Abstract
Two amino acids inserted between residues 69 and 70 of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase (RT) are rare mutations that may develop in viruses containing multiple thymidine analog (zidovudine [AZT], stavudine)-associated mutations and that confer high-level resistance to all currently approved chain-terminating nucleoside and nucleotide RT inhibitors (NRTIs). The two known mechanisms of resistance to NRTIs are decreased incorporation and increased excision. The mechanism used by RT insertion mutants has not been described for tenofovir (TFV), a recently approved agent in this class. A patient-derived HIV-1 strain (strain FS-SSS) that contained an insertion mutation in a background of additional resistance mutations M41L, L74V, L210W, and T215Y was obtained. A second virus (strain FS) was derived from FS-SSS. In strain FS the insertion and T69S were reverted but the other resistance mutations were retained. The FS virus showed strong resistance to AZT but low-level changes in susceptibilities to other NRTIs and TFV. The FS-SSS virus showed reduced susceptibilities to all NRTIs including TFV. Steady-state kinetics demonstrated that the relative binding or incorporation of TFV was slightly decreased for FS-SSS RT compared to those for wild-type RT. However, significant ATP-mediated excision of TFV was detected for both mutant RT enzymes and followed the order FS-SSS RT > FS RT > wild-type RT. The presence of physiological concentrations of the +1 nucleotide inhibited TFV excision by the wild-type RT and slightly inhibited excision by the FS RT, whereas the level of excision by the FS-SSS RT remained high. Computer modeling suggests that the increased mobility of the β3-β4 loop may contribute to the high-level and broad NRTI resistance caused by the T69 insertion mutation.
Human immunodeficiency virus type 1 (HIV-1) reverse transcriptase (RT) is the target for antiretroviral drugs such as nucleoside and nucleotide RT inhibitors (NRTIs). The nucleoside analogs zidovudine (AZT), didanosine (ddI), zalcitabine (ddC), stavudine (d4T), lamivudine (3TC), emtricitabine, and abacavir and the nucleotide analog tenofovir (TFV) are metabolized by cellular enzymes to their triphosphate and diphosphate active forms, respectively. Their mechanisms of action are competitive inhibition of incorporation of natural deoxynucleoside triphosphates (dNTPs) into proviral DNA and subsequent termination of polymerization of the viral DNA chain upon incorporation. Although NRTIs are effective at reducing viral loads in HIV-1-infected patients, their use may lead to the development of mutations in RT that contribute to HIV-1 drug resistance. In some cases, mutations that result in resistance to many NRTIs may develop.
Multi-NRTI resistance patterns fall into two classes of mutations, consisting of (i) the Q151M complex, which consists of the substitutions A62V, V75I, F77L, F116Y, and Q151M, and (ii) the insertion of two amino acids between positions 69 and 70 of RT (the 69-insertion mutation). Viruses containing the insertion mutation have reduced susceptibilities to all currently approved NRTIs, including TFV, but viruses containing the Q151M mutation remain susceptible to TFV and 3TC (42, 57; K. Wolf, H. Walter, N. Beerenwinkel, W. Keulen, R. Kaiser, D. Hoffmann, T. Langauer, J. Selbig, A.-M. Vandamme, K. Korn, and B. Schmidt, Abstr. 11th Int. HIV Drug Resist. Workshop: Basic Principles Clin. Implications, abstr. 20, 2002). The 69-insertion mutation typically consists of T69S plus the insertion of SS, SG, or SA between amino acids 69 and 70 of RT in a background of multiple thymidine analog-associated mutations (TAMs; consisting of substitutions at amino acids 41, 67, 70, 210, 215, and 219 of RT) that are associated with resistance to AZT and d4T (29, 35, 54, 59). The 69-insertion mutations can occur with various NRTI treatment regimens following prolonged periods of incomplete virus suppression. They have been observed most commonly after the use of AZT followed by the use of ddI or ddC, but the exact pattern(s) of drug selection has not been determined (6, 16, 29, 59). These mutations are rare, occurring in ≤1% of antiretroviral agent-experienced patients [6, 22, 54, 59; S. Bloor, S. D. Kemp, K. Hertogs, T. Alcorn, and B. A. Larder, Abstr. 4th Int. Workshop HIV Drug Resist. Treatment Strategies, Antivir. Ther. 5(Suppl. 3):169, 2000].
Two mechanisms are known to contribute to decreased NRTI susceptibility (47). Mutations that alter the relative binding affinities of inhibitors over the relative binding affinities of the natural substrates can result in decreased incorporation of the inhibitors. An example of resistance by this mechanism is the decreased binding of 3TC triphosphate (3TC-TP), the active metabolite of 3TC, to RT with the M184V mutation (49). Mutations that increase the levels of excision of chain-terminating inhibitors following incorporation into viral DNA by pyrophosphorolysis or ATP-mediated excision also contribute to drug resistance. RT mutants containing multiple TAMs excise AZT and d4T at increased levels compared to those for wild-type RT, which is the likely mechanism of AZT and d4T resistance (1, 3, 38, 45, 46).
TFV disoproxil fumarate (TDF) is an oral prodrug of TFV that has recently been licensed for use for the treatment of HIV-1 infection. TFV (9-[2-(phosphonomethoxypropyl)adenine]) is an acyclic nucleotide phosphonate analog of AMP that requires two steps of phosphorylation by cellular kinases to become the active triphosphate metabolite (TFV diphosphate [TFV-PP]). The primary mutation selected for by TFV in vitro and in vivo is K65R (32, 57). Although TFV does not select for TAMs, the presence of specific combinations of TAMs, namely, T215Y together with M41L and L210W, results in reduced susceptibility to TDF (36; M. D. Miller, N. A. Margot, and B. Lu, Program Abstr. Ninth Conf. Retrovir. Opportunistic Infect., abstr. 43, p. 68, 2002). It has previously been shown that the molecular mechanism of TFV resistance by K65R RT is the decreased ability of K65R RT to bind to or incorporate TFV-PP (16a, 57, 58). Multiple TAMs and the 69-insertion mutations, however, are known to cause resistance to other NRTIs through enhanced excision (5, 30, 31, 33, 34, 37); however, the mechanism of resistance to TFV by the 69-insertion mutant RT is unknown.
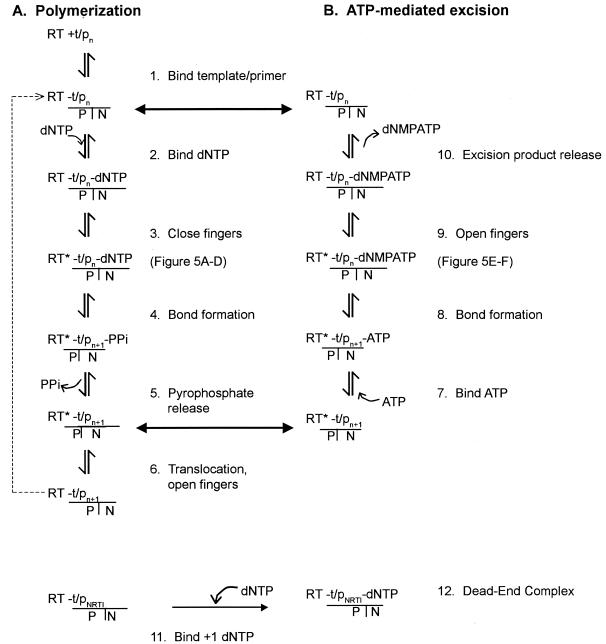
The 69-insertion mutations occur in the β3-β4 loop, comprised of amino acids 64 to 72 located in the fingers subdomain of HIV-1 RT (26). Molecular modeling based on crystal structures of HIV-1 RT and pre-steady-state kinetics suggests that during dNTP incorporation, substantial conformational changes that affect the positions of the subdomains with respect to each other occur: part of the fingers subdomain rotates toward the palm subdomain and inward toward the primer-template and the polymerase active site (25). The polymerization process is diagrammed in Fig. 1A and is reviewed elsewhere (53). In the first step, RT binds to the template-primer. The position of the 3′ terminal nucleotide of the primer in the active site resides at either the posttranslocation priming site (P site) or the pretranslocation nucleotide-binding site (N site) (3). When the primer terminus is in the P site, the N site is unoccupied. The incoming dNTP binds to the N site (step 2), followed by a rate-limiting conformational change (step 3) in which the β3-β4 fingers loop of RT closes from an open to a closed position (15, 24, 28). During this process close contacts are formed between the incoming dNTP and RT residues K65, R72, Y115, and Q151 (25, 26). Phosphodiester bond formation occurs in step 4 to generate pyrophosphate, which is released in step 5. The order of the mechanistic steps that follow are not well defined; however, in order for the next round of polymerization to occur, the primer must translocate such that its terminus is shifted from the N site to the P site and the fingers domain must return to the open position, in which it is primed for the next dNTP to bind to it.
FIG. 1.
Polymerization diagram and ATP-mediated excision model. (A) RT polymerization cycle. RT binds to the template-primer (t/pn) (step 1), in which the primer terminus resides in the P site and the N site is unoccupied. The incoming dNTP binds to the N site (step 2). A rate-limiting conformational change occurs in step 3, in which the fingers subdomain moves from an open to a closed position (RT*). The phosphodiester bond formation occurs in step 4 to form the primern + 1, in which the primer terminus is in the N site and pyrophosphate (PPi) is produced and then released in step 5. After incorporation, the deoxynucleoside monophosphate (dNMP) is denoted n + 1. This complex is also the substrate for the excision reaction shown in panel B. Translocation and an opening of the fingers domain occur before binding of the next dNTP for the next round of polymerization. (B) Model of ATP-mediated excision. In this diagram, n + 1 denotes the 3′ terminus of the primer that may be an incorporated chain terminator and is the substrate for ATP-mediated excision. ATP binds to RT prior to translocation, when the terminal residue of the primer resides in the N site and the fingers are still in the closed position (step 7). The bound ATP forms a phosphodiester bond with the n + 1 terminal primer to form dNMP-ATP (step 8). In order for the dNMP-ATP to be released, the RT fingers domain must undergo a putative conformational change to the open position (step 9). This is followed by the release of dNMP-ATP, leaving the RT-template-primern in the P site and an empty N site that it is available to bind to dNTP for polymerization (step 10). The bottom row depicts the formation of the excision-resistant dead-end complex. For this reaction, the NRTI-terminated primer terminus resides in the P site. The +1 dNTP binds (step 11), and this forms the salt-stable dead-end complex (step 12).
For pyrophosphorolysis (the reverse reaction of polymerization) or ATP-mediated excision to occur, the chain-terminated primer must reside in the N site in order to be excised by pyrophosphate or ATP, respectively (51). This process is diagrammed in Fig. 1B (steps 7 to 10). The product of pyrophosphorolysis is the triphosphate form of the NRTI, and the product of ATP-mediated excision is a larger dinucleoside tetraphosphate molecule (1, 3, 38). The binding of the nucleotide complementary to the next position in the template (next nucleotide) to a translocated chain-terminated primer-template-RT complex forms a stable, dead-end complex that is resistant to excision (Fig. 1, steps 11 to 12) (39, 55). The binding of physiological concentrations of the next nucleotide has been shown to decrease the excision of some NRTIs such as d4T and ddA, but not AZT (5, 33, 34, 38-40, 46). This has implications for the level of resistance observed in vivo compared to that observed in vitro, in which the dNTP concentrations in cell culture are relatively high (17, 23, 56) and can mask the level of resistance by excision mechanisms. The change of threonine to serine at amino acid 69 followed by the insertion of two serine residues likely affects the interaction between the enzyme and dNTP substrates and might therefore alter the nucleotide-binding specificity or phosphodiester bond formation efficiency of RT.
To investigate the effects of resistance mutations on RT function and the molecular mechanisms of the altered susceptibilities of these mutants to TFV, the viral susceptibility of a patient-derived HIV-1 strain containing multiple NRTI resistance mutations plus the 69-insertion mutation, as well as a control virus in which the insertion was repaired but which retained the multiple NRTI resistance mutations, was evaluated. The Michaelis-Menten constant (Km) for dATP and the inhibition constant (Ki) for TFV-PP of the wild-type and mutant RTs were determined. The excision of TFV from chain-terminated primers and the effect of the next complementary nucleotide on this process by the wild-type and mutant enzymes were measured. Finally, computational models of the RT with the 69-insertion mutation complexed to TFV-PP and a model of the dinucleoside tetraphosphate excision product bound to the RT-primer-template complex were constructed and examined.
MATERIALS AND METHODS
Recombinant HIV-1 production and antiviral susceptibility assays
Fragments corresponding to the first 1,000 bp of HIV-1 RT were amplified from HIV-1 in plasma by PCR and cotransfected with HIV-1 proviral molecular clone pHXB2Δ2-261RT (a gift from C. Boucher, Utrecht University, Utrecht The Netherlands) from which the sequence for RT has been deleted, as described previously (2, 43). The replication-competent viruses were generated by homologous recombination and the genotypes were determined as described elsewhere (58). HIV-1 subtype B strain FS-SSS was obtained in 1997 from a patient who had undergone extensive antiretroviral treatment that included 5 years of monotherapy with AZT, followed by dual therapy with AZT and ddI and the subsequent addition of treatment with d4T, ddC, 3TC, protease inhibitors, and nonnucleoside RT inhibitors. The FS description of this virus is an arbitrary designation. The FS-SSS virus contained the following RT amino acid substitutions compared to the sequence of strain HXB2D: V35M, T39A, M41L, T69S, SS insertion, L74V, R83K, A98S, V108I, E122K, D123E, I135V, Q145C, D177E, G196E, T200A, L210W, R211K, L214F, T215Y, L228H, K249N, P272A, R277K, and E297K (the NRTI-associated resistance mutations are underlined). The FS virus was constructed from FS-SSS by site-directed mutagenesis and contained all of the substitutions described above except T69S, which was reverted to T69T, and the SS insertion was removed.
The susceptibilities of the recombinant mutant viruses to TFV, ddI, abacavir, 3TC, ddC, d4T, and AZT were evaluated by the PhenoSense assay (ViroLogic, Inc., South San Francisco, Calif.), which measures luciferase expression in a single-cycle replication assay in 293 cells (48). In this assay, the cutoff values for reduced susceptibility (fold change limits) to TFV, ddI, abacavir, 3TC, ddC, d4T, and AZT are 1.4, 1.7, 4.5, 2.5, 1.7, 1.7, and 2.5, respectively. For abacavir, ddI, d4T, and TFV, these susceptibility cutoff values are also the clinical cutoff values. The susceptibilities of FS-SSS, FS, and wild-type HIV-1 molecular clone HXB2D to TFV were also evaluated by a multiple-cycle 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT)-based viability assay with MT-2 cells, as described previously (14).
Recombinant RT construction, purification, and kinetic analyses
The wild-type RT expression construct pRT66 (20) was a gift from M. Wainberg (McGill University, Montreal, Quebec, Canada). The pol sequences were amplified from the HXB2D molecular clone of HIV-1 or patient-derived viruses by PCR and cloned into the expression vector pKK223-3 (Amersham Pharmacia Biotech). The RT was expressed and purified from Escherichia coli strain JM109 as described previously (58). For all RT preparations, there were 80% p66/p66 homodimer and 20% p66/p51 heterodimer determined by gel filtration and sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis. The enzyme kinetic analyses were performed as described previously (44). The reaction mixtures for DNA-dependent DNA polymerase function contained 50 mM Tris-HCl (pH 7.8), 60 mM KCl, 10 mM MgCl2, 5 mM dithiothreitol, 50 μM each dNTP, 150 μg of activated calf thymus DNA (Amersham Pharmacia Biotech) per ml, and 30 to 100 Ci of the appropriate [3H]dNTP (Amersham, Arlington Heights, Ill.) per mmol. Kinetic constants were determined by plotting the substrate concentration (S) versus the initial rate (v) data in a Michaelis-Menten hyperbolic relationship curve and fitting by nonlinear regression to the equation v = Vmax × S/(Km + S) by using SigmaPlot (version 4.01) software. In these experiments, the template was present in excess, and competitive inhibition was observed on the basis of a constant Vmax when increasing concentrations of TFV-PP were assayed.
ATP-mediated primer rescue assay
A 31-nucleotide (nt) DNA primer (primer L31; 5′-CTACTAGTTTTCTCCATCTAGACGATACCAG-3′) (40) was 5′ end labeled with [γ-33P]ATP, annealed to a twofold excess of 50-nt DNA template WL50 (5′-GAGTGCTGAGGTCTTCATTCTGGTATCGTCTAGATGGAGAAAACTAGTAG-3′) (39), and chain terminated with TFV-PP to produce a chain-terminated 32-mer. End-labeled, chain-terminated primers were gel purified twice on 8 M urea-16% polyacrylamide sequencing gels, excised, eluted in 0.5 M ammonium acetate-10 mM Tris-HCl (pH 7.5)-0.1 mM EDTA-2 mM MgCl2-0.01% sodium dodecyl sulfate, and desalted by filtration through NAP10 columns (Amersham Pharmacia Biotech). Purified, chain-terminated primers were annealed to a fivefold excess of the WL50 DNA template. The chain-terminated primer-template (2.5 nM) was incubated with RT enzyme in excess at approximately 30 nM wild-type recombinant RT enzyme or mutant enzymes in the presence of no complementary next nucleotide or increasing concentrations of the next nucleotide (final concentration, 0.1, 0.5, 2.5, 12.5, 62.5, or 312.5 μM) for 5 min at 37°C in RT removal buffer (50 mM Tris [pH 7.8], 60 mM KCl, 5 mM MgCl2, 4.2% glycerol, 300 μg of bovine serum albumin per ml), followed by the addition of ATP (3.2 mM final) or NaPPi (150 μM final) to start the reaction. The reactions were stopped at 0.1, 5, 15, 30, and 45 min (ATP) or 0.1, 0.5, 1.5, 3, and 9 min (PPi) by heat inactivation of RT at 90°C for 5 min, followed by placement on ice for 5 min. The unblocked primers were extended to full-length 50-mers through the addition of 500 μM dNTPs and the Klenow fragment (exo−) of E. coli DNA polymerase I (New England Biolabs, Beverly, Mass.) at 37°C for 30 min, as described previously (40). The reactions were stopped by the addition of an equivalent amount of formamide stop solution (U.S. Biochemicals, Cleveland, Ohio) at 30 min. The samples were heated at 95°C and run on 8 M urea-16% polyacrylamide sequencing gels, and the bands were quantified with a Storm 860 PhosphorImager (Amersham Pharmacia Biotech) and ImageQuant (version 5.0) software (Molecular Dynamics, Sunnyvale, Calif.). Degradation and extension of the primer band were less than 4% when each RT enzyme was incubated with the primer-template for 45 min at 37°C in the absence of ATP or PPi. Several control experiments were performed to confirm that the enzyme concentrations used were in excess of the primer-template concentration, that the RT enzyme was fully inactivated after the 5-min heat inactivation step, that the Klenow fragment enzyme was not capable of removing TFV from chain-terminated primers in the presence or absence of these concentrations of ATP, and that greater than 95% of the starting primers used were blocked by TFV (data not shown). The rate of excision (the percentage of TFV removed per minute) was determined by linear regression analysis of values in the linear range (0.1 to 45 min for wild-type RT, 0.1 to 15 min for SF-SSS RT, and 0.1 to 30 min for SF RT) by using SigmaPlot (version 4.01) software. The 50% inhibitory concentrations (IC50s) for the next complementary nucleotide on ATP-mediated excision were calculated as the means from three experiments. Statistical significance (P < 0.05) was determined by two-tailed Student's t tests for the arrays of data.
Molecular modeling
The structure of the ternary complex of HIV-1 RT, primer-template, and dTTP of Huang et al. (25) was used as the starting point for all modeling studies. The model was converted into its corresponding minimized model by a force-field method (Sybyl molecular modeling software, version 6.8; Tripos Inc., St. Louis, Mo.). An iterative energy refinement protocol was applied to derive the final minimized RT structure (11, 12). These refinement methods have been used to produce modified models and homology models that have been successfully applied to prediction of the biological properties of proteins as well as to the discovery of inhibitor leads of therapeutic targets (8, 9, 11, 13).
The predicted modified model representing the HIV-1 RT Ser69-Ser-Ser insertion-Lys70 mutant was constructed by a loop search technique within Sybyl modeling software. Here, the wild-type K66-D67-S68-T69-K70 segment was replaced with the inserted K66-D67-S68-S69-S-S-K70 (mutant) segment taken from a library of known X-ray-determined loop structures in which K66 (wild type) and K70 (wild type) served as anchor positions. The final structure containing the insertion was constructed and minimized as described above. The original RT structure containing a dTTP terminator nucleotide within the N site was removed and replaced with TFV-PP; in addition, the corresponding base group of the template strand was also modified to a pyrimidine in order to match the purine base of the inhibitor. The final step involved similar iterative minimization procedures, as described above.
The RT-TFV-PP models were also converted to the RT-TFV-ATP models, which represented the product of ATP-mediated excision. First, the wild-type RT sequence was modified to include TAMs at positions 210 and 215. The modified side chain conformations of T215Y and L210W were placed such that both residues formed stable stacking interactions; these models were then minimized. Second, the phosphate group of AMP was joined to the beta phosphate of TFV-PP to form the TFV-ATP excision product. The AMP was then adjusted such that the purine base of AMP formed favorable stacking interactions with both T215Y and L210W. The final models of RT-TFV-ATP were energy minimized.
A second modeling method, molecular dynamics (MD), was applied in order to study the movement of the RT active-site residues based on the conformational space of static X-ray structures (7, 21). Unlike energy minimization, in which the most energy-stable complex is depicted, the MD method is designed to include the effects of entropy and thus yields a model that represents the putative positions of residues in solution and that simulates the type of data obtained from nuclear magnetic resonance imaging determinations. MD is also used to determine potential conformational changes that can occur in proteins, such as solvent-exposed loop segments (10). Here, MD was used to determine the effect of the insertion in a T69S-SS-L210W-T215Y RT compared to that in a L210W-T215Y RT. Constrained MD simulations were carried out with both the wild-type and mutant RT-TFV-PP models. A 7-Å sphere around the TFV-ATP, including the complete loop region, was allowed to move during the molecular dynamics trajectories. The rest of the RT complex was energetically constrained to remain fixed to its original position. All simulations were carried out by the Tripos force-field method. The simulation system was heated from 0 to 300 K by using 50-degree increments. After system equilibration, the simulation was carried out for a total of 2 ns by using a 2-fs time step. The final 50 trajectories during the last 100 ps of the molecular dynamics run were averaged to derive the final three-dimensional model.
RESULTS
Patient-derived HIV-1 containing the 69-insertion mutation
HIV-1 isolates containing multiple TAMs and the 69-insertion mutation typically show high-level resistance to all NRTIs. To investigate the susceptibility to TFV of a virus containing these mutations, we selected a patient-derived virus containing a 69-insertion mutation obtained in 1997 from the baseline plasma sample from a closed clinical trial (27). Recombinant viruses were constructed from the RT sequence of the virus from this patient's plasma in an HIV-1 HXB2D background. The virus, labeled FS-SSS, contained multiple TAMs (M41L, L210W, T215Y) associated with reduced susceptibilities to NRTIs and TFV, the ddI and abacavir resistance mutation L74V, and the multinucleoside resistance insertion mutation of T69S-SS, as well as several other substitutions (V35M, T39A, R83K, A98S, V108I, E122K, D123E, I135V, Q145C, D177E, G196E, T200A, R211K, L214F, L228H, K249N, P272A, R277K, E297K). A second virus, FS, was constructed from FS-SSS. In the virus FS, T69S-SS was reverted to T69 in order to address the specific role of the T69S substitution and insertion on the susceptibility of this virus to NRTIs.
Susceptibility in cell culture
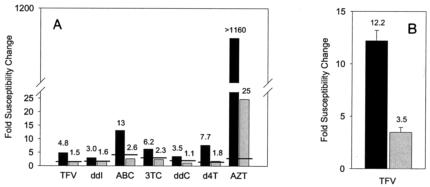
The cell-based antiviral drug susceptibilities of the FS-SSS and FS viruses were measured by the commercially available PhenoSense assay (Fig. 2A). The FS virus demonstrated high-level resistance to AZT (25-fold) and reduced susceptibilities that were near the cutoff values for clinical significance to TFV (1.5-fold), ddI (1.6-fold), d4T (1.8-fold), and 3TC (2.3-fold). The susceptibilities of FS to abacavir and ddC remained below the cutoff values of this assay. The FS-SSS virus showed more strongly reduced susceptibilities to all NRTIs: AZT, >1,160-fold; TFV, 4.8-fold; ddI, 3.0-fold; d4T, 7.7-fold; 3TC, 6.2-fold; abacavir, 13-fold; and ddC, 3.5-fold. The presence of the 69-insertion mutation contributed to a further 3.3-fold reduced TFV susceptibility of FS-SSS compared to that of FS. Susceptibility to TFV was also measured independently by an XTT-based assay with T lymphocytes. By this assay, the FS and FS-SSS viruses were 3.5- and 12.2-fold less susceptible, respectively, than the wild type (Fig. 2B). The second assay confirmed the decreased susceptibilities to TFV of viruses containing multiple TAMs and the further reduction in susceptibility to TFV in the presence of the 69-insertion mutation. The differences between the fold changes in drug susceptibility by these two assays may be explained by one or more differences between the assays, including the different cell types and the different reporter systems used and the single-round versus multiple-round assay lengths (14, 48). These viruses did not contain mutations associated with nonnucleoside RT or protease inhibitor resistance and remained susceptible to these drug classes (data not shown).
FIG. 2.
Cell culture assays for drug susceptibility of the FS-SSS and FS viruses. (A) The fold changes in drug susceptibilities of mutant viruses FS-SSS (black bars) and FS (gray bars) compared to those of wild-type HIV-1 were determined by the PhenoSense assay (ViroLogic), and the values are indicated above the bars. The reduced susceptibility cutoffs for the drugs are indicated by horizontal lines and were 1.4, 1.7, 4.5, 2.5, 1.7, 1.7, and 2.5-fold for TFV, ddI, abacavir (ABC), 3TC, ddC, d4T, and AZT, respectively. For ABC, ddI, d4T, and TFV, these susceptibility cutoff values are also clinical cutoffs for a reduced response. (B) The fold changes in TFV susceptibilities of mutant viruses FS-SSS (black bars) and FS (gray bars) compared to those of the wild type were determined by using MT-2 cells, with the cytopathic effect quantified by a modified XTT-based assay. The fold change values are mean values from two to three experiments, with mean ± standard deviation fold change values shown. The 50% effective concentration of TFV for wild-type virus by this assay was 3.1 μM.
Relative binding affinity measurements
One mechanism of resistance resulting from RT mutations is alteration of the binding or incorporation of the inhibitors with respect to that to their natural substrates (dNTPs). This mechanism allows the mutant RT to selectively discriminate between the NRTI and the natural substrate. Alterations in natural substrate and NRTI discrimination have been described for RTs with 69-insertion mutations (5, 33). We examined the abilities of the FS-SSS and FS RT enzymes to selectively bind to and incorporate dATP, the natural substrate mimicked by TFV-PP, compared to their abilities to bind to and incorporate TFV-PP itself. The Km for dATP, the Ki for the active metabolite of TFV (TFV-PP), and the relative inhibitory capacity (Ki/Km ratio) were measured for these mutants (Table 1). The Km value of wild-type RT for dATP was 0.28 μM and was consistent with the values presented in previous reports (19, 45, 58). The FS-SSS RT showed a slightly elevated Km value of 0.43 μM for dATP compared to that of wild-type RT (P = 0.046). The Km of FS RT for dATP was similar to that of wild-type RT (P > 0.05).
TABLE 1.
Km, Ki, and Ki/Km of wild-type and mutant HIV-1 RT enzymesa
| HIV-1 RT | Km (μM) for dATP | Ki (μM) TFV-PP | Ki/Km for TFV-PP/dATP |
|---|---|---|---|
| Wild type | 0.28 ± 0.09 (1.0) | 0.13 ± 0.04 (1.0) | 0.46 (1.0) |
| FS-SSS | 0.43 ± 0.13b (1.5) | 0.55 ± 0.16c (4.3) | 1.3c (2.8) |
| FS | 0.37 ± 0.13 (1.3) | 0.26 ± 0.10b (2.0) | 0.70 (1.5) |
Km and Ki values are the means ± standard deviations for four to eight experiments. Values in parentheses are the fold change from the value for the wild type.
P < 0.05 compared to the wild type by two-tailed Student's t test.
P < 0.01 compared to the wild type by two-tailed Student's t test.
When the Ki for TFV-PP was measured, FS-SSS RT yielded a Ki value of 0.55 μM, which was elevated 4.3-fold compared to the Ki of 0.13 μM of wild-type RT (P < 0.01) (Table 1). The Ki value of FS RT for TFV-PP was slightly increased to 0.26 μM compared to that of the wild-type RT (P < 0.05). The Ki/Km ratio for TFV-PP was also calculated for the wild-type and mutant RT enzymes (Table 1). FS-SSS RT had a Ki/Km ratio of 1.3, which was slightly, but significantly, increased 2.8-fold compared to that of the wild-type RT (P < 0.01). The Ki/Km ratio of FS RT was 0.7 and was only 1.5-fold increased over that of wild-type RT (P = 0.09). We detected no differences in the Ki and Km values of the wild-type RT enzyme preparation used in this study and those of a wild-type pure heterodimer RT (data not shown). The observed change in the level of discrimination by the FS-SSS RT, although statistically significant, was less than 3-fold, whereas the change in antiviral susceptibility by the MT-2 cell assay was 12.2-fold, suggesting that other resistance mechanisms may also be involved.
Excision of chain terminators by the ATP-mediated excision mechanism
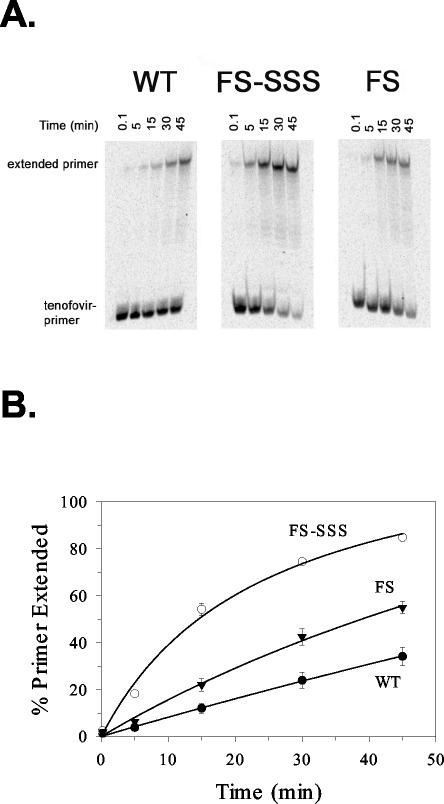
We analyzed the ATP-mediated excision of TFV in order to determine if this mechanism contributed to the susceptibility profiles of the FS and FS-SSS viruses. To measure excision, ATP was added at the physiological concentration of 3.2 mM (23, 56), in which it acts as a pyrophosphate donor to excise TFV from the primer terminus. In this assay, the excision step is followed by an extension reaction in the presence of all dNTPs to yield full-length primers. The mutant FS RT demonstrated significantly increased excision of TFV relative to that of the wild-type RT, and the FS-SSS insertion mutant showed the highest level of excision (Fig. 3). At 30 min, 24, 43, and 75% of the TFV was removed by the wild-type, FS, and FS-SSS RTs, respectively. For wild-type RT, we detected no differences in the levels of ATP-mediated excision for the enzyme preparation used in this study and a wild-type heterodimer RT (data not shown). When the rate of excision of this TFV-terminated primer-template by the mutated RT sequences was determined relative to that by the wild-type RT, the rate was increased 2.3-fold when multiple TAMs were present (FS RT) and was increased 4.5-fold with the addition of the 69-insertion mutation (FS-SSS RT). The excision of TFV by pyrophosphorolysis was also examined. The pyrophosphate-mediated excision by the wild-type enzyme was slightly more efficient than that by the mutant enzymes (data not shown).
FIG. 3.
ATP-dependent excision of TFV chain-terminated primers by wild-type and mutant RTs. (A) A 5′ end-labeled primer-template terminated with TFV was incubated with wild-type (WT), FS-SSS, or FS RT enzymes, followed by the addition of 3.2 mM ATP at 0.1, 5, 15, 30, and 45 min (indicated from left to right) at 37°C. The RT enzymes were heat inactivated, followed by the addition of dNTPs and the Klenow fragment to extend the unblocked primers to yield 50-nt full-length products (extended primer). The reaction products were separated through 8 M urea-16% polyacrylamide sequencing gels and exposed to PhosphorImager screens. (B) The percentages of rescued primers over time were determined for the wild type (WT), FS-SSS, and FS and are means ± standard deviations from three experiments.
Effect of next complementary nucleotide on chain terminator excision
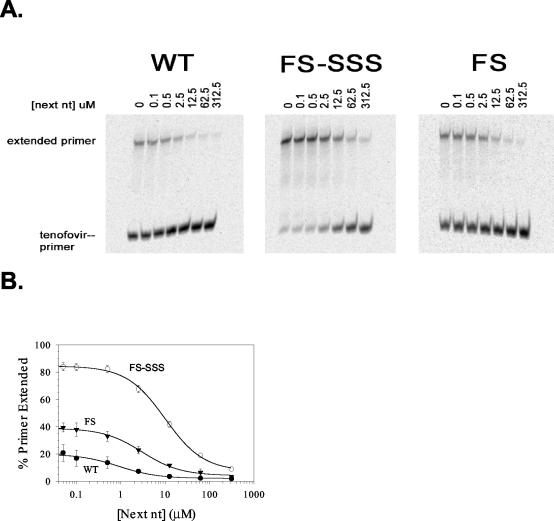
The effect of the next complementary nucleotide on TFV excision was determined (Fig. 4). In this assay, the next nucleotide (dATP) efficiently inhibited the excision of TFV by wild-type RT, with an IC50 of 1.2 μM (Table 2). The IC50s for the next nucleotide for TFV excision were 4.6 and 14.1 μM for the FS and FS-SSS RTs, respectively. This 12-fold increase in the IC50s for the next nucleotide for FS-SSS RT and the 4-fold increase in the IC50s for the next nucleotide for FS RT compared to the IC50 for the next nucleotide for the wild-type RT suggests that the inhibitory effect of the next nucleotide on TFV excision is reduced by the insertion mutations compared to the effect of the RT whose sequence contains only background mutations. Although the dNTP concentrations in replicating virion capsids in the cytoplasms of infected cells are not known, they vary by specific dNTP in primary peripheral blood lymphocytes and are low in resting cells (0.3 to 5 μM) but are increased when cells are activated (3 to 26 μM) (17, 56). At a concentration of the next nucleotide (dATP) of 5 μM, a concentration observed in activated lymphocytes (17), the levels of TFV excision at 30 min were 5% for the wild-type RT and 17% for the FS RT (Table 2). However, for the FS-SSS RT, the level of excision remained high (59%), despite the presence of a putative physiological concentration of the next nucleotide.
FIG. 4.
Effect of the next complementary nucleotide on the ATP-dependent rescue of TFV chain-terminated primers. (A) Rescue of polymerization after excision of TFV by wild-type (WT), FS-SSS, and FS RTs in the presence of the next complementary nucleotide (dATP) at 0, 0.1, 0.5, 2.5, 12.5, 62.5, and 312.5 μM. The excision reactions were initiated by the addition of 3.2 mM ATP for 30 min and heat inactivation of the RT, followed by the addition of 500 μM dNTPs and the Klenow fragment for the extension reaction. (B) The percentages of rescued primers over time were determined for the wild type (WT), FS-SSS, and FS and are means ± standard deviations from three experiments.
TABLE 2.
Next nucleotide inhibition of ATP-mediated TFV excision by wild-type and mutant HIV-1 RT enzymes
| HIV-1 RT | IC50 (μM) for next nucleotidea | % TFV removed in 5 μM next nucleotideb |
|---|---|---|
| Wild type | 1.2 ± 0.2 (1.0)c | 5 (1.0) |
| FS-SSS | 14.1 ± 1.9d (11.8) | 59 (12) |
| FS | 4.6 ± 1.3e (3.8) | 17 (3.4) |
IC50s were determined at 30 min and are the means ± standard deviations of three experiments.
Percentage of tenofovir removed at 30 min in 5 μM dATP.
Values in parentheses are the fold change from the value for the wild type.
P < 0.01 compared to the value for the wild type by two-tailed Student's t test.
P < 0.05 compared to the value for the wild type by two-tailed Student's t test.
Molecular modeling of mutant HIV-1 RT
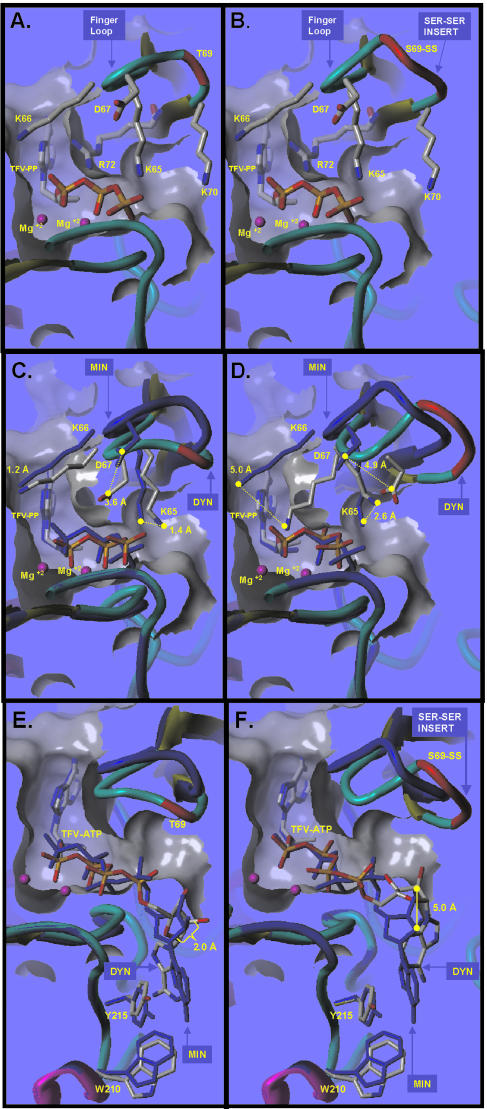
The molecular modeling studies were performed to visualize the interaction of the insertion mutation within the context of the enzyme-substrate complex and to aid in the generation of a hypothesis to describe the molecular mechanisms of resistance to TFV for this mutant. We modeled the wild-type and 69-insertion mutation RTs on the basis of the RT-DNA-dTTP crystal structure of Huang et al. (25). The models are shown in Fig. 5A and B, respectively. The ternary complexes of RT-DNA-TFV-PP (with TFV-PP binding to the N site) were examined. For this complex, the placement of the insertion mutation in the fingers loop resulted in an elongated loop; however, the positions of the key binding sites (residues K65 and R72) of the incoming substrate (TFV-PP) were maintained (compare Fig. 5A and B).
FIG. 5.
Molecular models of HIV-1 RT bound to TFV-PP and TFV-ATP. (A and B) The energy-minimized models of TFV-PP bound to wild-type RT (A) and T69S-SS RT (B) are shown. Residues important in stabilizing this complex are labeled and include K65, K66, K70, and R72. The minimized models show that the insertion does not directly break any critical interactions. The inserted segment occurs at the end of the finger loop and is solvent exposed. (C and D) MD-averaged molecular models of RT bound to TFV-PP are shown for the wild-type RT (C) and the T69S-SS RT (D). The energy-minimized models are colored blue, and the dynamics-averaged structures are shown in atom-type colors. The distances shown as dashed lines indicate the deviations of the polar functional groups between the minimized (MIN) and dynamic (DYN) structures. For wild-type RT, thedistances were modest: K65, 1.4 Å; K66, 1.2 Å; and D67, 3.6 Å. These distances were increased for the 69-insertion mutant RT structure: K65, 2.6 Å; K66, 5.0 Å; and D67, 4.9 Å. (E and F) The energy-minimized models (blue) and MD-averaged models (atom-type colors) of the TFV-ATP excision product complexed to L210W-T215Y RT (E) or T69S-SS-L210W-T215Y RT (F) are shown. The insertion does not directly break any critical interactions. The distance measured indicates the deviations of the cyclic ribose ring between the minimized and dynamic models. The dynamics-averaged models show a larger movement of the ribose ring for the insertion-containing structure (5.0 Å) than for the structure without the insertion (2.0 Å).
The energy-minimized models of wild-type and insertion mutant RTs suggest that the key active-site residues can reside in the positions defined in the crystal structure of Huang et al. (25). These models did not detect any steric conflict between TFV-PP and RT for either mutant, which is consistent with the lack of a large increase in the Ki/Km ratio for this mutant.
The energy-minimized models are not designed to depict the integrity or the entropy effects of these interactions. Therefore, MD models were created in which TFV-PP and the wild-type RT or the 69-insertion mutant RT dNTP-binding site were allowed to undergo the conformational movements that may occur when this protein is present in solution. The results from these analyses are shown in Fig. 5C and D, respectively. In the wild-type RT model, TFV-PP displays only minimal movement in the dNTP-binding pocket. The K65 and K66 side chain moieties remain stable (changes of <1.4 Å) and the D67 side chain shows moderate movement of 3.6 Å from its location in the minimized structure. When the RT of the insertion mutant was examined, TFV-PP also showed minimal movement, as was seen with the wild-type RT. In contrast, the fingers loop of the 69-insertion mutant RT showed increased mobility relative to that of the wild-type RT, as illustrated by the increased movements of K65, K66, and D67 (2.6, 5.0, and 4.9 Å, respectively). This putative increase in mobility of the fingers-loop domain and movement away from the dNTP may result in a decrease in the overall level of binding or the polymerization rate of the insertion RT compared to that of the wild-type RT and is in agreement with the subtle kinetic changes observed for this enzyme.
The effect of the 69-insertion mutation on the ATP-mediated excision complex was also examined (Fig. 5E and F). The T215Y mutation appears to stack in rings with ATP to increase the affinity of RT for ATP, thus facilitating the excision process (3). The product of excision is a dinucleoside tetraphosphate (TFV-ATP, in the case of TFV). Energy-minimized and MD models were created to examine the excision complex consisting of an RT with the T69S-SS, L210W, and T215Y mutations (insertion) and an RT with only L210W and T215Y (210/215 RT) complexed with a DNA primer-template and the TFV-ATP product of excision in the N site. These models depict the excision complex after the formation of the dinucleoside tetraphosphate and prior to its dissociation from the RT-primer-template complex (between steps 8 and 9 of Fig. 1B). The energy-minimized models of the 210/215 and insertion RTs were similar overall. When the MD simulation was applied to the energy-minimized structure of RT bound to TFV-ATP, the fingers loop of the 69-insertion mutant RT had increased mobility compared to that of the 210/215 RT in these simulations. In addition, possibly due to the increased mobility of the fingers-loop domain, the TFV-ATP molecule was additionally observed to show displacement at the ATP moiety. The displacement of the ribose ring of ATP was increased to 5 Å by the 69-insertion mutant RT, whereas it was increased to 2 Å in the fingers-loop model of the wild-type RT. These models suggest that the increased mobility of the β3-β4 loop of the 69-insertion mutant RT may facilitate the opening of the fingers-loop domain and the dissociation of the dinucleoside tetraphosphate from the RT-DNA complex and may decrease the level of formation or the stability of the next nucleotide-RT-DNA-TFV dead-end complex.
DISCUSSION
This study sought to describe the mechanisms of resistance of the 69-insertion mutant RT to TFV. The set of mutants described was particularly interesting because they also contained the M41L, L210W, and T215Y TAMs that are associated with decreased clinical responses to TDF (Miller et al., Program Abstr. Ninth Conf. Retrovir. Opportunistic Infect.). The addition of the diserine 69-insertion mutation was also of particular interest because it causes cross-resistance to all NRTIs and appears to use both mechanisms of resistance: decreased levels of binding or incorporation of ddA, ddC, and 3TC (5) and increased levels of ATP-mediated excision of AZT, d4T, ddT, and ddA (5, 30, 31, 33, 34, 37). However, these mechanisms do not appear to contribute equally to resistance for individual NRTIs. Decreased levels of binding and incorporation may contribute to resistance to ddI and 3TC, but the combination of the increased level of excision of NRTIs (AZT, d4T, ddA, and ddC) from chain-terminated primers and the decreased level of inhibition of excision by the next nucleotide appears to be the major mechanism involved in resistance to NRTIs for 69-insertion mutants (5, 30, 31, 33, 34, 37). The presence of the 69-insertion mutation alone does not confer broad cross-resistance to NRTIs but requires the addition of TAMs for this multidrug resistance phenotype (29, 33). Boyer et al. (5) have shown, for example, that the 69-insertion mutation by itself had little effect on the excision of AZT from chain-terminated primers but, rather, enhanced the level of AZT excision when it was present with the TAM T215Y. In the present work, decreased levels of binding or incorporation of TFV by insertion mutant FS-SSS had a minor role in the observed resistance to TFV.
Excision of chain terminators as a mechanism of resistance to AZT and, more recently, to d4T has been described as a second mechanism of resistance to NRTIs (1, 3, 30, 31, 38, 45, 46). The excision mechanism can be studied biochemically, in which a pyrophosphate donor (pyrophosphate or ATP) attacks the phosphodiester bond between the chain terminator and the previous nucleotide to release an unblocked primer terminus and an NRTI triphosphate or a dinucleoside tetraphosphate, respectively (Fig. 1B) (1, 3, 38). Several assays have been used to evaluate the role of the excision mechanism in resistance to the NRTI class of antiretroviral drugs. These excision assays have been quantified by examining either (i) the quantity of the remaining unblocked primer after excision of the chain terminator (1, 18, 33, 45, 46, 58), (ii) the direct products of excision (i.e., dinucleoside tetraphosphate) (37, 38, 40, 41), (iii) the appearance of the full-length extension products extended by either RT or other polymerases (18, 33, 34, 38-41), or (iv) the extension products after multiple rounds of incorporation and excision on homopolymeric or heteropolymeric templates (3-5, 30, 31, 38-40). TFV has been shown to be excised by wild-type RT from DNA annealed to an HIV RNA template in the presence of pyrophosphate, but excision was barely detectable when ATP was used as the pyrophosphate donor (45, 46, 58). In the present study we used the third method of analyzing the excision of TFV from DNA annealed to an HIV-1 DNA template using ATP as the pyrophosphate donor. Higher levels of excision of TFV by wild-type RT was measured by this assay than by previous assays, and the level was further increased by the mutant RTs studied. The differences in excision rates between these studies may be due to differences in the specific primer-template analyzed, the use of DNA versus RNA templates, the assay methodology, and/or the effects of the different RT mutations analyzed. The present study used RT enzymes containing TAMs M41L, L210W, and T215Y, whereas the previous studies of AZT-resistant RT lacked the M41L and L210W mutations (45, 46). Thus, in addition to TAMs that result in increased levels of excision of AZT, d4T, ddI, and abacavir (3, 5, 18, 38-41, 45, 46, 50), increased levels of excision of TFV can also be demonstrated with the pattern of TAMs used in the present study. The 69-insertion mutation further increased the level of excision of TFV, which was also consistent with the data for AZT, d4T, and ddI (5, 33, 34, 37). However, excision must be considered in the context of the physiologically relevant next nucleotide concentrations present in cells.
The next complementary nucleotide and the chain-terminated primer-template-RT complex can form a salt-stable dead-end complex that is resistant to chain-terminator excision (38, 39, 55). When dNTP concentrations are low, the rate of excision may be increased due to the lower level of inhibition by the next complementary nucleotide. The inhibition of excision by the next nucleotide likely occurs after translocation of the chain terminator from the N site to the P site, and dNTP binds at the N site (3, 52). This hypothesis is supported by several pieces of data, which can be summarized by comparison of the excisions of AZT and d4T. AZT is thought to reside primarily in the N site because the azido group interacts unfavorably with the active-site aspartic acid residues when it is modeled or retained at the P site in a crystal structure (3, 52). d4T, on the other hand, is not expected to show this steric conflict and should translocate efficiently to the P site (3, 46). AZT and d4T are removed efficiently by ATP, but higher levels of the next nucleotide are required for AZT than for d4T to achieve an inhibitory effect on excision. For TFV, only low levels of the next nucleotide (∼1 μM dATP) were required for inhibition of excision by wild-type RT. This inhibition required higher levels of the next nucleotide for RT containing multiple TAMs and even higher levels with the addition of the 69-insertion mutations. Similar observations have been made for TAMs and 69-insertion mutations with other NRTIs, such as ddA and AZT (5, 33, 34, 40). Because dNTP levels in cells can vary substantially between different cell types and during the cell cycle (56), cells with low dNTP concentrations would show increased levels of ATP-mediated excision as a result of the decreased levels of inhibition by the next nucleotide. As discussed in previous reports on this topic (34, 37), the dNTP concentrations in cells can vary greatly; however, for activated lymphocytes in vivo, this value lies in the low micromolar range (17). Although the dNTP concentrations in virion capsids undergoing reverse transcription in the cytoplasms of infected cells are not known, at concentrations ranging over 3 logs around the observed intracellular concentration, the rates of excision of TFV were low for the wild-type RT and moderate for the FS mutant RT containing multiple TAMs; the rate was efficient for the FS-SSS RT containing the 69-insertion mutation compared to that for the wild-type RT.
The 69-insertion mutation is located in the β3-β4 loop of the fingers domain of RT and contains residues that make important contacts between the incoming nucleotide and the RT active site. We hypothesized that the insertion in the FS-SSS RT created a larger solvent-exposed loop and, hence, should have greater flexibility or mobility, supporting the hypotheses of others (54). The MD models presented here further support this hypothesis. The proposed increased mobility of the fingers loop conferred by the 69-insertion mutations may influence the binding of the next complementary nucleotide or the stability of the ternary complex, as observed here and by others (5, 33, 34, 37). Our results further suggest that that the increased mobility of the fingers loop conferred by the 69-insertion mutation may allow the more efficient opening of the fingers-loop domain and release of the excision product, in addition to a destabilization effect on the dead-end complex (Fig. 1, steps 9 and 12). The amplitude of the motion of the fingers-loop domain would require a larger movement for release of the large dinucleoside tetraphosphate molecule than for dNTP or ATP binding, the release of pyrophosphate, or the release of the dNTP product of pyrophosphorolysis. Given that a conformational change at the active site to release the excision product may be rate limiting, this increased mobility would result in an increased rate of ATP-mediated excision. This model would support chain-terminator excision by the insertion mutation as a primary mechanism of resistance to NRTIs when resistance is measured in the presence of physiological dNTP concentrations. The mobility of the fingers domain may be further increased when the insertion mutation is Ser-Gly or Gly-Gly instead of Ser-Ser. In fact, this is supported by a study by Boyer et al. (5), in which a virus with the Ser-Ser insertion in a patient subsequently developed the Ser-Gly insertion. This change was further accompanied by an increased AZT excision rate (5). On the basis of the biochemical properties of amino acid side chains, the mobility of the fingers domain may be decreased by the insertion of Ser-Thr. The prediction would be that this mutant may show a decreased excision rate compared to that of a strain with a Ser-Ser insertion. The position of this residue in mutants with the less frequent pattern of insertion mutations that contain the fingers-loop K70R mutation may be altered, and this alteration may affect the mobility of the fingers loop. Overall, many steps in the excision process could be affected by the insertion mutation. These include translocation, binding, and the correct positioning of the ATP molecule; release of the excision product; the formation and stability of the next nucleotide complex; or the affinity of the RT to chain-terminated primers. We have presented evidence that the 69-insertion mutation affects excision product release and causes a decreased level of formation of the next nucleotide complex or affects the stability of the next nucleotide complex; however, the other steps may also be involved.
The mechanisms involved in drug resistance may differ for each NRTI, each pattern of RT mutations, and a combination of these variables. The 69-insertion mutation in the presence of multiple NRTI resistance mutations confers strong reductions in susceptibility to all NRTIs. In the present work, we have demonstrated that the 69-insertion mutation confers reduced susceptibility to TFV through a combination of slightly decreased level of binding or incorporation and a strongly increased level of ATP-mediated excision. Mechanistically, this pattern of resistance may be explained by the more flexible insertion-containing fingers-loop domain that would negatively affect substrate binding and incorporation, confer the less efficient formation or the decreased stability of the dead-end complex, and facilitate the release of the dinucleoside tetraphosphate product of excision. Although the mutants with insertion mutations show broad NRTI cross-resistance, they are infrequently observed in treated patients, and this may reflect genetic and fitness barriers to the development of this complex mutational pattern. Since the exact agents that select for these insertion mutations are not clear, the best deterrent for the development of these multinucleoside resistance mutations is the use of NRTIs in antiretroviral regimens that effectively suppress virus replication and hence reduce the overall level of resistance development.
Acknowledgments
We thank Mark Wainberg for the HXB2D RT construct and C. Boucher for the HXB2 proviral clone. We also thank Martin McDermott, Ruth Wang, and Martina Pavelko for technical assistance; Manuel Tsiang for regression analysis equation derivation; Craig Gibbs, Andrew Mulato, Shelly Xiong, William Delaney, Damian McColl, Gina Bahador, Adrian Ray, Joseph Vaccaro, Tomas Cihlar, and Janet Douglas for helpful discussions; and Justin Hendrix for assistance with the graphics.
REFERENCES
- 1.Arion, D., N. Kaushik, S. McCormick, G. Borkow, and M. A. Parniak. 1998. Phenotypic mechanism of HIV-1 resistance to 3′-azido-3′-deoxythymidine (AZT): increased polymerization processivity and enhanced sensitivity to pyrophosphate of the mutant viral reverse transcriptase. Biochemistry 37:15908-15917. [DOI] [PubMed] [Google Scholar]
- 2.Boucher, C. A. B., W. Keulen, T. van Bommel, M. Nijhuis, D. de Jong, M. D. de Jong, P. Schipper, and N. K. T. Back. 1996. HIV-1 drug susceptibility determination by using recombinant viruses generated from patient sera tested in a cell-killing assay. Antimicrob. Agents Chemother. 40:2404-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyer, P., S. Sarafianos, E. Arnold, and S. Hughes. 2001. Selective excision of AZTMP by drug-resistant human immunodeficiency virus reverse transcriptase. J. Virol. 75:4832-4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyer, P. L., S. G. Sarafianos, E. Arnold, and S. H. Hughes. 2002. The M184V mutation reduces the selective excision of zidovudine 5′-monophosphate (AZTMP) by the reverse transcriptase of human immunodeficiency virus type 1. J. Virol. 76:3248-3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyer, P. L., S. G. Sarafianos, E. Arnold, and S. H. Hughes. 2002. Nucleoside analog resistance caused by insertions in the fingers of human immunodeficiency virus type 1 reverse transcriptase involves ATP-mediated excision. J. Virol. 76:9143-9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briones, C., A. Mas, G. Gomez-Mariano, C. Altisent, L. Menendez-Arias, V. Soriano, and E. Domingo. 2000. Dynamics of dominance of a dipeptide insertion in reverse transcriptase of HIV-1 from patients subjected to prolonged therapy. Virus Res. 66:13-26. [DOI] [PubMed] [Google Scholar]
- 7.Brooks, B. R., R. E. Bruccoleri, B. D. Olafson, D. J. States, S. Swaminathan, and M. Karpus. 1983. CHARMM: a program for macromolecular energy, minimization, and dynamics calculations. J. Comp. Chem. 4:187-217. [Google Scholar]
- 8.Chen, J. M., G. Jin, A. Sung, and J. I. Levin. 2002. Anthranilate sulfonamide hydroxamate TACE inhibitors. Part 1. Structure-based design of novel acetylenic P1′ groups. Bioorg. Med. Chem. Lett. 12:1195-1198. [DOI] [PubMed] [Google Scholar]
- 9.Chen, J. M., F. C. Nelson, J. I. Levin, D. Mobilio, F. J. Moy, R. Nilakantan, A. Zask, and R. Powers. 2000. Structure-based design of a novel, potent, and selective inhibitor for MMP-13 utilizing NMR spectroscopy and computer-aided molecular design. J. Am. Chem. Soc. 122:9648-9654. [Google Scholar]
- 10.Chen, J. M., K. Rijhwani, F. K. Friedman, M. J. Hyde, and M. R. Pincus. 2000. Identification, using molecular dynamics, of an effector domain of the ras-binding domain of the raf-p74 protein that is uniquely involved in oncogenic ras-p21 signaling. J. Protein Chem. 19:545-551. [DOI] [PubMed] [Google Scholar]
- 11.Chen, J. M., A. Sheldon, and M. R. Pincus. 1993. Structure-function correlations of calcium binding and calcium channel activities based on 3-dimensional models of human annexins I, II, III, V and VII. J. Biomol. Struct. Dyn. 10:1067-1089. [DOI] [PubMed] [Google Scholar]
- 12.Chen, J. M., A. Sheldon, and M. R. Pincus. 1995. Three-dimensional energy-minimized model of human type II “Smith” collagen microfibril. J. Biomol. Struct. Dyn. 12:1129-1159. [DOI] [PubMed] [Google Scholar]
- 13.Chen, J. M., S. L. Xu, Z. Wawrzak, G. S. Basarab, and D. B. Jordan. 1998. Structure-based design of potent inhibitors of scytalone dehydratase: displacement of a water molecule from the active site. Biochemistry 37:17735-17744. [DOI] [PubMed] [Google Scholar]
- 14.Cherrington, J. M., M. D. Fuller, A. S. Mulato, S. J. W. Allen, S. C. Kunder, M. A. Ussery, Z. Lesnikowski, R. F. Schinazi, J.-P. Sommadossi, and M. S. Chen. 1996. Comparative kinetic analysis of interaction of inhibitors with Rauscher murine leukemia virus and human immunodeficiency virus reverse transcriptases. Antimicrob. Agents Chemother. 40:1270-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dahlberg, M. E., and S. J. Benkovic. 1991. Kinetic mechanism of DNA polymerase I (Klenow fragment): identification of a second conformational change and evaluation of the internal equilibrium constant. Biochemistry 30:4835-4843. [DOI] [PubMed] [Google Scholar]
- 16.de Jong, J. J., J. Goudsmit, V. V. Lukashov, M. E. Hillebrand, E. Baan, R. Huismans, S. A. Danner, J. H. ten Veen, F. de Wolf, and S. Jurriaans. 1999. Insertion of two amino acids combined with changes in reverse transcriptase containing tyrosine-215 of HIV-1 resistant to multiple nucleoside analogs. AIDS 13:75-80. [DOI] [PubMed] [Google Scholar]
- 16a.Deval, J., K. L. White, M. D. Miller, N. T. Parkin, J. Courcambeck, P. Halfon, B. Selmi, J. Boretto, and B. Canard.2004. Mechanistic basis for reduced viral and enzymatic fitness of HIV-1 reverse transcriptase containing both K65R and M184V mutations. J. Biol. Chem. 279:509-516. [DOI] [PubMed] [Google Scholar]
- 17.Gao, W. Y., A. Cara, R. C. Gallo, and F. Lori. 1993. Low levels of deoxynucleotides in peripheral blood lymphocytes: a strategy to inhibit human immunodeficiency virus type 1 replication. Proc. Natl. Acad. Sci. USA 90:8925-8928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gotte, M., D. Arion, M. A. Parniak, and M. A. Wainberg. 2000. The M184V mutation in the reverse transcriptase of human immunodeficiency virus type 1 impairs rescue of chain-terminated DNA synthesis. J. Virol. 74:3579-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu, Z., R. S. Fletcher, E. J. Arts, M. A. Wainberg, and M. A. Parniak. 1994. The K65R mutant reverse transcriptase of HIV-1 cross-resistant to 2′,3′-dideoxycytidine, 2′,3′-dideoxy-3′-thiacytidine, and 2′,3′-dideoxyinosine shows reduced sensitivity to specific dideoxynucleoside triphosphate inhibitors in vitro. J. Biol. Chem. 269:28118-28122. [PubMed] [Google Scholar]
- 20.Gu, Z., Q. Gao, H. Fang, H. Salomon, M. A. Parniak, E. Goldberg, J. Cameron, and M. A. Wainberg. 1994. Identification of a mutation of codon 65 in the IKKK motif of reverse transcriptase that encodes human immunodeficiency virus resistance to 2′,3′-dideoxycytidine and 2′,3′-dideoxy-3′-thiacytidine. Antimicrob. Agents Chemother. 38:275-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagler, A. T., D. J. Osguthorpe, P. Dauber-Osguthorpe, and J. C. Hempel. 1985. Dynamics and conformational energetics of a peptide hormone: vasopressin. Science 227:1309-1315. [DOI] [PubMed] [Google Scholar]
- 22.Harrigan, P. R., M. D. Miller, P. McKenna, Z. L. Brumme, and B. A. Larder. 2002. Phenotypic susceptibilities to tenofovir in a large panel of clinically derived human immunodeficiency virus type 1 isolates. Antimicrob. Agents Chemother. 46:1067-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hauschka, P. V. 1973. Analysis of nucleotide pools in animal cells. Methods Cell Biol. 7:361-462. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh, J. C., S. Zinnen, and P. Modrich. 1993. Kinetic mechanism of the DNA-dependent DNA polymerase activity of human immunodeficiency virus reverse transcriptase. J. Biol. Chem. 268:24607-24613. [PubMed] [Google Scholar]
- 25.Huang, H., R. Chopra, G. L. Verdine, and S. C. Harrison. 1998. Structure of a covalently trapped catalytic complex of HIV-1 reverse transcriptase: implications for drug resistance. Science 282:1669-1675. [DOI] [PubMed] [Google Scholar]
- 26.Jacobo-Molina, A., J. Ding, R. G. Nanni, A. D. Clark, Jr., X. Lu, C. Tantillo, R. L. Williams, G. Kamer, A. L. Ferris, P. Clark, A. Hizi, S. H. Hughes, and E. Arnold. 1993. Crystal structure of human immunodeficiency virus type 1 reverse transcriptase complexed with double-stranded DNA at 3.0 Å resolution shows bent DNA. Proc. Natl. Acad. Sci. USA 90:6320-6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahn, J., S. Lagakos, M. Wulfsohn, D. Cherng, M. Miller, J. Cherrington, D. Hardy, G. Beall, R. Cooper, R. Murphy, N. Basgoz, E. Ng, S. Deeks, D. Winslow, J. J. Toole, and D. Coakley. 1999. Efficacy and safety of adefovir dipivoxil with antiretroviral therapy. JAMA 282:2305-2312. [DOI] [PubMed] [Google Scholar]
- 28.Kati, W. M., K. A. Johnson, L. F. Jerva, and K. S. Anderson. 1992. Mechanism and fidelity of HIV reverse transcriptase. J. Biol. Chem. 267:25988-25997. [PubMed] [Google Scholar]
- 29.Larder, B. A., S. Bloor, S. D. Kemp, K. Hertogs, R. L. Desmet, V. Miller, M. Sturmer, S. Staszewski, J. Ren, D. K. Stammers, D. I. Stuart, and R. Pauwels. 1999. A family of insertion mutations between codons 67 and 70 of human immunodeficiency virus type 1 reverse transcriptase confer multinucleoside analog resistance. Antimicrob. Agents Chemother. 43:1961-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lennerstrand, J., K. Hertogs, D. K. Stammers, and B. A. Larder. 2001. Correlation between viral resistance to zidovudine and resistance at the reverse transcriptase level for a panel of human immunodeficiency virus type 1 mutants. J. Virol. 75:7202-7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lennerstrand, J., D. K. Stammers, and B. A. Larder. 2001. Biochemical mechanism of human immunodeficiency virus type 1 reverse transcriptase resistance to stavudine. Antimicrob. Agents Chemother. 45:2144-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Margot, N. A., E. Isaacson, I. McGowan, A. K. Cheng, R. T. Schooley, and M. D. Miller. 2002. Genotypic and phenotypic analyses of HIV-1 in antiretroviral-experienced patients treated with tenofovir DF. AIDS 16:1227-1235. [DOI] [PubMed] [Google Scholar]
- 33.Mas, A., M. Parera, C. Briones, V. Soriano, M. A. Martinez, E. Domingo, and L. Menendez-Arias. 2000. Role of a dipeptide insertion between codons 69 and 70 of HIV-1 reverse transcriptase in the mechanism of AZT resistance. EMBO J. 19:5752-5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mas, A., B. M. Vazquez-Alvarez, E. Domingo, and L. Menendez-Arias. 2002. Multidrug-resistant HIV-1 reverse transcriptase: involvement of ribonucleotide-dependent phosphorolysis in cross-resistance to nucleoside analogue inhibitors. J. Mol. Biol. 323:181-197. [DOI] [PubMed] [Google Scholar]
- 35.Masquelier, B., E. Race, C. Tamalet, D. Descamps, J. Izopet, C. Buffet-Janvresse, A. Ruffault, A. S. Mohammed, J. Cottalorda, A. Schmuck, V. Calvez, E. Dam, H. Fleury, and F. Brun-Vezinet. 2001. Genotypic and phenotypic resistance patterns of human immunodeficiency virus type 1 variants with insertions or deletions in the reverse transcriptase (RT): multicenter study of patients treated with RT inhibitors. Antimicrob. Agents Chemother. 45:1836-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McColl, D. J., and M. D. Miller. 2003. The use of tenofovir disoproxil fumarate for the treatment of nucleoside-resistant HIV-1. J. Antimicrob. Chemother. 51:219-223. [DOI] [PubMed] [Google Scholar]
- 37.Meyer, P. R., J. Lennerstrand, S. E. Matsuura, B. A. Larder, and W. A. Scott. 2003. Effects of dipeptide insertions between codons 69 and 70 of human immunodeficiency virus type 1 reverse transcriptase on primer unblocking, deoxynucleoside triphosphate inhibition, and DNA chain elongation. J. Virol. 77:3871-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer, P. R., S. E. Matsuura, A. M. Mian, A. G. So, and W. A. Scott. 1999. A mechanism of AZT resistance: an increase in nucleotide-dependent primer unblocking by mutant HIV-1 reverse transcriptase. Mol. Cell 4:35-43. [DOI] [PubMed] [Google Scholar]
- 39.Meyer, P. R., S. E. Matsuura, R. F. Schinazi, A. G. So, and W. A. Scott. 2000. Differential removal of thymidine nucleotide analogues from blocked DNA chains by human immunodeficiency virus reverse transcriptase in the presence of physiological concentrations of 2′-deoxynucleoside triphosphates. Antimicrob. Agents Chemother. 44:3465-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer, P. R., S. E. Matsuura, A. G. So, and W. A. Scott. 1998. Unblocking of chain-terminated primer by HIV-1 reverse transcriptase through a nucleotide-dependent mechanism. Proc. Natl. Acad. Sci. USA 95:13471-13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer, P. R., S. E. Matsuura, A. A. Tolun, I. Pfeifer, A. G. So, J. W. Mellors, and W. A. Scott. 2002. Effects of specific zidovudine resistance mutations and substrate structure on nucleotide-dependent primer unblocking by human immunodeficiency virus type 1 reverse transcriptase. Antimicrob. Agents Chemother. 46:1540-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller, M., N. Margot, K. Hertogs, B. Larder, and V. Miller. 2001. Antiviral activity of tenofovir (PMPA) against nucleoside-resistant clinical HIV samples. Nucleosides Nucleotides Nucleic Acids 20:1025-1028. [DOI] [PubMed] [Google Scholar]
- 43.Miller, M. D., K. E. Anton, A. S. Mulato, P. D. Lamy, and J. M. Cherrington. 1999. Human immunodeficiency virus type 1 expressing the lamivudine-associated M184V mutation in reverse transcriptase shows increased susceptibility to adefovir and decreased replication capability in vitro. J. Infect. Dis. 179:92-100. [DOI] [PubMed] [Google Scholar]
- 44.Miller, M. D., P. D. Lamy, M. D. Fuller, A. S. Mulato, N. A. Margot, T. Cihlar, and J. M. Cherrington. 1998. Human immunodeficiency virus type 1 reverse transcriptase expressing the K70E mutation exhibits a decrease in specific activity and processivity. Mol. Pharmacol. 54:291-297. [DOI] [PubMed] [Google Scholar]
- 45.Naeger, L. K., N. A. Margot, and M. D. Miller. 2001. Altered drug susceptibility of HIV-1 RT mutants containing M184V and zidovudine-resistance mutations: analysis of RT processivity, viral replication, and chain-terminator removal. Antivir. Ther. 6:111-123. [PubMed] [Google Scholar]
- 46.Naeger, L. K., N. A. Margot, and M. D. Miller. 2002. ATP-dependent removal of nucleoside reverse transcriptase inhibitors by human immunodeficiency virus type 1 reverse transcriptase. Antimicrob. Agents Chemother. 46:2179-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naeger, L. K., and M. D. Miller. 2001. Mechanisms of HIV-1 nucleoside reverse transcriptase inhibitor resistance: is it all figured out? Curr. Opin. Investig. Drugs 2:335-339. [PubMed] [Google Scholar]
- 48.Petropoulos, C. J., N. T. Parkin, K. L. Limoli, Y. S. Lie, T. Wrin, W. Huang, H. Tian, D. Smith, G. A. Winslow, D. J. Capon, and J. M. Whitcomb. 2000. A novel phenotypic drug susceptibility assay for human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 44:920-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quan, Y., Z. Gu, X. Li, Z. Li, C. D. Morrow, and M. A. Wainberg. 1996. Endogenous reverse transcription assays reveal high-level resistance to the triphosphate of (−)2′-dideoxy-3′-thiacytidine by mutated M184V human immunodeficiency virus type 1. J. Virol. 70:5642-5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ray, A. S., A. Basavapathruni, and K. S. Anderson. 2002. Mechanistic studies to understand the progressive development of resistance in human immunodeficiency virus type 1 reverse transcriptase to abacavir. J. Biol. Chem. 277:40479-40490. [DOI] [PubMed] [Google Scholar]
- 51.Sarafianos, S., A. D. Clark, Jr., S. Tuske, C. Squire, K. Das, D. Sheng, P. Ilankumaran, A. R. Ramesha, H. Kroth, J. M. Sayer, D. M. Jerina, P. L. Boyer, S. H. Hughes, and E. Arnold. 2003. Trapping HIV-1 reverse transcriptase before and after translocation on DNA. J. Biol. Chem. 278:16280-16288. [DOI] [PubMed] [Google Scholar]
- 52.Sarafianos, S. G., A. D. Clark, Jr., K. Das, S. Tuske, J. J. Birktoft, P. Ilankumaran, A. R. Ramesha, J. M. Sayer, D. M. Jerina, P. L. Boyer, S. H. Hughes, and E. Arnold. 2002. Structures of HIV-1 reverse transcriptase with pre- and post-translocation AZTMP-terminated DNA. EMBO J. 21:6614-6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sluis-Cremer, N., D. Arion, and M. A. Parniak. 2000. Molecular mechanisms of HIV-1 resistance to nucleoside reverse transcriptase inhibitors (NRTIs). Cell. Mol. Life Sci. 57:1408-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tamalet, C., N. Yahi, C. Tourres, P. Colson, A. M. Quinson, I. Poizot-Martin, C. Dhiver, and J. Fantini. 2000. Multidrug resistance genotypes (insertions in the beta3-beta4 finger subdomain and MDR mutations) of HIV-1 reverse transcriptase from extensively treated patients: incidence and association with other resistance mutations. Virology 270:310-316. [DOI] [PubMed] [Google Scholar]
- 55.Tong, W., C. D. Lu, S. K. Sharma, S. Matsuura, A. G. So, and W. A. Scott. 1997. Nucleotide-induced stable complex formation by HIV-1 reverse transcriptase. Biochemistry 36:5749-5757. [DOI] [PubMed] [Google Scholar]
- 56.Traut, T. 1994. Physiological concentrations of purines and pyrimidines. Mol. Cell. Biochem. 140:1-22. [DOI] [PubMed] [Google Scholar]
- 57.Wainberg, M. A., M. D. Miller, Y. Quan, H. Salomon, A. S. Mulato, P. D. Lamy, N. A. Margot, K. E. Anton, and J. M. Cherrington. 1999. In vitro selection and characterization of HIV-1 with reduced susceptibility to PMPA. Antivir. Ther. 4:87-94. [DOI] [PubMed] [Google Scholar]
- 58.White, K. L., N. A. Margot, T. Wrin, C. J. Petropoulos, M. D. Miller, and L. K. Naeger. 2002. Molecular mechanisms of resistance to human immunodeficiency virus type 1 with reverse transcriptase mutations K65R and K65R+M184V and their effects on enzyme function and viral replication capacity. Antimicrob. Agents Chemother. 46:3437-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Winters, M. A., K. L. Coolley, Y. A. Girard, D. J. Levee, H. Hamdan, R. W. Shafer, D. A. Katzenstein, and T. C. Merigan. 1998. A 6-basepair insert in the reverse transcriptase gene of human immunodeficiency virus type 1 confers resistance to multiple nucleoside inhibitors. J. Clin. Investig. 102:1769-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]