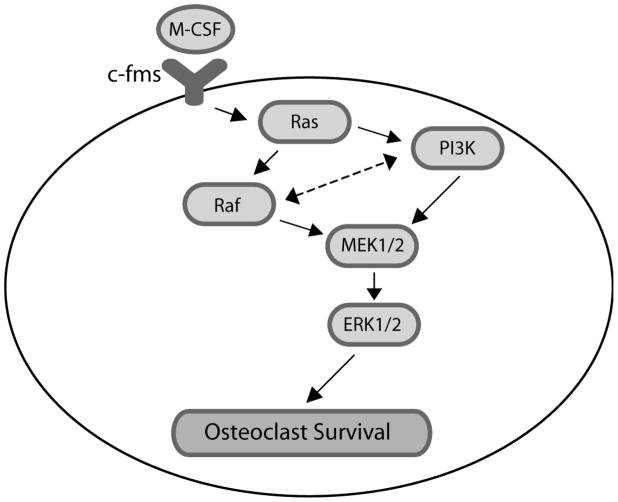
We also examined potential interactions between PI3K and Raf. Blocking PI3K decreased both activating site and inhibitory site phosphorylation of Raf, implying potential PI3K-mediated Raf regulation. As regulation of Raf activation is complex and mediated by multiple phosphorylation events, this possibility was further evaluated. Expression of caPI3K bypassed inhibition of Raf to promote M-CSF-mediated osteoclast survival as well as MEK/ERK activation induced by M-CSF. Blocking Raf also decreased PI3K activation with a corresponding decrease in MEK/ERK activation induced by M-CSF. These results suggest a role for Raf in regulation of PI3K. Raf-mediated PI3K regulation may be direct or mediated through additional components, such as feedback to Ras-mediated PI3K activation. Inhibition of PI3K also did not promote activation of the MEK/ERK pathway through an Akt-mediated decrease RafSer259 phosphorylation, again demonstrating positive and separate functions for PI3K and Raf in mediating MEK/ERK activation and M-CSF-promoted osteoclast survival. These findings document two separate mechanisms for M-CSF mediated MEK/ERK activation downstream of Ras activation. As shown in Figure 10, our data indicate that Ras acts to coordinately activate PI3K and Raf, which then act in parallel to mediate MEK/ERK activation.
Figure 10.
Model of signaling pathway.
In studies by Wennstrom and Downward, basal and not cytokine-stimulated PI3K activity promoted Ras/MEK/ERK activation in response to low levels of EGF [Wennstrom and Downward, 1999]. In the study reported here, we describe functions for both Ras- and PI3K-dependent activation of the MEK/ERK pathway in response to M-CSF, and show that Ras activation modulates PI3K function with a clear delineation in that signaling promoted by PI3K does not utilize Raf to activate MEK/ERK in response to M-CSF. This result correlates well with given the results shown by Bucher et al. demonstrating that Raf alone does not mediate activation of MEK/ERK in response to M-CSF in macrophages [Buscher et al., 1995].
Osteoclast survival has been previously shown to be dependent on both the Ras/Raf and PI3K/Akt pathways [Gingery et al., 2003; Glantschnig et al., 2003; Sato et al., 2004]. While considerable crosstalk between these pathways has been observed in other cell types, the interaction between these two signaling pathways in promotion of M-CSF-mediated osteoclast survival has not been addressed. Here we show an interaction between PI3K and Ras function in promotion of osteoclast survival upstream of activation of the MEK/ERK pathway. Collectively, these findings show that Ras functions as a mediator of M-CSF signaling, inducing activation of two signal transduction pathways culminating in MEK/ERK activation and suppression of osteoclast apoptosis.
Acknowledgments
Contract grant sponsor: Dr. Merry Jo Oursler; Contract grant number: DE14680
Support for this work was provided by the NIH grant R01 DE14680 and The Mayo Foundation. We thank Dr. Beth Lee for the gift of the GST RANKL expression construct. We also thank Drs. Patricia Collin-Osdoby and Philip Osdoby for their advice on the GST RANKL purification.
References
- Akiyama T, Miyazaki T, Bouillet P, Nakamura K, Strasser A, Tanaka S. In vitro and in vivo assays for osteoclast apoptosis. Biol Proced Online. 2005;7:48–59. doi: 10.1251/bpo105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi DR, Saito Y, Campbell DG, Cohen P, Sithanandam G, Rapp U, Ashworth A, Marshall CJ, Cowley S. Identification of the sites in MAP kinase kinase-1 phosphorylated by p74raf-1. Embo J. 1994;13:1610–9. doi: 10.1002/j.1460-2075.1994.tb06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai F, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K, Miyata T, Anderson DM, Suda T. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J Exp Med. 1999;190:1741–54. doi: 10.1084/jem.190.12.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscher D, Hipskind RA, Krautwald S, Reimann T, Baccarini M. Ras-dependent and -independent pathways target the mitogen-activated protein kinase network in macrophages. Mol Cell Biol. 1995;15:466–75. doi: 10.1128/mcb.15.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni JM, Yan N, Cox AD, Bustelo X, Graham SM, Lynch MJ, Weinmann R, Seizinger BR, Der CJ, Barbacid M, et al. Farnesyltransferase inhibitors are inhibitors of Ras but not R-Ras2/TC21, transformation. Oncogene. 1995;10:1905–13. [PubMed] [Google Scholar]
- Chong H, Lee J, Guan KL. Positive and negative regulation of Raf kinase activity and function by phosphorylation. Embo J. 2001;20:3716–27. doi: 10.1093/emboj/20.14.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox AD, Hisaka MM, Buss JE, Der CJ. Specific isoprenoid modification is required for function of normal, but not oncogenic, Ras protein. Mol Cell Biol. 1992;12:2606–15. doi: 10.1128/mcb.12.6.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coxon FP, Helfrich MH, Van’t Hof R, Sebti S, Ralston SH, Hamilton A, Rogers MJ. Protein geranylgeranylation is required for osteoclast formation, function, and survival: inhibition by bisphosphonates and GGTI-298. J Bone Miner Res. 2000;15:1467–76. doi: 10.1359/jbmr.2000.15.8.1467. [DOI] [PubMed] [Google Scholar]
- Dougherty MK, Muller J, Ritt DA, Zhou M, Zhou XZ, Copeland TD, Conrads TP, Veenstra TD, Lu KP, Morrison DK. Regulation of Raf-1 by direct feedback phosphorylation. Mol Cell. 2005;17:215–24. doi: 10.1016/j.molcel.2004.11.055. [DOI] [PubMed] [Google Scholar]
- Edme N, Downward J, Thiery JP, Boyer B. Ras induces NBT-II epithelial cell scattering through the coordinate activities of Rac and MAPK pathways. J Cell Sci. 2002;115:2591–601. doi: 10.1242/jcs.115.12.2591. [DOI] [PubMed] [Google Scholar]
- Fuller K, Owens JM, Jagger CJ, Wilson A, Moss R, Chambers TJ. Macrophage colony-stimulating factor stimulates survival and chemotactic behavior in isolated osteoclasts. J Exp Med. 1993;178:1733–44. doi: 10.1084/jem.178.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingery A, Bradley E, Shaw A, Oursler MJ. Phosphatidylinositol 3-kinase coordinately activates the MEK/ERK and AKT/NFkappaB pathways to maintain osteoclast survival. J Cell Biochem. 2003;89:165–79. doi: 10.1002/jcb.10503. [DOI] [PubMed] [Google Scholar]
- Glantschnig H, Fisher JE, Wesolowski G, Rodan GA, Reszka AA. M-CSF, TNFalpha and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ. 2003;10:1165–77. doi: 10.1038/sj.cdd.4401285. [DOI] [PubMed] [Google Scholar]
- Hume DA, Yue X, Ross IL, Favot P, Lichanska A, Ostrowski MC. Regulation of CSF-1 receptor expression. Mol Reprod Dev. 1997;46:46–52. doi: 10.1002/(SICI)1098-2795(199701)46:1<46::AID-MRD8>3.0.CO;2-R. discussion 52–3. [DOI] [PubMed] [Google Scholar]
- Jaworowski A, Christy E, Yusoff P, Byrne R, Hamilton JA. Differences in the kinetics of activation of protein kinases and extracellular signal-related protein kinase 1 in colony-stimulating factor 1-stimulated and lipopolysaccharide-stimulated macrophages. Biochem J. 1996;320(Pt 3):1011–6. doi: 10.1042/bj3201011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley TW, Graham MM, Doseff AI, Pomerantz RW, Lau SM, Ostrowski MC, Franke TF, Marsh CB. Macrophage colony-stimulating factor promotes cell survival through Akt/protein kinase B. J Biol Chem. 1999;274:26393–8. doi: 10.1074/jbc.274.37.26393. [DOI] [PubMed] [Google Scholar]
- Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351(Pt 2):289–305. [PMC free article] [PubMed] [Google Scholar]
- Lee AW, States DJ. Both src-dependent and -independent mechanisms mediate phosphatidylinositol 3-kinase regulation of colony-stimulating factor 1-activated mitogen-activated protein kinases in myeloid progenitors. Mol Cell Biol. 2000;20:6779–98. doi: 10.1128/mcb.20.18.6779-6798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AW, States DJ. Colony-stimulating factor-1 requires PI3-kinase-mediated metabolism for proliferation and survival in myeloid cells. Cell Death Differ. 2006;13:1900–14. doi: 10.1038/sj.cdd.4401884. [DOI] [PubMed] [Google Scholar]
- Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13:581–9. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- Marais R, Light Y, Paterson HF, Mason CS, Marshall CJ. Differential regulation of Raf-1, A-Raf, and B-Raf by oncogenic ras and tyrosine kinases. J Biol Chem. 1997;272:4378–83. doi: 10.1074/jbc.272.7.4378. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Katagiri H, Kanegae Y, Takayanagi H, Sawada Y, Yamamoto A, Pando MP, Asano T, Verma IM, Oda H, Nakamura K, Tanaka S. Reciprocal role of ERK and NF-kappaB pathways in survival and activation of osteoclasts. J Cell Biol. 2000;148:333–42. doi: 10.1083/jcb.148.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyssonnaux C, Eychene A. The Raf/MEK/ERK pathway: new concepts of activation. Biol Cell. 2001;93:53–62. doi: 10.1016/s0248-4900(01)01125-x. [DOI] [PubMed] [Google Scholar]
- Ponzetto C, Bardelli A, Maina F, Longati P, Panayotou G, Dhand R, Waterfield MD, Comoglio PM. A novel recognition motif for phosphatidylinositol 3-kinase binding mediates its association with the hepatocyte growth factor/scatter factor receptor. Mol Cell Biol. 1993;13:4600–8. doi: 10.1128/mcb.13.8.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–14. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- Sato S, Fujita N, Tsuruo T. Involvement of 3-phosphoinositide-dependent protein kinase-1 in the MEK/MAPK signal transduction pathway. J Biol Chem. 2004;279:33759–67. doi: 10.1074/jbc.M402055200. [DOI] [PubMed] [Google Scholar]
- Sells Galvin RJ, Gatlin CL, Horn JW, Fuson TR. TGF-beta enhances osteoclast differentiation in hematopoietic cell cultures stimulated with RANKL and M-CSF. Biochem Biophys Res Commun. 1999;265:233–9. doi: 10.1006/bbrc.1999.1632. [DOI] [PubMed] [Google Scholar]
- Stoyanov B, Volinia S, Hanck T, Rubio I, Loubtchenkov M, Malek D, Stoyanova S, Vanhaesebroeck B, Dhand R, Nurnberg B, et al. Cloning and characterization of a G protein-activated human phosphoinositide-3 kinase. Science. 1995;269:690–3. doi: 10.1126/science.7624799. [DOI] [PubMed] [Google Scholar]
- Sun H, King AJ, Diaz HB, Marshall MS. Regulation of the protein kinase Raf-1 by oncogenic Ras through phosphatidylinositol 3-kinase, Cdc42/Rac and Pak. Curr Biol. 2000;10:281–4. doi: 10.1016/s0960-9822(00)00359-6. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Akatsu T, Udagawa N, Sasaki T, Yamaguchi A, Moseley JM, Martin TJ, Suda T. Osteoblastic cells are involved in osteoclast formation. Endocrinology. 1988;123:2600–2. doi: 10.1210/endo-123-5-2600. [DOI] [PubMed] [Google Scholar]
- Udagawa N, Takahashi N, Akatsu T, Tanaka H, Sasaki T, Nishihara T, Koga T, Martin TJ, Suda T. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci U S A. 1990;87:7260–4. doi: 10.1073/pnas.87.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennstrom S, Downward J. Role of phosphoinositide 3-kinase in activation of ras and mitogen-activated protein kinase by epidermal growth factor. Mol Cell Biol. 1999;19:4279–88. doi: 10.1128/mcb.19.6.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MA, Nicolette C, Minden A, Polverino A, Van Aelst L, Karin M, Wigler MH. Multiple Ras functions can contribute to mammalian cell transformation. Cell. 1995;80:533–41. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- Yang FC, Chen S, Robling AG, Yu X, Nebesio TD, Yan J, Morgan T, Li X, Yuan J, Hock J, Ingram DA, Clapp DW. Hyperactivation of p21ras and PI3K cooperate to alter murine and human neurofibromatosis type 1-haploinsufficient osteoclast functions. J Clin Invest. 2006;116:2880–91. doi: 10.1172/JCI29092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang M, Hayne C, Luo Z. Interaction between active Pak1 and Raf-1 is necessary for phosphorylation and activation of Raf-1. J Biol Chem. 2002;277:4395–405. doi: 10.1074/jbc.M110000200. [DOI] [PubMed] [Google Scholar]
- Zang M, Waelde CA, Xiang X, Rana A, Wen R, Luo Z. Microtubule integrity regulates Pak leading to Ras-independent activation of Raf-1. insights into mechanisms of Raf-1 activation. J Biol Chem. 2001;276:25157–65. doi: 10.1074/jbc.M100152200. [DOI] [PubMed] [Google Scholar]
- Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B) Science. 1999;286:1741–4. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]