Abstract
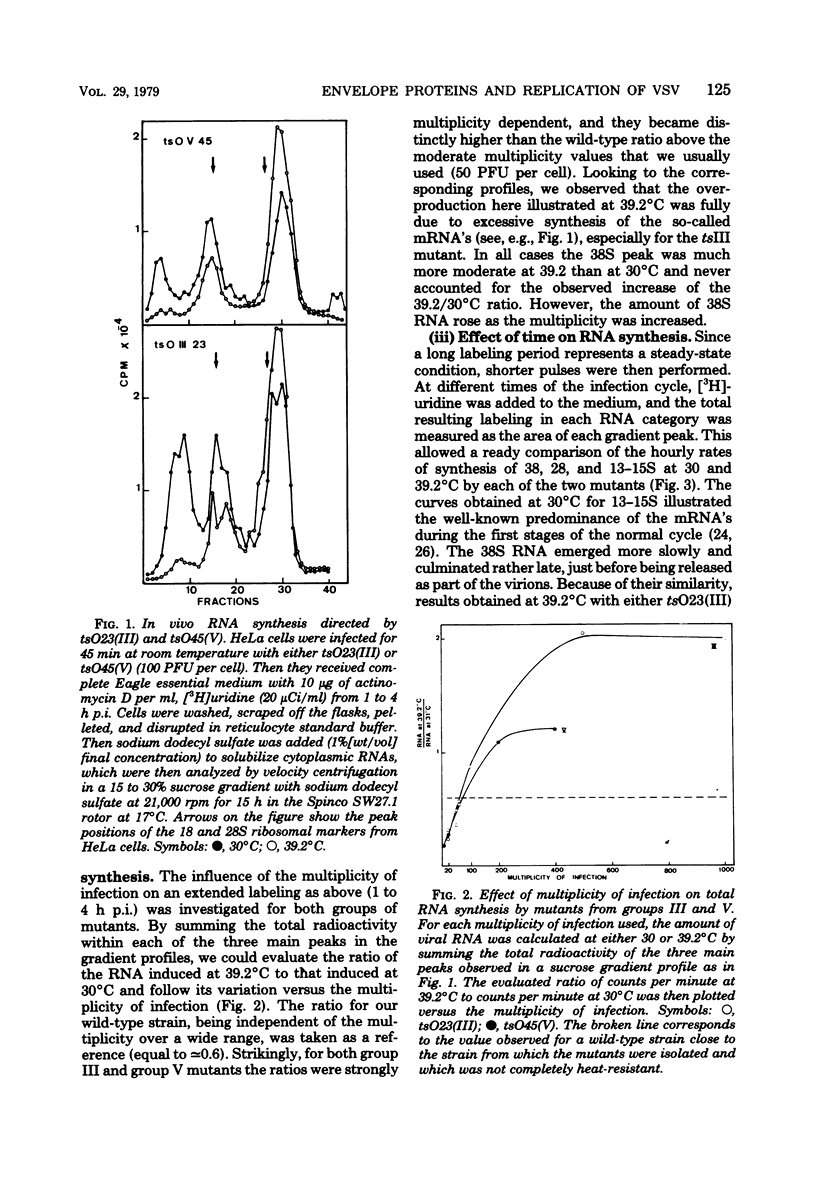
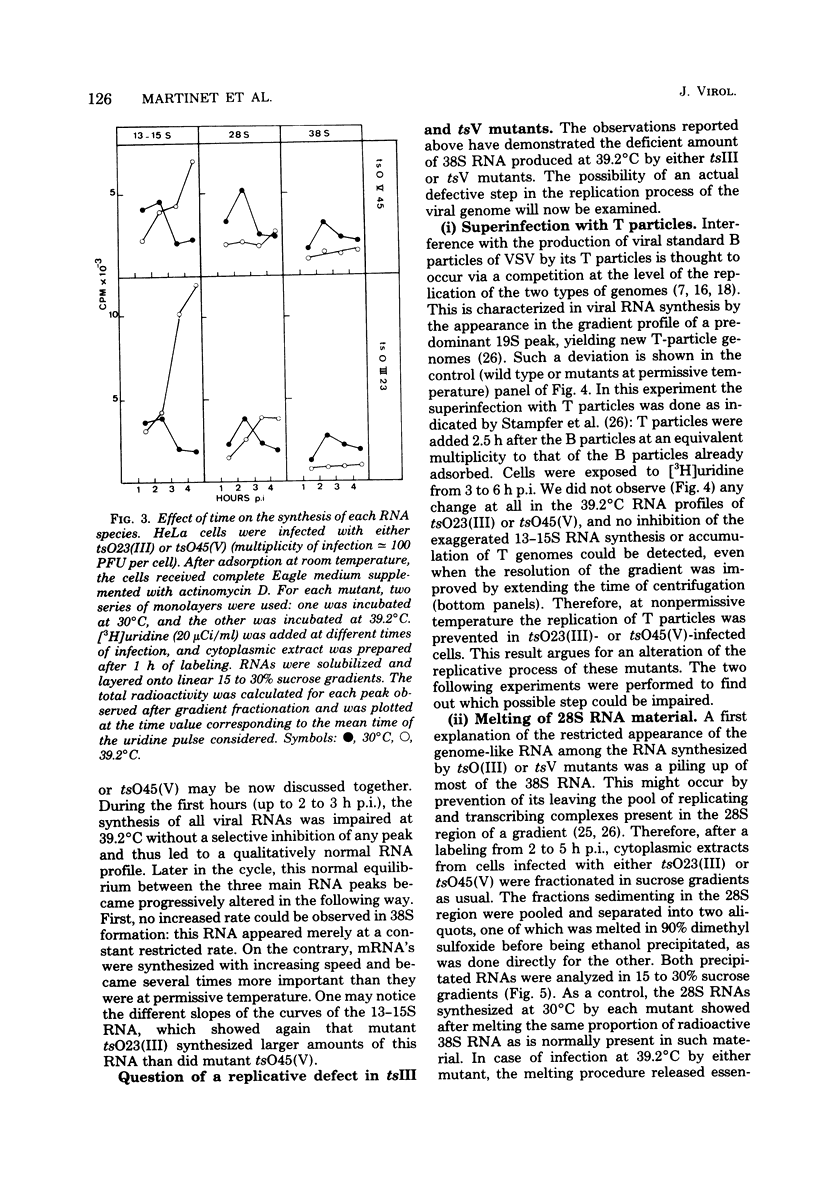
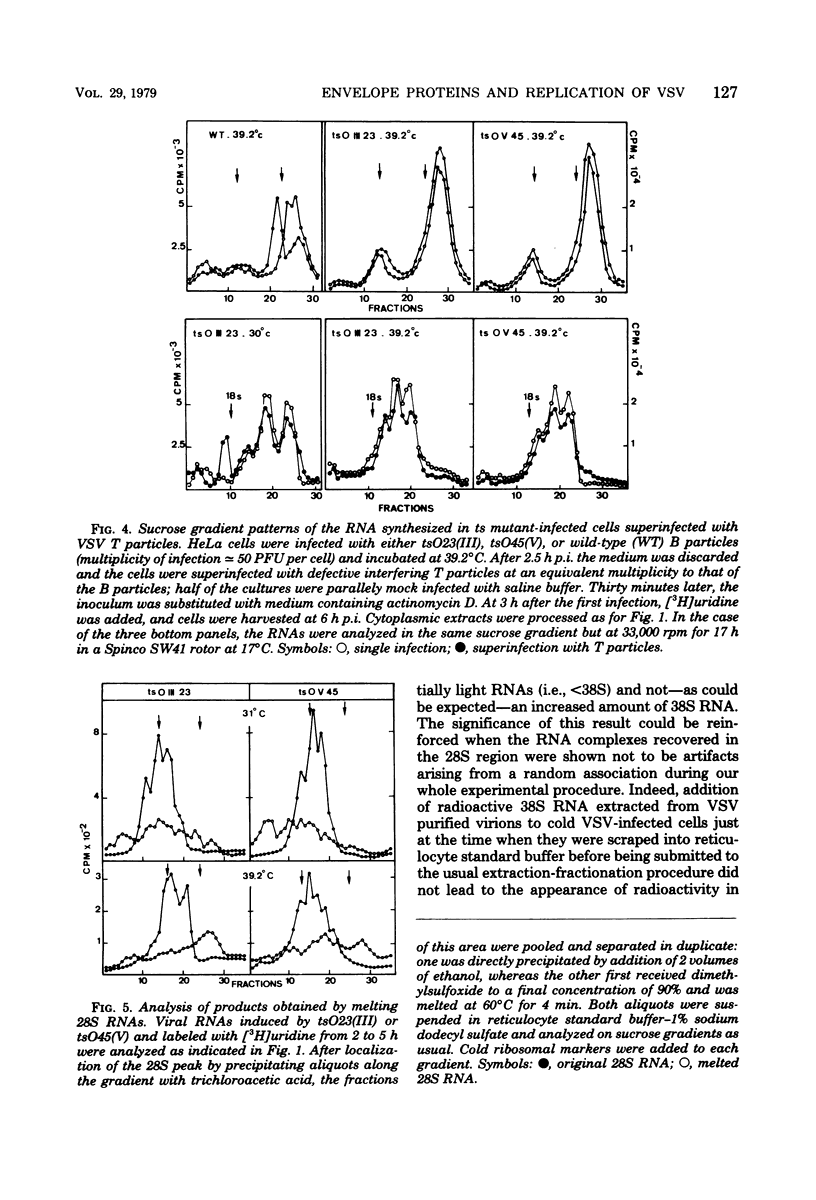
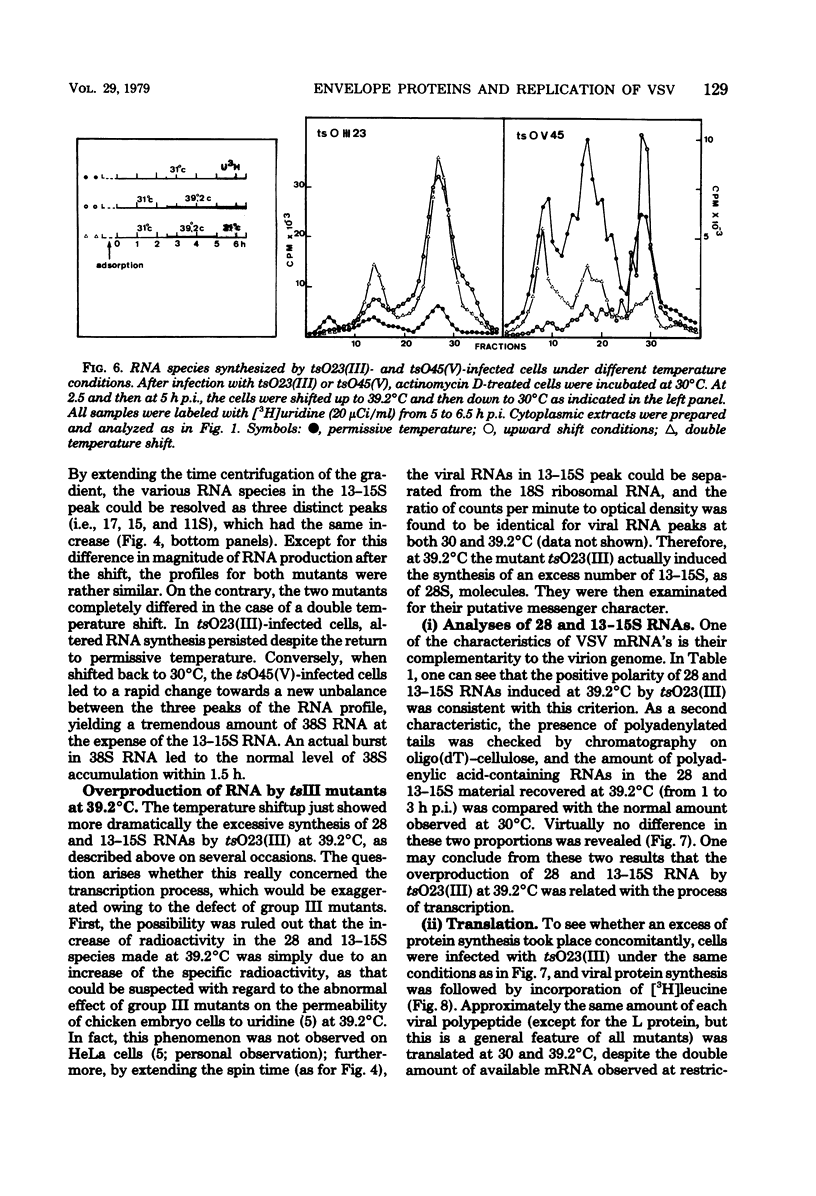
Temperature-sensitive (ts) mutants of vesicular stomatitis virus belonging to complementation groups III and V were investigated for their in vivo RNA synthesis. The sucrose gradient patterns of the RNA species which they produced at nonpermissive temperature (39.2 degrees C) were systematically compared under different experimental conditions: variation of input multiplicity and of time of infection, superinfection with T particles, and temperature shifts. Finally, a more precise analysis of the various RNA species synthesized was carried out. It appeared that the characteristics of RNA synthesis specified at 39.2 degrees C by tsIII or tsV mutants differed from the normal RNA synthesis of vesicular stomatitis virus wild type. Their common depression at 39.2 degrees C in virion-like RNA (38S) production--i.e., so-called genome replication--was tentatively paralleled with the concomitant ts events which have been previously shown to affect the two viral envelope proteins. An overproduction of the RNA transcripts was described for mutants in group III and posed the question of a regulation process to determine the amount of RNA to be transcribed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Both G. W., Banerjee A. K., Shatkin A. J. Methylation-dependent translation of viral messenger RNAs in vitro. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1189–1193. doi: 10.1073/pnas.72.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combard A., Martinet C., Printz Ane C., Friedman A., Printz P. Transcription and replication of vesicular stomatitis virus: effects of temperature-sensitive mutations in complementation group IV. J Virol. 1974 Apr;13(4):922–930. doi: 10.1128/jvi.13.4.922-930.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combard A., Printz-Ane C., Martinet C., Printz P. Temperature-sensitive defect of vesicular stomatitis virus in complementation group II. J Virol. 1977 Mar;21(3):913–923. doi: 10.1128/jvi.21.3.913-923.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch V. Parental G protein reincorporation by a vesicular stomatitis virus temperature-sensitive mutant of complementation group V at nonpermissive temperature. Virology. 1976 Feb;69(2):607–616. doi: 10.1016/0042-6822(76)90489-x. [DOI] [PubMed] [Google Scholar]
- Genty N. Analysis of uridine incorporation in chicken embryo cells infected by vesicular stomatitis virus and its temperature-sensitive mutants: uridine transport. J Virol. 1975 Jan;15(1):8–15. doi: 10.1128/jvi.15.1.8-15.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus. 3. Multiple complementary messenger RNA molecules. Virology. 1970 Dec;42(4):946–957. doi: 10.1016/0042-6822(70)90343-0. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Manders E. K. Ribonucleic acid synthesis of vesicular stomatitis virus. IV. Transcription by standard virus in the presence of defective interfering particles. J Virol. 1972 Jun;9(6):909–916. doi: 10.1128/jvi.9.6.909-916.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Lodish H. F., Baltimore D. Localization of two cellular forms of the vesicular stomatitis viral glycoprotein. J Virol. 1977 Mar;21(3):1121–1127. doi: 10.1128/jvi.21.3.1121-1127.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D., Lodish H. F., Baltimore D. Analysis of the defects of temperature-sensitive mutants of vesicular stomatitis virus: intracellular degradation of specific viral proteins. J Virol. 1977 Mar;21(3):1140–1148. doi: 10.1128/jvi.21.3.1140-1148.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafay F., Berkaloff A. Etude des mutants thermosensibles du virus de la stomatite vésiculaire (VSV). Mutants de maturation. C R Acad Sci Hebd Seances Acad Sci D. 1969 Sep 15;269(11):1031–1034. [PubMed] [Google Scholar]
- Lafay F. Envelope proteins of vesicular stomatitis virus: effect of temperature-sensitive mutations in complementation groups III and V. J Virol. 1974 Nov;14(5):1220–1228. doi: 10.1128/jvi.14.5.1220-1228.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafay F. Etude des fonctions du virus de la stomatite vésiculaire altérées par une mutation thermosensible: mise en evidence dela protéine structurale affectée par la mutation ts 23. J Gen Virol. 1971 Dec;13(3):449–453. doi: 10.1099/0022-1317-13-3-449. [DOI] [PubMed] [Google Scholar]
- Lafay F. Etude des mutants thermosensibles du Virus de la Stomatite Vésiculaire (VSV). Classification de quelques mutants d'après des critères de fonctionnement. C R Acad Sci Hebd Seances Acad Sci D. 1969 May 12;268(19):2385–2388. [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D., Gurdon J. B., Partington G. A. Protein synthesis in oocytes of Xenopus laevis is not regulated by the supply of messenger RNA. Cell. 1977 Jun;11(2):345–351. doi: 10.1016/0092-8674(77)90051-4. [DOI] [PubMed] [Google Scholar]
- Marx P. A., Portner A., Kingsbury D. W. Sendai virion transcriptase complex: polyeptide composition and inhibition by virion envelope proteins. J Virol. 1974 Jan;13(1):107–112. doi: 10.1128/jvi.13.1.107-112.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma E. L., Perlman S. M., Huang A. S. Ribonucleic acid synthesis of vesicular stomatitis virus. VI. Correlation of defective particle RNA synthesis with standard RNA replication. J Mol Biol. 1974 May 5;85(1):127–136. doi: 10.1016/0022-2836(74)90133-8. [DOI] [PubMed] [Google Scholar]
- Perlman S. M., Huang A. S. RNA synthesis of vesicular stomatitis virus. V. Interactions between transcription and replication. J Virol. 1973 Dec;12(6):1395–1400. doi: 10.1128/jvi.12.6.1395-1400.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault J., Holland J. J. Absence of transcriptase activity or transcription-inhibiting ability in defective interfering particles of vesicular stomatitis virus. Virology. 1972 Oct;50(1):150–170. [PubMed] [Google Scholar]
- Perrault J., Kingsbury D. T. Inhibitor of vesicular stomatitis virus transcriptase in purified virions. Nature. 1974 Mar 1;248(5443):45–47. doi: 10.1038/248045a0. [DOI] [PubMed] [Google Scholar]
- Pringle C. R., Duncan I. B. Preliminary physiological characterization of temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1971 Jul;8(1):56–61. doi: 10.1128/jvi.8.1.56-61.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Printz-Ané C., Combard A., Martinet C. Study of the transcription and the replication of vesicular stomatitis virus by using temperature-sensitive mutants. J Virol. 1972 Nov;10(5):889–895. doi: 10.1128/jvi.10.5.889-895.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Printz P., Wagner R. R. Temperature-sensitive mutants of vesicular stomatitis virus: synthesis of virus-specific proteins. J Virol. 1971 May;7(5):651–662. doi: 10.1128/jvi.7.5.651-662.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Lodish H. F. Synchronised transmembrane insertion and glycosylation of a nascent membrane protein. Nature. 1977 Oct 27;269(5631):775–780. doi: 10.1038/269775a0. [DOI] [PubMed] [Google Scholar]
- Schincariol A. L., Howatson A. F. Replication of vesicular stomatitis virus. I. Viral specific RNA and nucleoprotein in infected L cells. Virology. 1970 Nov;42(3):732–743. doi: 10.1016/0042-6822(70)90319-3. [DOI] [PubMed] [Google Scholar]
- Schincariol A. L., Howatson A. F. Replication of vesicular stomatitis virus. II. Separation and characterization of virus-specific RNA species. Virology. 1972 Sep;49(3):766–783. doi: 10.1016/0042-6822(72)90533-8. [DOI] [PubMed] [Google Scholar]
- Stampfer M., Baltimore D., Huang A. S. Ribonucleic acid synthesis of vesicular stomatitis virus. I. Species of ribonucleic acid found in Chinese hamster ovary cells infected with plaque-forming and defective particles. J Virol. 1969 Aug;4(2):154–161. doi: 10.1128/jvi.4.2.154-161.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilágyi J. F., Uryvayev L. Isolation of an infectious ribonucleoprotein from vesicular stomatitis virus containing an active RNA transcriptase. J Virol. 1973 Feb;11(2):279–286. doi: 10.1128/jvi.11.2.279-286.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toneguzzo F., Ghosh H. P. Characterization and translation of methylated and unmethylated vesicular stomatitis virus mRNA synthesized in vitro by ribonucleoprotein particles from vesicular stomatitis virus-infected L cells. J Virol. 1976 Feb;17(2):477–491. doi: 10.1128/jvi.17.2.477-491.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger J. T., Reichmann M. E. RNA synthesis in temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1973 Sep;12(3):570–578. doi: 10.1128/jvi.12.3.570-578.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz G. W., Levine M. RNA synthesis by vesicular stomatitis virus and a small plaque mutant: effects of cycloheximide. J Virol. 1973 Aug;12(2):253–264. doi: 10.1128/jvi.12.2.253-264.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild T. F. Replication of vesicular stomatitis virus: characterization of the virus-induced RNA. J Gen Virol. 1971 Nov;13(2):295–310. doi: 10.1099/0022-1317-13-2-295. [DOI] [PubMed] [Google Scholar]
- Wunner W. H., Pringle C. R. Protein synthesis in BHK21 cells infected with vesicular stomatitis virus. I. ts Mutants of the Indiana serotype. Virology. 1972 Apr;48(1):104–111. doi: 10.1016/0042-6822(72)90118-3. [DOI] [PubMed] [Google Scholar]
- Závada J. Pseudotypes of vesicular stomatitis virus with the coat of murine leukaemia and of avian myeloblastosis viruses. J Gen Virol. 1972 Jun;15(3):183–191. doi: 10.1099/0022-1317-15-3-183. [DOI] [PubMed] [Google Scholar]



