Abstract
Purpose
The Quantitative Assay Database (QuAD), http://proteome.moffitt.org/QUAD/, facilitates widespread implementation of quantitative mass spectrometry in cancer biology and clinical research through sharing of methods and reagents for monitoring protein expression and modification.
Experimental Design
Liquid chromatography coupled to multiple reaction monitoring mass spectrometry (LC-MRM) assays are developed using SDS-PAGE fractionated lysates from cancer cell lines. Pathway maps created using GeneGO Metacore provide the biological relationships between proteins and illustrate concepts for multiplexed analysis; each protein can be selected to examine assay development at the protein and peptide level.
Results
The coupling of SDS-PAGE and LC-MRM screening has been used to detect 876 peptides from 218 cancer-related proteins in model systems including colon, lung, melanoma, leukemias, and myeloma, which has led to the development of 95 quantitative assays including stable-isotope labeled peptide standards. Methods are published online and peptide standards are made available to the research community. Protein expression measurements for heat shock proteins, including a comparison with ELISA and monitoring response to the HSP90 inhibitor, 17-DMAG, are used to illustrate the components of the QuAD and its potential utility.
Conclusions and Clinical Relevance
This resource enables quantitative assessment of protein components of signaling pathways and biological processes and holds promise for systematic investigation of treatment responses in cancer.
Keywords: Cancer Biology, LC-MRM, Pathways, Quantification, Signaling
1. Introduction
Quantitative proteomics is coming of age with rapid advances in liquid chromatography coupled to selected reaction monitoring mass spectrometry; the assessment of panels of analytes has been termed multiple reaction monitoring (LC-MRM). This capability may soon enable the measurement of thousands of proteins in a single LC-MRM analysis, enabling rapid biomarker profiling, assessment, and selection using large clinical cohorts, bridging previous gaps in discovery and clinical assay development.1 Prior literature has focused on panels of specific biomarkers, typically for detection in plasma.2–4 Strategies have also been developed that use shotgun sequencing for protein discovery prior to development of targeted quantitative assays for peptides that are differentially detected.5,6 Proteome-wide initiatives are currently underway, including translation of shotgun sequencing data into quantitative assays,7,8 broad scale assessment of MRM transitions using triggered tandem mass spectra to develop peptide targets for quantification of a significant portion of the yeast proteome,9 and plans for a Human Proteome Detection and Quantitation Project (hPDQ).10 In addition, the National Cancer Institute’s Clinical Proteomics Technology Assessment for Cancer Program has placed emphasis on standardization of these assays in the same plasma samples with comparisons of performance on different instrument platforms at multiple sites.11 This technology holds great promise for elucidation of cancer biology and patient assessment; LC-MRM is being adopted into many clinical and translational research programs.12,13 However, LC-MRM assay design, development and methods of distributing reagents remain a critical bottleneck for widespread implementation, particularly for investigators interested in current cancer drug targets and signaling pathways or biological processes linked to tumorigenesis and disease progression.
Hypothesis-driven experiments targeting specific signaling proteins, low abundance components of biological processes, or multiple components of key biological pathways are often difficult to develop, because these proteins are not typically detected in broad scale proteomics. However, affinity capture of selected proteins prior to liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) peptide sequencing has proven to be a powerful tool for basic science paradigms in cancer biology because detailed sequence characterization enables the localization of specific biologically relevant molecular changes (e.g., mutations or post-translational modifications). Conversion of these data for LC-MRM implementation can likewise revolutionize our approaches to studying disease, enabling systems biology or integrative biology approaches that are focused on relevant signaling pathways13 or biological processes, such as adhesion, apoptosis, metastasis, and proliferation.14 Furthermore, analysis of these targets coincides with current therapeutic modalities that inhibit the function of specific molecules. The goal of the Quantitative Assay Database (QuAD, available at http://proteome.moffitt.org/QUAD) is both to provide a framework for investigators to share assays and to enable multiplexed measurements for exploring hypotheses in systems biology. Quantitative assays are developed with SDS-PAGE coupled to LC-MRM (GeLC-MRM); this platform has been chosen based on prior successful implementation in the absolute quantification methodology15–18 and the emergence of SDS-PAGE coupled with LC-MS/MS (as in GeLC-MS/MS as well as more complex fractionation strategies) for proteome cataloging and analysis.19–24 Using the methods described in the QuAD or their own analytical protocols, investigators can use these developed assays to probe multiple pathways and biological processes in a single analysis with LC-MRM.
Because of their facile translation from cell line models to clinical samples, LC-MRM assays may be the optimal method for translation from the bench to the bedside and vice versa, enabling testing of basic science hypotheses in patient specimens. As an ongoing part of this project, assays are systematically developed to quantify expression and biologically relevant post-translational modifications. The QuAD has been constructed to provide a resource for the analysis of proteins implicated in tumorigenesis and cancer progression; it presents the results of these efforts to date, including pathway interaction maps, protein and peptide level information for MRM design, methods for protein fractionation, transitions for targeted detection, and stable-isotope labeled peptide standards (which are made available in a cost recovery model). Examples of biological measurements have been provided, which include a comparison with enzyme linked immunosorbent assays (ELISA) in measuring heat shock protein 90α (HSP90α) as well as relative and absolute quantification of the changes in the expression of heat shock proteins in multiple myeloma cells after treatment with the HSP90 inhibitor, 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin (17-DMAG).
2. Materials and Methods
2.1 Reagents
Chemicals are purchased from Sigma-Aldrich (St. Louis, MO) at their highest available purity unless otherwise noted. HPLC solvents (water and acetonitrile) are supplied by Burdick and Jackson (Honeywell, Muskegon, MI). Stable-isotope labeled FMOC amino acids were purchased from Cambridge Isotope Laboratories (Andover, MA).
2.2 Cell Culture, Lysis, and Sample Preparation for Mass Spectrometry
Cell lines are obtained from American Type Culture Collection (ATCC, Manassas, VA) and cultured as directed. Typical conditions are RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, with incubation in a 5% CO2 atmosphere at 37 °C. Cell lines are included in Table 1.
Table 1. Selected Pathways and Processes Targeted For Quantitative Assay Development.
Each cancer-related pathway or process is presented with its model cell line(s) used for generating protein for the LC-MRM screening and initial assay implementation. At least one assay has been developed or is currently under investigation to quantify the expression of each protein. Subsequent to the development of assays for expression, additional attention is paid to quantification of isoforms, truncations, mutations, and post-translational modifications of selected proteins.
| Pathway/Process | Target Proteins, Peptides, (Assays) | Cancer | Cell Line(s) used for Development |
|---|---|---|---|
| Heat Shock Proteins | 10, 36 (8) | Acute Myeloid Leukemia, Myeloma | U937, RPMI8226 |
| BCR-Abl Signaling | 7, 24 (11) | Chronic Myelogenous Leukemia | K562 |
| Wnt Signaling | 23, 87 (17) | Colon | HT29, HCT116, KM12, KM12C, KM12SM, KM12L4A, SW480, SW620 |
| Notch Signaling | 23, 37 (0) | ||
| TGF/SMAD/BMP Signaling | 22, 44 (0) | ||
| Receptor Tyrosine Kinases (e.g., EGFR)/Substrates | 23, 135 (9) | Lung, Melanoma, Colon, Breast | HCC827, H292, WM3670, WM3629, WM3130, HCT116, SKBR3, inter al. |
| Ras/Raf/Mek/Erk Signaling | 11, 74 (0) | Melanoma | WM3670, WM3629, WM3130, inter al. |
| PI3K/AKT/MTOR Signaling | 5, 45 (0) | ||
| Cyclins/Cyclin-dependent Kinases | 4, 23 (0) | ||
| Apoptosis (Bcl-2 Family) | 18, 30 (14) | Multiple Myeloma | RPMI8226, 8226/LR5 |
| BRCA/Fanconi Anemia DNA Damage Response | 13, 90 (13) | ||
| NFκB Signaling | 3, 25 (8) | ||
| Other | 59, 229 (23) | Myeloma, Breast, Bladder, Sarcoma, Melanoma, inter al. | Various Cells, Serum, Plasma, Urine |
The detection of 100 attomoles of yeast alcohol dehydrogenase I (ADH1_YEAST) against a background of lysate from RPMI-8226 myeloma cells is used to establish the sensitivity as well as the variability of quantification of low amounts of protein from biological samples. Triplicate samples of 500 attomoles of ADH1 is spiked into lysate prepared from 105 cells from the RPMI-8226 multiple myeloma line (~10 micrograms of total protein). This number of cells was selected because it is at least two-fold higher than our experiments usually use. Proteins are fractionated with SDS-PAGE prior to gel excision (based on the migration of higher amounts of the standard protein in adjacent lanes), reduction, alkylation, trypsin digestion, and LC-MRM analysis of 20% of the sample (100 attomoles injected). A control of RPMI-8226 cell lysate without ADH1 analyzed in parallel to enable detection of interference. Each LC-MRM analysis was repeated three times. Two peptides were used to measure the amount of the protein: ANELLINVK (m/z 507.3), and DIVGAVLK (m/z 407.8). Each peptide was quantified using an internal standard, which contained a stable-isotope label: ANELLINV(13C515N1)K (m/z 510.3) and DIVGAVL(13C615N1)K (m/z 411.3).
For LC-MRM screens, cells are lysed in aqueous 8M urea/100mM ammonium bicarbonate buffer on ice or aqueous 50 mM Tris-HCl pH 7.4, 0.1% NP-40, 1M NaCl, protease inhibitor cocktail (Roche), 25 mM NaF, 2 mM Na3VO4, and 0.1M Na2HPO4. After lysis, the cell supernatants are clarified by centrifugation and decanted. The equivalent of 25,000 to 100,000 cells or 2.5 to 10 micrograms (μg) of protein is loaded for SDS-PAGE separations, using 10% or 4–12% Criterion XT Bis-Tris gels, which is then visualized with colloidal Coomassie BrilliantBlue G-250 (Bio-Rad, Hercules, CA).
Because the bands corresponding to the endogenous proteins of interest are not specifically visualized (as they would be in immunoprecipitates), gel regions containing proteins of interest are excised using the molecular weight markers as the first guide and, if necessary, by banding patterns observed for the whole cell lysate. For LC-MRM screens, the MW markers are used exclusively and adjacent gel regions are also screened for the endogenous protein of interest. Proteins are reduced with 2 mM tris-carboxyethylphosphine (TCEP) and alkylated with 20 mM iodoacetamide (IAA) prior to in-gel digestion with sequencing grade trypsin (Promega, Madison, WI). Following digestion at 37°C overnight, samples are concentrated by vacuum centrifugation. The peptides are resuspended in 2% acetonitrile with 0.1% formic acid (LC solvent A) for mass analysis.
2.3 Peptide and Transition Selection for LC-MRM Screening
The peptides and transitions are predicted by SRM Builder,7 now called Pinpoint (Thermo, San Jose, CA) or Skyline.25 Doubly-protonated molecules corresponding to all peptides between 7 and 25 amino acids in length are probed; cysteine and methionine containing peptides are excluded unless there are few other choices (n < 5). In addition, each peptide is reviewed using the existing literature and online databases to examine whether tandem mass spectrometry data is available and if the sequence contains sites of mutation or post-translational modification. Protein isoforms are also examined to determine peptides that are common in all sequences and to identify peptides that are unique to each isoform. Then, all y fragment ions for each peptide starting from y3 or yx > peptide m/z and ending with y(n-1) are monitored in the initial screens. Methods are developed for Xcalibur 2.0 SR2 and TSQ Quantum 1.4 software versions; no scheduling is used for LC-MRM screening. To date, this strategy has not been limiting. For high molecular weight proteins, one experiment is used to monitor the best candidates; for lower molecular weight proteins with fewer peptide candidates, the screening is multiplexed. Therefore, each screen contains less than 140 transitions (conservatively 28 peptides with 5 fragment ions each) at 20 milliseconds sampling and still obtain at least 7 points in each peak. Because the peptides are limited to those expected to be optimal for LC-MRM based on length, amino acid content, and flanking sequences, scheduling or multiple screens for any protein have not been necessary.
2.4 Liquid Chromatography coupled to Multiple Reaction Monitoring
A nanoflow liquid chromatograph (U3000, Dionex, Sunnyvale, CA or EasynLC, Proxeon, Denmark) is coupled with the triple quadrupole mass spectrometer (TSQ Quantum Ultra, Thermo, San Jose, CA). Aliquots of each sample are loaded onto a C18 reversed-phase trap column and washed for 20 minutes, prior to switching in line with C18 analytical column (PepMap100, Dionex, Sunnyvale, CA) with 75 μm inner diameter, 15 cm length, 3 μm particle size, and 100 Å pore size. Peptides are eluted at 300 nL/min using a 35 minute gradient from 5% B to 50% B. The solvent system is composed of aqueous 2% acetonitrile with 0.1% formic acid (A) and aqueous 90% acetonitrile with 0.1% formic acid (B). LC-MRM is performed with 2,500 V nanoelectrospray from 10 μm tips (New Objective, Woburn, MA) with 200 °C transfer tube temperature, and 12 V skimmer offset. The Q1 setting is m/z 0.2 or m/z 0.4 in width, and Q3 is set to filter m/z 0.7 in width. Fragmentation is achieved with 1.5 mTorr argon. Each transition is monitored for 20 milliseconds. Consistent coelution of transitions and ratios of signal intensity are used to verify peptide candidates; these data are compared with LC-MS/MS data, when available. Currently, one peptide standard is synthesized for quantification of each protein’s expression, but all peptides detected in these screens can be monitored in biological experiments. Additional peptides are being synthesized to improve the quantification of expression and enable the assessment of post-translational modifications.
2.5 Peptide Synthesis and Evaluation
Solid state peptide synthesis (Symphony, Protein Technologies, Tucson, AZ) is used to make standards at the 25 micromole scale using standard FMOC chemistry. Purified peptides are analyzed with MALDI MS to verify coupling efficiency and stable-isotope incorporation level (as specified by Cambridge Isotope Laboratories) and sequenced with MS/MS (4700, Applied Biosystems, Framingham, MA). Peptides in 2% ACN with 0.1% formic acid are mixed 1:1 with α-cyano-4-hydroxycinnamic acid (10 mg/ml) in 50% ACN and deposited in 1 μl aliquots on the MALDI target. Peptide standards are quantified by amino acid analysis using 6N HCl-phenol hydrolysis; amino acids are derivatized by FMOC and OPA (to label both primary and secondary amino acids) and then detected after reversed-phase HPLC separation (AminoQuant, Hewlett Packard).
2.6 Verification of Coelution and Transition Patterns Comparing Endogenous and Standard Peptides
Biological samples are prepared from cell lysates as described above. Stable-isotope labeled internal standards are added to the samples after in-gel digestion, but prior to vacuum centrifugation, for triplicate LC-MRM analyses. The coelution of the peptide standard and the endogenous peptide of interest and the similarity of their composite tandem mass spectra are used to verify the identity of the peptide and its utility in the assay.
2.7 Website Development for the Quantitative Assay Database
QuAD is a web application developed using JavaServer Pages (JSP) deployed on an Apache Tomcat 6.0 application server. Links are provided to other online resources, including SwissProt/UniProt, IPI, and NCBI protein databases as well as Peptide Atlas26 and PhosphoSite (http://www.phosphosite.org/homeAction.do). Summary statistics for target proteins, detected peptides, and completed assays is kept on the Index and Statistics page. Users can search for proteins of interest by selecting different pathways or processes, typing in the UniProt accession number (e.g., HS90A_HUMAN) in the Search dialog box, clicking the protein name on the Index page, or clicking on the protein symbol in maps of each signaling pathway or biological process.
Within the database (http://proteome.moffitt.org/QUAD/), information is shown at three levels starting at the whole pathway or biological process, then focusing on each individual protein, and its representative peptides. Pathway maps are created with MapEditor in MetaCore from GeneGO Inc. Relevant proteins with entries in the database are shown with a key listing their icon, name, and UniProt Accession number. On the protein pages, the name, accession number, and sequence are shown with a description of relevant separation methods, typically SDS-PAGE, example tandem mass spectra from LC-MS/MS, and the results of the initial LC-MRM screening. From the protein sequence, the user is linked to peptides that were successfully detected; each of these has its own page including the sequence, position in the protein, tables of peptide m/z values, fragment m/z values, isoelectric point (pI), and the peptide synthesis report for the internal standard (when applicable). Additional steps of the assay workup, including tandem mass spectra acquired on the triple quadrupole mass spectrometer, optimization of selected transitions, calibration curves of the peptide standard, are presented along with the example verifying the match between endogenous and standard peptides, which displays the ion chromatograms for the biological peptide and its internal standard as well as their composite tandem mass spectra (an illustration of the data from all monitored transitions). These data then verify the utility of the peptide standard in SDS-PAGE coupled to LC-MRM and serve as a checkpoint for amino acid analysis and further assay characterization.
2.8 Comparison of Absolute Quantification by ELISA and LC-MRM
Enzyme linked immunosorbent assays (ELISA) were performed in 96-well plates for expression of HSP90α according to the manufacturer’s instructions (EKS-895, Assay Designs-Stressgen, Ann Arbor, MI). This sandwich assay uses horseradish peroxidase modification of the tetramethylbenzidine substrate for colorimetric monitoring at 450 nm using a microplate reader (Versa Max, Molecular Devices, Sunnyvale, CA). Serial dilutions of the provided protein standard were analyzed with ELISA to make a standard curve. In addition, aliquots of the standard were denatured and digested in solution prior to LC-MRM quantification to create a calibration curve for the peptide-based assay. Then, the amounts of HSP90α were measured in RPMI-8226 multiple myeloma cells (n = 500 to 50,000 or 50 ng to 5 μg of protein) using both methods. For ELISA, cells were lysed in the buffer provided by the kit. For LC-MRM, cells were lysed in 8 M urea with 30 mM ammonium bicarbonate. After reduction with TCEP and alkylation with iodoacetamide, the clarified lysate was diluted tenfold and digested with trypsin. LC-MRM analysis was performed for the peptide, ALLFVPR, using Q1 to select peptides with a width of m/z 0.4 and Q3 filtering fragment ions with a width of m/z 0.7. Quantification is performed using the stable-isotope labeled peptide, ALLFVP(13C515N1)R, spiked in at a known concentration. Other instrument parameters are identical to those described above.
2.9 Monitoring Heat Shock Proteins in Multiple Myeloma Cells
RPMI-8226 multiple myeloma cells were cultured in suspension as described above with the addition of 100 units/ml penicillin/streptomycin. Stock 17-dimethylaminoethylamino-17-demethoxy-geldanamycin (17-DMAG) was dissolved in DMSO at a concentration of 10 μM. Based on prior IC50 measurements, the RPMI-8226 cells were treated with 106 nM 17-DMAG for 24 hours.
RPMI-8226 cells (n = 106) were lysed using 50 μL aqueous 8 M urea with 100 mM ammonium bicarbonate. Protein concentration was measured by Bradford assay and 0.38μg of protein (equivalent to 3,750 cells) was injected for LC-MRM analysis of control and drug treated samples. The proteins were reduced with 2 mM TCEP, alkylated with 20 mM IAA, and digested overnight in solution with trypsin at 37°C. Corresponding stable-isotope labeled internal standard peptides were added to the samples after digestion. Then, peptides were extracted using pipette tips packed with C18 reversed-phase resin (Ziptip, Millipore) and concentrated to dryness by vacuum centrifugation (Speedvac, Thermo), and resuspended in aqueous 2% acetonitrile with 0.1% formic acid prior to mass spectrometry. LC-MRM was performed as described above. Quantification is achieved by using the sum of the peak areas for all detected transitions using QuanBrowser (Xcalibur, Thermo, San Jose, CA) or Skyline. The ratio of peak area of the endogenous peptide over corresponding internal standard was calculated for each peptide in the sample to enable absolute and relative quantification. Relative protein expression after treatment is normalized to the pretreatment control for each cell line and plotted to show the change in expression after drug treatment. Bubble plots were created using SigmaPlot 10 (SPSS).
3. Results and Discussion
3.1 Target Protein Selection
A list of representative signaling pathways and biological processes targeted for assay development are given in Table 1. These clusters of interacting proteins were chosen due to relevance to numerous cancer models;14 frequently, these individual proteins do not have high quality antibodies or antibody-based assays available for detection and quantification. Most importantly, these proteins represent drug targets, signaling pathways, and biological processes that are well-characterized in cancer biology; quantitative assays could be used to translate basic science hypotheses into clinical research with the ultimate goal of patient assessment.27,28 LC-MRM peptide-based assays can be used for quantifying panels of proteins, enabling multiplexed analysis of protein expression and modification across entire pathways and processes, rather than just focusing on the hubs of protein interaction and activity. This database can also indicate when proteins are interactive in multiple pathways, e.g., HSP90 proteins in heat shock response and EGFR signaling.
3.2 Strategy
The coupling of SDS-PAGE with LC-MRM was selected for numerous reasons, many of which are based on the experiments combining SDS-PAGE and mass spectrometry in protein quantification and proteome cataloging. 15–22 For assay development, lysates are prepared from cancer cell lines that are known to contain the protein of interest from prior Westerns, which can be used to predict the migration of the protein. The coupling of SDS-PAGE with LC-MRM enables cancer biologists to view this technology as a direct complement to or enhancement of Westerns. While the sensitivity of SDS-PAGE coupled to LC-MRM has been demonstrated to be sufficient for attomole level detection of proteins,17 additional data was acquired for replicates of 100 attomoles of alcohol dehydrogenase I from yeast (ADH1_YEAST) spiked into a background of lysate from RPMI-8226 multiple myeloma cells prior to SDS-PAGE. These data also illustrate that SDS-PAGE coupled with LC-MRM is a sensitive technique for protein detection. Furthermore, in-gel digestion and subsequent peptide recovery are consistent, because coefficient of variation (CV) values were 10.7% for measuring ANELLINVK and 7.1% for measuring DIVGAVLK. Furthermore, the ease of use, reproducibility, and tolerance for contamination (e.g., buffers, salts, and detergents) make SDS-PAGE a methodology that can be implemented in every lab, enabling each collaborating investigator to prepare their own samples for LC-MRM. These methods are easily disseminated to the scientific community as well. The molecular weight fractionation reduces potential interferences for selected transitions and is also useful to isolate isoforms or active/inactive proteins (e.g., NFκB and Notch).
Tryptic digests of gel bands or gel regions delineated by the molecular weight markers are then probed with LC-MRM screens to identify peptides that can be used to quantify the protein of interest, as shown in the workflow (Figure 1). LC-MRM screening of SDS-PAGE separated lysates has few false positives in determining appropriate peptides for monitoring the low-abundance, hypothesis-driven proteins targeted to date. In other words, it is rare that the retention time and fragmentation pattern of synthetic stable-isotope labeled peptides does not match the data for the endogenous peptide observed in the LC-MRM screen.
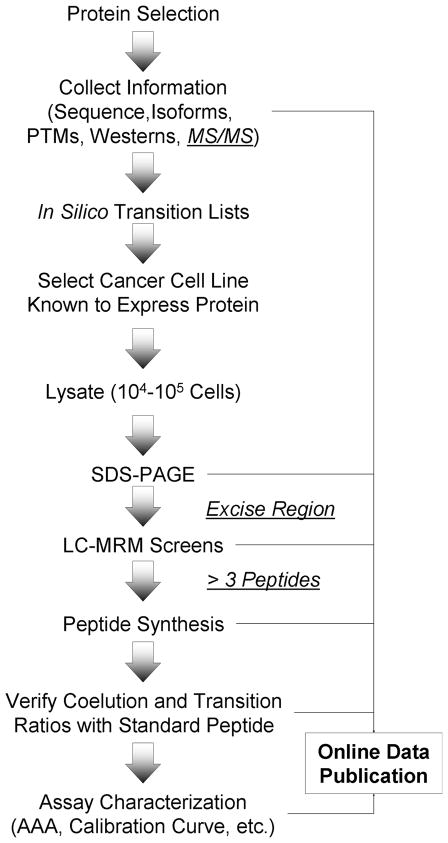
Figure 1. Workflow for LC-MRM Assay Development and Online Data Publication.
Endogenous proteins of interest are selected; information is gathered to inform assay development, which must include the protein sequence, post-translational modification sites, isoforms, and prior Western blots to inform gel excision. Tandem mass spectra are optional, typically due to a lack of availability. Transition lists are created for LC-MRM screening. Lysates are prepared from appropriate cell lines, which are known to contain the protein. The coupling of SDS-PAGE with LC-MRM screening can be used to identify peptides for quantitative assay development. Gel regions are determined by comparison to Western blots and reproducibly cut using the molecular weight markers. When more than 3 peptides are detected in the LC-MRM screens, subsequent assay development is expected to be successful. If 3 peptides are not detected, additional protein may be loaded or immunoprecipitation may be necessary to enrich the protein. After peptide synthesis, the coelution of the endogenous and synthetic peptide and the similarity of the transition ratios are used for verification as a checkpoint to further assay development. Data are published to the QuAD at each step; at the present time, LC-MRM screens are completed for 218 proteins that have been selected because of their roles in particular pathways and biological processes listed in Table 1. Synthetic stable-isotope labeled peptide standards have been created for 95 assays to enable relative and absolute quantification.
3.3 Pathway Displays
Protein interaction networks are displayed using MapEditor from GeneGO, which demonstrate the relationships between the target proteins. Components of the heat shock response and protein chaperoning process are illustrated as an example of the pathway maps in the QuAD (Figure 2A). Because of their role in folding and chaperoning proteins (particularly mutant forms), the heat shock proteins (HSPs) have emerged as promising drug targets29–31 in diverse cancer types, including multiple myeloma,32–38 leukemias,39–43 and solid tumors.44–46 Phase 1 clinical trials have recently been completed for the HSP 90 inhibitor, 17-DMAG.47–49 The ability to monitor numerous components of the chaperone network will enable the basic scientists and clinicians to systematically observe the response to HSP treatment and detect changes indicative of drug sensitivity or drug resistance.
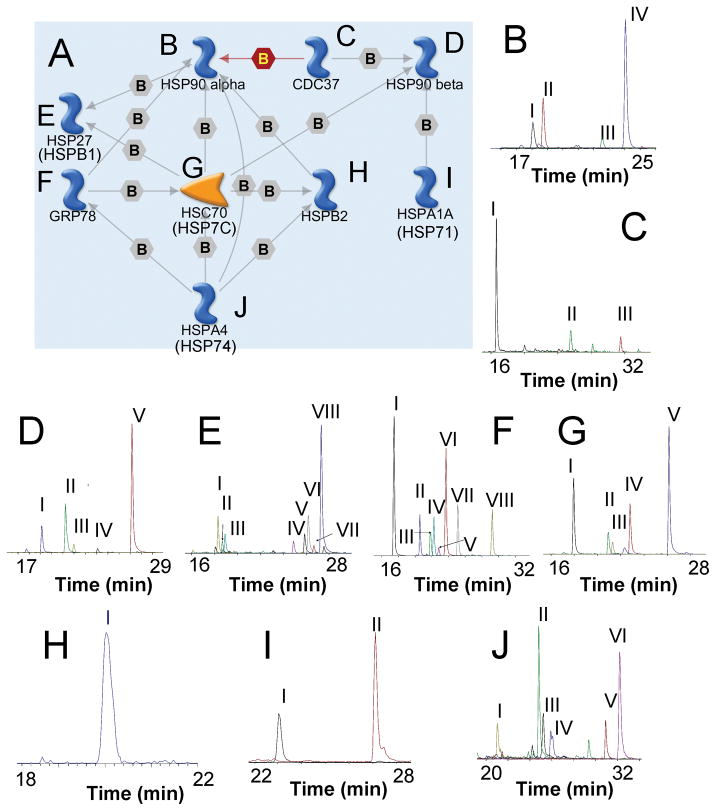
Figure 2. LC-MRM Screens for Heat Shock Proteins.
A protein interaction map created GeneGO MapEditor is used to show the biological relationships between the different components. Each arrow describes the nature of the interaction using the following codes: green for activation, red for inactivation, gray for other known associations, B for binding, P for phosphorylation, and Cn for competition. For clarity, only selected interactions are shown. LC-MRM screening data (B–K), which plot the sum of the ion signal for all transitions in a peptide against time, are displayed for each protein in this signaling network. The Roman numeral codes linking the corresponding peptide to each peak are identified in Table 2. These peptide sequences are candidates for quantitative assay development.
The main purpose of the pathway maps is to define the clusters of interacting proteins and show how multiplexed assays could be constructed and implemented to address biological hypotheses. In addition to the map, a table listing the protein icons in the pathway map with their corresponding names and UniProt accession numbers is provided at the bottom of the webpage. Each protein icon in the map is linked to the corresponding webpage with data for that protein target. Here, the results of the LC-MRM screens are shown for each protein (Figure 2B–J); peptides selected for assay development are listed in Table 2. For the initial analysis of HSPs and other protein chaperones, the following proteins were selected based on literature curation: heat shock protein 90α (HS90A_HUMAN), heat shock protein 90β (HS90B_HUMAN), heat shock protein 90β2 (H90B2_HUMAN), the inhibitory co-chaperone CDC-37 (CDC37_HUMAN), heat shock protein 27 (HSPB2_HUMAN), heat shock protein β3 (HSPB3_HUMAN), heat shock protein 70 isoform 1 (HSP71_HUMAN), heat shock protein 70 isoform 2 (HSP72_HUMAN), heat shock protein 70 isoform 4 (HSP74_HUMAN), heat shock cognate 71 (HSP7C_HUMAN), and heat shock 70 kDa protein 5 (GRP78_HUMAN). Potential peptide matches detected by the screens are reported in the online database; peptides chosen for assay development are listed in Table 2.
Table 2. Endogenous Peptides, Corresponding Internal Standards, and Selected Transitions For Quantification of Heat Shock Proteins in RPMI-8226 Multiple Myeloma Cells.
Underlined amino acid residues are stable-isotope labeled with 13C and 15N in the corresponding synthetic standards. Student’s t-tests were used for calculation of p values. Roman numerals indicate the corresponding ion signals in Figure 2, panels B–J.
| Protein (UniProt Identifier) | Endogenous Peptide | Transitions | Pre-Treatment (amol/cell) | Post-Treatment (amol/cell) | Ratio | p value |
|---|---|---|---|---|---|---|
| HS90A | ALLFVPR (IV) | y3 - y6 | 11.2 | 20.9 | 1.87 | 2.9E-5 |
| HS90B | ALLFIPR (V) | y3 - y6 | 7.90 | 14.5 | 1.84 | 4.4E-4 |
| H90B2 | HSQFLGYPITLYLEK (IV) | y3 -y12 | 16.1 | 25.3 | 1.57 | 3.2E-4 |
| CDC37 | LQAEAQQLR (I) | y4 – y7 | 1.08 | 0.91 | 1.07 | 0.038 |
| HSPB3 | ADLINNLR (I) | y3 - y7 | 0.11 | 0.14 | 1.25 | 0.026 |
| HSP71 | NQVALNPQNTVFDAK (I) | y3, y4, y9 - y12 | 0.013 | 0.051 | 3.95 | 1.2E-5 |
| HSP72 | EIAEAYLGGK (I) | y3 - y8 | 0.27 | 0.39 | 1.21 | 0.11 |
| HSP7C | GTLDPVEK (I) | y3 - y7 | 7.72 | 14.9 | 1.93 | 2.2E-6 |
| HSP74 | AFSDPFVEAEK (III) | y4, y6 - y9 | 0.73 | 0.89 | 1.23 | 1.3E-3 |
| GRP78 | VEIIANDQGNR (I) | y4 - y9 | 48.8 | 110 | 2.25 | 2.7E-4 |
3.4 Protein-Based Display for Hypothesis-Driven Targets
On each protein page, the protein name, UniProt accession number, and sequence are listed. Links are placed in the protein sequence to connect to each webpage that describes a particular peptide that passed the LC-MRM screening criteria; these links are highlighted in yellow when a quantitative assay has been developed. Sites of post-translational modification are highlighted in green, when assays for these molecular changes are under development. Selected items from the protein display are shown in Figure 3, which contains the sequence, SDS-PAGE gel image, LC-MS/MS data, and LC-MRM screen for heat shock protein 90α. A description of the methods used for protein separation is provided with an image of the results; currently, most of these illustrations are from SDS-PAGE (as in Figure 3B), but maps using monolithic reversed-phase chromatography for one-dimensional protein separation are also being generated. When available, LC-MS/MS data from previous analyses are included for each target peptide (Figure 3C) in annotated raw data or Scaffold displays (proteomesoftware.com). Finally, the LC-MRM screens, as shown in Figure 3D, illustrate all peptides that can be used to quantify the protein in the small amounts of sample injected into the mass spectrometer (0.5 to 2.5 micrograms of protein from 5,000 to 25,000 cells fractionated by SDS-PAGE). These peptides should prove to have the most robust ion signals with little interference from other molecules. For HSP90α, four peptides could be detected in the initial screen: NPDDITNEEYGEFYK, ALLFVPR, FYEQFSK, and DQVANSAFVER (assays have been developed for the underlined peptides).
Figure 3. Data Included on the Protein Web Page for Heat Shock Protein 90α (HS90A_HUMAN).
The sequence of the protein is listed (A); the sequence of each peptide that passed the initial LC-MRM screen is linked to the corresponding peptide page (as shown here in underlined blue text). The data from the protein separation, in this case the gel image (B), are shown with a box indicating the region that was excised for LC-MRM analysis. Example LC-MS/MS data are displayed for each peptide (C); here, the fragment ion spectrum obtained on a Q-TOF instrument is shown for the peptide, DQVANSAFVER. Finally, a plot of the LC-MRM screen (D) is included to enable ranking of target peptides by ion signal intensity and the selectivity of their transitions. Four peptides were detected: DQVANSAFVER (I), FYEQFSK (II), NPDDITNEEYGEFYK (III), and ALLFVPR (IV). Peptides I and IV were selected for assay development.
3.5 Peptide-Based Display for Developed Assays
In all LC-MRM screens, successfully detected peptides are ranked by the intensity of the sum of all detected transitions and the amount of interference observed in the ion chromatograms. Using this information, the highest intensity peptides are selected as candidate internal standards, which are then synthesized with stable-isotope labels.
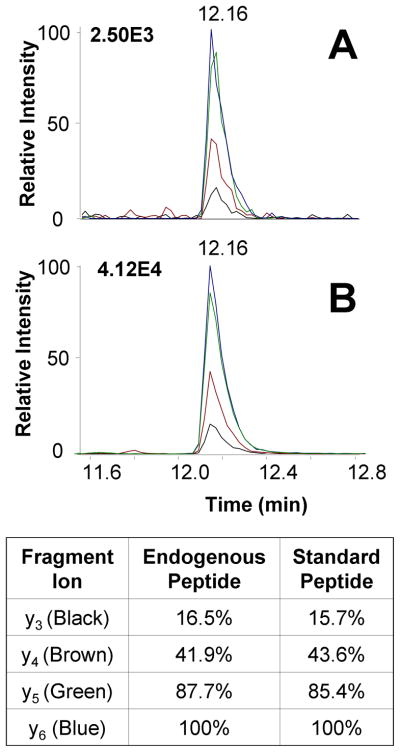
On each peptide webpage, the sequence, location in the protein (noted by amino acid residue numbers), peptide m/z values, isoelectric points, and tables of fragment ions are presented for the native and labeled synthetic sequences. Further characterization of the standard peptide is presented in the peptide synthesis report (linked in Portable Document Format), which shows the results of purification with reversed-phase HPLC, purity assessment with matrix assisted laser desorption ionization mass spectrometry (MALDI MS), and sequence verification with tandem mass spectrometry (MS/MS). Each synthetic standard is then analyzed on the triple quadrupole mass spectrometer for assay development, which includes the following steps: MS1 to identify the most prominent charge state(s), full scan MS/MS, optimization of the y ion transitions used to screen the peptide, and optimization of any additional high-intensity and high-specificity (typically high-m/z) transitions. Calibration curves are then created to assess the limit of detection and limit of quantification in order to define the sensitivity of the assay. Finally, the verification of each assay in a cell line model is displayed on the peptide page, as shown by the example in Figure 4, for Heat Shock Protein 90α. Five peptides for HSP90α passed the initial LC-MRM screen: NPDDITNEEYGEFYK, LGIHEDSQNR, DQVANSAFVER, ALLFVPR, and FYEQFSK. Of these, ALLFVPR and DQVANSAFVER were selected for synthesis due to their intensities (stable-isotope labeled amino acids are underlined in bold text). After the synthesis of the peptide standard is complete, the assay is tested in a biological sample derived from the cell line used for assay development. To verify the identity of the ion signal in the screen, LC-MRM data must match the retention time of the endogenous peptide detected during screening to the synthetic standard within 0.05 minutes (Figure 4); plots of detected transitions are created to compare the fragmentation patterns of the synthetic standard and the biological peptide further increasing confidence in its assignment (Figure 4). The transition ratios, expressed as a percentage of the base peak, should agree within 5%. In the example shown in Figure 4, all ratios for the endogenous peptide are within 2.5% of the values observed for the synthetic stable-isotope labeled standard. The co-elution of the biological peptide and internal standard as well as similar fragmentation patterns verify the accuracy of each assay and serve as a checkpoint before any further assay development is performed, including calibration curves and amino acid analysis of the synthetic peptide.
Figure 4. Example of Standard Peptide Verification for Quantification of HSP90α in RPMI-8226 Myeloma Cells.
The coelution of the biological peptide (A) and stable-isotope labeled standard (B) is detected at 12.16 minutes for this peptide, ALLFVPR. Overlay plots of the ion signals for each transition illustrate similar fragmentation of the endogenous and stable-isotope labeled peptides; the transition ratios match within 2.5%. These data are presented on the peptide web page, along with tables of peptide and fragment m/z values, breakdown curves, calibration curve, and a link to the peptide synthesis report, which includes preparative HPLC traces as well as MALDI MS and MS/MS verification of the peptide purity and sequence.
3.6 Comparison with ELISA for Absolute Quantification
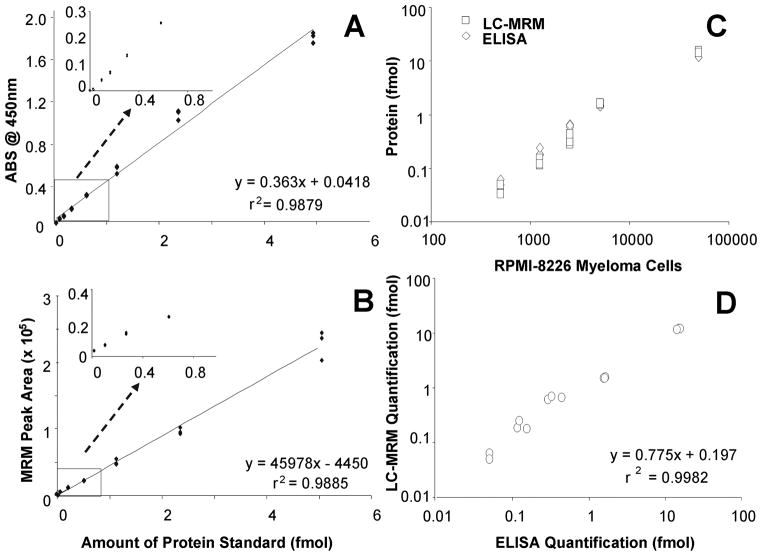
In addition to providing an example of absolute quantification, LC-MRM was compared to an existing method for protein quantification (ELISA). Calibration curves were created using serial dilutions of protein standard using both ELISA measurements of standard protein (Figure 5A) and LC-MRM monitoring of the ALLFVPR peptide (Figure 5B). The concentration of the protein standard was determined using the concentration of the heavy labeled peptide internal standard as calculated by amino acid analysis; the amount determined was higher than the specified value by a factor of 1.9. Equations and r2 values are provided for best fit lines on both plots to enable comparison and illustrate the fact that similar linear responses could be obtained for both methods across this range of protein amounts. Then, HSP90α was then measured in 500 to 50,000 multiple myeloma cells from the RPMI-8226 line using both techniques (Figure 5C). The absolute quantification by ELISA was based on the measurements of standard protein, while LC-MRM data were quantified by the use of an internal stable-isotope labeled peptide standard. Using lysates from 50,000 cells, the amounts detected by ELISA, 14.9 ± 0.7 fmol, and the value obtained by LC-MRM, 12.0 ± 0.2 fmol, were systematically offset by a factor of 0.775 (Figure 5D). The difference could be due to incomplete digestion and peptide recovery prior to LC-MRM analysis. The limits of detection and quantification were slightly better for LC-MRM when compared with ELISA. The median CV values for both sets of measurements were similar: 5.9% for LC-MRM and 10.4% for ELISA. The variability in both methods increased with decreasing sample amount: for example, the values ranged from 1.5% for 50,000 cells to 17% for 500 cells in LC-MRM. The highest CV values observed for ELISA were 22% when analyzing amounts between 500 to 2,500 cells. Using this example for illustration, the LC-MRM methods and reagents described in the QuAD can provide utility for absolute quantification that compares well with antibody-based techniques in amounts of cells expected from patient samples (e.g., bone marrow aspirates).
Figure 5. Comparison of ELISA and LC-MRM Quantification of HSP90a in RPMI-8226 Multiple Myeloma Cells.
Serial dilution curves were created using ELISA (A) and LC-MRM (B) where either absorbance at 450 nm or total peak area of all monitored transitions are plotted against the amount of protein standard analyzed. Then, the amounts of HSP90a protein expressed in RPMI-8226 cells are measured for a range of inputs between 500 and 50,000 cells using both ELISA and LC-MRM (C); here, the total amount of protein in femtomoles in the sample is plotted against the number of myeloma cells. Correlation of the ELISA and LC-MRM data is shown (D). Equations and r2 values are provided for linear fits of the data in each plot.
3.7 Implementation in Relative and Absolute Quantification of Heat Shock Proteins in Multiple Myeloma Cells and their Modulation by 17-DMAG Treatment
To illustrate the utility of these assays in monitoring response to treatment, the expression of selected heat shock proteins was measured before and 24 hours after treatment of RPMI-8226 multiple myeloma cells with 17-DMAG. Although the LC-MRM screens for assay development were carried out using SDS-PAGE for protein fractionation, these endogenous proteins are expressed at sufficient levels that they can be monitored in tryptic digests of whole cell lysate, enabling higher-throughput analysis. The endogenous and standard peptides monitored in each assay, the data for absolute quantification, and the changes in expression with treatment are reported in Table 2; p values were calculated using Student’s t-tests. Relative expression is also shown in Figure 6A using plots analogous to “dot” immunoblots or reversed-phase protein arrays,50,51 which enable rapid detection of the most significant changes. The mean and standard deviation of each measurement is plotted in bar-graph format in Figure 6B to examine the assay performance as well as the fold change in expression. Significant upregulation (p < 0.01) is noted for several heat shock proteins, including HSP90α and HSP90β as well as HSP71. Changes in the expression of HSP90α and HSP90β were not visualized in traditional Western blotting analysis of heat shock protein expression in RPMI-8226 cells treated with 17-allylamino-17-demethoxygeldanamycin (17-AAG), but could be measured as significantly different using quantitative Western blotting with an Odyssey Infrared Imaging System.34,35 Here, the precise quantification from LC-MRM enables detection of 1.5 to 1.8 fold changes in protein expression at 24 hours after treatment when compared with controls. Quantification of increases in HSP7134,35 and HSC7134 expression, which are among the compensatory mechanisms for HSP90 inhibition, were also possible using LC-MRM, which indicated modulation of expression by factors of 3.95 and 1.93, respectively. The modulations in HSP expression as measured by LC-MRM appear to be in agreement with the prior data acquired by quantitative Western blotting.34 Additional changes were observed for other chaperones, including GRP78.
Figure 6. LC-MRM Quantification of HSP Expression in RPMI-8226 Multiple Myeloma Cells Before and After Treatment with 17-DMAG.
Protein expression was measured prior to treatment (0 hours) and 24 hours after administration of 17-DMAG at its IC50. Bubble plots of protein expression enable rapid visual interpretation (A), similar to “dot” immunoblots or reversed-phase protein array data. Bar graphs are also presented (B) to illustrate the protein modulation in response to drug and to display the variability in the measurements.
To define advantages of this change in technology, these LC-MRM experiments used less than 0.5 μg of protein, whereas Western blots typically use 50 to 100 μg of protein. Furthermore, the multiplexed analysis of numerous analytes in LC-MRM enables detection of the proteins in a single sample, as opposed to aliquots of the same cell lysate and this set of assays could be further expanded to include other endogenous proteins of interest.
4. Concluding Remarks
The QuAD is an online resource enabling portability of LC-MRM method development and dispersal of the experimental designs and techniques. Because this resource is continually evolving, additional assays, peptides, proteins, and pathways will be added as the LC-MRM screens and standard peptide syntheses are completed. Furthermore, distribution of these reagents is a critical service required for each assay, because the creation of labeled peptide standards is the most expensive component of assay development, and it presents a major barrier to acceptance of LC-MRM as a widespread method for protein quantification. Synthesis of each peptide at the 25 micromole scale costs approximately $500 (USD), but produces enough material for as many as 109 assays. If each investigator requested 100 picomoles, one synthesis could supply labeled peptide standard for approximately 20,000 laboratories. Global implementation of these standards should be possible, and the cost would not be prohibitive. Therefore, reagents developed as part of this project will be made available on a cost-recovery basis. To further develop the resource, request forms are available to suggest additional targets or refinement of specific assays; data from other investigators can also be uploaded for curation and incorporation into the QuAD.
Furthermore, the sample preparation and protein separation, SDS-PAGE, can be implemented in any lab. These assays are also being examined with other separation schemes and in tryptic digests of whole cell lysate. Preliminary in silico analysis with Pinpoint7 and Skyline25 indicates that these transitions should be specific, but these programs do not typically include missed cleavages or common/artifactual post-translational modifications, like methionine oxidation. Our goal is to encourage other investigators to try these assays in their analytical workflows to generate data about their versatility and applicability in other methods and biological systems.
Quantitative interrogation of signaling networks in patient tissues using LC-MRM has the potential to impact personalized medicine by addressing critical issues in patient care including selection of therapy regimens and elucidation of drug resistance. The QuAD is an existing information and peptide reagent resource that will enable assessment of the selected signaling pathways and biological processes in minimal amounts of precious patient samples, as shown both in prior publications and here in the comparison with ELISA and in the relative and absolute quantification of HSPs following HSP90 inhibitor treatment. This capability, in turn, enables protein quantification from patient samples acquired by numerous collection methods, including fine needle aspirates or core biopsies. In addition, the resources available in the QuAD can also provide the capability to analyze cells that are only present in low numbers, such as cancer stem cells. Implementation of these assays in translational or clinical research projects (e.g., to measure biological correlates in clinical trials) will enable us to judge their utility and further gauge the potential of LC-MRM analysis in translational research.
Statement of Clinical Relevance.
This database of quantitative mass spectrometry assays has been developed to share methods and reagents for monitoring the expression of proteins that function as components in signaling pathways and biological processes relevant to cancer biology. The structure of the database also indicates possibilities for combining assays in attempts to explore systems biology: examining all of the components of the process rather than assessing only the hubs of activity as is typically done with antibody-based techniques (e.g., immunoblotting/Western). These assays can elucidate cancer biology and enable basic science hypotheses to be tested in clinical specimens for patient assessment. Because many of these endogenous proteins are drug targets, these assays have the potential to contribute in tailoring therapeutic regimens and assessing drug response and drug resistance in cancer patients.
Statement of Novelty.
The Quantitative Assay Database supports elucidation of cancer biology as well as selection and evaluation of candidate biomarkers by providing methods and reagents for assessing cancer biology in model systems with the ultimate goal of translation to patients. The potential use of selected assays is illustrated in a model of heat shock protein inhibition in multiple myeloma.
Acknowledgments
The authors would like to thank Ted Gauthier (University of South Florida Chemistry Department) for discussions on peptide synthesis, Rajesh Nair for assistance with ELISA, and Amy Koomen for editing the manuscript. Amino acid analysis is performed by Virginia Johnson and Lawrence Dangott at the Texas A&M University Protein Chemistry Laboratory.
Moffitt Proteomics is supported by the US Army Medical Research and Materiel Command under Award No. DAMD17-02-2-0051, continuing as W81XWH-08-2-0101, for a National Functional Genomics Center, the National Cancer Institute under Award No. P30-CA076292 as a Cancer Center Support Grant, and the Moffitt Foundation. The triple quadrupole mass spectrometer was purchased with a shared instrument grant from the Bankhead-Coley Cancer Research program of the Florida Department of Health (06BS-02-9614). This work has been funded by the University of Florida-Moffitt Collaborative Partnership, Moffitt’s Hematological Oncology Program, two subcontracts to EH and JK from the institutional National Functional Genomics Center awarded by the Department of Defense (see above), and the National Cancer Institute, R21CA141285-01 (JK) and RO1CA077859 (WSD). KSMS is supported by The Melanoma Research Foundation, The Bankhead-Coley Research Program of the State of Florida (09BN-14), an Institutional Research Grant from the American Cancer Society #93-032-13 and the NIH/National Cancer Institute PSOC grant U54 CA143970-01.
Abbreviations
- MRM
Multiple Reaction Monitoring
- GeLC-MRM
SDS-PAGE coupled to liquid chromatography-multiple reaction monitoring mass spectrometry
- TCEP
tris-carboxyethylphosphine
- IAA
iodoacetamide
Footnotes
Conflict of Interest Statement
The authors report no conflicts of interest.
References
- 1.Rifai N, Gillette MA, Carr SA. Protein biomarker discovery and validation: the long and uncertain path to clinical utility. Nat Biotechnol. 2006;24(8):971–83. doi: 10.1038/nbt1235. [DOI] [PubMed] [Google Scholar]
- 2.Anderson L, Hunter CL. Quantitative mass spectrometric multiple reaction monitoring assays for major plasma proteins. Mol Cell Proteomics. 2006;5(4):573–88. doi: 10.1074/mcp.M500331-MCP200. [DOI] [PubMed] [Google Scholar]
- 3.Kuzyk MA, Smith D, Yang J, Cross TJ, Jackson AM, Hardie DB, Anderson NL, Borchers CH. MRM-based, multiplexed, absolute quantitation of 45 proteins in human plasma. Mol Cell Proteomics. 2009;8(8):1860–77. doi: 10.1074/mcp.M800540-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keshishian H, Addona T, Burgess M, Kuhn E, Carr SA. Quantitative, multiplexed assays for low abundance proteins in plasma by targeted mass spectrometry and stable isotope dilution. Mol Cell Proteomics. 2007;6(12):2212–29. doi: 10.1074/mcp.M700354-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whiteaker JR, Zhang H, Zhao L, Wang P, Kelly-Spratt KS, Ivey RG, Piening BD, Feng LC, Kasarda E, Gurley KE, Eng JK, Chodosh LA, Kemp CJ, McIntosh MW, Paulovich AG. Integrated pipeline for mass spectrometry-based discovery and confirmation of biomarkers demonstrated in a mouse model of breast cancer. J Proteome Res. 2007;6(10):3962–75. doi: 10.1021/pr070202v. [DOI] [PubMed] [Google Scholar]
- 6.Yocum AK, Gratsch TE, Leff N, Strahler JR, Hunter CL, Walker AK, Michailidis G, Omenn GS, O’Shea KS, Andrews PC. Coupled global and targeted proteomics of human embryonic stem cells during induced differentiation. Mol Cell Proteomics. 2008;7(4):750–67. doi: 10.1074/mcp.M700399-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prakash A, Tomazela DM, Frewen B, Maclean B, Merrihew G, Peterman S, Maccoss MJ. Expediting the development of targeted SRM assays: using data from shotgun proteomics to automate method development. J Proteome Res. 2009;8(6):2733–9. doi: 10.1021/pr801028b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh GM, Lin S, Evans DM, Khosrovi-Eghbal A, Beavis RC, Kast J. Implementation of a data repository-driven approach for targeted proteomics experiments by multiple reaction monitoring. J Proteomics. 2009;72(5):838–52. doi: 10.1016/j.jprot.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Picotti P, Lam H, Campbell D, Deutsch EW, Mirzaei H, Ranish J, Domon B, Aebersold R. A database of mass spectrometric assays for the yeast proteome. Nat Methods. 2008;5(11):913–4. doi: 10.1038/nmeth1108-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson NL, Anderson NG, Pearson TW, Borchers CH, Paulovich AG, Patterson SD, Gillette M, Aebersold R, Carr SA. A human proteome detection and quantitation project. Mol Cell Proteomics. 2009;8(5):883–6. doi: 10.1074/mcp.R800015-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Addona TA, Abbatiello SE, Schilling B, Skates SJ, Mani DR, Bunk DM, Spiegelman CH, Zimmerman LJ, Ham AJ, Keshishian H, Hall SC, Allen S, Blackman RK, Borchers CH, Buck C, Cardasis HL, Cusack MP, Dodder NG, Gibson BW, Held JM, Hiltke T, Jackson A, Johansen EB, Kinsinger CR, Li J, Mesri M, Neubert TA, Niles RK, Pulsipher TC, Ransohoff D, Rodriguez H, Rudnick PA, Smith D, Tabb DL, Tegeler TJ, Variyath AM, Vega-Montoto LJ, Wahlander A, Waldemarson S, Wang M, Whiteaker JR, Zhao L, Anderson NL, Fisher SJ, Liebler DC, Paulovich AG, Regnier FE, Tempst P, Carr SA. Multi-site assessment of the precision and reproducibility of multiple reaction monitoring-based measurements of proteins in plasma. Nat Biotechnol. 2009;27(7):633–41. doi: 10.1038/nbt.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koomen JM, Haura EB, Bepler G, Sutphen R, Remily-Wood ER, Benson K, Hussein M, Hazlehurst LA, Yeatman TJ, Hildreth LT, Sellers TA, Jacobsen PB, Fenstermacher DA, Dalton WS. Proteomic contributions to personalized cancer care. Mol Cell Proteomics. 2008;7(10):1780–94. doi: 10.1074/mcp.R800002-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolf-Yadlin A, Hautaniemi S, Lauffenburger DA, White FM. Multiple reaction monitoring for robust quantitative proteomic analysis of cellular signaling networks. Proc Natl Acad Sci U S A. 2007;104:5860–5865. doi: 10.1073/pnas.0608638104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 15.Gerber SA, Kettenbach AN, Rush J, Gygi SP. The absolute quantification strategy: application to phosphorylation profiling of human separase serine 1126. Methods Mol Biol. 2007;359:71–86. doi: 10.1007/978-1-59745-255-7_5. [DOI] [PubMed] [Google Scholar]
- 16.Kirkpatrick DS, Gerber SA, Gygi SP. The absolute quantification strategy: a general procedure for the quantification of proteins and post-translational modifications. Methods. 2005;35:265–73. doi: 10.1016/j.ymeth.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 17.Gerber SA, Rush J, Stemman O, Kirschner MW, Gygi SP. Absolute quantification of proteins and phosphoproteins from cell lysates by tandem MS. Proc Natl Acad Sci U S A. 2003;100:6940–5. doi: 10.1073/pnas.0832254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnidge DR, Dratz EA, Martin T, Bonilla LE, Moran LB, Lindall A. Absolute quantification of the G protein-coupled receptor rhodopsin by LC/MS/MS using proteolysis product peptides and synthetic peptide standards. Anal Chem. 2003;75:445–451. doi: 10.1021/ac026154+. [DOI] [PubMed] [Google Scholar]
- 19.Fang Y, Robinson DP, Foster LJ. Quantitative analysis of proteome coverage and recovery rates for upstream fractionation methods in proteomics. J Proteome Res. 2010;9:1902–1912. doi: 10.1021/pr901063t. [DOI] [PubMed] [Google Scholar]
- 20.Piersma SR, Fiedler U, Span S, Lingnau A, Pham TV, Hoffmann S, Kubbutat MH, Jiménez CR. Workflow comparison for label-free, quantitative secretome proteomics for cancer biomarker discovery: method evaluation, differential analysis, and verification in serum. J Proteome Res. 2010;9(4):1913–1922. doi: 10.1021/pr901072h. [DOI] [PubMed] [Google Scholar]
- 21.Tang HY, Ali-Khan N, Echan LA, Levenkova N, Rux JJ, Speicher DW. A novel four-dimensional strategy combining protein and peptide separation methods enables detection of low-abundance proteins in human plasma and serum proteomes. Proteomics. 2005;5:3329–42. doi: 10.1002/pmic.200401275. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Chang-Wong T, Tang HY, Speicher DW. Comparison of extensive protein fractionation and repetitive LC-MS/MS analyses on depth of analysis for complex proteomes. J Proteome Res. 2010;9:1032–1040. doi: 10.1021/pr900927y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol. 1999;19:1720–1730. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schirle M, Heurtier M, Kuster B. Profiling Core Proteomes of Human Cell Lines by One-dimensional PAGE and Liquid Chromatography-Tandem Mass Spectrometry. Mol Cell Biol. 2003;2:1297–1305. doi: 10.1074/mcp.M300087-MCP200. [DOI] [PubMed] [Google Scholar]
- 25.MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, Kern R, Tabb DL, Liebler DC, MacCoss MJ. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 2010;26:966–8. doi: 10.1093/bioinformatics/btq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deutsch Eric W, Eng Jimmy K, Zhang Hui, King Nichole L, Nesvizhskii Alexey I, Lin Biaoyang, Lee Hookeun, Yi Eugene C, Ossola Reto, Aebersold Ruedi. Human Plasma PeptideAtlas. Proteomics. 2005;5(13):3497–500. doi: 10.1002/pmic.200500160. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Gruidl M, Remily-Wood E, Liu RZ, Eschrich S, Lloyd M, Nasir A, Bui MM, Huang E, Shibata D, Yeatman T, Koomen JM. Quantification of beta-catenin signaling components in colon cancer cell lines, tissue sections, and microdissected tumor cells using reaction monitoring mass spectrometry. J Proteome Res. 2010;9:4215–27. doi: 10.1021/pr1005197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang G, Fang B, Liu RZ, Lin H, Kinose F, Bai Y, Oguz U, Remily-Wood ER, Li J, Altiok S, Eschrich S, Koomen J, Haura EB. Mass Spectrometry Mapping of Epidermal Growth Factor Receptor Phosphorylation Related to Oncogenic Mutations and Tyrosine Kinase Inhibitor Sensitivity. J Proteome Res. 2010 Dec 3; doi: 10.1021/pr1006203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davenport EL, Morgan GJ, Davies FE. Untangling the unfolded protein response. Cell Cycle. 2008 Apr 1;7(7):865–9. doi: 10.4161/cc.7.7.5615. [DOI] [PubMed] [Google Scholar]
- 30.Goetz MP, Toft DO, Ames MM, Erlichman C. The Hsp90 chaperone complex as a novel target for cancer therapy. Ann Oncol. 2003 Aug;14(8):1169–76. doi: 10.1093/annonc/mdg316. [DOI] [PubMed] [Google Scholar]
- 31.Banerji U. Heat shock protein 90 as a drug target: some like it hot. Clin Cancer Res. 2009 Jan 1;15(1):9–14. doi: 10.1158/1078-0432.CCR-08-0132. [DOI] [PubMed] [Google Scholar]
- 32.Okawa Y, Hideshima T, Steed P, Vallet S, Hall S, Huang K, Rice J, Barabasz A, Foley B, Ikeda H, Raje N, Kiziltepe T, Yasui H, Enatsu S, Anderson KC. SNX-2112, a selective Hsp90 inhibitor, potently inhibits tumor cell growth, angiogenesis, and osteoclastogenesis in multiple myeloma and other hematologic tumors by abrogating signaling via Akt and ERK. Blood. 2009 Jan 22;113(4):846–55. doi: 10.1182/blood-2008-04-151928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chatterjee M, Jain S, Stühmer T, Andrulis M, Ungethüm U, Kuban RJ, Lorentz H, Bommert K, Topp M, Krämer D, Müller-Hermelink HK, Einsele H, Greiner A, Bargou RC. STAT3 and MAPK signaling maintain overexpression of heat shock proteins 90alpha and beta in multiple myeloma cells, which critically contribute to tumor-cell survival. Blood. 2007 Jan 15;109(2):720–8. doi: 10.1182/blood-2006-05-024372. [DOI] [PubMed] [Google Scholar]
- 34.Cervantes-Gomez F, Nimmanapalli R, Gandhi V. Transcription inhibition of heat shock proteins: a strategy for combination of 17-allylamino-17-demethoxygeldanamycin and actinomycin d. Cancer Res. 2009 May 1;69(9):3947–54. doi: 10.1158/0008-5472.CAN-08-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stühmer T, Zöllinger A, Siegmund D, Chatterjee M, Grella E, Knop S, Kortüm M, Unzicker C, Jensen MR, Quadt C, Chène P, Schoepfer J, García-Echeverría C, Einsele H, Wajant H, Bargou RC. Signalling profile and antitumour activity of the novel Hsp90 inhibitor NVP-AUY922 in multiple myeloma. Leukemia. 2008 Aug;22(8):1604–12. doi: 10.1038/leu.2008.111. [DOI] [PubMed] [Google Scholar]
- 36.Nimmanapalli R, Gerbino E, Dalton WS, Gandhi V, Alsina M. HSP70 inhibition reverses cell adhesion mediated and acquired drug resistance in multiple myeloma. Br J Haematol. 2008 Aug;142(4):551–61. doi: 10.1111/j.1365-2141.2008.07217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huston A, Leleu X, Jia X, Moreau AS, Ngo HT, Runnels J, Anderson J, Alsayed Y, Roccaro A, Vallet S, Hatjiharissi E, Tai YT, Sportelli P, Munshi N, Richardson P, Hideshima T, Roodman DG, Anderson KC, Ghobrial IM. Targeting Akt and heat shock protein 90 produces synergistic multiple myeloma cell cytotoxicity in the bone marrow microenvironment. Clin Cancer Res. 2008 Feb 1;14(3):865–74. doi: 10.1158/1078-0432.CCR-07-1299. [DOI] [PubMed] [Google Scholar]
- 38.Patterson J, Palombella VJ, Fritz C, Normant E. IPI-504, a novel and soluble HSP-90 inhibitor, blocks the unfolded protein response in multiple myeloma cells. Cancer Chemother Pharmacol. 2008 May;61(6):923–32. doi: 10.1007/s00280-007-0546-0. [DOI] [PubMed] [Google Scholar]
- 39.Flandrin P, Guyotat D, Duval A, Cornillon J, Tavernier E, Nadal N, Campos L. Significance of heat-shock protein (HSP) 90 expression in acute myeloid leukemia cells. Cell Stress Chaperones. 2008 Sep;13(3):357–64. doi: 10.1007/s12192-008-0035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas X, Campos L, Mounier C, Cornillon J, Flandrin P, Le QH, Piselli S, Guyotat D. Expression of heat-shock proteins is associated with major adverse prognostic factors in acute myeloid leukemia. Leuk Res. 2005 Sep;29(9):1049–58. doi: 10.1016/j.leukres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 41.Chant ID, Rose PE, Morris AG. Analysis of heat-shock protein expression in myeloid leukaemia cells by flow cytometry. Br J Haematol. 1995 May;90(1):163–8. doi: 10.1111/j.1365-2141.1995.tb03395.x. [DOI] [PubMed] [Google Scholar]
- 42.Chant ID, Rose PE, Morris AG. Susceptibility of AML cells to in vitro apoptosis correlates with heat shock protein 70 (hsp 70) expression. Br J Haematol. 1996 Jun;93(4):898–902. doi: 10.1046/j.1365-2141.1996.d01-1737.x. [DOI] [PubMed] [Google Scholar]
- 43.Thomas X, Campos L, Le QH, Guyotat D. Heat shock proteins and acute leukemias. Hematology. 2005 Jun;10(3):225–35. doi: 10.1080/10245330500093120. [DOI] [PubMed] [Google Scholar]
- 44.Zaarur N, Gabai VL, Porco JA, Jr, Calderwood S, Sherman MY. Targeting heat shock response to sensitize cancer cells to proteasome and Hsp90 inhibitors. Cancer Res. 2006 Feb 1;66(3):1783–91. doi: 10.1158/0008-5472.CAN-05-3692. [DOI] [PubMed] [Google Scholar]
- 45.McCleese JK, Bear MD, Fossey SL, Mihalek RM, Foley KP, Ying W, Barsoum J, London CA. The novel HSP90 inhibitor STA-1474 exhibits biologic activity against osteosarcoma cell lines. Int J Cancer. 2009 Dec 15;125(12):2792–801. doi: 10.1002/ijc.24660. [DOI] [PubMed] [Google Scholar]
- 46.Song D, Chaerkady R, Tan AC, García-García E, Nalli A, Suárez-Gauthier A, López-Ríos F, Zhang XF, Solomon A, Tong J, Read M, Fritz C, Jimeno A, Pandey A, Hidalgo M. Antitumor activity and molecular effects of the novel heat shock protein 90 inhibitor, IPI-504, in pancreatic cancer. Mol Cancer Ther. 2008 Oct;7(10):3275–84. doi: 10.1158/1535-7163.MCT-08-0508. [DOI] [PubMed] [Google Scholar]
- 47.Ramanathan RK, Egorin MJ, Erlichman C, Remick SC, Ramalingam SS, Naret C, Holleran JL, TenEyck CJ, Ivy SP, Belani CP. Phase I pharmacokinetic and pharmacodynamic study of 17-dimethylaminoethylamino-17-demethoxygeldanamycin, an inhibitor of heat-shock protein 90, in patients with advanced solid tumors. J Clin Oncol. 2010 Mar 20;28(9):1520–6. doi: 10.1200/JCO.2009.25.0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lancet JE, Gojo I, Burton M, Quinn M, Tighe SM, Kersey K, Zhong Z, Albitar MX, Bhalla K, Hannah AL, Baer MR. Phase I study of the heat shock protein 90 inhibitor alvespimycin (KOS-1022, 17-DMAG) administered intravenously twice weekly to patients with acute myeloid leukemia. Leukemia. 2010 Apr;24(4):699–705. doi: 10.1038/leu.2009.292. [DOI] [PubMed] [Google Scholar]
- 49.Kummar S, Gutierrez ME, Gardner ER, Chen X, Figg WD, Zajac-Kaye M, Chen M, Steinberg SM, Muir CA, Yancey MA, Horneffer YR, Juwara L, Melillo G, Ivy SP, Merino M, Neckers L, Steeg PS, Conley BA, Giaccone G, Doroshow JH, Murgo AJ. Phase I trial of 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG), a heat shock protein inhibitor, administered twice weekly in patients with advanced malignancies. Eur J Cancer. 2010 Jan;46(2):340–7. doi: 10.1016/j.ejca.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang L, Wei Q, Mao L, Liu W, Mills GB, Coombes K. Serial dilution curve: a new method for analysis of reverse phase protein array data. Bioinformatics. 2009 Mar 1;25(5):650–4. doi: 10.1093/bioinformatics/btn663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park ES, Rabinovsky R, Carey M, Hennessy BT, Agarwal R, Liu W, Ju Z, Deng W, Lu Y, Woo HG, Kim SB, Cheong JH, Garraway LA, Weinstein JN, Mills GB, Lee JS, Davies MA. Integrative analysis of proteomic signatures, mutations, and drug responsiveness in the NCI 60 cancer cell line set. Mol Cancer Ther. 2010 Feb;9(2):257–67. doi: 10.1158/1535-7163.MCT-09-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]