Abstract
Congenital heart defects often include altered conduction as well as morphological changes. Model organisms, like the frog Xenopus laevis, offer practical advantages for the study of congenital heart disease. X. laevis embryos are easily obtained free living, and the developing heart is readily visualized. Functional and morphological evidence for a conduction system is available for adult frog hearts, but information on the normal properties of embryonic heart contraction is lacking, especially in intact animals. With the use of fine glass microelectrodes, we were able to obtain cardiac recordings and make standard electrophysiological measurements in 1-wk-old embryos (stage 46). In addition, a system using digital analysis of video images was adapted for measurement of the standard cardiac intervals and compared with invasive measurements. Video images were obtained of the heart in live, pharmacologically paralyzed, stage 46 X. laevis embryos. Normal values for the timing of the cardiac cycle were established. Intervals determined by video analysis (n = 53), including the atrial and ventricular cycle lengths (473 ± 10 ms and 464 ± 19 ms, respectively) and the atrioventricular interval (169 ± 5 ms) were not statistically different from those determined by intrathoracic cardiac recordings. We also present the data obtained from embryos treated with standard medications that affect the human conduction system. We conclude that the physiology of embryonic X. laevis cardiac conduction can be noninvasively studied by using digital video imaging. Additionally, we show the response of X. laevis embryonic hearts to chronotropic agents is similar but not identical to the response of the human heart.
Proper cardiac function requires an organized electrical conduction system to coordinate contraction. In humans, this system consists of the cardiac pacemaker, the sinoatrial node, a conduction relay from the atria to the ventricles, the atrioventricular node, and the His-Purkinje system, which synchronizes ventricular contraction. Formation of the conduction network begins early in organogenesis (23). Defects in this network are sometimes coupled with anatomic abnormalities (20). Accordingly, congenital heart disease is often complicated by defects in the cardiac conduction system. A complete understanding of congenital heart disease requires a thorough understanding of conduction system development.
Humans and many organisms typically used for laboratory studies share common patterns of gene expression and morphological detail during heart development. For example, the mouse is frequently used as a model for studying the effects of genetic mutations on conduction system development (6). Mutations associated with congenital heart disease have demonstrated associated atrioventricular conduction defects in early infancy as is seen in mutations of the transcription factor Nkx 2–5 (14, 20). Significant drawbacks to studying development of the conduction system in the mouse include the difficulty of assessing the embryo and the labor required to create murine mutations. Developmental consequences are typically analyzed after birth. Ideally, analysis of the effects of genetic mutations on the conduction system would be performed during embryogenesis and correlated with other developmental events.
Xenopus laevis is an animal model that lends itself to studies on the regulation of early development. Experimental manipulations can be readily performed on the embryo including injection of mRNA encoding normal or mutant forms of protein of interest, injection of oligonucleotides to reduce expression of protein, and generation of transgenic embryos. The developmental effects of experimental manipulations can be closely monitored through embryogenesis, because development occurs outside of the mother. Historical descriptions (17) of cardiovascular system development have been recently augmented by using modern techniques. Computer-assisted reconstruction of serial sections of the developing heart (16) and whole-mount immunohistochemistry imaged using confocal microscopy (15) allow a ready source of anatomic information. In X. laevis embryos, the developing heart can be viewed, starting with formation of the precardiac field through the establishment of a fully functional three-chambered heart [Nieuwkoop and Faber (17), stage 46] in ~1 wk after fertilization of the egg.
The present morphological understanding of embryonic heart formation is not matched by an equal level of information about the development and physiology of the conduction system in X. laevis. Properties of a formal conduction system are present in X. laevis and other amphibians (11). Recent studies by Sedmera et al. (21) using adult frogs and fish provide strong evidence that, although conduction system morphology is not identical when one compares frogs to mammals, functional similarities do exist.
The goal of this study was to develop a nondestructive method of assessing the conduction system physiology in stage 46 X. laevis embryos when the functional heart is present. Imaging techniques have been successfully used to assess temporal physiology in other settings including jejunal pacemakers and can be used to advantage in cardiac studies (4). Noninvasive cardiac imaging should allow investigation of electrophysiological function related to experimental manipulations during embryogenesis, when anatomic changes from the treatments are apparent. This study analyzes relevant physiological properties of the developing X. laevis conduction system and the cardiac response to pharmacological agents that typically alter cardiac electrophysiology.
MATERIALS AND METHODS
Animals
X. laevis were purchased from Xenopus I (Ann Arbor, MI). Females were induced to lay eggs by injection with 800 units of human chorionic gonadotropin (Sigma-Aldrich). Testes were removed from male frogs that had been euthanized with 1 ml of 10 mg/ml solution of tricaine (Sigma-Aldrich). A small piece of testes was crushed in 0.1 Marc’s modified Ringer [MMR; 1 × MMR is (in mM) 100 NaCl, 2 KCl, 1 MgCl, 2 CaCl2, and 5 HEPES, pH 7.4]. Eggs were collected and immediately fertilized with the testes solution and flooded with 0.1 MMR. The embryos were allowed to develop at room temperature. Embryonic stages were identified by criteria described in Nieuwkoop and Faber (17). Animal care and use for these studies conform to the Animal Welfare Assurance guidelines and were reviewed by the University of Iowa Institutional Animal Care and Use Committee.
Paralysis
Stage 46 X. laevis embryos were paralyzed utilizing neuromuscular blockade. The embryos were placed in a 3 ml, room temperature (22°C) bath of 0.1 MMR containing 0.33 mg/ml cisatracurium (Abbott) for 15 min, resulting in paralysis. Cisatracurium was selected, because there are no significant cardiovascular effects in humans and the drug is metabolized by Hoffman elimination (22). To assess any cardiac effect of cisatracurium, an alternate agent was used for comparison. Pancuronium (Organon; 0.17 mg/ml) was used in a similar method. Pancuronium bromide is an aminosteroidal competitive neuromuscular blocker that requires renal excretion and is partially metabolized by the liver. In humans, pancuronium bromide causes a dose-dependent increase in heart rate accompanied by increases in cardiac output and blood pressure due to sympathomimetic properties (5, 8). Doses of the paralytic medications were chosen to achieve rapid onset of paralysis while minimizing the duration of effect. Embryos were exposed to both paralyzing agents in a crossover experimental design. Each embryo was placed in 0.1 MMR for 1 h between treatments and fully recovered gross motor function before paralysis and evaluation with the alternate agent.
Intrathoracic cardiac recording
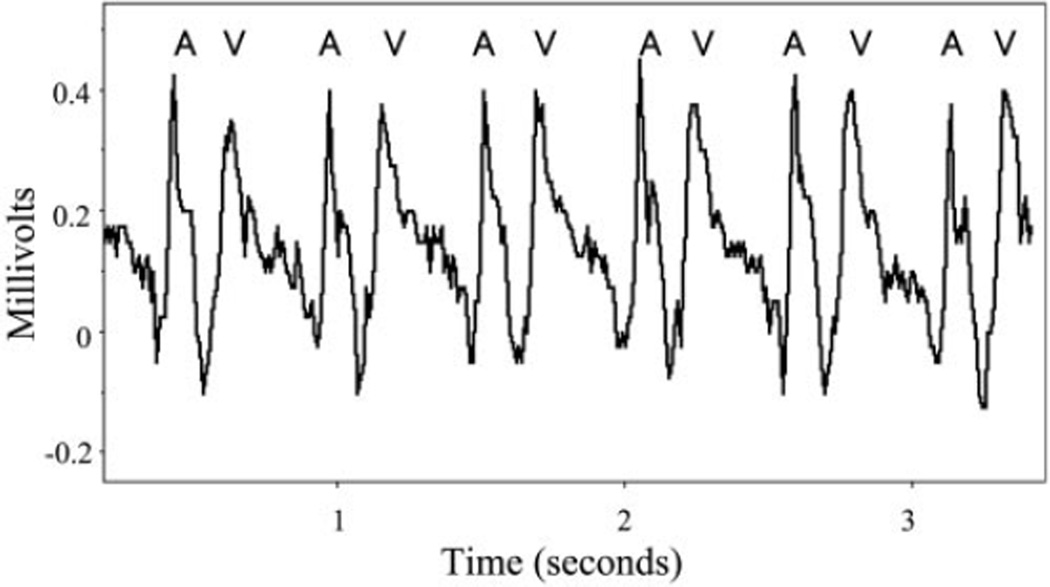
Stage 46 X. laevis embryos were paralyzed by using cisatracurium, as described above. The embryos were then stabilized in a supine position with the dental adhesive, cyanoacrylate (Aron Alpha Industrial Krazy Glue, Type 202; Chembond Adhesives) to a 22-mm glass slide and submerged in 0.1× MMR. Glass microelectrodes were pulled by using a Sutter Instrument micropipette puller filled with 100 mM KCl and attached to a silver wire floating microelectrode holder. The recording electrode was placed in the thorax at the right anterior margin of the heart near the atrioventricular groove. A similar reference microelectrode was positioned at the left lateral aspect of the atria. Cardiac tracings were recorded at 100 Hz using an A-M Systems model 1600 neuroprobe amplifier. No filtering was required. The cardiac intervals were analyzed for three cardiac cycles per embryo and averaged (Fig. 1). The rapid upstroke of the recording suggests the signal is from extracellular field potentials. However, a contribution from mechanical displacement cannot be excluded. Regardless of the genesis of the cardiac signals, they allow for clear measurement of cardiac intervals. These include three key measurements: the AA interval, the rate of atrial contraction that assesses the function of the cardiac pacemaker; the VV interval, the rate of ventricular contraction that measures the functional heart rate that directly correlates to the cardiac output; and the AV interval, which measures the time delay between atrial and ventricular contractions and evaluates the conduction system coordinating the sequential nature of atrial and ventricular contractions, akin to the atrioventricular node in humans and also includes atrial conduction time.
Fig. 1.
Intrathoracic extracellular cardiac recording of a stage 46 Xenopus laevis embryo. A and V, atrial and ventricular waveform in millivolt scale over time.
Video acquisition and analysis
Live, pharmacologically paralyzed, semitransparent stage 46 X. laevis embryos were placed in a right anterior oblique position with appropriate back illumination to optimize visualization of the posteriorly positioned atria. In this position, filling and contraction of the atria and ventricle can be detected with increasing and decreasing opacity of the chambers, respectively. With the use of a Zeiss dissecting microscope attached to a Panasonic charge-coupled device video camera, video images of the heart were acquired at a rate of 29.97 frames/s (33.3 ms/frame) and converted to a QuickTime movie format (data supplement at http://ajpheart.physiology.org/cgi/content/full/00807.2003/DC1). This nondrop frame conversion adds freeze frames to the movie to adjust the frame rate to 30 frames/s. This process slightly alters the time code, introducing a temporal error of ~0.1%. The QuickTime movies were then imported into ImageJ analysis software (Wayne Rasband, National Institutes of Health, Bethesda, MD) as 8 bits of grayscale. Circular areas of ~40 pixels were designated in the posterior atria and the anterior ventricle just inferior to the outflow tract (Fig. 2). Grayscale changes were first detected in these locations and allowed consistent visualization. In addition, measurements from these locations were not compromised by normal cardiac translation or AV valve insufficiency. The average grayscale value of the pixels within the designated circular areas was measured in each video frame. The grayscale value was plotted versus time to obtain waveforms depicting filling and emptying of the cardiac chambers. Measurement was made of the atrial cycle length (the AA interval), the ventricular cycle length (the VV interval), the delay between onset of the atrial and ventricular contractions (AV interval), and the durations of the atrial and ventricular contractions (Fig. 3). Measurements were made for 10 cardiac cycles per embryo and averaged to minimize the significance of time code conversion or physiological variability.
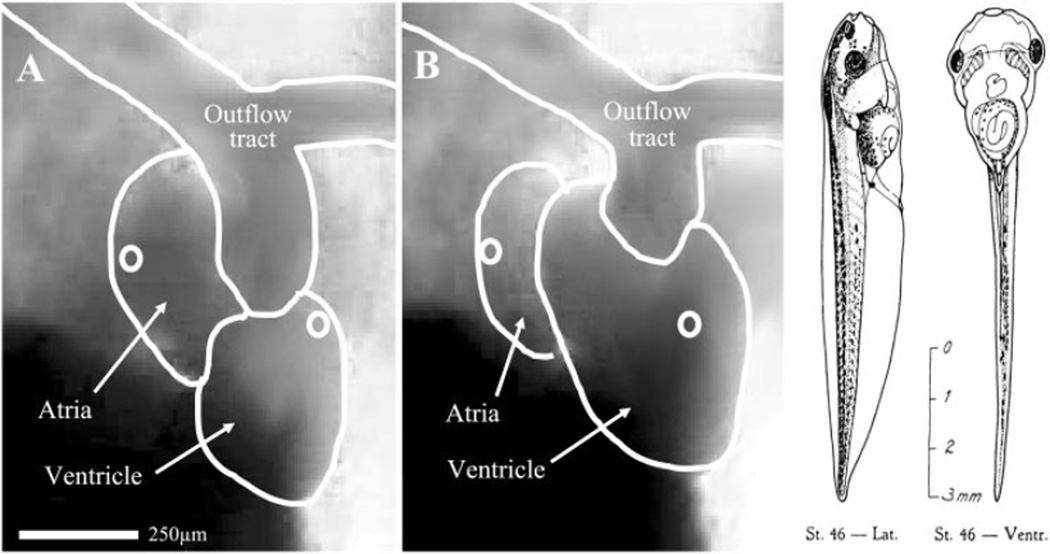
Fig. 2.
Still images captured from digitized video of the stage 46 X. laevis embryonic heart. Major cardiac structures are traced in white and labeled. White circles, areas in which the grayscale value was measured to depict filling and contraction of the cardiac chambers. A: image during ventricular systole with filled atria and a contracted ventricle. Note the darkness in the circular atrial area. B: still image during ventricular diastole with contracted atria and a filled ventricle. Note the lighter color of the circular atrial area of measurement with concurrent darkening of the ventricular area of measurement. Diagram to the right depicts the size and gross morphological features of a stage 46 X. laevis embryo (17). Lat, lateral view; Ventr, ventral view.
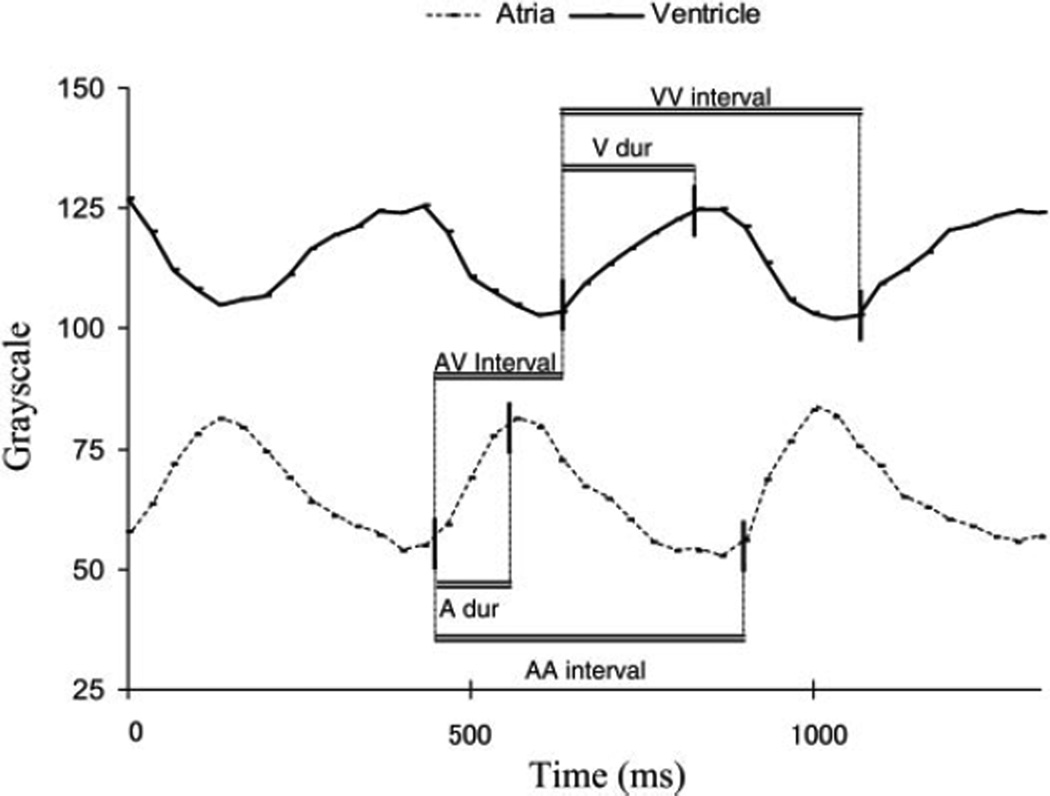
Fig. 3.
Waveforms of grayscale values of the cardiac chambers relative to time, which is calculated from the video acquisition frame rate. A higher value represents a value closer to white or a relatively empty cardiac chamber. A lower value represents a darker color of a filled cardiac chamber. Vertical solid lines denote where measurements were taken. Measurements bracketed by the horizontal double lines include the atrial cycle length (AA interval), the duration of the atrial contraction (A dur), the AV interval or the delay between atrial and ventricular contractions, the duration of the ventricular contraction (V dur), and the ventricular cycle length (VV interval). Note that as the atria contract, the ventricle completes filling.
Pharmacological treatment
The stage 46 X. laevis embryos were treated with isoproterenol, propranolol, atropine, and adenosine to assess the electrophysiological response of the X. laevis embryonic cardiac conduction system to common human chronotropic agents. Medications were dosed by placing the embryo in a 3-ml bath of the medication in 0.1× MMR. The concentrations for isoproterenol, propranolol, and adenosine were of 10−3 M in 0.1× MMR pH 7.4. Atropine was used at a concentration of 0.1 mg/ml. The duration of exposure to the medication was 10 min for isoproterenol, propranolol, and atropine and 30 min for adenosine. Doses were selected to account for transcutaneous diffusion as the route of medication delivery. During drug exposure, the stage 46 embryos were paralyzed and analyzed by video imaging as described above.
Statistical analysis
All data are presented as means ± SE. Cardiac intervals within each group were compared by using a paired or unpaired t-test with P < 0.05 considered significant. Cardiac conduction intervals between the groups of stage 46 embryos treated with medication and the controls were compared by ANOVA with a P value of <0.05 considered significant. Rates at baseline and with pharmacological stimulation were considered separately. If the overall ANOVA calculations indicated a significant difference, pairwise comparisons were made by using Tukey’s F test with a P value of <0.05 considered significant.
RESULTS
Invasive cardiac recordings
Cardiac recordings were obtained on six stage 46 X. laevis embryos paralyzed with cisatracurium and secured with cyanoacrylate. Measurements were made of the AA interval, VV interval, and the AV interval. Data are listed in Table 1. The atrial cycle length was 464 ± 19 ms with a 1:1 ratio of atrial to ventricular contraction. This equates to an average heart rate of ~130 beats/min. The atrioventricular interval was 174 ± 14 ms. Of the six embryos, two were injected at the single cell stage with 20 nl of sterile water. These embryos were included, because embryos injected with aqueous solutions of mRNA or DNA are commonly used to address questions of development in X. laevis embryos.
Table 1.
Cardiac interval measurements in stage 46 Xenopus laevis determined by video analysis and intrathoracic extracellular cardiac recordings
| Video | ICR | |
|---|---|---|
| n | 53 | 6 |
| AA interval | 473±10 | 464±19 (ns) |
| VV interval | 473±10 | 465±22 (ns) |
| AV interval | 169±5 | 174±14 (ns) |
| A duration | 105±3 | na |
| V duration | 148±4 | na |
Values are means ± SE. Video, video analysis; ICR, intrathoracic extracellular cardiac recordings; AA interval, the atrial cycle length; VV interval, the ventricular cycle length; AV interval, delay between onset of atrial and ventricular events; A duration, duration of the atrial contraction by video; V duration, duration of the ventricular contraction by video; ns, no significant difference between the two techniques by unpaired t-test evaluation; na, not applicable
Video data
Utilizing digital analysis of video images, measurements were made in stage 46 X. laevis embryos. Measurements of the AA, VV, and AV intervals, and the duration of atrial and ventricular contractions were consistent and reproducible. Fifty-three stage 46 embryos paralyzed with cisatracurium were assessed. The atrial cycle length was 473 ± 10 ms with a 1:1 ratio of atrial to ventricular contraction. The AV interval was 169 ± 5 ms. The duration of atrial contraction was 105 ± 3 ms, and the duration of ventricular contraction was 148 ± 4 ms. These measurements were compared with results of corresponding measurements made by invasive cardiac recordings. In comparing the two techniques, there was no statistical difference in the measurements of the cardiac intervals (Table 1).
Paralytic effect
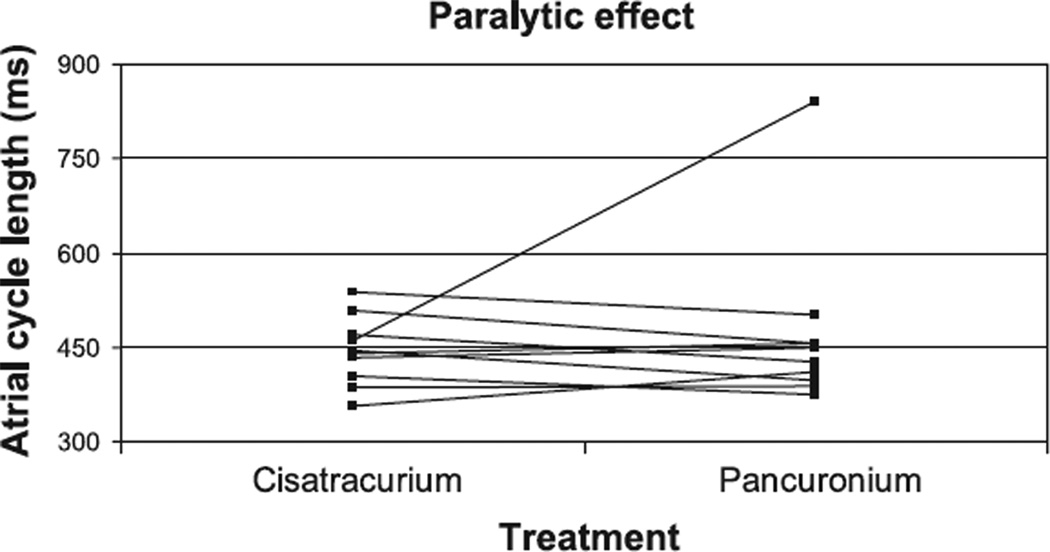
Comparisons were made in embryos when paralyzed with cisatracurium versus pancuronium to assess the paralytic effect on conduction. Ten embryos were serially paralyzed with the two agents. There was no significant difference in the duration of the cardiac contraction intervals between the two groups. The average AA interval was 444 ± 17 ms with cisatracurium treatment and 470 ± 43 ms with pancuronium treatment (Fig. 4). The AV intervals were 163 ± 11 and 193 ± 13 ms, respectively. All embryos had a 1:1 ratio of atrial to ventricular contraction. Pancuronium was noted in one embryo to be associated with bradycardia, which was not noted when the same embryo was subsequently treated with cisatracurium. This embryo demonstrated an AA interval of 842 ± 62 ms with a 1:1 ratio of atrial to ventricular contraction and an AV interval of 224 ms.
Fig. 4.
Comparison between the effect of the paralytic agents, cisatracurium and pancuronium, on the atrial cycle length in individual stage 46 X. laevis embryos. The drugs were tested sequentially in 10 embryos. The response of the atrial cycle length in individual embryos is plotted relative to the paralytic treatment.
Drug treatment
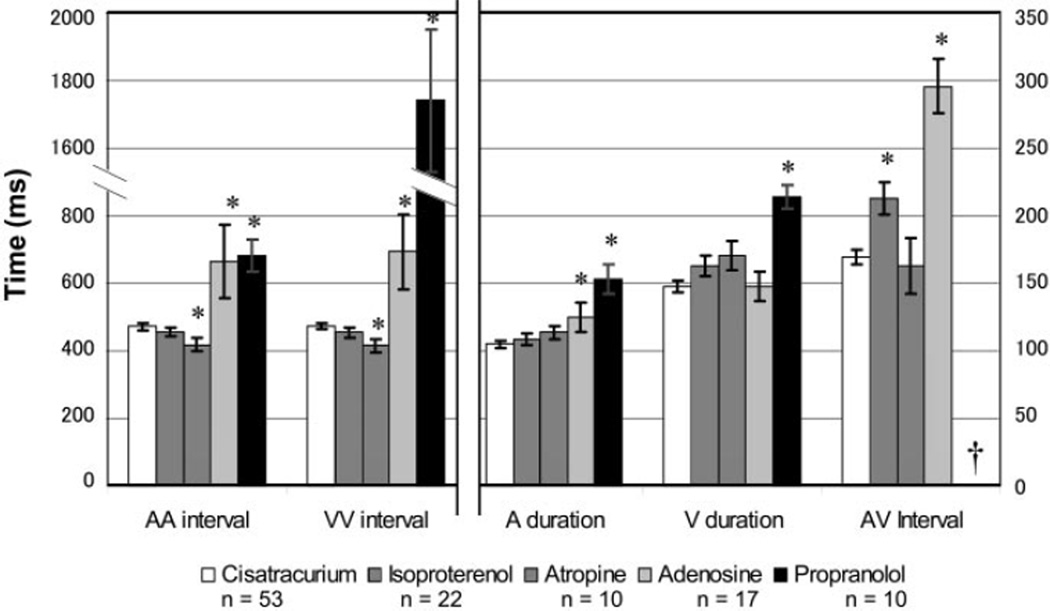
X. laevis embryos were treated with medications known to affect the human conduction system to assess sensitivity and response to these drugs. Drugs that affect the cardiac pacemaker and atrioventricular node function in humans include β-receptor agonists, β-receptor antagonists, antimuscarinics, and adenosine receptor agonists. Isoproterenol, a β-adrenergic receptor agonist, is used to increase the heart rate and decrease the refractory period of the conduction system (13). Propranolol has the opposite effect, i.e., slowing the heart rate and atrioventricular conduction (18). Atropine depresses parasympathetic effects, increasing the heart rate (1). Adenosine stimulates adenosine A1 receptors, slowing or blocking conduction through the atrioventricular node and decreasing the rate of cardiac pacemakers (3). Embryos were treated with these medications, and the cardiac intervals were measured by video analysis. All embryos in this study were tested while paralyzed with cisatracurium (Fig. 5).
Fig. 5.
Cardiac intervals in stage 46 X. laevis embryos treated with chronotropic medications as measured by video analysis. *Statistically significant difference (P < 0.05 by Tukey’s F test) compared with the cisatracurium paralyzed control group (n = 53; open bars). Left, time scale AA interval and VV interval. Right, time scale for the A duration, V duration, and AV interval. †No measurable AV interval due to second- or third-degree heart block.
X. laevis embryos had a mixed response to β-adrenergic receptor modulation. The β-adrenergic receptor agonist isoproterenol had no demonstrable effect on heart rate in the 22 embryos treated. The atrial cycle length averaged 455 ± 13 ms with a 1:1 ratio of atrial to ventricular contraction. There was an unexpected increase in the AV interval by 26% to 213 ± 12 ms, which was statistically significant. The other intervals were unaffected with the duration of atrial contraction measuring 109 ± 4 ms and the duration of ventricular contraction measuring 163 ± 7 ms.
Ten embryos were treated with the β-adrenergic receptor antagonist propranolol, which resulted in a significant effect on all cardiac intervals. The atrial cycle length was 45% longer than controls, averaging 683 ± 48 ms. Embryos exhibited second- or third-degree heart block, and thus had no measurable AV interval. Five embryos had Mobitz type II second-degree heart block with intermittent failure of conduction from the atria to the ventricle without prior prolongation of the AV interval (9). These embryos most commonly had a 2:1 ratio of atrial to ventricular contraction with one embryo demonstrating a ratio of 3:1. No episodes of progressive prolongation of the AV interval before blocked conduction, known as Mobitz type I (Wenckebach) second-degree heart block, were seen (9). With the high-grade heart block, the ventricular cycle length averaged 1,742 ± 212 ms. The other five embryos exhibited complete atrioventricular dissociation with an average VV interval of 1,931 ± 277 ms. Durations of atrial and ventricular contractions were both significantly prolonged, measuring 153 ± 11 and 214 ± 9 ms, respectively.
Embryos treated with atropine became tachycardic. Atrial and ventricular cycle lengths were significantly shortened with intervals of 418 ± 20 ms. The other cardiac intervals were unaffected with the AV interval measuring 163 ± 21 ms, the duration of atrial contraction measuring 114 ± 6 ms, and the duration of ventricular contraction measuring 171 ± 11 ms. Embryos did exhibit paradoxical bradycardia at lower doses of 0.03 mg/ml (data not shown). This response is thought to be related to vagal stimulation before peripheral cholinergic blockade (1).
Treatment with adenosine also had a significant electrophysiological effect as demonstrated by analysis of 17 embryos treated with the drug. Atrial and ventricular cycle lengths were significantly prolonged exhibiting intervals of 666 ± 108 and 694 ± 110 ms, respectively. There was generally a 1:1 ratio of atrial to ventricular contraction ratio with a significantly prolonged atrioventricular interval of 296 ± 20 ms. The duration of atrial contraction was prolonged at 125 ± 11 ms, which although statistically significant was a smaller increase than the analysis frequency. The duration of ventricular contraction was unchanged at 148 ± 11 ms.
DISCUSSION
Although many types of congenital heart disease are associated with cardiac conduction abnormalities, examining these defects as the embryonic heart forms has been difficult. X. laevis offers an opportunity to examine the conduction system in living embryos given the well-programmed and easily visualized development of the heart. However, simple protocols and normative baseline values for these embryos are lacking. This study describes ways to measure features of the embryonic conduction system using video microscopy and compared the values to measurements made by modified electrocardiograms. Similar values were found by using either approach but highlight that video analysis preserves the animal for additional study. The normative baseline data and measurements after pharmacological interventions provide important reference values for future studies of the genetic structure-function relationship during cardiac development.
One of the first obstacles to making measurements of the heart was immobilizing the embryos without compromising normal cardiac conduction. The anesthetic, tricaine (ethyl 3-aminobenzoate methanesulfonate; Sigma-Aldrich), traditionally used in amphibian and fish studies, resulted in a high mortality rate with prolonged exposure as well as dysrhythmias (this study and Ref. 2). Mechanical restraint resulted in significant trauma to the animal and was not a viable method for these studies. Thus another class of pharmacological agents was chosen. Cisatracurium was chosen for its simple metabolism and lack of cardiovascular effects in humans (22). In stage 46 X. laevis embryos, cisatracurium treatment was well tolerated with no obvious cardiovascular effects and no notable long-term consequences. Paralysis was not as well tolerated in older embryos. Stage 50 embryos were more sensitive to cisatracurium with quicker onset and longer duration of paralysis even at lower dosing concentrations (data not shown). We also had success using pancuronium, a common, readily available nondepolarizing paralytic agent. The benefits of pancuronium are its rapid onset of action, longer duration of action, extended shelf life, and low cost (5, 8). Pancuronium showed no significant cardiac effects as long as exposure was limited to 30 min or less. We found that exposure to pancuronium for >45 min generally resulted in death of the embryos.
Video analysis provided a reliable, noninvasive estimate of conduction intervals that accurately reflected intrathoracic cardiac recording data. Video analysis avoided trauma to the animal and distortion of cardiac physiology and anatomy while preserving the animal for continued evaluation. The temporal fidelity of this technique, limited to 33.3 ms, was balanced by measuring multiple cardiac cycles per analysis and multiple embryos. The frequency, duration, and interrelationship of chamber contraction were detected by measuring the grayscale value of pixels in the video images over time. The pixels darken as the cardiac chambers fill. On initiation of contraction, the ejection of blood results in increased whiteness of the pixels in the cardiac chamber. Waveforms of the darkening and lightening of the cardiac chambers accurately reflected the periods of atrial and ventricular filling and ejection. Although a limitation of video analysis is the temporal resolution, this technique has been successfully used to detect pacemakers in other organ systems (4).
Data were also presented, which may represent the first electrocardiograms recorded from the 0.3-mm hearts of X. laevis embryos. Although the signal recorded may contain some contribution of mechanical distortion, this technique may be advantageous in embryos displaying looping abnormalities or extracardiac anomalies that obscure clear visualization of cardiac structures. The procedure, however, is invasive and ultimately lethal and thus is not optimal for routine use. These experiments allowed verification of the cardiac interval values obtained through video analysis. The two methods provided similar results and established estimates of normal cardiac intervals for the cardiac cycle in stage 46 X. laevis embryos. The spontaneous, regular atrial contractions occurred every 473 ± 10 ms. After a standard delay of 169 ± 5 ms, ventricular contraction followed. This delay, the atrioventricular interval, relates to the PR interval used to diagnose heart block by surface electrocardiogram in humans. The atrioventricular interval contains interrelated data regarding atrial conduction and atrioventricular conduction. To help discern the impact of atrial conduction on the AV interval, measurements were made of the duration of atrial contraction using video analysis.
Response to common chronotropic agents was also tested in stage 46 embryos. Isoproterenol, which quickens mammalian heart conduction, is a mixed β1/β2-adrenergic receptor agonist that decreases the atrial cycle length in humans (13). There was no demonstrable effect on the atrial cycle length in X. laevis embryos at the doses and delivery methods tested. Further studies are needed to explore alternate treatment methods, including the cardiac response to epinephrine and norepinephrine, because uptake of catecholamines by the heart can vary through embryogenesis as demonstrated in another model system, the chick (10). The unexpected prolongation of the AV interval implies that inadequate medication delivery cannot entirely explain the lack of increased heart rate and may represent stage-specific responses to this dosing. Furthermore, the lack of heart rate increase with catecholamines was also demonstrated by Jacobsson and Fritsche (12) and attributed to high baseline adrenergic tonus.
Propranolol, a β-adrenergic receptor antagonist, slows mammalian heart conduction and in X. laevis embryos had the expected effect of inducing bradycardia. The effects on AV conduction were profound causing second- or third-degree heart block. The durations of atrial and ventricular contraction were prolonged, which may relate to the increased chamber volume associated with bradycardia or may represent delayed depolarization of intrachamber conduction systems. These data demonstrate the importance of sympathetic drive for normal cardiac function in X. laevis embryos.
Atropine interferes with parasympathetic tone. The cardiac effects of antimuscarinics are dose dependent in humans and result in an increase in heart rate when an adequate dose is given (1). Atropine treatment did result in tachycardia in X. laevis embryos. This suggests that parasympathetic control is present even at this early embryonic stage.
The effect of adenosine was studied to further detail the properties of the cardiac pacemaker and the atrioventricular conduction system. Adenosine slows the cardiac pacemaker and induces third-degree heart block in humans (3). Treatment with adenosine prolonged the AA interval and the AV interval. However, a 1:1 ratio of atrial to ventricular contraction persisted. Thus the response of atrioventricular conduction to adenosine is different than the postembryonic human atrioventricular node. The response of the stage 46 X. laevis embryo to adenosine suggests that their conduction system may be more comparable to the physiology of human accessory pathways. Accessory pathways are thought to be similar to atrioventricular connections in lower vertebrates and have a low incidence of complete conduction block with adenosine (7).
One key issue in X. laevis relates to the cardiac anatomy. Atrioventricular conduction tissue in higher vertebrates is typically located in the ventricular septum, which is not present in the three-chambered frog heart. Sedmera et al. (21) have elegantly documented a focused electrical communication between the atria and the ventricle using optical mapping in adult X. laevis. Some electrophysiological parameters in adult X. laevis hearts have been detailed; however, these measurements were made in despinalized mature animals. Myocardial dysfunction has been associated with brain death, possibly related to alterations in sympathetic tone, hormone regulation, or perfusion pressure (19), which adds to the value of making these measurements in living embryos.
Overall, the present study confirmed that noninvasive image analysis can be used to accurately depict the physiology of cardiac conduction in X. laevis embryos. Normative data were obtained that can be used as a reference, particularly to analyze the effects of experimental manipulation on cardiac conduction. Additionally, this study demonstrated that internal electrocardiograms can be obtained and may be useful for further studies on cardiac conduction, particularly when video analysis is difficult. Pharmacological techniques used to temporarily paralyze the embryos were effective and provided a nontoxic method to immobilize the embryo with minimal physiological effects. Mixed responses to common chronotropic medications were demonstrated with propranolol inducing the most profound effects, including bradycardia. These data demonstrate that X. laevis cardiac electrophysiology has many characteristics similar to the human conduction system and prepare the way for physiological analysis of congenital heart defects using X. laevis embryos. We also thank Bobby Thompson (Medical University of South Carolina) for helpful discussions and advice.
ACKNOWLEDGMENTS
The authors express appreciation to Shweta Padmanabha, Sandra Kolker, and Charles Greaves for their technical support; Greyson Purcell and Steven Beck for their expertise with video conversion and software configuration; and Thomas Barna for his assistance with electrocardiogram acquisition. We also thank Bobby Thompson (Medical University of South Carolina) for helpful discussions and advice.
GRANTS
The project was supported by National Heart, Lung, and Blood Institute Grants HL-62178, HL-62483, and NIH-T32-HL-07413 (from the Research Training Program in Pediatric Cardiology).
Footnotes
DISCLOSURE
The contents of this study are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.American Society of Hospital Pharmacists, and American Society of Health System Pharmacists. AHFS Drug Information. Bethesda, MD: American Society of Hospital Pharmacists; 2003. [Google Scholar]
- 2.Applebaum D, Halperin E. Asystole following a conventional therapeutic dose of lidocaine. Am J Emerg Med. 1986;4:143–145. doi: 10.1016/0735-6757(86)90160-9. [DOI] [PubMed] [Google Scholar]
- 3.Belardinelli L, Isenberg G. Actions of adenosine and isoproterenol on isolated mammalian ventricular myocytes. Circ Res. 1983;53:287–297. doi: 10.1161/01.res.53.3.287. [DOI] [PubMed] [Google Scholar]
- 4.Bercik P, Bouley L, Dutoit P, Blum AL, Kucera P. Quantitative analysis of intestinal motor patterns: spatiotemporal organization of nonneural pacemaker sites in the rat ileum. Gastroenterology. 2000;119:386–394. doi: 10.1053/gast.2000.9306. [DOI] [PubMed] [Google Scholar]
- 5.Duvaldestin P, Agoston S, Henzel D, Kersten UW, Desmonts JM. Pancuronium pharmacokinetics in patients with liver cirrhosis. Br J Anaesth. 1978;50:1131–1136. doi: 10.1093/bja/50.11.1131. [DOI] [PubMed] [Google Scholar]
- 6.Franco D, Icardo JM. Molecular characterization of the ventricular conduction system in the developing mouse heart: topographical correlation in normal and congenitally malformed hearts. Cardiovasc Res. 2001;49:417–429. doi: 10.1016/s0008-6363(00)00252-2. [DOI] [PubMed] [Google Scholar]
- 7.Garratt CJ, Griffith MJ, O’Nunain S, Ward DE, Camm AJ. Effects of intravenous adenosine on antegrade refractoriness of accessory atrioventricular connections. Circulation. 1991;84:1962–1968. doi: 10.1161/01.cir.84.5.1962. [DOI] [PubMed] [Google Scholar]
- 8.Gramstad L. Atracurium, vecuronium and pancuronium in end-stage renal failure. Dose-response properties and interactions with azathioprine. Br J Anaesth. 1987;59:995–1003. doi: 10.1093/bja/59.8.995. [DOI] [PubMed] [Google Scholar]
- 9.Harrison TR, Braunwald E. Harrison’s Principles of Internal Medicine. New York: McGraw-Hill; 2001. [Google Scholar]
- 10.Ignarro LJ, Shideman FE. Norepinephrine and epinephrine in the embryo and embryonic heart of the chick: uptake and subcellular distribution. J Pharmacol Exp Ther. 1968;159:49–58. [PubMed] [Google Scholar]
- 11.Irisawa H. Comparative physiology of the cardiac pacemaker mechanism. Physiol Rev. 1978;58:461–498. doi: 10.1152/physrev.1978.58.2.461. [DOI] [PubMed] [Google Scholar]
- 12.Jacobsson A, Fritsche R. Development of adrenergic and cholinergic cardiac control in larvae of the African clawed frog Xenopus laevis. Physiol biochem zool. 1999;72:328–338. doi: 10.1086/316669. [DOI] [PubMed] [Google Scholar]
- 13.Josephson ME. Clinical Cardiac Electrophysiology: Techniques and Interpretation. Philadelphia, PA: Lea & Febiger; 1993. [Google Scholar]
- 14.Kasahara H, Wakimoto H, Liu M, Maguire CT, Converso KL, Shioi T, Huang WY, Manning WJ, Paul D, Lawitts J, Berul CI, Izumo S. Progressive atrioventricular conduction defects and heart failure in mice expressing a mutant Csx/Nkx2.5 homeoprotein. J Clin Invest. 2001;108:189–201. doi: 10.1172/JCI12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolker SJ, Tajchman U, Weeks DL. Confocal imaging of early heart development in Xenopus laevis. Dev Biol. 2000;218:64–73. doi: 10.1006/dbio.1999.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohun TJ, Leong LM, Weninger WJ, Sparrow DB. The morphology of heart development in Xenopus laevis. Dev Biol. 2000;218:74–88. doi: 10.1006/dbio.1999.9559. [DOI] [PubMed] [Google Scholar]
- 17.Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin): a Systematical and Chronological Survey of the Development from the Fertilized Egg Till the End of Metamorphosis. Amsterdam: North-Holland; 1956. [Google Scholar]
- 18.Puech P, Wainwright RJ. Clinical electrophysiology of atrioventricular block. Cardiol Clin. 1983;1:209–224. [PubMed] [Google Scholar]
- 19.Rosendale JD, Kauffman HM, McBride MA, Chabalewski FL, Zaroff JG, Garrity ER, Delmonico FL, Rosengard BR. Hormonal resuscitation yields more transplanted hearts, with improved early function. Transplantation. 2003;75:1336–1341. doi: 10.1097/01.TP.0000062839.58826.6D. [DOI] [PubMed] [Google Scholar]
- 20.Schott JJ, Benson DW, Basson CT, Pease W, Silberbach GM, Moak JP, Maron BJ, Seidman CE, Seidman JG. Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science. 1998;281:108–111. doi: 10.1126/science.281.5373.108. [DOI] [PubMed] [Google Scholar]
- 21.Sedmera D, Reckova M, deAlmeida A, Sedmerova M, Biermann M, Volejnik J, Sarre A, Raddatz E, McCarthy RA, Gourdie RG, Thompson RP. Functional and morphological evidence for a ventricular conduction system in zebra fish and Xenopushearts. Am J Physiol Heart Circ Physiol. 2003;284:H1152–H1160. doi: 10.1152/ajpheart.00870.2002. [DOI] [PubMed] [Google Scholar]
- 22.Sorooshian SS, Stafford MA, Eastwood NB, Boyd AH, Hull CJ, Wright PM. Pharmacokinetics and pharmacodynamics of cisatracurium in young and elderly adult patients. Anesthesiology. 1996;84:1083–1091. doi: 10.1097/00000542-199605000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Van Mierop LH. Location of pacemaker in chick embryo heart at the time of initiation of heartbeat. Am J Physiol. 1967;212:407–415. doi: 10.1152/ajplegacy.1967.212.2.407. [DOI] [PubMed] [Google Scholar]