Abstract
The prognosis of pancreatic cancer (PC) patients is very poor with a five-year survival of less than 5%. One of the major challenges in developing new therapies for PC is the lack of expression of specific markers by pancreatic tumor cells. Mucins are heavily O-glycosylated proteins characterized by the presence of short stretches of amino acid sequences repeated several times in tandem. The expression of several mucins including MUC1, MUC4, MUC5AC, and MUC16 is strongly upregulated in PC. Recent studies have also demonstrated a link between the aberrant expression and differential overexpression of mucin glycoproteins to the initiation, progression, and poor prognosis of the disease. These studies have led to increasing recognition of mucins as potential diagnostic markers and therapeutic targets in PC. In this focused review we present an overview of the therapies targeting mucins in PC, including immunotherapy (i.e. vaccines, antibodies, and radioimmunoconjugates), gene therapy, and other novel therapeutic strategies.
Keywords: Pancreatic cancer, mucins, targeted therapy
INTRODUCTION
Pancreatic cancer (PC), a malignancy arising from the small and medium sized exocrine pancreatic ducts, has consistently been among the top five human cancers with the poorest prognosis for the last three decades [1,2]. While the definitive treatment of PC is surgery, it is only possible in about 15% of cases [3,4]. The other therapeutic modalities employed to treat PC are chemotherapy and radiation. Most PC patients will require chemotherapy, either in a neoadjuvant or adjuvant setting. Gemcitabine has been the first line therapy for PC since its introduction in the late 1990's and is currently administered in combination with Erlotinib, an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor. However, multiple clinical trials have failed to demonstrate a significant survival advantage in PC patients treated with combination chemotherapy (combination of two or more chemotherapy agents) beyond the median survival of 3–6 months observed with Gemcitabine alone [4,5]. It is now widely accepted that several factors contribute to the failure of chemotherapy in PC, including the intrinsic resistance of pancreatic tumors to these agents, the strong desmoplastic response that prevents delivery of the drugs in therapeutic doses to the tumor cells, and more recently, the existence of progenitor cells (cancer stem cells) that are highly resistant to both chemotherapy and radiation [6,7].
Only recently, with the availability of genetically engineered mouse models of PC, we were able to obtain a clear understanding of the mechanisms that might contribute to chemoresistance in PC cells [8,9]. Studies in these models suggest that a combination of vascular deficiency and the presence of a dense stromal matrix are the main factors responsible for the poor delivery of chemotherapeutic drugs to pancreatic tumors. As the complex stromal microenvironment deforms the normal architecture of the pancreas, it is very likely that several molecules, including mucins, which are the focus of the current article, have a role in both the progression [10–13] and chemoresistance [14–16] of PC.
MUCINS
All the epithelial surfaces in our bodies have a viscous protective layer that covers them, constituting a much needed barrier to harsh environmental onslaughts. A major component of this protective barrier is a class of glycoproteins whose importance in both benign and malignant human diseases is only now being fully elucidated. These glycoproteins are called “mucins” and are expressed by epithelial cells in a number of normal human organs. For a protein to be categorized as a mucin, it must possess a dense content of oligosaccharide side chains (comprising of N-acetyl-galactosamine) linked via an O-glycosidic linkage to specific amino acid residues (serine, threonine and proline) that occur in repetitive short stretches (termed as “tandem repeats”) in the protein backbone [17,18]. In fact, the carbohydrate component can make up between 50–90% of the weight of a mucin glycoprotein. Mucins are distinguished from another class of glycoproteins called proteoglycans in that they lack uronic acid and xylose. Genes encoding the protein backbone of mucins (also called the apomucin) are denoted by the three letter code MUC followed by a number. The mucin genes were numbered in the chronological order in which they were discovered. To date, 17 mucins have been discovered. In some cases, two mucins that originally shared the same gene name (e.g. MUC5 and MUC3) were given separate names, e.g. MUC5AC and MUC5B (for MUC5) and MUC3A and MUC3B (for the two proteins with the gene name MUC3). Some genes originally included as mucins have now been renamed following the recognition that they do not fulfill the classic criteria for a mucin. One such protein is MUC18 (now known as melanoma cell adhesion molecule or MCAM) that belongs to the immunoglobulin superfamily of proteins [19].
MUC proteins are classified based on their structure and function into two main categories: membrane-bound/transmembrane and secreted/gel-forming mucins [20]. The membrane-bound mucins, which include MUC1, MUC3A, MUC3B, MUC4, MUC12 and MUC17, possess an extracellular portion that makes up the major part of the molecule, followed by a transmembrane portion and a short C-terminal cytoplasmic portion. The cytoplasmic segment of these proteins is important from the standpoint of signal transduction as it contains several phosphorylation sites that may be important for interaction with other scaffolding and signaling proteins. The N-terminal extracellular portions of mucins also contain several domains, including EGF-like (epidermal growth factor-like), AMOP (adhesion associated domain in MUC4 and other proteins), VWD (Von willebrand factor D), SEA (Sea urchin sperm protein Enterokinase and Agrin) and NIDO (nidogen-like) domains. However, the function of these domains is, at present, only speculative [21]. The membrane-bound mucins are characterized by another unique characteristic: the existence of multiple isoforms that are generated by the process of alternative splicing of their mRNAs. For instance, MUC1 has a full-length isoform (MUC1/TM) in addition to at least two other isoforms, MUC1/Y and MUC1/SEC. These isoforms are generated by the process of alternative splicing of the mucin mRNA and may play a role in signal transduction. As an example, MUC1/Y which lacks the transmembrane segment, interacts with MUC1/SEC, a soluble form of the glycoprotein, to form a heterodimer which can then transduce intracellular signals [22]. Similar alternative splicing has also been demonstrated for other mucins including MUC4. However, these forms comprise only a small percentage of the total amount of mucin expressed by a cell and, hence, their functional significance remains a matter of debate.
Secreted (or gel-forming) mucins are unique as these are all encoded by genes located contiguously on chromosome 11 in the following order: MUC6, MUC2, MUC5AC, and MUC5B. This clustering of genes on the same chromosome suggests that these genes may be evolutionarily related to one another. Like their membrane-bound counterparts, these secreted mucins also have short stretches of amino acids repeated several times in tandem (“tandem repeats” or TRs). A feature common to mucins is the variation in the number of these TRs. Termed as a variable number of tandem repeat (VNTR) polymorphism, this unique characteristic has been suggested to have diagnostic, prognostic, and even therapeutic significance [23,24]. Two unique domains that characterize secreted mucins are the VWD domain and the cysteine knot (CK) motif [22,25]. The former is hypothesized to be important for the association of mucins into oligomeric structures, while the latter is believed to be crucial for the formation of mucin-mucin homo and heterodimers. The biochemistry of mucins has been described elegantly in several previous articles [19,21,26,27]. The focus of this review will be on their significance in the therapy of PC, one of the deadliest malignancies known to man.
MUCINS AND THERAPEUTIC RESISTANCE IN PC CELLS
Only a handful of mucins are expressed in the non-neoplastic pancreatic ducts including MUC1, MUC5B, and MUC6. However, several mucins are expressed de novo during the transformation of the normal ductal epithelium into malignant adenocarcinoma cells. MUC4, MUC5AC, and more recently MUC16 (unpublished data) are key mucins whose expression is noted de novo (i.e. not expressed by the normal ductal epithelium but expressed in the dys-plastic lesions) in the pre-malignant/dysplastic stages preceding invasive carcinoma. In frankly malignant adenocarcinoma cells, the expression of MUC1, MUC4, MUC5AC and MUC16 is significantly increased [28]. The pattern of expression of other mucins during the development and progression of PC, however, remains unclear. In addition to their aberrant (i.e. de novo) expression or overexpression in PC cells, several mucins have also been shown to modulate the behavior of PC cells. Both MUC1 [29,30] and MUC4 [31,32], for instance, have been demonstrated to promote proliferation, invasion, and metastasis in PC cells. While these studies suggest that the expression of mucins is significantly altered in PC and may contribute to the aggressiveness of PC cells, very little is known about the contribution of specific mucins to the resistance of PC cells to either chemotherapy or radiotherapy.
Recent advances from our laboratory suggest that MUC4 may be a crucial factor in modulating the resistance of PC cells to Gemcitabine, the front line therapy for PC [14]. Using a highly metastatic PC cell line, CD18/HPAF, we have demonstrated that down-regulation of MUC4 can sensitize these cells to Gemcitabine in vitro [14]. Of particular interest is our observation that MUC4 is expressed by PC stem (or progenitor) cells, suggested to be responsible for not just maintaining the growth of PC cells but also their extreme resistance to chemotherapy [16]. Silencing of MUC4 in these cancer stem cells using siRNA resulted in a significant increase in their sensitivity to the cytotoxic effect of Gemcitabine [16]. In melanoma cells, an overexpression of MUC4 has been observed to increase their resistance in vitro to other cytotoxic drugs, including Docetaxel, Doxorubicin, and Vinblastine [33]. While these observations have yet to be confirmed in vivo, they suggest that MUC4 modulates chemoresistance in multiple solid tumors and, hence, could be an important therapeutic target in PC.
The importance of other mucins in regulating chemoresistance in PC cells has not been well studied. MUC1, perhaps the best studied mucin, has been shown to modulate the resistance of thyroid cancer cells to Cisplatin, Docetaxel, and Doxorubicin [34], while in breast cancer cells its downregulation has been shown to sensitize breast cancer cells to Trastuzumab (a monoclonal antibody targeting the epidermal growth factor receptor-2 or EGFR-2/HER2) [35]. These results suggest that MUC1 may be important in regulating chemoresistance in PC cells as well, and thus, make it a potential target for downregulation. The role of mucins in the resistance of PC cells to radiation therapy, however, remains unresolved.
PANCREATIC CANCER THERAPIES TARGETING MUCINS
Therapies against PC have not been significantly improved over the last 30 years [1]. In the last decade, the main effort in the area of anti-PC therapy has been to enhance the effect of the standard chemotherapeutic drug Gemcitabine by including other cytotoxic drugs in the therapeutic regimen [9]. Nevertheless, most of these studies have failed in offering a significant overall survival benefit to PC patients and, therefore, novel therapeutic approaches are still needed.
The expression of mucins has been related to the poor prognosis, progression, and chemoresistance of human PC. Among all the transmembrane mucins that have been related to the progression of PC, MUC1 has been thoroughly investigated as a target for PC therapy. In the following section, we review some of the strategies that have been used to target mucins in PC.
Immunotherapy
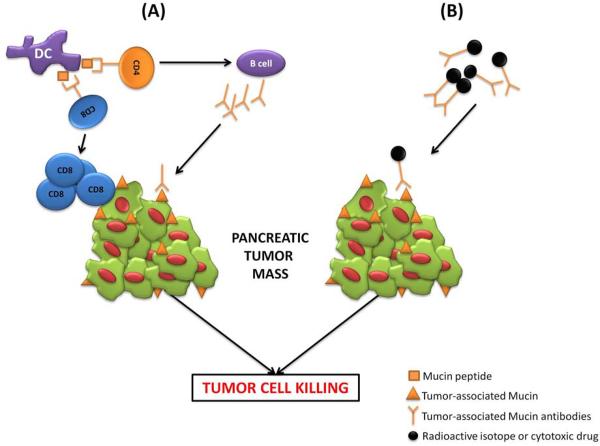
Immunotherapy is an anti-cancer therapeutic strategy where exogenous agents (e.g. antibodies) or the body's own immune system (e.g. T-helper cells, cytotoxic T-cells, and dendritic cells) are used to enhance the immune response of the cancer patient to target and kill tumor cells more efficiently (immunotherapy for PC reviewed excellently in [36]). Mucin-based immunotherapy for PC (Fig. (1)) has seen the testing of several novel strategies including vaccines, antibodies, radioimmunoconjugates, and gene therapy.
Fig. (1). Immunotherapeutic strategies targeting mucins in PC.
(A) Vaccination with Mucin-peptides or Mucin-pulsed dendritic cells enhances the activation of humoral and cell-mediated adaptive immune responses. Mucin-peptide loaded dendritic cell (DC) activate both CD4 T cells, which eventually leads to the activation of B cells to produce tumor-associated Mucin antibodies (i.e. humoral response), and CD8 T cells that target and kill Mucin-expressing tumors (i.e. cell-mediated response). (B) Tumor-associated Mucin antibodies are conjugated to radioactive isotopes or cytotoxic drugs to target and kill PC cells more efficiently.
Vaccines
Vaccination approaches for PC rely on strategies to enhance the tumor-antigen presentation of dendritic cells, which, in turn, activate T-helper cells that can activate cytotoxic T-cells (cell-mediated immunity) or B cells to produce tumor-specific antibodies (e.g. humoral immunity) (Fig. (1A)). The most relevant strategies evaluating Mucin-based vaccinations in PC are presented below.
MUC1
The clinical feasibility of MUC1 in targeted therapies against PC has been evaluated for over 15 years Table 1. Studies in murine models have provided a better understanding of the immunogenicity of this glycoprotein. In 1998, a C57BL/6 mouse transgenic for human MUC1 (MUC1.Tg) was developed by Gendler et al., to evaluate MUC1-specific tumor immunity [37]. These MUC1.Tg mice expressed the human transgene in a pattern similar to that observed in PC patients. Further, the mice were reported to be tolerant to exogenously administered human MUC1 protein. Immune responses induced in MUC1.Tg and wild type mice after tumor challenge revealed that wild type mice developed protective tumor immunity mediated by MUC1-specific CD4+ T lymphocytes, while MUC1.Tg mice were functionally tolerant to recombinant human MUC1 and their tumor growth could not be controlled [38]. This suggests that by tuning the immune response to actively recognize MUC1 as a non-self-protein on cancer cells, it is possible to induce the immune system to reject the tumor. This study prompted the subsequent evaluation of several MUC1 vaccines in these MUC1.Tg mice and formed the basis for several clinical trials which are summarized in Table 1.
Table 1.
Completed Clinical Trials Evaluating Mucin Vaccines in PC Patients
| Year | Phase | Mucin Formulation | Adjuvant | Major Outcomes | References |
|---|---|---|---|---|---|
| 1996 | I | MUC1 105 AA | BCG |
|
Goydos et al. [39] |
| 2002 | II | Adoptive transfer of autologous DCs transfected with MUC1 |
|
Pecher et al. [45] | |
| 2005 | I | MUC1 100 AA | SB-AS2 |
|
Ramanathan et al. [41] |
| 2005 | I | MUC1 100 AA | Incomplete Freund's |
|
Yamamoto et al. [43] |
| 2007 | I | Vaccinia and Fowlpox viral vectors expressing CEA and MUC1 | TRICOM™ + GM-CSF |
|
Kaufman et al. [86] |
| 2008 | I/II | Adoptive transfer of MUCl-peptide pulsed DCs |
|
Lepisto et al. [40] | |
| 2008 | I | Adoptive transfer of in vitro activated CTLs with MUC1-expressing cell line |
|
Kawaoka et al. [46, 47] | |
| 2008 | I | Adoptive transfer of MUC1 peptide pulsed DCs + activated CTLs |
|
Kondo et al. [48] |
MUC1, Mucinl; AA, amino acid; DCs, dendritic cells; IFNγ, Interferon gamma; BCG, Bacillus-Calmette-Guérin; SB-AS2, adjuvant composed of monophosphoryl lipid A and QS-21; CEA, Carcinoembryonic antigen; TRICOM™, formulation including the costimulatory molecules B7.1, ICAM-1, and LFA-3; GM-CSF, granulocyte-macrophage colony-stimulating factor; CTLs, cytotoxic T lymphocytes
One of the research groups that have evaluated the efficacy of MUC1 vaccines in several clinical trials is directed by Dr. Olivera Finn and her colleagues at the University of Pittsburgh [39–42]. In one of the earliest patient trials of a mucin vaccine in cancer, her group reported in a Phase I trial that the vaccine was relatively safe in humans. The study included 24 PC, nine breast cancer, and 30 colorectal cancer patients, all with adenocarcinomas, who were immunized with a MUC1 peptide (105 amino acids long), that contained five immunologic epitopes. The Bacillus-Calmette-Guérin (BCG) bacterium, which is a live attenuated Mycobacterium bovis strain specifically used for vaccinations, was used as an adjuvant to boost the immune response in these patients [39]. As in a typical phase I clinical trial, the primary goal was to determine the safety of the vaccine formulation with the secondary goal being to investigate if a delayed-type hypersensitive (DTH) response against MUC1 was induced at the vaccination site. Although the vaccine was well tolerated and strong T cell infiltration was reported in most cases, none of the patients included in the study exhibited a partial or complete response to the vaccine. Nearly a decade later, in an attempt to improve the response of PC patients to MUC1 vaccines, the same research group investigated the potential of a 100-amino acid MUC1 peptide co-injected with the potential adjuvant SB-AS2 (composed of monophosphoryl lipid A and QS-21 in an oil-in-water emulsion) [41] in 16 patients with locally advanced PC. The dose of the immunizing peptide ranged from 100–3000μg and it was administered once every three weeks. Each patient received three vaccination cycles. The adverse effects reported were relatively mild and included flu-like symptoms, erythema, and tenderness at the injection site. Although it is well known that the adjuvant SB-AS2 has the potential to enhance the immunogenicity of both arms of the immune system (i.e. humoral and adaptive), the anti-MUC1 antibody responses seen in five patients included in the study was not significant. Further, there was a significant increase in non-specific T cell activation that impeded the identification and quantification of MUC1-specific T cell responses. A similar clinical approach employing a 100-amino acid MUC1-peptide vaccine was reported by Yamamoto et.al using incomplete Freund's adjuvant [43]. Although the vaccine was proven to be safe, of the eight patients that were eligible for clinical evaluation, seven had progressive disease after immunization treatment.
Three years after the first MUC1-peptide vaccine clinical trial, an alternate strategy was assessed by Finn and collaborators. The vaccine formulation was chosen based on the results of animal studies done on wild type and MUC1.Tg mice, where three different formulations of MUC1 peptide vaccines were evaluated [44]. The vaccine that was successful in both prophylactic and therapeutic experiments consisted of MUC1 peptide-loaded dendritic cells, and, thus, it was chosen for the subsequent Phase I/II clinical trial in 10 PC and two biliary cancer patients with resected tumors [40]. Similar to the previous clinical trials, patients injected with MUC1 peptide-loaded autologous dendritic cells presented a high non-specific activation of T cells and none of the patients exhibited an increase in anti-MUC1 antibodies. The new vaccine formulation was well tolerated and four out of 12 patients were still alive after four years without evidence of tumor recurrence.
In order to optimize the immune response to MUC1 vaccines in cancer patients, a research group from Germany immunized 10 patients with metastatic breast, pancreatic, or ampullary cancers with autologous dendritic cells transfected with 22 tandem repeats region of MUC1 [45]. They suggested that their approach was superior to peptide-pulsed dendritic cells, as the transfected dendritic cells present more MUC1 epitopes at their surface (compared to the peptide pulsed dendritic cells), which in turn would be expected to elicit a stronger immune response. The study, however, included only two PC patients, who interestingly, had a higher number of interferon gamma (IFNγ)-secreting CD8+ T cells compared to patients with other malignancies that also received the vaccine. Three out of 10 patients in this trial had a vaccine-specific DTH response. Unfortunately, all of the patients presented had advanced cancer, and nine out of 10 patients had progressive disease after the two to three doses of the vaccine.
Other attempts to design MUC1 vaccines have been led by Dr. Masaaki Oka, who has evaluated MUC1 vaccines in clinical trials of PC patients over the past five years [46–48]. The earliest clinical attempt consisted of an adoptive transfer immunotherapy of cytotoxic T lymphocytes stimulated by the MUC1-expressing human PC cell line YPK-1 [46,47]. The adoptive transfer immunotherapy consisted of the injection of cytotoxic T cells generated from healthy donors into PC patients. The MUC-1-specific cytotoxicity of the cytotoxic T cells was validated on in vitro experiments on MUC1-expressing PC cell lines. The stimulated lymphocytes were subsequently injected into eight patients with unresectable PC and 20 patients with resectable PC post-surgically. Although no improvement in survival was reported in this study, the vaccine was found to be safe in all patients tested. The research group suggested that cytotoxic T lymphocytes may distribute in the liver, and based on this assumption they designed a clinical trial where the vaccine consisted of autologous cytotoxic T lymphocytes co-administered with autologous MUC1 peptide-loaded dendritic cells [48]. In this particular study, 20 patients with unresectable or recurrent PC were enrolled. Promising results from this clinical study included a mean survival time of 9.8 months, one patient with lung metastasis that experienced a complete response, and five patients that presented a stable disease after vaccinations with the MUC1 vaccine. Similar to the previous studies reported, this approach did not induce any major toxicity in the PC patients.
MUC4
Although the full potential of MUC4 in targeted PC therapies has not been explored yet and no clinical trials have been reported, some promising immunology therapies have been designed. The research group led by Dr. Yi Miao in Nanjing Medical University designed MUC4 vaccines that have been evaluated on in vitro experiments [49,50]. Their approach was based on enhancing antigen presentation in the context of human leukocyte antigen (HLA), HLA-A1 and HLA-A2, which functions to present antigens to activate the appropriate immune cells of humoral and cell-mediated immune responses pathways, respectively. The first study was based on dendritic cell transduced with the universal pan HLA DR-reactive epitope (PADRE) combined with HLA-A1 and HLA-A2 MUC4 epitopes [49]. The combined epitope DNA vaccine induced potent cytotoxic in vitro responses that were HLA-A2-restricted and MUC4-specific. Subsequently, the same research group sought to identify several HLA-A2 restricted epitopes that could activate cytotoxic T lymphocytes [50]. In this study, mature dendritic cells were pulsed with the predicted peptides and MUC4-specific cytotoxic responses were measured on in vitro experiments. Similar to the previous study, MUC4-specific and HLA-A2-restricted cytotoxic (CD8+ T cells) responses were shown against MUC4-expressing cancer cell lines. It is important to mention that both of these studies evaluated the in vitro responses on CD8+ T cells and dendritic cells derived from peripheral blood mononuclear cells (PBMCs) from HLA-A2+ healthy donors. The data from these in vitro experiments clearly show the potential of priming immune responses against MUC4-expressing PC cells. Whether these results will translate into the inhibition of PC cell growth in vivo will need to be tested in genetic models of PC (reviewed in [51–53]) prior to evaluation in clinical trials.
Importance of Glycosylation Patterns
One possible reason for the marginally successful attempts of MUC1 vaccines in PC patients is that, prior to 2008, most of these vaccines were based on unglycosylated MUC1 peptides or purified MUC1 from malignant sources with unknown glycosylation patterns [54]. As was pointed out in a recent review, it is of prime importance to know the altered glycosylation patterns of the mucins in cancer cells in order to increase the possibility of targeting the specific immunogenic epitopes expressed in tumors [54]. In this regard, some strategies have been tested in animal models, including one where MUC1 glycopeptides with different densities of cancer-associated O-glycosylation were evaluated for their immune-activation potency [55]. In one particular study done in a breast cancer model in MUC1.Tg mice, the glycopeptides that elicited stronger antibody responses were the ones with complete O-glycan occupancy. Other approaches that have addressed the importance of glycosylation have reported that synthetic immunostimulating glycopeptides of MUC1 and MUC4 carrying tumor-associated glycans can be potent inducers of the immune system [56]. These reports suggest that peptide sequences, glycosylation positions, and types of glycosylation are important determinants in the strength of the anti-tumor immune response and must be optimized for each mucin antigen.
Antibodies
Specific antibodies that recognize tumor associated antigens have also been tested in cancer therapeutics for their ability to directly bind to and lyse target cells by the activation of death receptors, blockage of growth signals, and/or making the cancer cell more recognizable to the immune system. Additionally, the use of antibodies for the targeted delivery of cytotoxic drugs has been a promising approach to increase the selective toxicity of cancer cells. Some of the anticancer therapeutics using Mucin antibodies to target PC are discussed below (Fig. (1B)).
MUC1
A comprehensive study done 15 years ago analyzed a panel of 56 MUC1 antibodies and found that all have unique specificity patterns [57]. This study done by 16 research groups confirmed that a majority of the epitopes recognized by MUC1 antibodies were located within the tandem repeat sequence of the glycoprotein, whereas the remaining antibodies recognized carbohydrate side chains. Although this study reported that there was not a relationship between the immunogen and the specificity of a given MUC1 antibody, several studies have since refuted this claim. Karsten and Goletz et al. described a carbohydrate-induced conformational tumor epitope on MUC1 based on the effect of peptide length of tandem repeats and O-glycosylation patterns [58]. In essence, MUC1 antibodies generated from tumor-derived MUC1 protein recognized peptides with highly glycosylated tandem repeats, whereas antibodies generated from non-tumor sources did not differentiate between glycosylated and non-glycosylated peptides. Based on this observation, the research group generated a novel MUC1 antibody with promising characteristics, which they named PankoMab [59]. PankoMab was designed to bind specifically to the carbohydrate-induced conformational epitope in tumors. After coupling the antibody to the toxin β-amanitin, the conjugate was able to induce specific cytotoxicity of tumor cells on in vitro experiments. Moreover, it was shown that PankoMab induced potent antibody-dependent cell cytotoxicity of MUC1 expressing PC cells, which was not possible with the clinically tested MUC1 antibody HMFG-1. A recent study analyzing 137 surgical specimens of different types of carcinomas by immunohistochemistry reported that a humanized form of PankoMab reacted strongly, even without an antigen retrieval step, with carcinomas from glandular or squamous epithelia origin [60]. All of these promising characteristics of PankoMab are being evaluated in a phase I clinical trial, where a dose escalation of the antibody is given to patients with advanced MUC1-expressing solid malignancies.
MUC16
Although the feasibility of MUC16 (CA125) has not been evaluated directly in PC treatment, therapies that target this glycoprotein have shown potential in other MUC16-expressing malignancies. One of the most promising therapies is the use of the murine monoclonal anti-CA125 B43.13, known as oregovomab or OvaRex®MAb [61,62]. The antibody forms immune complexes with CA125 in circulation within 30 minutes of injection, which improves antigen presentation to the immune system. Not surprisingly, clinical studies have shown that the generation of immune responses against MUC16 (CA125) after administration of oregovomab to ovarian cancer patients correlated with an improved overall survival [62].
Indirect therapeutic approaches targeting MUC16 in PC have relied on targeting the interaction partners (e.g. mesothelin) of this mucin glycoprotein [63,64]. There is compelling evidence of a strong binding between MUC16 and mesothelin which appears to be crucial for the metastasis of ovarian cancer cells to the peritoneum [65]. In a recent report, a humanized single chain variable antibody fragment specific for tumor-associated mesothelin (HN1) functionally blocked the interaction of mesothelin and MUC16 (CA125), an interaction that is known to be responsible for the metastatic properties of cancer cells [63]. Furthermore, there is an ongoing clinical trial of a mouse-human chimeric mesothelin antibody (MORAb-009) on patients with pancreatic, mesothelioma, non-small cell lung, and ovarian cancers [66]. This trial is based on preclinical studies that showed that MORAb-009 prevented the adhesion of mesothelin- expressing tumor cells to MUC16-positive cells [64]. These reports clearly indicate the therapeutic relevance of disrupting the interaction of MUC16 and mesothelin in MUC16-expressing PC.
Radioimmunoconjugates
Another strategy to target and kill PC cells is the conjugation of mucin antibodies to radioactive isotopes (Fig. (1B)). One of the most investigated strategies in this area is the radiolabeled antibody PAM4 to target MUC1 in pancreatic tumors [67–71]. Tumor targeting of 131Iodine (131I)-PAM4 or 99mTechnetium (99mTc)-PAM4 was evidenced in a small clinical evaluation where tumors of four out of five PC patients were efficiently targeted [69] Table 2. Nevertheless, 131I-PAM4 was apparently unstable and high levels of radioactivity were present in the blood of the patients after 24 h of administration. Further studies done by the same research group evaluated the efficacy of 90Yttrium (90Y) versus 131I labeled PAM4 and there was an evident superiority of 90Y-PAM4 to inhibit tumor growth in a xenograft model [68]. Following these studies, a clinical trial of the safety and efficacy of the humanized form of 90Y-PAM4 (h90Y-PAM4) on patients with advanced PC has just been reported Table 2 [71]. The study, done in four PC patients with stage III and 17 PC patients with stage IV cancer analyzed the biodistribution of the radiation in the tumors, bone marrow, and solid organs. Overall, the h90Y-PAM4 targeted PC tumors in 12 PC patients and the doses were well tolerated. Although grade 3–4 neutropenia (i.e. low number of neutrophils) and thrombocytopenia (i.e. low number of platelets) were reported during treatment, most of these recovered to grade 1 within 12 weeks. Unfortunately, most of the patients included in the study had progressive disease by the end of treatment and only three patients had partial responses (i.e. 32–52% tumor diameter shrinkage). This study reports that PAM4 antibody binds to mucin derived from pancreatic adenocarcinoma, therefore the antibody does not bind exclusively to MUC1 alone.
Table 2.
Clinical Trials Evaluating Mucin Radioimmunocongujates in PC Therapy
| Year | Radioimmunoconjugate (Radioisotope/Antibody) | Target Antigen | Additional Drugs | Major Outcomes/Characteristics of patients | References |
|---|---|---|---|---|---|
| 2000 | 90Y-CC49 | Sialyl T/Tn expressed in multiple mucins |
|
Tempero et al. [72] | |
| 2001 |
I31I-PAM4 99mTc-PAM4 |
MUC1 |
|
Gold et al. [69] | |
| 2011 | 90Y-hPAM4 | Mucin derived from PDA | Gemcitabine |
|
Gulec et al. [71] |
90Y-CC49, 90Yttrium-CC49; 131I-PAM4, 131Iodine-PAM4; 99mTc-PAM4, 99mTechnetium -PAM4; 90Y-hPAM4, 90Yttrium- humanized PAM4; PDA, pancreatic adenocarcinoma
Another radioimmunoconjugate tested on PC patients in the last decade is 90Y-CC49, a murine monoclonal antibody that recognizes Sialyl-Tn antigen present on tumor-associated mucin, TAG-72 [72]. This phase I trial, which intended to evaluate the tumor accumulation and potential toxicity of the radioimmunoconjugate, reported a suboptimal radiation absorbed in tumor even at the highest dose administered, whereas, hematopoietic toxicity levels were high.
More recently, a novel radionuclide strategy of targeted PC therapies involving alpha immunoconjugate of the monoclonal antibody (C595) which recognizes the tandem repeat of MUC1, has been designed [73]. The alpha immunoconjugate consists of C595 labeled with 213Bi using the chelator CHX.A and it was shown that it suppressed the tumor growth of MUC1-expressing pancreatic tumor xenografts for a period of 16 weeks. Targeted alpha therapy of cancer has proven to inhibit the growth of micrometastasis in patients with other malignancies [74], and, therefore, it represents a valuable approach to design PC therapies.
Gene Therapy
The U.S. Food and Drug Administration (FDA) have not yet approved any human gene therapy product for sale. However, the amount of gene-related research and development continues to rise, as evidenced by the more than 1,000 gene therapy clinical trials in the U.S. and 1,700 worldwide [75]. Although gene therapy often refers to strategies meant to increase the expression of normal (wild type) gene products, gene silencing by RNA interference (RNAi) or antisense oligonuclueotides can loosely be considered gene therapy. A number of preclinical studies suggest that decreasing select mucin tumor expression by RNA interference could be a novel molecular approach for the treatment of PC. For instance, knockdown of MUC1 by short interfering RNA (siRNA) in the S2-013 PC cell line resulted in decreased proliferation (in vitro and in vivo), while implantation of the cells into the cecum or pancreas in athymic mice resulted in a significantly reduced incidence of metastasis to the lymph nodes, lung, and peritoneum in the siMUC1-transfected cells compared to the control cells [76]. Similar results were also seen in MUC1-knockdown Panc1 cells [77], suggesting that MUC1 could be an important target for therapy against PC. The knockdown of MUC4 expression by siRNA in the highly metastatic CD18/HPAF PC cell line restrained tumor growth and metastasis in an orthotopic mouse model [31,32], decreased proliferation and invasiveness, and increased apoptosis in vitro [14]. Similarly, the knockdown of MUC5AC by siRNA in SW1990 and BxPC3 PC cell lines resulted in reduced adhesion and invasion in vitro and significantly decreased tumorigenecity upon subcutaneous implantation in athymic mice [78,79].
While the practical feasibility of siRNAs remains a matter of debate [80], the results of the aforementioned studies suggest that downregulation of specific mucins could be a novel therapeutic strategy in PC. Alternatively, these mucins can be employed as “readout proteins” to choose promising anti-PC drugs (e.g. small molecule inhibitors from a drug library) for subsequent global studies on PC function.
In addition to targeting mucins themselves for downregulation, a different strategy seeks to use the mucin gene promoter to drive the expression of genes that, in turn, will induce tumor cell death. The technique, called suicide gene therapy, or gene-directed enzyme prodrug therapy (GDEPT), is a technique of targeted chemotherapy in which tumor cells are genetically modified to express suicide genes and produce enzymes capable of converting nontoxic prodrugs into powerful cytotoxic agents [81]. Preclinical studies of suicide gene therapy under the control of MUC1 promoter sequences have been evaluated both in vitro and in vivo. In many of these studies, the MUC1 promoter was linked to the herpes simplex virus thymidine kinase (HSV-TK) gene. This GDEPT approach conferred greater sensitivity to the prodrug ganciclovir in MUC1-expressing breast [82–84] and pancreatic [84] cancer cell lines and showed that it was possible to induce tumor cell death using mucin (MUC1) promoters.
In a 2005 study, recombinant vectors expressing the suicide gene E. coli purine nucleoside phosphorylase (ePNP) under control of MUC1 promoter were designed and stably transfected into MUC1-positive (BxPC3) and MUC1-negative (Panc-1) cells lines [85]. Upon treatment with the prodrug 6-methylpurine deoxyribose (MePdR), high in vitro cytotoxicity was induced in the MUC1-producing cells. Furthermore, prodrug treatment induced a significant tumor reg ression on transfected MUC1-positiv e BxPC3 xenografts, but not in the transfected MUC1-negative Panc1 tumors. A high bystander killing effect was also seen, and the authors suggested that 10–25% of ePNP-transduced cells are sufficient to induce a complete tumor regression and eradicate most of the tumors. Although this study did not overcome the major challenge of efficient in vivo gene delivery, it did demonstrate the practical feasibility of a transcriptional targeting strategy under the control of the MUC1 promoter, thereby suggesting the possibility of a clinically applicable method to preferentially kill MUC1-producing pancreatic tumor cells. Since these MUC1 promoter preclinical studies show some promise in targeting gene delivery specifically to MUC1-expressing cancer cells, we suggest that using other mucin promoters (e.g. MUC4, MUC5AC, MUC16) may have the same targeting ability.
A clinical trial of mucin-targeted gene therapy in PC was reported four years ago [86] Table 1. The formulation, which consisted of the co-expression of the tumor antigens MUC1 and carcinoembryonic antigen (CEA) and costimulatory molecules within poxviruses, was evaluated in a phase I clinical trial given to 10 patients with advanced PC. The vaccine, also known as PANVACVF, consisted of attenuated recombinant vaccine and fowl pox viral vectors expressing CEA and MUC1 and the costimulatory molecules B7.1, ICAM-1 (inter-cellular adhesion molecule 1), and LFA-3 (Lymphocyte function associated antigen 3), and was co-injected with GM-CSF (granulocyte-macrophage colony-stimulating factor). A significant increase in overall survival was observed in patients that presented CEA and/or MUC1 antigen-specific T cell immune responses. Although the results of the study were promising, the company that synthesized the vaccine filed for bankruptcy protection after failed attempts to significantly improve clinical results [87].
CONCLUSIONS AND PERSPECTIVES
PC is a lethal disease and its management is an ongoing challenge. Mucins have been implicated in the diagnosis, prognosis, and progression of PC. Deregulation of expression, differential glycosylation, and alternative splicing of MUCs is being investigated and has potentials for the development of new generation of therapeutics against this disease. MUC1, MUC4, MUC5AC, and MUC16 are finding unique functions in the early diagnosis and progression of the disease.
The preceding discussion suggests that while several attempts have been made to target MUC1, only a handful have shown promise and some of these are still ongoing. Clearly, two things are desperately needed: first, studies that target other mucins, particularly mucins like MUC4 that are unique in that they are not expressed in any of the normal pancreatic components (ducts, acini or islets), and second, novel therapeutic approaches (including small molecule inhibitors and natural-derived compounds) to target mucins in PC. We and others are actively studying the potential of MUC4, MUC5AC, and some new MUCs in targeted PC therapies. Recently, we reported the potential of the natural-derived drug thymoquinone in downregulating MUC4 expression in PC cells [88] and are currently screening other drugs that target this mucin in order to optimize PC therapies. Nevertheless, appropriate animal models to evaluate these therapies are still needed.
A recent overview of clinically-tested cancer vaccines indicates that immunotherapies are more likely to be successful either in patients with early stage tumors or in those in whom tumors have been reduced as a result of surgeries or chemotherapies [87]. Unfortunately, most of the mucin-based clinical trials are conducted in immune-compromised patients at an advanced stage of the malignancy and with a large tumor burden, possibly contributing to the failure of cancer vaccines. This is probably one of the main reasons behind the poor transferability of preclinical evaluations of mucin-based targeted PC therapies to the clinic. At present, however, the availability of genetically-engineered mouse models that bear high resemblance with human PC will greatly advance our understanding of PC in order to design more suitable therapies against this malignant disease. Perhaps one of the main advancements of using these animal models is the understanding of the contribution of the pancreatic tumor stromal microenvironment to tumor progression and therapeutic resistance, which until now has remained poorly understood [89].
A recent genetically-engineered mouse model of spontaneous pancreatic adenocarcinoma, which expresses MUC1 (PC.MUC1) has been developed [90]. It was reported that MUC1 enhanced the progression of pre-malignant pancreatic ductal lesions (termed as pancreatic intraepithelial neoplasia or PanINs) to adenocarcinomas. More specifically, 80% of PC.MUC1 mice developed invasive carcinomas by 26 weeks of age, as compared to the age-matched PC mice, where only 10% developed the same malignant phenotype. This spontaneous PC model clearly indicates that MUC1 plays a major role in PC progression and the availability of this animal model is ideal for preclinical evaluation of therapies against MUC1. In this regard, a novel vaccine formulation that consisted of MUC1-based vaccine and a cyclooxygenase-2 inhibitor (COX-2), commonly known as Celecoxib, was evaluated on the PC.MUC1 triple transgenic model [91]. The study reported that PC.MUC1 mice receiving the MUC1 vaccine in combination with Celecoxib were able to evade the natural progression of PanIN lesions to invasive PC. Interestingly, the MUC1 vaccine was only effective against PC development if it was given in combination with the COX-2 inhibitor, even if strong anti-MUC1 immune responses were present. These results clearly indicate that an optimum PC therapy must target different malignant pathways simultaneously in order to improve the efficacy.
The relationship between mucins and PC has been investigated over the last four decades and there is absolutely no doubt that these glycoproteins represent potential therapeutic targets against PC. As we discussed in this review, MUC1 has been the mucin glycoprotein most evaluated on clinical trials in PC patients and the recent availability of animal models of spontaneous pancreatic adenocarcinoma expressing MUC1 represents a great advance to improve its evaluation on clinical studies. Additionally, the potential of other mucins related to PC progression, such as MUC4, MUC5AC, and MUC16, must also be evaluated extensively. Perhaps, an optimum PC therapy must involve a combination of chemotherapeutic drugs administered along with formulations that target several mucins in pancreatic tumors. Although PC therapy has not improved over the last 30 years, there is clear evidence that we are getting closer to a more suitable design of novel formulations to improve the poor survival rates of this malignancy in the coming years and mucins have proven to be valuable targets against this malignancy.
ACKNOWLEDGMENTS
The authors on this work are, in part, supported by grants from the National Institutes of Health (T32CA009479, RO1 CA78590, UO1 CA111294, RO1 CA133774, RO1 CA131944, and P50 CA127297) and the Department of Defense (PC074289 and BC074639). We thank Dr. Maneesh Jain, UNMC for his valuable comments and Ms. Kristi L. Berger for editing the manuscript.
ABBREVIATIONS
- PC
Pancreatic cancer
- MUC
Mucin
- EGFR
Epidermal growth factor receptor
- EGF
Epidermal growth factor
- MCAM
Melanoma cell adhesion molecule
- AMOP
Adhesion associated domain in MUC4 and other proteins
- VWD
Von willebrand factor D
- SEA
Sea urchin sperm protein Enterokinase and Agrin
- NIDO
Nidogen domains
- VNTR
Variable number of tandem repeat
- CK
Cysteine knot
- BCG
Bacillus-Calmette-Guérin
- DTH
Delayed-type hypersensitive
- DCs
Dendritic cells
- IFNγ
Interferon gamma
- HLA
Human leukocyte antigen
- PADRE
Pan HLA DR-reactive epitope
- PBMCs
Peripheral blood mononuclear cells
- FDA
The U.S. Food and Drug Administration
- RNAi
RNA interference
- GDEPT
Gene-directed enzyme prodrug therapy
- HSV-TK
Herpes simplex virus thymidine kinase
- ePNP
E. coli purine nucleoside phosphorylase
- ICAM-1
Inter-cellular adhesion molecule 1
- LFA-3
Lymphocyte function associated antigen 3
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- COX-2
Cyclooxygenase-2 inhibitor
REFERENCES
- [1].Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- [2].Wong HH, Lemoine NR. Pancreatic cancer: molecular pathogenesis and new therapeutic targets. Nature Rev Gastroenterol Hepatol. 2009;6(7):412–22. doi: 10.1038/nrgastro.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chakraborty S, Baine MJ, Sasson AR, Batra SK. Current status of molecular markers for early detection of sporadic pancreatic cancer. Biochim Biophys Acta. 2011;1815(1):44–64. doi: 10.1016/j.bbcan.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cartwright T, Richards DA, Boehm KA. Cancer of the pancreas: are we making progress? A review of studies in the US Oncology Research Network. Cancer Control. 2008;15(4):308–13. doi: 10.1177/107327480801500405. [DOI] [PubMed] [Google Scholar]
- [5].Lockhart AC, Rothenberg ML, Berlin JD. Treatment for pancreatic cancer: current therapy and continued progress. Gastroenterology. 2005;128(6):1642–54. doi: 10.1053/j.gastro.2005.03.039. [DOI] [PubMed] [Google Scholar]
- [6].El Maalouf G, Le Tourneau C, Batty GN, Faivre S, Raymond E. Markers involved in resistance to cytotoxics and targeted therapeutics in pancreatic cancer. Cancer Treat Rev. 2009;35(2):167–74. doi: 10.1016/j.ctrv.2008.10.002. [DOI] [PubMed] [Google Scholar]
- [7].Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J Am Coll Surg. 1999;189(1):1–7. doi: 10.1016/s1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- [8].Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Neesse A, Michl P, Frese KK, et al. Stromal biology and therapy in pancreatic cancer. Gut. 2010 doi: 10.1136/gut.2010.226092. In press. [DOI] [PubMed] [Google Scholar]
- [10].Andrianifahanana M, Moniaux N, Schmied BM, et al. Mucin (MUC) gene expression in human pancreatic adenocarcinoma and chronic pancreatitis: a potential role of MUC4 as a tumor marker of diagnostic significance. Clin Cancer Res. 2001;7(12):4033–40. [PubMed] [Google Scholar]
- [11].Hollingsworth MA, Strawhecker JM, Caffrey TC, Mack DR. Expression of MUC1, MUC2, MUC3 and MUC4 mucin mRNAs in human pancreatic and intestinal tumor cell lines. uInt J Cancer. 1994;57(2):198–203. doi: 10.1002/ijc.2910570212. [DOI] [PubMed] [Google Scholar]
- [12].McDermott KM, Crocker PR, Harris A, et al. Overexpression of MUC1 reconfigures the binding properties of tumor cells. International journal of cancer. 2001;94(6):783–91. doi: 10.1002/ijc.1554. [DOI] [PubMed] [Google Scholar]
- [13].Swartz MJ, Batra SK, Varshney GC, et al. MUC4 expression increases progressively in pancreatic intraepithelial neoplasia. Am J Clin Pathol. 2002;117(5):791–6. doi: 10.1309/7Y7N-M1WM-R0YK-M2VA. [DOI] [PubMed] [Google Scholar]
- [14].Bafna S, Kaur S, Momi N, Batra SK. Pancreatic cancer cells resistance to gemcitabine: the role of MUC4 mucin. Br J Cancer. 2009;101(7):1155–61. doi: 10.1038/sj.bjc.6605285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kalra AV, Campbell RB. Mucin overexpression limits the effectiveness of 5-FU by reducing intracellular drug uptake and antineo-plastic drug effects in pancreatic tumours. Eur J Cancer. 2009;45(1):164–73. doi: 10.1016/j.ejca.2008.10.008. [DOI] [PubMed] [Google Scholar]
- [16].Mimeault M, Johansson SL, Senapati S, Momi N, Chakraborty S, Batra SK. MUC4 down-regulation reverses chemoresistance of pancreatic cancer stem/progenitor cells and their progenies. Cancer Lett. 2010;295(1):69–84. doi: 10.1016/j.canlet.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rachagani S, Torres MP, Moniaux N, Batra SK. Current status of mucins in the diagnosis and therapy of cancer. Biofactors. 2009;35(6):509–27. doi: 10.1002/biof.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Corfield AP, Myerscough N, Longman R, Sylvester P, Arul S, Pignatelli M. Mucins and mucosal protection in the gastrointestinal tract: new prospects for mucins in the pathology of gastrointestinal disease. Gut. 2000;47(4):589–94. doi: 10.1136/gut.47.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chakraborty S, Bonthu N, Swanson BJ, Batra SK. Role of mucins in the skin during benign and malignant conditions. Cancer Lett. 2011;301(2):127–41. doi: 10.1016/j.canlet.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23(1–2):77–99. doi: 10.1023/a:1025815113599. [DOI] [PubMed] [Google Scholar]
- [21].Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4(1):45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- [22].Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23(1–2):77–99. doi: 10.1023/a:1025815113599. [DOI] [PubMed] [Google Scholar]
- [23].Singh AP, Senapati S, Ponnusamy MP, et al. Clinical potential of mucins in diagnosis, prognosis, and therapy of ovarian cancer. Lancet Oncol. 2008;9(11):1076–85. doi: 10.1016/S1470-2045(08)70277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Singh AP, Chaturvedi P, Batra SK. Emerging roles of MUC4 in cancer: a novel target for diagnosis and therapy. Cancer Res. 2007;67(2):433–6. doi: 10.1158/0008-5472.CAN-06-3114. [DOI] [PubMed] [Google Scholar]
- [25].Perez-Vilar J, Hill RL. The structure and assembly of secreted mucins. J Biol Chem. 1999;274(45):31751–4. doi: 10.1074/jbc.274.45.31751. [DOI] [PubMed] [Google Scholar]
- [26].Senapati S, Das S, Batra SK. Mucin-interacting proteins: from function to therapeutics. Trends Biochem Sci. 2010;35(4):236–45. doi: 10.1016/j.tibs.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wittel UA, Goel A, Varshney GC, Batra SK. Mucin antibodies - new tools in diagnosis and therapy of cancer. Front Biosci. 2001;6:D1296–310. doi: 10.2741/wittel. [DOI] [PubMed] [Google Scholar]
- [28].Jonckheere N, Van S I. The membrane-bound mucins: From cell signalling to transcriptional regulation and expression in epithelial cancers. Biochimie. 2010;92(1):1–11. doi: 10.1016/j.biochi.2009.09.018. [DOI] [PubMed] [Google Scholar]
- [29].Kohlgraf KG, Gawron AJ, Higashi M, et al. Contribution of the MUC1 tandem repeat and cytoplasmic tail to invasive and metastatic properties of a pancreatic cancer cell line. Cancer Res. 2003;63(16):5011–20. [PubMed] [Google Scholar]
- [30].Tsutsumida H, Swanson BJ, Singh PK, et al. RNA interference suppression of MUC1 reduces the growth rate and metastatic phenotype of human pancreatic cancer cells. Clin Cancer Res. 2006;12(10):2976–87. doi: 10.1158/1078-0432.CCR-05-1197. [DOI] [PubMed] [Google Scholar]
- [31].Chaturvedi P, Singh AP, Moniaux N, et al. MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins. Mol Cancer Res. 2007;5(4):309–20. doi: 10.1158/1541-7786.MCR-06-0353. [DOI] [PubMed] [Google Scholar]
- [32].Singh AP, Moniaux N, Chauhan SC, Meza JL, Batra SK. Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res. 2004;64(2):622–30. doi: 10.1158/0008-5472.can-03-2636. [DOI] [PubMed] [Google Scholar]
- [33].Hu YP, Haq B, Carraway KL, Savaraj N, Lampidis TJ. Multidrug resistance correlates with overexpression of Muc4 but inversely with P-glycoprotein and multidrug resistance related protein in transfected human melanoma cells. Biochem Pharmacol. 2003;65(9):1419–25. doi: 10.1016/s0006-2952(03)00086-8. [DOI] [PubMed] [Google Scholar]
- [34].Siragusa M, Zerilli M, Iovino F, et al. MUC1 oncoprotein promotes refractoriness to chemotherapy in thyroid cancer cells. Cancer Res. 2007;67(11):5522–30. doi: 10.1158/0008-5472.CAN-06-4197. [DOI] [PubMed] [Google Scholar]
- [35].Fessler SP, Wotkowicz MT, Mahanta SK, Bamdad C. MUC1* is a determinant of trastuzumab (Herceptin) resistance in breast cancer cells. Breast Cancer Res Treat. 2009;118(1):113–24. doi: 10.1007/s10549-009-0412-3. [DOI] [PubMed] [Google Scholar]
- [36].Laheru D, Jaffee EM. Immunotherapy for pancreatic cancer - science driving clinical progress. Nat Rev Cancer. 2005;5(6):459–67. doi: 10.1038/nrc1630. [DOI] [PubMed] [Google Scholar]
- [37].Rowse GJ, Tempero RM, VanLith ML, Hollingsworth MA, Gendler SJ. Tolerance and immunity to MUC1 in a human MUC1 transgenic murine model. Cancer Res. 1998;58(2):315–21. [PubMed] [Google Scholar]
- [38].Tempero RM, VanLith ML, Morikane K, Rowse GJ, Gendler SJ, Hollingsworth MA. CD4+ lymphocytes provide MUC1-specific tumor immunity in vivo that is undetectable in vitro and is absent in MUC1 transgenic mice. J Immunol. 1998;161(10):5500–6. [PubMed] [Google Scholar]
- [39].Goydos JS, Elder E, Whiteside TL, Finn OJ, Lotze MT. A phase I trial of a synthetic mucin peptide vaccine. Induction of specific immune reactivity in patients with adenocarcinoma. The J Surg Res. 1996;63(1):298–304. doi: 10.1006/jsre.1996.0264. [DOI] [PubMed] [Google Scholar]
- [40].Lepisto AJ, Moser AJ, Zeh H, et al. A phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Ther. 2008;6(B):955–64. [PMC free article] [PubMed] [Google Scholar]
- [41].Ramanathan RK, Lee KM, McKolanis J, et al. Phase I study of a MUC1 vaccine composed of different doses of MUC1 peptide with SB-AS2 adjuvant in resected and locally advanced pancreatic cancer. Cancer Immunol Immunother. 2005;54(3):254–64. doi: 10.1007/s00262-004-0581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Vlad AM, Kettel JC, Alajez NM, Carlos CA, Finn OJ. MUC1 immunobiology: from discovery to clinical applications. Adv Immunol. 2004;82:249–93. doi: 10.1016/S0065-2776(04)82006-6. [DOI] [PubMed] [Google Scholar]
- [43].Yamamoto K, Ueno T, Kawaoka T, et al. MUC1 peptide vaccination in patients with advanced pancreas or biliary tract cancer. Anticancer Res. 2005;25(5):3575–9. [PubMed] [Google Scholar]
- [44].Soares MM, Mehta V, Finn OJ. Three different vaccines based on the 140-amino acid MUC1 peptide with seven tandemly repeated tumor-specific epitopes elicit distinct immune effector mechanisms in wild-type versus MUC1-transgenic mice with different potential for tumor rejection. J Immunol. 2001;166(11):6555–63. doi: 10.4049/jimmunol.166.11.6555. [DOI] [PubMed] [Google Scholar]
- [45].Pecher G, Haring A, Kaiser L, Thiel E. Mucin gene (MUC1) transfected dendritic cells as vaccine: results of a phase I/II clinical trial. Cancer Immunol Immunother. 2002;51(11-12):669–73. doi: 10.1007/s00262-002-0317-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kawaoka T, Oka M, Takashima M, et al. Adoptive immunotherapy for pancreatic cancer: cytotoxic T lymphocytes stimulated by the MUC1-expressing human pancreatic cancer cell line YPK-1. Oncol Rep. 2008;20(1):155–63. [PubMed] [Google Scholar]
- [47].Kawaoka T, Takashima M, Yamamoto K, Ueno T, Oka M. [Adoptive immunotherapy using MUC1--specific CTLs for unresectable pancreatic cancer] Nippon Rinsho. 2006;64(Suppl 1):279–82. [PubMed] [Google Scholar]
- [48].Kondo H, Hazama S, Kawaoka T, et al. Adoptive immunotherapy for pancreatic cancer using MUC1 peptide-pulsed dendritic cells and activated T lymphocytes. Anticancer Res. 2008;28(1B):379–87. [PubMed] [Google Scholar]
- [49].Wei J, Gao W, Wu J, et al. Dendritic cells expressing a combined PADRE/MUC4-derived polyepitope DNA vaccine induce multiple cytotoxic T-cell responses. Cancer Biother Radiopharm. 2008;23(1):121–8. doi: 10.1089/cbr.2007.0427. [DOI] [PubMed] [Google Scholar]
- [50].Wu J, Wei J, Meng K, et al. Identification of an HLA-A*0201-restrictive CTL epitope from MUC4 for applicable vaccine therapy. Immunopharmacol Immunotoxicol. 2009;31(3):468–76. doi: 10.1080/08923970902795203. [DOI] [PubMed] [Google Scholar]
- [51].Fernandez-Zapico ME, Kaczynski JA, Urrutia R. Pancreatic cancer research: challenges, opportunities, and recent developments. Curr Opin Gastroenterol. 2002;18(5):563–7. doi: 10.1097/00001574-200209000-00007. [DOI] [PubMed] [Google Scholar]
- [52].Hansen K, Khanna C. Spontaneous and genetically engineered animal models; use in preclinical cancer drug development. Eur J Cancer. 2004;40(6):858–80. doi: 10.1016/j.ejca.2003.11.031. [DOI] [PubMed] [Google Scholar]
- [53].Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4(6):437–50. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- [54].Tarp MA, Clausen H. Mucin-type O-glycosylation and its potential use in drug and vaccine development. Biochim Biophys Acta. 2008;1780(3):546–63. doi: 10.1016/j.bbagen.2007.09.010. [DOI] [PubMed] [Google Scholar]
- [55].Sorensen AL, Reis CA, Tarp MA, et al. Chemoenzymatically synthesized multimeric Tn/STn MUC1 glycopeptides elicit cancer-specific anti-MUC1 antibody responses and override tolerance. Glycobiology. 2006;16(2):96–107. doi: 10.1093/glycob/cwj044. [DOI] [PubMed] [Google Scholar]
- [56].Dziadek S, Kunz H. Synthesis of tumor-associated glycopeptide antigens for the development of tumor-selective vaccines. Chem Rec. 2004;3(6):308–21. doi: 10.1002/tcr.10074. [DOI] [PubMed] [Google Scholar]
- [57].Price MR, Rye PD, Petrakou E, et al. Summary report on the ISOBM TD-4 Workshop: analysis of 56 monoclonal antibodies against the MUC1 mucin. San Diego, Calif., November 17–23, 1996. Tumour Biol. 1998;19(Suppl 1):1–20. doi: 10.1159/000056500. [DOI] [PubMed] [Google Scholar]
- [58].Karsten U, Serttas N, Paulsen H, Danielczyk A, Goletz S. Binding patterns of DTR-specific antibodies reveal a glycosylation-conditioned tumor-specific epitope of the epithelial mucin (MUC1) Glycobiology. 2004;14(8):681–92. doi: 10.1093/glycob/cwh090. [DOI] [PubMed] [Google Scholar]
- [59].Danielczyk A, Stahn R, Faulstich D, et al. PankoMab: a potent new generation anti-tumour MUC1 antibody. Cancer Immunol Immunother. 2006;55(11):1337–47. doi: 10.1007/s00262-006-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Fan XN, Karsten U, Goletz S, Cao Y. Reactivity of a humanized antibody (hPankoMab) towards a tumor-related MUC1 epitope (TA-MUC1) with various human carcinomas. Pathol Res Pract. 2010;206(8):585–9. doi: 10.1016/j.prp.2010.03.006. [DOI] [PubMed] [Google Scholar]
- [61].Ehlen TG, Hoskins PJ, Miller D, et al. A pilot phase 2 study of oregovomab murine monoclonal antibody to CA125 as an immuno-therapeutic agent for recurrent ovarian cancer. Int J Gynecol Cancer. 2005;15(6):1023–34. doi: 10.1111/j.1525-1438.2005.00483.x. [DOI] [PubMed] [Google Scholar]
- [62].Noujaim AA, Schultes BC, Baum RP, Madiyalakan R. Induction of CA125-specific B and T cell responses in patients injected with MAb-B43.13--evidence for antibody-mediated antigen-processing and presentation of CA125 in vivo. Cancer Biother Radiopharm. 2001;16(3):187–203. doi: 10.1089/10849780152389384. [DOI] [PubMed] [Google Scholar]
- [63].Ho M, Feng M, Fisher RJ, Rader C, Pastan I. A novel high affinity human monoclonal antibody to mesothelin. Int J Cancer. 2010 doi: 10.1002/ijc.25557. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hassan R, Ebel W, Routhier EL, et al. Preclinical evaluation of MORAb-009, a chimeric antibody targeting tumor-associated mesothelin. Cancer Immun. 2007;7:20. [PMC free article] [PubMed] [Google Scholar]
- [65].Gubbels JA, Belisle J, Onda M, et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer. 2006;5(1):50. doi: 10.1186/1476-4598-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hassan R, Cohen SJ, Phillips M, et al. Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. Clin Cancer Res. 2010;16(24):6132–8. doi: 10.1158/1078-0432.CCR-10-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Cardillo TM, Blumenthal R, Ying Z, Gold DV. Combined gemcitabine and radioimmunotherapy for the treatment of pancreatic cancer. Int J Cancer. 2002;97(3):386–92. doi: 10.1002/ijc.1613. [DOI] [PubMed] [Google Scholar]
- [68].Cardillo TM, Ying Z, Gold DV. Therapeutic advantage of (90)yttrium- versus (131)iodine-labeled PAM4 antibody in experimental pancreatic cancer. Clin Cancer Res. 2001;7(10):3186–92. [PubMed] [Google Scholar]
- [69].Gold DV, Cardillo T, Goldenberg DM, Sharkey RM. Localization of pancreatic cancer with radiolabeled monoclonal antibody PAM4. Crit Rev Oncol Hematol. 2001;39(1–2):147–54. doi: 10.1016/s1040-8428(01)00114-7. [DOI] [PubMed] [Google Scholar]
- [70].Gold DV, Lew K, Maliniak R, Hernandez M, Cardillo T. Characterization of monoclonal antibody PAM4 reactive with a pancreatic cancer mucin. Int J Cancer. 1994;57(2):204–10. doi: 10.1002/ijc.2910570213. [DOI] [PubMed] [Google Scholar]
- [71].Gulec SA, Cohen SJ, Pennington KL, et al. Treatment of advanced pancreatic carcinoma with 90Y-clivatuzumab tetraxetan: a phase I single dose escalation trial. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-10-2579. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Tempero M, Leichner P, Baranowska-Kortylewicz J, et al. High-dose therapy with 90Yttrium-labeled monoclonal antibody CC49: a phase I trial. Clin Cancer Res. 2000;6(8):3095–102. [PubMed] [Google Scholar]
- [73].Qu CF, Songl YJ, Rizvi SM, et al. In vivo and in vitro inhibition of pancreatic cancer growth by targeted alpha therapy using 213Bi-CHX.A”-C595. Cancer Biol Ther. 2005;4(8):848–53. doi: 10.4161/cbt.4.8.1892. [DOI] [PubMed] [Google Scholar]
- [74].Allen BJ, Raja C, Rizvi S, et al. Targeted alpha therapy for cancer. Phys Med Biol. 2004;49(16):3703–12. doi: 10.1088/0031-9155/49/16/016. [DOI] [PubMed] [Google Scholar]
- [75].Gene Therapy Clinical Trials Worldwide website. J Gene Med. 2011 http://www.wiley.com/legacy/wileychi/genmed/clinical/. (viewed 5/1/11)
- [76].Tsutsumida H, Swanson BJ, Singh PK, et al. RNA interference suppression of MUC1 reduces the growth rate and metastatic phenotype of human pancreatic cancer cells. Clin Cancer Res. 2006;12(10):2976–87. doi: 10.1158/1078-0432.CCR-05-1197. [DOI] [PubMed] [Google Scholar]
- [77].Yuan Z, Wong S, Borrelli A, Chung MA. Down-regulation of MUC1 in cancer cells inhibits cell migration by promoting E-cadherin/catenin complex formation. Biochem Biophys Res Commun. 2007;362(3):740–6. doi: 10.1016/j.bbrc.2007.08.074. [DOI] [PubMed] [Google Scholar]
- [78].Yamazoe S, Tanaka H, Sawada T, et al. RNA interference suppression of mucin 5AC (MUC5AC) reduces the adhesive and invasive capacity of human pancreatic cancer cells. J Exp Clin Cancer Res. 2010;29:53. doi: 10.1186/1756-9966-29-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Hoshi H, Sawada T, Uchida M, et al. Tumor-associated MUC5AC stimulates in vivo tumorigenicity of human pancreatic cancer. Int J Oncol. 2011;38(3):619–27. doi: 10.3892/ijo.2011.911. [DOI] [PubMed] [Google Scholar]
- [80].Pecot CV, Calin GA, Coleman RL, Lopez-Berestein G, Sood AK. RNA interference in the clinic: challenges and future directions. Nat Rev Cancer. 2011;11(1):59–67. doi: 10.1038/nrc2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Aghi M, Hochberg F, Breakefield XO. Prodrug activation enzymes in cancer gene therapy. J Gene Med. 2000;2(3):148–64. doi: 10.1002/(SICI)1521-2254(200005/06)2:3<148::AID-JGM105>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- [82].Chen L, Chen D, Manome Y, Dong Y, Fine HA, Kufe DW. Breast cancer selective gene expression and therapy mediated by recombinant adenoviruses containing the DF3/MUC1 promoter. J Clin Invest. 1995;96(6):2775–82. doi: 10.1172/JCI118347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Manome Y, Abe M, Hagen MF, Fine HA, Kufe DW. Enhancer sequences of the DF3 gene regulate expression of the herpes simplex virus thymidine kinase gene and confer sensitivity of human breast cancer cells to ganciclovir. Cancer Res. 1994;54(20):5408–13. [PubMed] [Google Scholar]
- [84].Ring CJ, Blouin P, Martin LA, Hurst HC, Lemoine NR. Use of transcriptional regulatory elements of the MUC1 and ERBB2 genes to drive tumour-selective expression of a prodrug activating enzyme. Gene Ther. 1997;4(10):1045–52. doi: 10.1038/sj.gt.3300510. [DOI] [PubMed] [Google Scholar]
- [85].Deharvengt S, Wack S, Aprahamian M, Hajri A. Transcriptional tumor-selective promoter targeting of E. coli purine nucleoside phosphorylase for pancreatic cancer suicide gene therapy. J Gene Med. 2005;7(5):672–80. doi: 10.1002/jgm.701. [DOI] [PubMed] [Google Scholar]
- [86].Kaufman HL, Kim-Schulze S, Manson K, et al. Poxvirus-based vaccine therapy for patients with advanced pancreatic cancer. J Transl Med. 2007;5:60. doi: 10.1186/1479-5876-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Goldman B, DeFrancesco L. The cancer vaccine roller coaster. Nat Biotechnol. 2009;27(2):129–39. doi: 10.1038/nbt0209-129. [DOI] [PubMed] [Google Scholar]
- [88].Torres MP, Ponnusamy MP, Chakraborty S, et al. Effects of thymoquinone in the expression of mucin 4 in pancreatic cancer cells: implications for the development of novel cancer therapies. Mol Cancer Ther. 2010;9(5):1419–31. doi: 10.1158/1535-7163.MCT-10-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Guturu P, Shah V, Urrutia R. Interplay of tumor microenvironment cell types with parenchymal cells in pancreatic cancer development and therapeutic implications. J Gastrointest Cancer. 2009;40(1–2):1–9. doi: 10.1007/s12029-009-9071-1. [DOI] [PubMed] [Google Scholar]
- [90].Tinder TL, Subramani DB, Basu GD, et al. MUC1 enhances tumor progression and contributes toward immunosuppression in a mouse model of spontaneous pancreatic adenocarcinoma. J Immunol. 2008;181(5):3116–25. doi: 10.4049/jimmunol.181.5.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Mukherjee P, Basu GD, Tinder TL, et al. Progression of pancreatic adenocarcinoma is significantly impeded with a combination of vaccine and COX-2 inhibition. J Immunol. 2009;182(1):216–24. [PMC free article] [PubMed] [Google Scholar]