Abstract
Mitochondrial disease refers to a heterogenous group of genetic disorders that result from dysfunction of the final common pathway of energy metabolism. Mitochondrial DNA mutations affect key components of the respiratory chain and account for the majority of mitochondrial disease in adults. Owing to critical dependence of the heart on oxidative metabolism, cardiac involvement in mitochondrial disease is common and may occur as the principal clinical manifestation or part of multisystem disease. Recent advances in our understanding of the clinical spectrum and genetic aetiology of cardiac involvement in mitochondrial DNA disease have important implications for cardiologists in terms of the investigation and multi-disciplinary management of patients.
Keywords: Mitochondrial DNA disease, Cardiac involvement, Cardiomyopathy, Conduction system disease, Ventricular pre-excitation
Introduction
Mitochondrial disease includes various clinical disorders that occur as a result of dysfunctional cellular oxidative phosphorylation (OXPHOS), due to a primary genetic defect. Such mitochondrial disease can be caused by defects in either mitochondrial or nuclear DNA, but mitochondrial DNA (mtDNA) mutations are the commonest cause of mitochondrial disease in adults, identified in ∼70% patients, and present unique challenges in diagnosis and management. Clinical disease-based prevalence studies suggest that mtDNA disease affects 9.2/100 000 adults aged <65 years, with a further 16.5/100 000 children and adults aged <65 years at risk of development of disease.1 These figures derive from regional referral patterns and are likely an underestimation of the true prevalence of mtDNA disease. The m.3243A>G mutation is present in ∼1 in 300 of the general population and, while many individuals will possess low levels of mutation and remain asymptomatic, mtDNA disease appears more common than previously thought, causing disease in ∼1 in 5000 individuals.2
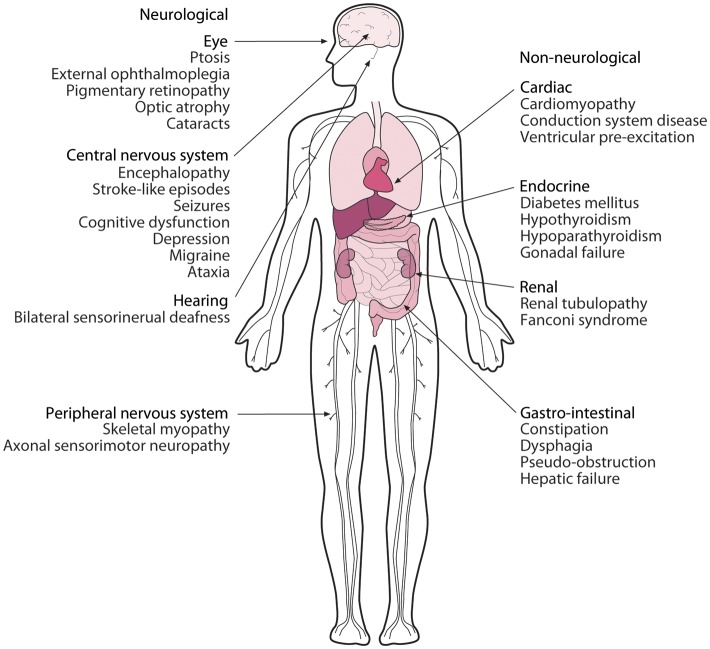
The clinical spectrum in mtDNA disease is wide (Figure 1) with both isolated organ involvement and more frequent multisystem disease recognized. Presentation may be at any age and in almost any organ, but those with high energy requirements, including the brain, eye, skeletal muscle, and heart, are most frequently involved.3,4 Indeed natural history studies have demonstrated that cardiac involvement in mtDNA disease is progressive and an independent predictor of morbidity and early mortality.5,6 Cardiac and neurological diseases are the commonest causes of early death in patients with mitochondrial disease due to the m.3243A>G mutation, while sudden death, often with a suspected cardiac aetiology, is frequently reported.7 Hence, cardiologists are likely to become increasingly involved in the multi-disciplinary care of these patients. They should be familiar with the unique non-Mendelian inheritance pattern and distinctive pathophysiology, the clinical spectrum of cardiac manifestations, and the challenges of diagnosis and management in patients with mtDNA disease.
Figure 1.
Clinical features of mitochondrial DNA disease. Diverse organ systems can be affected in mitochondrial DNA disease either within an individual or a family. Patterns of distant organ involvement (e.g. diabetes and deafness) or a relevant family history may prompt consideration of a mitochondrial aetiology.
Clinical features
The manifestations of mtDNA disease vary from oligosymptomatic states (e.g. type 2 diabetes mellitus or migraine) to complex syndromes often involving neurological, ophthalmological, cardiological, gastroenterological, or endocrine features.4 Proximal skeletal myopathy may be slowly progressive, while ophthalmological manifestations, including ptosis, ophthalmoplegia, cataracts, and optic atrophy, are common presenting symptoms. Central nervous system involvement is often associated with more severe disease, including deafness, migraine, epilepsy, ataxia, encephalopathy, stroke, or dementia. Diabetes is common in patients with mtDNA disease, while liver, renal, and other endocrinological abnormalities are more rarely described.
Clinical syndromes of mtDNA disease (Table 1), originally described in individual families, have permitted investigations of well-characterized groups of patients.5,7,8 However, it is now recognized that many patients with mtDNA disease do not fit into such clinical categories. Patients may present with features suggestive of mtDNA disease, such as involvement of distant organs (e.g. deafness and diabetes) or a family history of isolated organ involvement [e.g. hypertrophic cardiomyopathy (HCM)].9
Table 1.
Clinical syndromes associated with mitochondrial DNA mutations
| Syndrome | Principal clinical features | Mitochondrial DNA mutation |
|---|---|---|
| CPEO | External ophthalmoplegia, myopathy | Single or multiple mtDNA deletions |
| Kearns-Sayre syndrome | Pigmentary retinopathy, ataxia, cardiac conduction defects | Single, large-scale mtDNA deletion |
| Leigh syndrome | Subacute necrotizing encephalopathy, basal ganglia lesions | Complex I, IV, and V gene mutations |
| LHON | Acute or sub-acute visual loss | Complex I gene mutations |
| MELAS | Myopathy, encephalopathy, lactic acidosis, stroke-like episodes | mt-tRNA gene mutations |
| MERRF | Myoclonus, epilepsy, ataxia | mt-tRNA gene mutations |
| NARP | Neuropathy, ataxia, pigmentary retinopathy | Complex V mutations |
| Pearson's marrow-pancreas syndrome | Sideroblastic anaemia, exocrine pancreatic insufficiency, hepatopathy, nephropathy | Single, large-scale mtDNA deletion |
CPEO, chronic progressive external ophthalmoplegia; LHON, Leber's hereditary optic neuropathy; MELAS, myopathy, encephalopathy, and lactic acidosis with stroke-like episodes; MERRF, mitochondrial encephaolopathy with ragged red fibres; mtDNA, mitochondrial DNA; NARP, neurogenic ataxia and retinitis pigmentosa.
Mitochondrial genetics
Mitochondria are involved in essential cellular processes including calcium signalling, apoptosis, and generation of reactive oxygen species (ROS) but their principal function is adenosine triphsophate (ATP) synthesis via OXPHOS. The transfer of electrons between respiratory chain enzyme complexes I–IV drives proton transfer across the inner mitochondrial membrane, forming an electro-chemical gradient that is utilized by complex V to generate ATP. The mitochondrial genome encodes 22 transfer RNAs (mt-tRNAs), 2 ribosomal RNAs (mt-rRNAs), and 13 polypeptides that are all critical components of OXPHOS enzyme complexes. All other proteins involved in mitochondrial function are encoded by the nuclear genome. This bi-genomic control of the mitochondrial proteome is an important feature of mitochondrial biology.
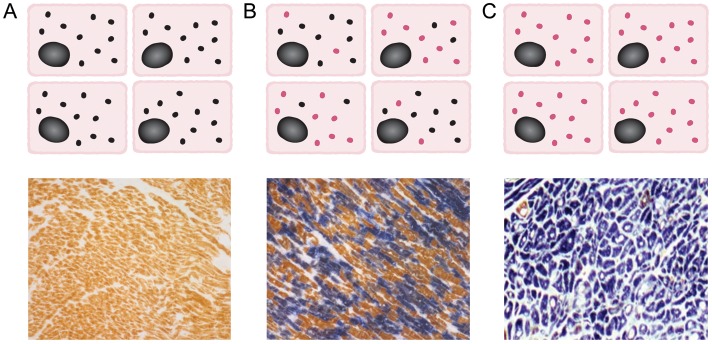
Mitochondrial genetics are complex and display a number of unique characteristics.10,11 Multiple copies of mtDNA exist within each cell. In the general population, although a small number of mtDNA molecules may contain mutations, their proportion is usually so small (<1%) that the tissue can be regarded as uniform for the normal mitochondrial genome (homoplasmy).4 In contrast, for most pathogenic mtDNA mutations, two or more distinct mitochondrial genomes exist within the same tissue at high percentage (heteroplasmy). Most mtDNA mutations behave recessively, only manifesting when the proportion of mutated mtDNA exceeds a threshold level, typically ∼60–90% (Figure 2). Tissue mtDNA mutation load and threshold may affect the onset and extent of clinical disease.12 The recognition of pathogenic homoplasmic mtDNA mutations, which frequently result in isolated organ phenotypes including cardiomyopathy, emphasizes the fact that other genetic (e.g. expression of aminoacyl tRNA synthetases) or environmental factors can modulate the phenotype.9,13,14
Figure 2.
Mitochondrial DNA mutations and patterns of cellular respiratory function. (A) In normal individuals, all cardiomyocytes contain multiple copies of wild-type mitochondrial DNA (black circles, upper panel), with sequential cytochrome c oxidase/succinate dehydrogenase histochemistry showing all cardiomyocytes as cytochrome c oxidase-positive (brown, lower panel). (B) In patients with heteroplasmic mitochondrial DNA mutations, different proportions of wild-type (black) and mutated mitochondrial DNA (red) are present in individual cardiomyocytes (upper panel); cytochrome c oxidase/succinate dehydrogenase histochemistry reveals a mosaic pattern of cytochrome c oxidase-deficient and cytochrome c oxidase-positive cardiomyocytes, with cellular respiratory deficiency only apparent when a threshold proportion of mutated mitochondrial DNA is reached (lower panel). (C) In patients with homoplasmic mitochondrial DNA mutations, all cardiomyocytes contain multiple copies of mutated mitochondrial DNA (red, upper panel), with the majority of cells displaying cytochrome c oxidase deficiency (blue, lower panel).
Human mtDNA exhibits strict maternal inheritance. Clinical disease exclusively in maternal relatives raises suspicion of mtDNA disease, and genetic counselling is manifestly different to that in nuclear genetic disorders. The nature of the defect also affects the likelihood of maternal transmission such that single, large-scale deletions are rarely transmitted from females to their offspring, while point mutations are frequently transmitted.15 Indeed, during female germline development, the number of mtDNA molecules within each oocyte is dramatically reduced before being re-amplified to a final number >100 000. This ‘genetic bottleneck’ accounts for shifts in mtDNA mutation load between generations and partly explains variations in clinical disease severity.16
The mitochondrial genome acquires mutations at a rate 10–17-fold higher than nuclear DNA due to proximity to the OXPHOS system and deficiency in DNA repair mechanisms.4 Owing to the continuous replicative nature of mtDNA, the proportion of mutated mtDNA molecules can be increased by clonal expansion, even in post-mitotic cells including cardiomyocytes.17 Although the clinical importance of these acquired mtDNA mutations in the general population is debated, in patients possessing high levels of a mtDNA mutation, this process can lead to profound changes in mtDNA mutation load and contribute to clinical progression.
Cardiac disease
More than 250 different pathogenic mtDNA mutations have been reported in humans, many in association with cardiac disease, which ranges from cardiomyopathy to electropathy, including conduction disease and ventricular pre-excitation. This diversity and the absence of a cardiac phenotype that is unique to patients with mtDNA disease present challenges to the cardiologist.
Prevalence and natural history
The true prevalence of mtDNA-related cardiomyopathy is unknown although, based on the prevalence of mtDNA disease and the frequency of cardiac involvement, at least ∼1 in 10–15 000 of the general population will be affected. Public databases of mtDNA mutations associated with human disease exist and will be an important resource in determining prevalence.18,19 However, such databases are currently not completely accurate as, owing to extensive variability of the mitochondrial genome and a lack of adherence to strict canonical criteria for determining pathogenicity, some non-disease causing variants are listed. Challenges lie ahead with regard to the analysis of such bio-informatic data.20–22
Natural history studies have demonstrated both the high prevalence of cardiac disease and the deleterious effects on patient outcome of a cardiac presentation. A significant difference in survival to age 16 years was noted in 113 children with mitochondrial disease (18 and 92%, respectively, in those with and without cardiomyopathy).6 This result, in a cohort including patients with mitochondrial and nuclear DNA mutations, has subsequently been confirmed in other large paediatric cohorts.23,24 Adult studies, in patients with mtDNA mutations exclusively, have established the progressive nature of cardiac involvement,8,25,26 with important impacts on morbidity and early mortality.7,27 In common with many newly recognized disorders, early reports of cardiac involvement in mtDNA disease featured patients with severe phenotypes. Family genetic screening has undoubtedly broadened the spectrum of mtDNA disease to include more asymptomatic or oligosymptomatic adults, perhaps limiting the applicability of early studies. A recent study of 32 adult patients demonstrated that, although cardiac involvement was apparent in 78% patients, minor electrocardiogram (ECG) abnormalities represented the most common manifestation, with cardiomyopathy present in 25% patients.5 Progressive systolic dysfunction and high-grade atrio-ventricular (AV) block did occur in a minority but the incidence of severe cardiovascular complications was relatively low over a median follow-up of 4 years. Large multi-centre prospective clinical cohort studies are underway and will provide novel insights into the natural history and response to intervention of adult mtDNA disease.
Pathogenetic mechanisms
The molecular events linking mtDNA defects to cardiac dysfunction are poorly understood. Although several factors, including rarity of the disorder, limited access to human cardiac tissue and an absence of reliable animal models of mtDNA disease play a role in limiting investigation, the weak nature of genotype–phenotype correlations is a critical factor. The development of cardiomyocyte cell lines from patients with mtDNA disease using inducible pluripotent stem cell technology will undoubtedly be an important step forward in this area.
Early mechanistic insights developed from observation of patterns of disease. Although patients with specific mtDNA mutations may present with different cardiac phenotypes,25 and similar cardiac involvement can occur in patients with different mtDNA mutations,25,28 cross-sectional studies suggest patterns of cardiac involvement do exist (Table 2). For example, cardiomyopathies, often with a hypertrophic phenotype, are more frequently reported in association with mt-tRNA gene mutations, while AV block is a feature of Kearns-Sayre syndrome (KSS), which is commonly caused by single, large-scale deletions in mtDNA.5 The only cardiac phenotype reported in association with the m.1555A>G mt-rRNA gene mutation is a restrictive cardiomyopathy.29 Although differential effects of mutations in mt-tRNA, mt-rRNA, and polypeptide genes on mitochondrial transcription, translation, and protein function may be expected, the mechanisms underlying this apparent genotype–phenotype relationship are unclear. Tissue specificity of mutation load is widely recognized as a factor in the diverse clinical features of mtDNA disease generally; a similar phenomenon occurring within, rather than between, tissues may be equally important. Higher mutation load of a single, large-scale mtDNA deletion has been reported in post-mortem AV nodal and His-Purkinje system tissue than in contractile myocardium from a patient with KSS, suggesting a reason for the apparent sensitivity of the conduction system.30
Table 2.
Cardiac phenotypes associated with pathogenic mtDNA mutations
| Electropathy |
Cardiomyopathy |
|||||||
|---|---|---|---|---|---|---|---|---|
| Gene | mtDNA mutation | Ventricular pre-excitation | Conduction disease | Hypertrophic | Dilated | Restrictive | Left ventricular non-compaction | Histiocytoid |
| Common | ||||||||
| MTTL1 | m.3243A>G | ++ | + | ++ | + | + | + | − |
| MTTI | m.4300A>G | − | − | ++ | + | − | − | − |
| MTTK | m.8344A>G | ++ | + | ++ | ++ | − | − | + |
| MTND4 | m.11778G>A | ++ | − | + | − | − | − | − |
| single, large-scale mtDNA deletion | − | ++ | − | + | − | − | − | |
| Rare | ||||||||
| MTRNR1 | m.1555A>G | − | − | − | − | + | − | − |
| MTTV | m.1624C>T | − | − | + | + | − | − | − |
| MTTL1 | m.3252T>C | − | + | − | + | − | − | − |
| m.3260A>G | + | − | + | + | − | − | − | |
| m.3303T>C | − | + | + | + | − | − | − | |
| MTND1 | m.3337G>A | − | − | + | + | − | − | − |
| m.3460G>A | + | − | + | − | − | + | − | |
| MTTI | m.4269A>G | − | − | − | + | − | − | − |
| m.4277T>C | − | − | + | − | − | − | − | |
| m.4284G>A | − | + | + | + | − | − | − | |
| m.4317A>G | − | − | + | + | − | − | − | |
| m.4320C>T | − | − | + | − | − | − | − | |
| MTTK | m.8363G>A | − | − | + | + | − | − | − |
| MTATP8/MTATP6 | m.8528T>C | − | − | + | − | − | − | − |
| m.8529G>A | − | − | + | − | − | − | − | |
| MTATP6 | m.8993T>G | − | − | + | − | − | − | − |
| MTTG | m.9997T>C | − | − | + | − | − | − | − |
| MTND4 | m.11778A>G | − | − | − | + | − | − | − |
| MTTL2 | m.12297T>C | − | − | − | + | − | − | − |
| MTND5 | m.13513G>A | + | + | − | − | − | − | − |
| MTND6 | m.14484T>C | − | − | − | + | − | − | − |
| MTCYB | m.14849T>C | − | − | + | − | − | − | − |
| m.15498G>A | − | − | − | − | − | − | + | |
Pathogenic mitochondrial DNA mutations were identified from a search of online databases,18,19 together with the cumulative experience of the authors, excluding rare single nucleotide polymorphisms, and haplogroup markers. mtDNA, mitochondrial DNA; ++, reported in cross-sectional cohort study with ≥10% frequency; +, reported in single case report(s)/family series only; −, not reported.
Marked induction of mitochondrial biogenesis is a prominent feature of end-stage mtDNA-related cardiomyopathy,31–33 and has been demonstrated in diverse tissues from patients with mtDNA disease. Although in skeletal muscle this response can partially compensate for OXPHOS dysfunction, experimental, and clinical evidence suggests that it may have a detrimental effect in cardiac muscle.34,35 Proliferation of intermyofibrillar mitochondria mechanically interferes with sarcomeric function, contributing to adverse cardiac remodelling.32,34 Induction of genes involved in mitochondrial biogenesis and fatty acid oxidation (FAO) in mtDNA-related cardiomyopathy increases oxygen consumption and contrasts with other pathologies, including left ventricular hypertrophy (LVH), where cardiac energy metabolism shifts from FAO to glucose oxidation to reduce oxygen consumption.36 Moreover, in the absence of induction of antioxidants, an increased mass of mutated mitochondria causes increased ROS.32,37 The pathogenetic role of ROS has been confirmed in animal models of nuclear mitochondrial disease, but data on mtDNA disease are lacking.38,39
Cardiomyopathy
Hypertrophic cardiomyopathy/left ventricular hypertrophy
Hypertrophic remodelling is the dominant pattern of cardiomyopathy in all forms of mitochondrial disease,5,28,40,41 occurring in up to ∼40% patients,5,6 and can mimic HCM. The prevalence of HCM within the general population is ∼1 in 500 yet sarcomeric protein mutations are identified in only ∼60% of HCM patients. mtDNA-related cardiomyopathy represents a potential phenocopy of HCM and may partly account for this discrepancy similar to single gene disorders that have already been identified in HCM cohorts such as Anderson-Fabry and glycogen storage diseases.42,43 Cardiologists should be alert to the presence of extra-cardiac features (Figure 1), or possible maternal inheritance patterns, in this population.
Point mutations in mtDNA can cause sporadic or maternally inherited cardiomyopathy, which may be the only or presenting feature. Recent cohort studies using echocardiography have identified LVH in 38–56% patients harbouring the m.3243A>G mutation and have revealed a correlation between skeletal muscle mutant load and indexed left ventricular mass.28,41 Patients with high mutation load may therefore be at increased risk of development of cardiomyopathy. Left ventricular hypertrophy is recognized in patients with other mtDNA mutations including several mt-tRNA genes (e.g. m.8344A>G in MTTK, m.4269A>G and m.4317A>G in MTTI) and infrequent polypeptide genes (e.g. m.8993T>G in MTATP6 and m.8528T>C in the MTATP6/MTATP8 overlap region).25,44–47 Indeed the mt-tRNA genes appear to be a particularly sensitive location in the mitochondrial genome for mutations associated with the hypertrophic phenotype (Table 2). Although LVH is reported in patients with mitochondrial disease due to mutations in genes encoding mt-rRNAs and polypeptides, it appears to be a much less common clinical finding in this subset of patients than in patients with mt-tRNA gene mutations. Homoplasmic mtDNA mutations, which characteristically cause organ-specific phenotypes, have also been reported in patients with cardiac disease.9,14 The homoplasmic m.4300A>G mutation, in the mt-tRNAIle (MTTI) gene has now been identified in several families with isolated mtDNA-related cardiomyopathy and may play a more important role in inherited cardiomyopathy than previously appreciated, although this is yet to be confirmed through systematic analysis of HCM cohorts.9
There are important differences in the cardiac phenotype and natural history of HCM and mtDNA-related cardiomyopathy. Left ventricular outflow tract (LVOT) obstruction is rarely observed in mtDNA-related cardiomyopathy, yet it appears that the likelihood of progression to ventricular dilatation and heart failure is higher than in HCM.41 A longitudinal study with 6.9 years mean follow-up duration demonstrated that the degree of LVH correlated positively with chamber dilatation and negatively with systolic function in patients harbouring the m.3243A>G mutation.26 Heart failure with ventricular dilatation and impaired systolic function has been reported in patients with LVH and the m.3243A>G or m.8344A>G mutations.25,26
Dilated cardiomyopathy
Although dilated cardiomyopathy (DCM) can be the initial pattern of cardiac involvement in mtDNA disease,48 it more commonly represents progression of pre-existing hypertrophy with chamber dilation and systolic dysfunction.25,49,50 One patient with DCM was identified among 17 patients with mitochondrial disease, while a recent study of 18 patients with the m.8344A>G mutation confirmed DCM in 22% patients.25,27 Dilated cardiomyopathy is rarer than the hypertrophic phenotype in association with other mt-tRNA point mutations, including m.3243A>G, m.4269A>G, and m.4317A>G,45,46,48 and appears to be an infrequent and late phenomenon in KSS, described in only 2% of published patients.51,52
Due primarily to phenotypic rarity, data are lacking concerning natural history in patients with mtDNA disease and DCM phenotype. Mouse models of DCM and mitochondrial disease do exist, 39,53,54 but do not feature mtDNA point mutations or single deletions and have little direct relevance to patients with these specific mutations. Cardiac symptoms may be limited in patients with multisystem mtDNA disease due to progressive skeletal myopathy restricting physical activity. However, limited echocardiographic studies in adults suggest that progression of DCM may be slow and, at least in some patients, responsive to conventional heart failure therapies.25,48
Rarer cardiomyopathies
Restrictive cardiomyopathy is a rare presentation of cardiac involvement in mtDNA disease but has been reported in association with maternally inherited deafness and diabetes due to the m.3243A>G mutation55 and as the only clinical finding in a subject with the m.1555A>G mutation.29
Left ventricular non-compaction (LVNC) is caused by abnormal compaction of myofibrils during cardiac development and results in progressive ventricular dilatation and systolic dysfunction. Differentiation from normal variants can be difficult, diagnosis remains controversial, and the natural history is unclear.56,57 Mutations in sarcomeric or ion channel genes account for only a small proportion of LVNC cases.58 Left ventricular non-compaction has recently been recognized as a cardiac manifestation of mtDNA disease, particularly in paediatric populations, and most commonly as part of multisystem disease.6,59 A recent report of an association between a m.3398T>C MTND1 variant and LVNC supports the assertion that mtDNA mutations may be important in pathogenesis.60
Histiocytoid cardiomyopathy is another rare cardiomyopathy characterized by pathognomonic histiocyte-like cells within the subendocardium. Reported cases frequently document aggregates of structurally abnormal mitochondria,61 and have been linked to the m.8344A>G mutation and a mutation in the MTCYB gene that encodes an complex III enzyme subunit.62,63
Electropathy
Conduction system disease and bradyarrhythmias
Conduction system disease occurs commonly in patients with mtDNA disease, and prevalence increases with age as in the general population. Atrio-ventricular block forms part of the diagnostic criteria of KSS such that a review of the published literature suggests a prevalence of conduction system disease of 84%.51 Conduction system disease occurs, albeit less commonly, in ∼5–10% of patients in other forms of mtDNA disease with AV or intra-ventricular conduction disturbances reported in association with the m.3243A>G and m.8344A>G mutations.25,28 Although mechanisms are currently unknown, differences in mutation load or in sensitivity of different cardiac cell types to different mtDNA mutations (threshold) may account for this phenotypic discrepancy.30
Importantly in patients with neuromuscular disease, including mtDNA disease, progression to high-grade AV block is often unpredictable necessitating prompt recognition of any conduction system disease and consideration of early intervention.64,65 Early deaths in patients with KSS may be directly attributable to infra-nodal heart block.66 Risks of progression and clinical outcomes associated with conduction system disease in other forms of mtDNA disease are unknown.
Ventricular pre-excitation and tachyarrhythmias
Ventricular pre-excitation and Wolff–Parkinson–White syndrome may be more common in patients with mtDNA disease than in the general population. First observed in association with Leber's hereditary optic neuropathy, ventricular pre-excitation has been reported in 10% patients and 8% maternal relatives compared with 1.6% of paternal relatives.67 Although supported by several studies, the failure of some groups to replicate this finding has stimulated debate as to whether these results represent chance findings or evidence of a direct aetiological link.40 Evidence in support of the latter is provided by reports of ventricular pre-excitation occurring in association with the m.8344A>G and m.3243A>G mutations, where manifest pre-excitation was observed in 3–27% of patients.25–27,68 Although ventricular pre-excitation has been reported in association with mtDNA-related cardiomyopathy,69 this combination does not appear as common as in other forms of inherited disease such as that caused by PRKAG2 gene mutations.70 Symptomatic patients with mtDNA disease and manifest ventricular pre-excitation have undergone successful radio-frequency ablation (RFA) of accessory pathways, but natural history remains unclear and invasive management of asymptomatic patients is controversial.
Supraventricular and ventricular tachyarrhythmias have both been reported in patients with mtDNA disease, particularly in children and in those with cardiomyopathy.6,71 Although prolongation of the QT interval has been identified in some patient groups,72 determination of the true incidence of this finding and the risk of ventricular arrhythmia requires larger longitudinal studies.
Diagnosis
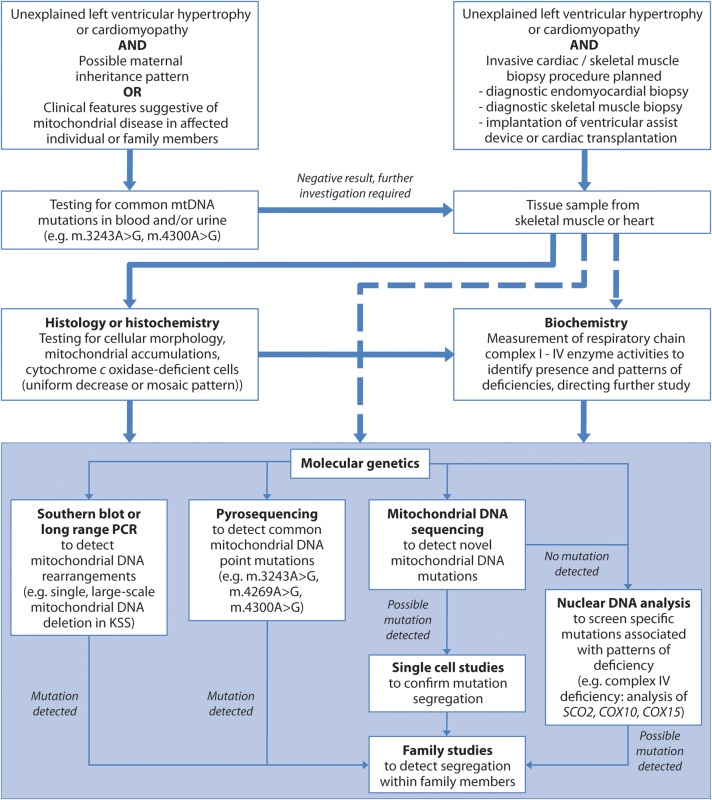
The diagnosis of mtDNA disease is complex and requires a multidisciplinary approach (Figure 3). A maternal inheritance pattern or the presence of extra-cardiac features of mtDNA disease may raise suspicion of the diagnosis. Although these extra-cardiac manifestations include common or non-specific features (Figure 1), particular patterns of organ involvement (e.g. diabetes and deafness) should alert the cardiologist to the possibility of mtDNA disease.
Figure 3.
Algorithm for investigation of mitochondrial DNA disease. Ideally, mitochondrial disease should be assessed in the most affected tissue. However, this is often not possible and skeletal muscle biopsy can serve as an alternative even without clinical myopathy. Histochemical analysis, although not always possible due to tissue availability, can direct genetic investigation but it may be necessary to perform biochemical or molecular genetic analysis directly (dashed arrows). mtDNA, mitochondrial DNA; PCR, polymerase chain reaction.
Molecular genetic testing
Emerging evidence supports screening of peripheral lymphocytes or urine samples for mtDNA mutations (e.g. 3243A>G, m.4300A>G) in specific clinical scenarios. In patients with unexplained LVH not fulfilling standard criteria for HCM, symmetrical hypertrophy and the absence of LVOT obstruction may favour an alternative diagnosis, such as mtDNA-related cardiomyopathy.73 Sequencing of the mitochondrial genome may be an appropriate next step in investigation. However, with more pronounced variation than the nuclear genome, challenges exist in the determination of pathogenesis.21 Comparison with published databases is necessary but true determination of the pathogenicity of novel mtDNA mutations is complex and reliant on canonical criteria involving segregation of mutation within tissues and families, evolutionary conservation of affected nucleotides or amino acids, and occasionally biochemical studies in cultured cells.
Invasive biopsy analysis
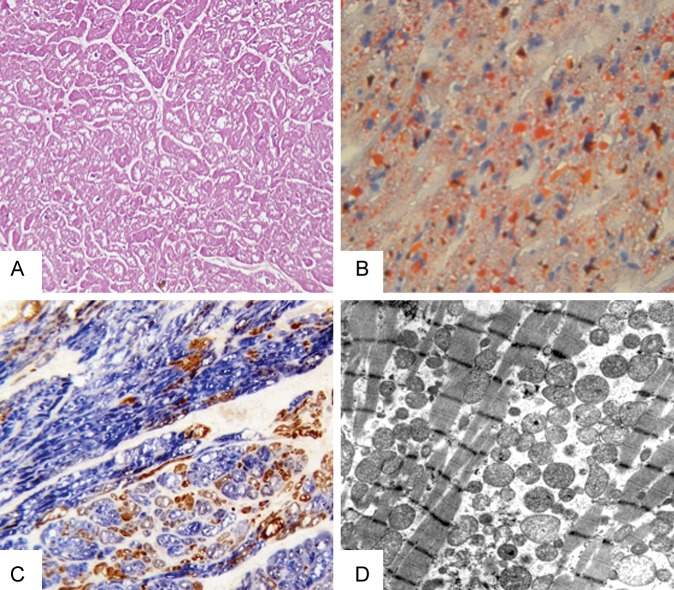
Although molecular genetic testing may expedite diagnosis of mitochondrial disease in some patients, in many, particularly those with novel mutations, analysis of invasive biopsy tissue remains important. Pathological studies of the myocardium are available from a small number of patients with mtDNA-related cardiomyopathy.9,32 Common but relatively non-specific histological findings are diffuse cellular hypertrophy with swollen, often vacuolated, cardiomyocytes (Figure 4). Interstitial fibrosis varies but myofibre disarray, typical of HCM, is absent and ultrastructural examination reveals proliferation of abnormal mitochondria with sarcomere displacement.74 On cardiac frozen sections, the sequential assay of cytochrome c oxidase (COX)/succinate dehydrogenase (SDH) activities can demonstrate the typical mosaic appearance of COX deficiency (Figure 4). Skeletal muscle biopsy is a low-risk procedure that can provide similar evidence for mtDNA disease, even in patients without evidence of myopathy. However, the tissue specificity of biochemical defects due to mtDNA mutations is such that in isolated or prominent cardiomyopathy, examination of endomyocardial biopsy (EMB) tissue may be relevant. This procedure is associated with a serious complication rate of ∼1% and remains controversial.75 International guidelines suggest pathological methodologies and clinical scenarios where EMB can reasonably be performed, including in the investigation of possible mtDNA-related cardiomyopathy.74,76 Indeed, in such patients, opportunistic assessment of cardiac tissue obtained during other invasive cardiac procedures (e.g. ventricular assist device implantation) should be considered.77 A recent consensus statement supports attempts to maximize the diagnostic utility of such specimens.78
Figure 4.
Histological, histochemical, and ultrastructural features of mitochondrial DNA-related cardiomyopathy. (A) Histological examination of explanted left ventricular tissue from a patient with a homoplasmic mt-tRNAIle mutation reveals enlarged cardiomyocytes with prominent cytoplasmic vacuolization (H&E, 20×). (B) Vacuoles contain lipid droplets that stain with Oil Red O (40×). (C) Sequential cytochrome c oxidase/succinate dehydrogenase histochemistry shows several cytochrome c oxidase-deficient cardiomyocytes (blue) with scattered cytochrome c oxidase-positive cells (brown, 40×). (D) Ultrastructural analysis reveals proliferation of polymorphic mitochondria and displacement of sarcomeres (uracyl acetate lead citrate, 3150×).
Cardiac investigations
Cardiac involvement in mtDNA disease can remain asymptomatic until an advanced stage is reached, often due to limited mobility of patients. Although the utility of screening is debated in mtDNA disease given variability in clinical course, best practice supports a high index of suspicion and instigation of regular surveillance. Multi-disciplinary care is essential given potential involvement of organs that can cause symptoms associated with cardiac disease. Exercise intolerance, for example, may result from skeletal myopathy or respiratory muscle weakness, as well as cardiomyopathy or arrhythmia. A cardiologist with an understanding of mtDNA disease should be involved in the care of all patients with confirmed cardiac involvement (Figure 5).
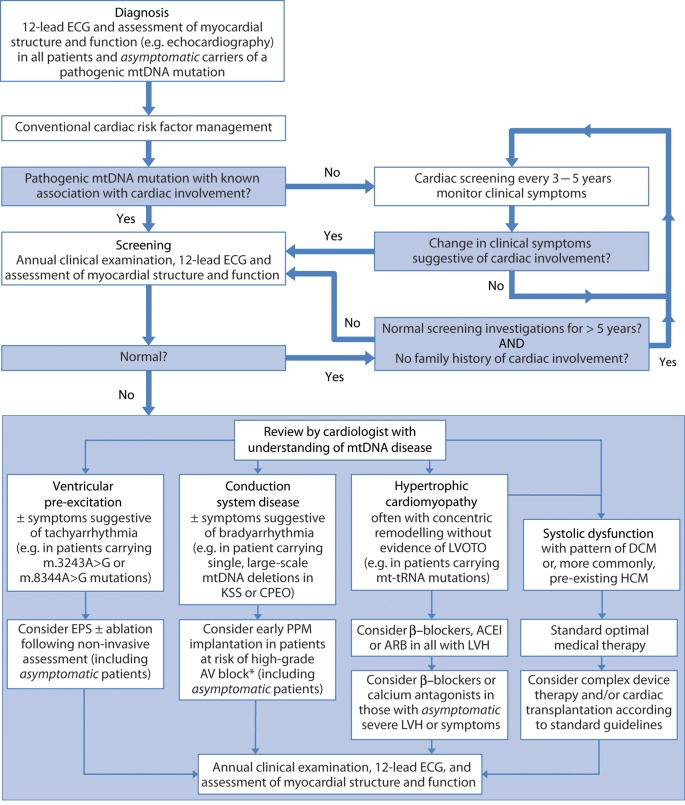
Figure 5.
Clinical algorithm for cardiac screening and management in mitochondrial DNA disease. International guidelines support early intervention for conduction disease in patients with mitochondrial DNA disease.64,65 ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; AV, atrio-ventricular; CPEO, chronic progressive external ophthalmoplegia; DCM, dilated cardiomyopathy; EPS, electrophysiological study; HCM, hypertrophic cardiomyopathy; KSS, Kearns-Sayre syndrome; LVH, left ventricular hypertrophy; LVOTO, left ventricular outflow tract obstruction; mtDNA, mitochondrial DNA; PPM, permanent pacemaker; *many experienced centres regard rapid progression of conduction disease, severe surface ECG abnormalities, and/or HV interval >70 ms as high-risk features for progression to AV block.92,93
In common with a number of other rare neuromuscular or metabolic conditions, there are few clear recommendations for disease management. There is general agreement that all patients with mtDNA disease, unaffected carriers of a known mutation, and obligate carriers should have baseline cardiac assessment. This should include clinical history and examination, 12-lead ECG and an assessment of cardiac structure and function, typically echocardiography, as a minimum standard in all forms of mtDNA disease as, although specific cardiac phenotypes are associated with different mtDNA mutations (e.g. single, large-scale mtDNA deletion and AV block), diverse cardiac phenotypes can occur. Although the initiation, nature, and frequency of cardiac screening has not been subject to specific study, many experienced centres use an initial 12-month interval for repeated ECG and functional assessments, consistent with guidelines for HCM and different forms of neuromuscular disease, with extension of this interval to 3–5 years if normal findings are repeated (Figure 5). Magnetic resonance imaging (MRI) may reveal cardiac involvement when standard evaluation is unremarkable,79 and permits imaging without reliance on acoustic windows, often absent in patients with skeletal or respiratory muscle disease. Cardiac MRI also permits accurate tissue characterization using late gadolinium enhancement,48 an area where ongoing studies may reveal important features of mtDNA-related cardiomyopathy. Several lines of evidence suggest a central role of disrupted energy metabolism in HCM and phenocopies, including mtDNA disease.80,81 Abnormal cardiac bioenergetics have been demonstrated in patients with m.3243A>G mutation and structurally normal hearts on echocardiography.82 Contemporaneous assessments of myocardial bioenergetics, fibrosis, and myocardial deformation, using cardiac tagging may permit early identification of patients at risk of developing cardiomyopathy. The preferred approach may therefore involve both cardiac MRI and echocardiography at diagnosis to establish a baseline, with subsequent screening performed with echocardiography alone. However, larger longitudinal studies are necessary to clarify the role of such investigations in patients with mtDNA disease.
Management
Although clinical trials are underway, a recent Cochrane review suggests that there is no current drug treatment that has shown clear clinical benefit in the primary outcome in patients with mtDNA disease.83 Resistance and endurance exercise training programmes both improve symptoms in mtDNA disease but effects on cardiac structure and function are currently unknown, and benefits are lost on cessation of exercise with deconditioning.84,85 Patients with mtDNA disease remain at risk of common acquired cardiac disorders and current guidelines to address conventional risk factors should be followed.
Cardiomyopathy
Recommendations for the management of hypertrophic remodelling in mtDNA disease are reliant on clinical studies in HCM and LVH, with interventions based on reasonable clinical assumptions of similar treatment effects, together with reports of successful outcomes.48,86,87 Non-dihydropyridine calcium channel antagonists and β-blockers are recommended in symptomatic patients or those with asymptomatic severe LVH in HCM.73,88 β-blockers, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers have been demonstrated to reduce LVH in the general population. Given the progressive nature of hypertrophic remodelling in mtDNA disease, these drugs are often started with the first appreciation of LVH.
Standard optimal medical therapies for heart failure with systolic dysfunction are used in mtDNA disease, with reports of both clinical improvement and progression despite therapy.48,79 Angiotensin-converting enzyme inhibitors have been shown to slow the onset and progression of cardiomyopathy associated with Duchenne muscular dystrophy, and reduce mortality.89 Complex device therapy including the use of implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy should be considered in patients with mtDNA disease provided conventional guidelines are met, including life expectancy of >1 year. Cardiac transplantation, although controversial in metabolic disease with potential multisystem involvement, has been performed successfully in patients with mtDNA disease.86,87,90,91 Clinical outcomes appear to be dependent on the extent of extra-cardiac involvement in addition to complications of transplantation itself, although data are lacking.
Electropathy
International guidelines recommend permanent pacemaker (PPM) implantation at an earlier stage of conduction system dysfunction in patients with neuromuscular disease, including mtDNA disease, than in the general population due to unpredictable progression.64,65 In patients with neuromuscular disease, any degree of AV block, including first-degree block, and/or any degree of fascicular block are class IIb indications for PPM implantation, irrespective of symptoms. Such prophylactic PPM implantation, however, remains controversial. Severe surface ECG abnormalities (PR interval >240 ms, QRS duration >120 ms, rhythm other than sinus, or high-grade AV block) and an HV interval >70 ms are high-risk features for sudden death in myotonic dystrophy.92,93 Recent evidence suggests that an invasive strategy to assess AV conduction in those with high-risk non-invasive features is associated with improved survival.94 Many centres use similar criteria in patients with mtDNA disease, particularly KSS. Although sudden deaths have been reported in patients with functioning PPMs and a variety of neuromuscular diseases, there are no data to guide ICD implantation in patients with mtDNA disease out with standard primary and secondary indications.
Conventional medications for symptomatic supraventricular arrhythmias can be used in patients with mtDNA disease. Ventricular pre-excitation can lead to symptomatic re-entrant tachyarrhythmia in patients with mtDNA disease and is, in other patients, associated with a small risk of sudden cardiac death. Consistent with international guidelines, and following non-invasive assessment including an exercise ECG, consideration should therefore be given to invasive electrophysiological study (EPS) in all patients with mtDNA disease and non-intermittent pre-excitation.95,96 Asymptomatic pre-excitation is a class IIa indication for EPS ± RFA in adults and class IIb in children >5 years of age, but class III (i.e. not indicated) in those <5 years of age.
Conclusions
Cardiac involvement in mtDNA disease is common and an important predictor of morbidity and early mortality. Specific disease-modifying therapies do not yet exist, and data are scarce concerning natural history, screening, and management. Comprehensive clinical algorithms for cardiac disease are vitally needed, and considerable international collaborative efforts will be required to achieve this aim. Nevertheless, cardiologists will become more involved in the care of patients with mtDNA disease as recognition of these disorders increases. Appreciation of the clinical spectrum of cardiac involvement in mtDNA disease and risks of disease progression will enable appropriate input to the multi-disciplinary care of patients.
Funding
This work was supported by the Wellcome Trust (BH092142 to M.G.D.B., 096919Z/11/Z and 074454/Z/04/Z to D.M.T. and R.W.T.); the Medical Research Council (G0601943 to D.M.T., G0800674 to D.M.T. and R.W.T.); and the Newcastle upon Tyne Hospitals NHS Foundation Trust and NHS Specialized Services that support the ‘Rare Mitochondrial Disorders of Adults and Children’ Diagnostic Service (http://www.mitochondrialncg.nhs.uk).
References
- 1.Schaefer AM, McFarland R, Blakely EL, He L, Whittaker RG, Taylor RW, Chinnery PF, Turnbull DM. Prevalence of mitochondrial DNA disease in adults. Ann Neurol. 2008;63:35–39. doi: 10.1002/ana.21217. doi:10.1002/ana.21217. [DOI] [PubMed] [Google Scholar]
- 2.Elliott HR, Samuels DC, Eden JA, Relton CL, Chinnery PF. Pathogenic mitochondrial DNA mutations are common in the general population. Am J Hum Genet. 2008;83:254–260. doi: 10.1016/j.ajhg.2008.07.004. doi:10.1016/j.ajhg.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McFarland R, Taylor RW, Turnbull DM. A neurological perspective on mitochondrial disease. Lancet Neurol. 2010;9:829–840. doi: 10.1016/S1474-4422(10)70116-2. doi:10.1016/S1474-4422(10)70116-2. [DOI] [PubMed] [Google Scholar]
- 4.Tuppen HAL, Blakely EL, Turnbull DM, Taylor RW. Mitochondrial DNA mutations and human disease. Biochim Biophys Acta. 2010;1797:113–128. doi: 10.1016/j.bbabio.2009.09.005. doi:10.1016/j.bbabio.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Limongelli G, Tome-Esteban M, Dejthevaporn C, Rahman S, Hanna M, Elliott P. Prevalence and natural history of heart disease in adults with primary mitochondrial respiratory chain disease. Eur J Heart Fail. 2010;12:114–121. doi: 10.1093/eurjhf/hfp186. doi:10.1093/eurjhf/hfp186. [DOI] [PubMed] [Google Scholar]
- 6.Scaglia F, Towbin JA, Craigen WJ, Belmont JW, Smith EOB, Neish SR, Ware SM, Hunter JV, Fernbach SD, Vladutiu GD, Wong L-JC, Vogel H. Clinical spectrum, morbidity, and mortality in 113 pediatric patients with mitochondrial disease. Pediatrics. 2004;114:925–931. doi: 10.1542/peds.2004-0718. doi:10.1542/peds.2004-0718. [DOI] [PubMed] [Google Scholar]
- 7.Majamaa-Voltti K, Turkka J, Kortelainen ML, Huikuri H, Majamaa K. Causes of death in pedigrees with the 3243A>G mutation in mitochondrial DNA. J Neurol Neurosurg Psychiatry. 2008;79:209–211. doi: 10.1136/jnnp.2007.122648. doi:10.1136/jnnp.2007.122648. [DOI] [PubMed] [Google Scholar]
- 8.Majamaa-Voltti KA, Winqvist S, Remes AM, Tolonen U, Pyhtinen J, Uimonen S, Karppa M, Sorri M, Peuhkurinen K, Majamaa K, Majamaa-Voltti K, Makikallio TH, Huikuri HV, Kortelainen ML, Hassinen IE, Moilanen JS, Salmela PI, Rusanen H, Peuhkurinen KJ, Herva R. A 3-year clinical follow-up of adult patients with 3243A>G in mitochondrial DNA. Neurology. 2006;66:1470–1475. doi: 10.1212/01.wnl.0000216136.61640.79. doi:10.1212/01.wnl.0000216136.61640.79. [DOI] [PubMed] [Google Scholar]
- 9.Taylor RW, Giordano C, Davidson MM, d'Amati G, Bain H, Hayes CM, Leonard H, Barron MJ, Casali C, Santorelli FM, Hirano M, Lightowlers RN, DiMauro S, Turnbull DM. A homoplasmic mitochondrial transfer ribonucleic acid mutation as a cause of maternally inherited hypertrophic cardiomyopathy. J Am Coll Cardiol. 2003;41:1786–1796. doi: 10.1016/s0735-1097(03)00300-0. doi:10.1016/S0735-1097(03)00300-0. [DOI] [PubMed] [Google Scholar]
- 10.Taylor RW, Turnbull DM. Mitochondrial DNA mutations in human disease. Nat Rev Genet. 2005;6:389–402. doi: 10.1038/nrg1606. doi:10.1038/nrg1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duchen MR, Szabadkai G. Roles of mitochondria in human disease. Essays Biochem. 2010;47:115–137. doi: 10.1042/bse0470115. doi:10.1042/bse0470115. [DOI] [PubMed] [Google Scholar]
- 12.Chinnery PF, Howell N, Lightowlers RN, Turnbull DM. Molecular pathology of MELAS and MERRF. The relationship between mutation load and clinical phenotypes. Brain. 1997;120:1713–1721. doi: 10.1093/brain/120.10.1713. doi:10.1093/brain/120.10.1713. [DOI] [PubMed] [Google Scholar]
- 13.Carelli V, Giordano C, d'Amati G. Pathogenic expression of homoplasmic mtDNA mutations needs a complex nuclear-mitochondrial interaction. Trends Genet. 2003;19:257–262. doi: 10.1016/S0168-9525(03)00072-6. doi:10.1016/S0168-9525(03)00072-6. [DOI] [PubMed] [Google Scholar]
- 14.Perli E, Giordano C, Tuppen HA, Montopoli M, Montanari A, Orlandi M, Pisano A, Catanzaro D, Caparrotta L, Musumeci B, Autore C, Morea V, Di Micco P, Campese AF, Leopizzi M, Gallo P, Francisci S, Frontali L, Taylor RW, d'Amati G. Isoleucyl-tRNA synthetase levels modulate the penetrance of a homoplasmic m.4277T>C mitochondrial tRNAIle mutation causing hypertrophic cardiomyopathy. Hum Mol Genet. 2012;21:85–100. doi: 10.1093/hmg/ddr440. doi:10.1093/hmg/ddr440. [DOI] [PubMed] [Google Scholar]
- 15.Chinnery PF, DiMauro S, Shanske S, Schon EA, Zeviani M, Mariotti C, Carrara F, Lombes A, Laforet P, Ogier H, Jaksch M, Lochmuller H, Horvath R, Deschauer M, Thorburn DR, Bindoff LA, Poulton J, Taylor RW, Matthews JN, Turnbull DM. Risk of developing a mitochondrial DNA deletion disorder. Lancet. 2004;364:592–596. doi: 10.1016/S0140-6736(04)16851-7. doi:10.1016/S0140-6736(04)16851-7. [DOI] [PubMed] [Google Scholar]
- 16.Cree LM, Samuels DC, de Sousa Lopes SC, Rajasimha HK, Wonnapinij P, Mann JR, Dahl H-HM, Chinnery PF. A reduction of mitochondrial DNA molecules during embryogenesis explains the rapid segregation of genotypes. Nat Genet. 2008;40:249–254. doi: 10.1038/ng.2007.63. doi:10.1038/ng.2007.63. [DOI] [PubMed] [Google Scholar]
- 17.Muller-Hocker J. Cytochrome-c-oxidase deficient cardiomyocytes in the human heart—an age-related phenomenon. A histochemical ultracytochemical study. Am J Pathol. 1989;134:1167–1173. [PMC free article] [PubMed] [Google Scholar]
- 18.MITOMAP. 2012. A human mitochondrial genome database http://mitomap.org .
- 19.Online Mendelian Inheritance in Man, OMIM. Baltimore, MD: 2012. McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University http://omim.org . [Google Scholar]
- 20.Zaragoza M, Fass J, Diegoli M, Lin D, Arbustini E. Mitochondrial DNA variant discovery and evaluation in human cardiomyopathies through next-generation sequencing. PLoS. 2010;5:e12295. doi: 10.1371/journal.pone.0012295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaragoza MV, Brandon MC, Diegoli M, Arbustini E, Wallace DC. Mitochondrial cardiomyopathies: how to identify candidate pathogenic mutations by mitochondrial DNA sequencing, MITOMASTER and phylogeny. Eur J Hum Genet. 2011;19:200–207. doi: 10.1038/ejhg.2010.169. doi:10.1038/ejhg.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elson JL, Sweeney MG, Procaccio V, Yarham JW, Salas A, Kong QP, van der Westhuizen FH, Pitceathly RD, Thorburn DR, Lott MT, Wallace DC, Taylor RW, McFarland R. Towards a mtDNA locus-specific mutation database using the LOVD platform. Hum Mutat. 2012;33:1352–1358. doi: 10.1002/humu.22118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmgren D, Wahlander H, Eriksson B, Oldfors A, Holme E, Tulinius M. Cardiomyopathy in children with mitochondrial disease; clinical course and cardiological findings. Eur Heart J. 2003;24:280–288. doi: 10.1016/s0195-668x(02)00387-1. doi:10.1016/S0195-668X(02)00387-1. [DOI] [PubMed] [Google Scholar]
- 24.Debray FG, Lambert M, Chevalier I, Robitaille Y, Decarie JC, Shoubridge EA, Robinson BH, Mitchell GA. Long-term outcome and clinical spectrum of 73 pediatric patients with mitochondrial diseases. Pediatrics. 2007;119:722–733. doi: 10.1542/peds.2006-1866. doi:10.1542/peds.2006-1866. [DOI] [PubMed] [Google Scholar]
- 25.Wahbi K, Larue S, Jardel C, Meune C, Stojkovic T, Ziegler F, Lombes A, Eymard B, Duboc D, Laforet P. Cardiac involvement is frequent in patients with the m.8344A>G mutation of mitochondrial DNA. Neurology. 2010;74:674–677. doi: 10.1212/WNL.0b013e3181d0ccf4. doi:10.1212/WNL.0b013e3181d0ccf4. [DOI] [PubMed] [Google Scholar]
- 26.Okajima Y, Tanabe Y, Takayanagi M, Aotsuka H. A follow up study of myocardial involvement in patients with mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) Heart. 1998;80:292–295. doi: 10.1136/hrt.80.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anan R, Nakagawa M, Miyata M, Higuchi I, Nakao S, Suehara M, Osame M, Tanaka H. Cardiac involvement in mitochondrial diseases. A study on 17 patients with documented mitochondrial DNA defects. Circulation. 1995;91:955–961. doi: 10.1161/01.cir.91.4.955. doi:10.1161/01.CIR.91.4.955. [DOI] [PubMed] [Google Scholar]
- 28.Majamaa-Voltti K, Peuhkurinen K, Kortelainen ML, Hassinen IE, Majamaa K. Cardiac abnormalities in patients with mitochondrial DNA mutation 3243A>G. BMC Cardiovasc Disord. 2002;2:12. doi: 10.1186/1471-2261-2-12. doi:10.1186/1471-2261-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santorelli FM, Tanji K, Manta P, Casali C, Krishna S, Hays AP, Mancini DM, DiMauro S, Hirano M. Maternally inherited cardiomyopathy: an atypical presentation of the mtDNA 12S rRNA gene A1555G mutation. Am J Hum Genet. 1999;64:295–300. doi: 10.1086/302188. doi:10.1086/302188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller-Hocker J, Jacob U, Seibel P. The common 4977 base pair deletion of mitochondrial DNA preferentially accumulates in the cardiac conduction system of patients with Kearns-Sayre syndrome. Mod Pathol. 1998;11:295–301. [PubMed] [Google Scholar]
- 31.Heddi A, Stepien G, Benke PJ, Wallace DC. Coordinate induction of energy gene expression in tissues of mitochondrial disease patients. J Biol Chem. 1999;274:22968–22976. doi: 10.1074/jbc.274.33.22968. doi:10.1074/jbc.274.33.22968. [DOI] [PubMed] [Google Scholar]
- 32.Sebastiani M, Giordano C, Nediani C, Travaglini C, Borchi E, Zani M, Feccia M, Mancini M, Petrozza V, Cossarizza A, Gallo P, Taylor RW, d'Amati G. Induction of mitochondrial biogenesis is a maladaptive mechanism in mitochondrial cardiomyopathies. J Am Coll Cardiol. 2007;50:1362–1369. doi: 10.1016/j.jacc.2007.06.035. doi:10.1016/j.jacc.2007.06.035. [DOI] [PubMed] [Google Scholar]
- 33.Hansson A, Hance N, Dufour E, Rantanen A, Hultenby K, Clayton DA, Wibom R, Larsson NG. A switch in metabolism precedes increased mitochondrial biogenesis in respiratory chain-deficient mouse hearts. Proc Natl Acad Sci U S A. 2004;101:3136–3141. doi: 10.1073/pnas.0308710100. doi:10.1073/pnas.0308710100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russell LK, Mansfield CM, Lehman JJ, Kovacs A, Courtois M, Saffitz JE, Medeiros DM, Valencik ML, McDonald JA, Kelly DP. Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circ Res. 2004;94:525–533. doi: 10.1161/01.RES.0000117088.36577.EB. doi:10.1161/01.RES.0000117088.36577.EB. [DOI] [PubMed] [Google Scholar]
- 35.Wredenberg A, Wibom R, Wilhelmsson H, Graff C, Wiener HH, Burden SJ, Oldfors A, Westerblad H, Larsson NG. Increased mitochondrial mass in mitochondrial myopathy mice. Proc Natl Acad Sci U S A. 2002;99:15066–15071. doi: 10.1073/pnas.232591499. doi:10.1073/pnas.232591499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lehman JJ, Kelly DP. Gene regulatory mechanisms governing energy metabolism during cardiac hypertrophic growth. Heart Fail Rev. 2002;7:175–185. doi: 10.1023/a:1015332726303. doi:10.1023/A:1015332726303. [DOI] [PubMed] [Google Scholar]
- 37.Esposito LA, Melov S, Panov A, Cottrell BA, Wallace DC. Mitochondrial disease in mouse results in increased oxidative stress. Proc Natl Acad Sci U S A. 1999;96:4820–4825. doi: 10.1073/pnas.96.9.4820. doi:10.1073/pnas.96.9.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. doi:10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 39.Dai DF, Chen T, Wanagat J, Laflamme M, Marcinek DJ, Emond MJ, Ngo CP, Prolla TA, Rabinovitch PS. Age-dependent cardiomyopathy in mitochondrial mutator mice is attenuated by overexpression of catalase targeted to mitochondria. Aging Cell. 2010;9:536–544. doi: 10.1111/j.1474-9726.2010.00581.x. doi:10.1111/j.1474-9726.2010.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sorajja P, Sweeney M, Chalmers R, Sachdev B, Syrris P, Hanna M, Wood N, McKenna W, Elliott P. Cardiac abnormalities in patients with Leber's hereditary optic neuropathy. Heart. 2003;89:791–792. doi: 10.1136/heart.89.7.791. doi:10.1136/heart.89.7.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vydt TCG, de Coo RFM, Soliman OI, Ten Cate FJ, van Geuns R-JM, Vletter WB, Schoonderwoerd K, van den Bosch BJC, Smeets HJM, Geleijnse ML. Cardiac involvement in adults with m.3243A>G MELAS gene mutation. Am J Cardiol. 2007;99:264–269. doi: 10.1016/j.amjcard.2006.07.089. doi:10.1016/j.amjcard.2006.07.089. [DOI] [PubMed] [Google Scholar]
- 42.Sachdev B, Takenaka T, Teraguchi H, Tei C, Lee P, McKenna WJ, Elliott PM. Prevalence of Anderson-Fabry disease in male patients with late onset hypertrophic cardiomyopathy. Circulation. 2002;105:1407–1411. doi: 10.1161/01.cir.0000012626.81324.38. doi:10.1161/01.CIR.0000012626.81324.38. [DOI] [PubMed] [Google Scholar]
- 43.Arad M, Maron BJ, Gorham JM, Johnson WH, Jr, Saul JP, Perez-Atayde AR, Spirito P, Wright GB, Kanter RJ, Seidman CE, Seidman JG. Glycogen storage diseases presenting as hypertrophic cardiomyopathy. N Engl J Med. 2005;352:362–372. doi: 10.1056/NEJMoa033349. doi:10.1056/NEJMoa033349. [DOI] [PubMed] [Google Scholar]
- 44.Pastores G, Santorelli F, Shanske S, Gelb B, Fyfe B, Wolfe D, Willner J. Leigh syndrome and hypertrophic cardiomyopathy in an infant with a mitochondrial DNA point mutation (T8993G) Am J Med Genet. 1994;50:265–271. doi: 10.1002/ajmg.1320500310. doi:10.1002/ajmg.1320500310. [DOI] [PubMed] [Google Scholar]
- 45.Taniike M, Fukushima H, Yanagihara I, Tsukamoto H, Tanaka J, Fujimura H, Nagai T, Sano T, Yamaoka K, Inui K, Okada S. Mitochondrial tRNA(Ile) mutation in fatal cardiomyopathy. Biochem Biophys Res Commun. 1992;186:47–53. doi: 10.1016/s0006-291x(05)80773-9. doi:10.1016/S0006-291X(05)80773-9. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka M, Ino H, Ohno K, Hattori K, Sato W, Ozawa T, Tanaka T, Itoyama S. Mitochondrial mutation in fatal infantile cardiomyopathy. Lancet. 1990;336:1452. doi: 10.1016/0140-6736(90)93162-i. doi:10.1016/0140-6736(90)93162-I. [DOI] [PubMed] [Google Scholar]
- 47.Ware SM, El-Hassan N, Kahler SG, Zhang Q, Ma YW, Miller E, Wong B, Spicer RL, Craigen WJ, Kozel BA, Grange DK, Wong LJ. Infantile cardiomyopathy caused by a mutation in the overlapping region of mitochondrial ATPase 6 and 8 genes. J Med Genet. 2009;46:308–314. doi: 10.1136/jmg.2008.063149. doi:10.1136/jmg.2008.063149. [DOI] [PubMed] [Google Scholar]
- 48.Stalder N, Yarol N, Tozzi P, Rotman S, Morris M, Fellmann F, Schwitter J, Hullin R. Mitochondrial A3243G mutation with manifestation of acute dilated cardiomyopathy. Circ Heart Fail. 2012;5:e1–e3. doi: 10.1161/CIRCHEARTFAILURE.111.963900. doi:10.1161/CIRCHEARTFAILURE.111.963900. [DOI] [PubMed] [Google Scholar]
- 49.Seibel P, Degoul F, Bonne G, Romero N, Francois D, Paturneau-Jouas M, Ziegler F, Eymard B, Fardeau M, Marsac C, Kadenbach B. Genetic biochemical and pathophysiological characterization of a familial mitochondrial encephalomyopathy (MERRF) J Neurol Sci. 1991;105:217–224. doi: 10.1016/0022-510x(91)90148-z. doi:10.1016/0022-510X(91)90148-Z. [DOI] [PubMed] [Google Scholar]
- 50.Ito T, Hattori K, Tanaka M, Sugiyama S, Ozawa T. Mitochondrial cytopathy. Jpn Circ J. 1990;54:1214–1220. doi: 10.1253/jcj.54.1214. doi:10.1253/jcj.54.1214. [DOI] [PubMed] [Google Scholar]
- 51.Hirano M, DiMauro S. Clinical features of mitochondrial myopathies and encephalomyopathies. In: Lane RJM, editor. Handbook of Muscle Disease. New York: Marcel Dekker Inc; 1996. pp. 479–504. [Google Scholar]
- 52.Tveskov C, Angelo-Nielsen K. Kearns-Sayre syndrome and dilated cardiomyopathy. Neurology. 1990;40:553–554. doi: 10.1212/wnl.40.3_part_1.553. doi:10.1212/WNL.40.3_Part_1.553. [DOI] [PubMed] [Google Scholar]
- 53.Ashrafian H, Docherty L, Leo V, Towlson C, Neilan M, Steeples V, Lygate CA, Hough T, Townsend S, Williams D, Wells S, Norris D, Glyn-Jones S, Land J, Barbaric I, Lalanne Z, Denny P, Szumska D, Bhattacharya S, Griffin JL, Hargreaves I, Fernandez-Fuentes N, Cheeseman M, Watkins H, Dear TN. A mutation in the mitochondrial fission gene Dnm1l leads to cardiomyopathy. PLoS Genet. 2010;6:e1001000. doi: 10.1371/journal.pgen.1001000. doi:10.1371/journal.pgen.1001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Narula N, Zaragoza MV, Sengupta PP, Li P, Haider N, Verjans J, Waymire K, Vannan M, Wallace DC. Adenine nucleotide translocase 1 deficiency results in dilated cardiomyopathy with defects in myocardial mechanics, histopathological alterations, and activation of apoptosis. JACC Cardiovasc Imaging. 2011;4:1–10. doi: 10.1016/j.jcmg.2010.06.018. doi:10.1016/j.jcmg.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thebault C, Ollivier R, Leurent G, Marcorelles P, Langella B, Donal E. Mitochondriopathy: a rare aetiology of restrictive cardiomyopathy. Eur J Echocardiogr. 2008;9:840–845. doi: 10.1093/ejechocard/jen189. doi:10.1093/ejechocard/jen189. [DOI] [PubMed] [Google Scholar]
- 56.Kohli SK, Pantazis AA, Shah JS, Adeyemi B, Jackson G, McKenna WJ, Sharma S, Elliott PM. Diagnosis of left-ventricular non-compaction in patients with left-ventricular systolic dysfunction: time for a reappraisal of diagnostic criteria? Eur Heart J. 2008;29:89–95. doi: 10.1093/eurheartj/ehm481. doi:10.1093/eurheartj/ehm481. [DOI] [PubMed] [Google Scholar]
- 57.Oechslin EN, Attenhofer Jost CH, Rojas JR, Kaufmann PA, Jenni R. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol. 2000;36:493–500. doi: 10.1016/s0735-1097(00)00755-5. doi:10.1016/S0735-1097(00)00755-5. [DOI] [PubMed] [Google Scholar]
- 58.Klaassen S, Probst S, Oechslin E, Gerull B, Krings G, Schuler P, Greutmann M, Hurlimann D, Yegitbasi M, Pons L, Gramlich M, Drenckhahn JD, Heuser A, Berger F, Jenni R, Thierfelder L. Mutations in sarcomere protein genes in left ventricular noncompaction. Circulation. 2008;117:2893–2901. doi: 10.1161/CIRCULATIONAHA.107.746164. doi:10.1161/CIRCULATIONAHA.107.746164. [DOI] [PubMed] [Google Scholar]
- 59.Finsterer J. Cardiogenetics, neurogenetics, and pathogenetics of left ventricular hypertrabeculation/noncompaction. Pediatr Cardiol. 2009;30:659–681. doi: 10.1007/s00246-008-9359-0. doi:10.1007/s00246-008-9359-0. [DOI] [PubMed] [Google Scholar]
- 60.Tang S, Batra A, Zhang Y, Ebenroth ES, Huang T. Left ventricular noncompaction is associated with mutations in the mitochondrial genome. Mitochondrion. 2010;10:350–357. doi: 10.1016/j.mito.2010.02.003. doi:10.1016/j.mito.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 61.Shehata BM, Patterson K, Thomas JE, Scala-Barnett D, Dasu S, Robinson HB. Histiocytoid cardiomyopathy: three new cases and a review of the literature. Pediatr Dev Pathol. 1998;1:56–69. doi: 10.1007/s100249900007. doi:10.1007/s100249900007. [DOI] [PubMed] [Google Scholar]
- 62.Vallance HD, Jeven G, Wallace DC, Brown MD. A case of sporadic infantile histiocytoid cardiomyopathy caused by the A8344G (MERRF) mitochondrial DNA mutation. Pediatric Cardiology. 2004;25:538–540. doi: 10.1007/s00246-003-0446-y. doi:10.1007/s00246-003-0446-y. [DOI] [PubMed] [Google Scholar]
- 63.Andreu A, Checcarelli N, Iwata S, Shanske S, DiMauro S. A missense mutation in the mitochondrial cytochrome b gene in a revisited case with histiocytoid cardiomyopathy. Pediatr Cardiol. 2000;48:311–314. doi: 10.1203/00006450-200009000-00008. doi:10.1203/00006450-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 64.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, III, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Faxon DP, Halperin JL, Hiratzka LF, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Ornato JP, Riegel B, Tarkington LG, Yancy CW. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51:e1–62. doi: 10.1016/j.jacc.2008.02.032. doi:10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 65.Vardas PE, Auricchio A, Blanc JJ, Daubert JC, Drexler H, Ector H, Gasparini M, Linde C, Morgado FB, Oto A, Sutton R, Trusz-Gluza M. Guidelines for cardiac pacing and cardiac resynchronization therapy: the Task Force for Cardiac Pacing and Cardiac Resynchronization Therapy of the European Society of Cardiology. Developed in collaboration with the European Heart Rhythm Association. Eur Heart J. 2007;28:2256–2295. doi: 10.1093/eurheartj/ehm305. doi:10.1093/eurheartj/ehm305. [DOI] [PubMed] [Google Scholar]
- 66.Roberts NK, Perloff JK, Kark RA. Cardiac conduction in the Kearns-Sayre syndrome (a neuromuscular disorder associated with progressive external ophthalmoplegia and pigmentary retinopathy). Report of 2 cases and review of 17 published cases. Am J Cardiol. 1979;44:1396–1400. doi: 10.1016/0002-9149(79)90459-4. doi:10.1016/0002-9149(79)90459-4. [DOI] [PubMed] [Google Scholar]
- 67.Nikoskelainen E, Savontaus M, Huoponen K, Antila K, Hartiala J. Pre-excitation syndrome in Leber's hereditary optic neuropathy. Lancet. 1994;344:857–858. doi: 10.1016/s0140-6736(94)92830-4. doi:10.1016/S0140-6736(94)92830-4. [DOI] [PubMed] [Google Scholar]
- 68.Sproule DM, Kaufmann P, Engelstad K, Starc TJ, Hordof AJ, De Vivo DC. Wolff-Parkinson-White syndrome in patients with MELAS. Arch Neurol. 2007;64:1625–1627. doi: 10.1001/archneur.64.11.1625. doi:10.1001/archneur.64.11.1625. [DOI] [PubMed] [Google Scholar]
- 69.Finsterer J, Stollberger C, Kopsa W, Jaksch M. Wolff-Parkinson-White syndrome and isolated left ventricular abnormal trabeculation as a manifestation of Leber's hereditary optic neuropathy. Can J Cardiol. 2001;17:464–466. [PubMed] [Google Scholar]
- 70.Arad M, Moskowitz IP, Patel VV, Ahmad F, Perez-Atayde AR, Sawyer DB, Walter M, Li GH, Burgon PG, Maguire CT, Stapleton D, Schmitt JP, Guo XX, Pizard A, Kupershmidt S, Roden DM, Berul CI, Seidman CE, Seidman JG. Transgenic mice overexpressing mutant PRKAG2 define the cause of Wolff-Parkinson-White syndrome in glycogen storage cardiomyopathy. Circulation. 2003;107:2850–2856. doi: 10.1161/01.CIR.0000075270.13497.2B. doi:10.1161/01.CIR.0000075270.13497.2B. [DOI] [PubMed] [Google Scholar]
- 71.Oginosawa Y, Abe H, Nagatomo T, Mizuki T, Nakashima Y. Sustained polymorphic ventricular tachycardia unassociated with QT prolongation or bradycardia in the Kearns-Sayre syndrome. Pac Clin Electrophysiol. 2003;26:1911–1912. doi: 10.1046/j.1460-9592.2003.00292.x. doi:10.1046/j.1460-9592.2003.00292.x. [DOI] [PubMed] [Google Scholar]
- 72.Karanikis P, Korantzopoulos P, Kountouris E, Dimitroula V, Patsouras D, Pappa E, Siogas K. Kearns-Sayre syndrome associated with trifascicular block and QT prolongation. Int J Cardiol. 2005;101:147–150. doi: 10.1016/j.ijcard.2004.01.027. doi:10.1016/j.ijcard.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 73.Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58:e212–e260. doi: 10.1016/j.jacc.2011.06.011. doi:10.1016/j.jacc.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 74.Leone O, Veinot JP, Angelini A, Baandrup UT, Basso C, Berry G, Bruneval P, Burke M, Butany J, Calabrese F, d'Amati G, Edwards WD, Fallon JT, Fishbein MC, Gallagher PJ, Halushka MK, McManus B, Pucci A, Rodriguez ER, Saffitz JE, Sheppard MN, Steenbergen C, Stone JR, Tan C, Thiene G, van der Wal AC, Winters GL. 2011 Consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc Pathol. 2011;21:245–274. doi: 10.1016/j.carpath.2011.10.001. doi:10.1016/j.carpath.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 75.From AM, Maleszewski JJ, Rihal CS. Current status of endomyocardial biopsy. Mayo Clin Proc. 2011;86:1095–1102. doi: 10.4065/mcp.2011.0296. doi:10.4065/mcp.2011.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, Levine GN, Narula J, Starling RC, Towbin J, Virmani R. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. Eur Heart J. 2007;28:3076–3093. doi: 10.1093/eurheartj/ehm456. doi:10.1093/eurheartj/ehm456. [DOI] [PubMed] [Google Scholar]
- 77.Bates MG, Nesbitt V, Kirk R, He L, Blakely EL, Alston CL, Brodlie M, Hasan A, Taylor RW, McFarland R. Mitochondrial respiratory chain disease in children undergoing cardiac transplantation: a prospective study. Int J Cardiol. 2012;155:305–306. doi: 10.1016/j.ijcard.2011.11.063. doi:10.1016/j.ijcard.2011.11.063. [DOI] [PubMed] [Google Scholar]
- 78.Stone JR, Basso C, Baandrup UT, Bruneval P, Butany J, Gallagher PJ, Halushka MK, Miller DV, Padera RF, Radio SJ, Sheppard MN, Suvarna K, Tan CD, Thiene G, van der Wal AC, Veinot JP. Recommendations for processing cardiovascular surgical pathology specimens: a consensus statement from the Standards and Definitions Committee of the Society for Cardiovascular Pathology and the Association for European Cardiovascular Pathology. Cardiovasc Pathol. 2012;21:2–16. doi: 10.1016/j.carpath.2011.01.001. doi:10.1016/j.carpath.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 79.Nakanishi M, Harada M, Tadamura E, Kotani H, Kawakami R, Kuwahara K, Nakagawa Y, Usami S, Kinoshita H, Fujiwara M, Hosoda K, Ueshima K, Nakao K. Images in cardiovascular medicine. Mitochondrial cardiomyopathy evaluated with cardiac magnetic resonance. Circulation. 2007;116:e25–e26. doi: 10.1161/CIRCULATIONAHA.107.691808. doi:10.1161/CIRCULATIONAHA.107.691808. [DOI] [PubMed] [Google Scholar]
- 80.Ashrafian H, Redwood C, Blair E, Watkins H. Hypertrophic cardiomyopathy:a paradigm for myocardial energy depletion. Trends Genet. 2003;19:263–268. doi: 10.1016/S0168-9525(03)00081-7. doi:10.1016/S0168-9525(03)00081-7. [DOI] [PubMed] [Google Scholar]
- 81.Crilley JG, Boehm EA, Blair E, Rajagopalan B, Blamire AM, Styles P, McKenna WJ, Ostman-Smith I, Clarke K, Watkins H. Hypertrophic cardiomyopathy due to sarcomeric gene mutations is characterized by impaired energy metabolism irrespective of the degree of hypertrophy. J Am Coll Cardiol. 2003;41:1776–1782. doi: 10.1016/s0735-1097(02)03009-7. doi:10.1016/S0735-1097(02)03009-7. [DOI] [PubMed] [Google Scholar]
- 82.Lodi R, Rajagopalan B, Blamire AM, Crilley JG, Styles P, Chinnery PF. Abnormal cardiac energetics in patients carrying the A3243G mtDNA mutation measured in vivo using phosphorus MR spectroscopy. Biochim Biophys Acta. 2004;1657:146–150. doi: 10.1016/j.bbabio.2004.05.003. doi:10.1016/j.bbabio.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 83.Pfeffer G, Majamaa K, Turnbull DM, Thorburn D, Chinnery PF. Treatment for mitochondrial disorders. Cochrane Database Syst Rev. 2012;4:CD004426. doi: 10.1002/14651858.CD004426.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murphy JL, Blakely EL, Schaefer AM, He L, Wyrick P, Haller RG, Taylor RW, Turnbull DM, Taivassalo T. Resistance training in patients with single, large-scale deletions of mitochondrial DNA. Brain. 2008;131:2832–2840. doi: 10.1093/brain/awn252. doi:10.1093/brain/awn252. [DOI] [PubMed] [Google Scholar]
- 85.Taivassalo T, Gardner JL, Taylor RW, Schaefer AM, Newman J, Barron MJ, Haller RG, Turnbull DM. Endurance training and detraining in mitochondrial myopathies due to single large-scale mtDNA deletions. Brain. 2006;129:3391–3401. doi: 10.1093/brain/awl282. doi:10.1093/brain/awl282. [DOI] [PubMed] [Google Scholar]
- 86.Tranchant C, Mousson B, Mohr M, Dumoulin R, Welsch M, Weess C, Stepien G, Warter JM. Cardiac transplantation in an incomplete Kearns-Sayre syndrome with mitochondrial DNA deletion. Neuromuscul Disord. 1993;3:561–566. doi: 10.1016/0960-8966(93)90116-2. doi:10.1016/0960-8966(93)90116-2. [DOI] [PubMed] [Google Scholar]
- 87.Bonnet D, Rustin P, Rotig A, Le Bidois J, Munnich A, Vouhe P, Kachaner J, Sidi D. Heart transplantation in children with mitochondrial cardiomyopathy. Heart. 2001;86:570–573. doi: 10.1136/heart.86.5.570. doi:10.1136/heart.86.5.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maron BJ, McKenna WJ, Danielson GK, Kappenberger LJ, Kuhn HJ, Seidman CE, Shah PM, Spencer WH, III, Spirito P, Ten Cate FJ, Wigle ED. American College of Cardiology/European Society of Cardiology Clinical Expert Consensus Document on Hypertrophic Cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. Eur Heart J. 2003;24:1965–1991. doi: 10.1016/s0195-668x(03)00479-2. doi:10.1016/S0195-668X(03)00479-2. [DOI] [PubMed] [Google Scholar]
- 89.Duboc D, Meune C, Lerebours G, Devaux JY, Vaksmann G, Becane HM. Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. J Am Coll Cardiol. 2005;45:855–857. doi: 10.1016/j.jacc.2004.09.078. doi:10.1016/j.jacc.2004.09.078. [DOI] [PubMed] [Google Scholar]
- 90.Morgan KG, Hasleton PS, Brooks NH, Curry A, Walter J, Cumming WJ. Mitochondrial cardiomyopathy. Eur Heart J. 1996;17:1600. doi: 10.1093/oxfordjournals.eurheartj.a014727. doi:10.1093/oxfordjournals.eurheartj.a014727. [DOI] [PubMed] [Google Scholar]
- 91.Schmauss D, Sodian R, Klopstock T, Deutsch MA, Kaczmarek I, Roemer U, Reichart B, Daebritz SH. Cardiac transplantation in a 14-yr-old patient with mitochondrial encephalomyopathy. Pediatr Transplant. 2007;11:560–562. doi: 10.1111/j.1399-3046.2007.00719.x. doi:10.1111/j.1399-3046.2007.00719.x. [DOI] [PubMed] [Google Scholar]
- 92.Groh WJ, Groh MR, Saha C, Kincaid JC, Simmons Z, Ciafaloni E, Pourmand R, Otten RF, Bhakta D, Nair GV, Marashdeh MM, Zipes DP, Pascuzzi RM. Electrocardiographic abnormalities and sudden death in myotonic dystrophy type 1. N Engl J Med. 2008;358:2688–2697. doi: 10.1056/NEJMoa062800. doi:10.1056/NEJMoa062800. [DOI] [PubMed] [Google Scholar]
- 93.Lazarus A, Varin J, Babuty D, Anselme F, Coste J, Duboc D. Long-term follow-up of arrhythmias in patients with myotonic dystrophy treated by pacing: a multicenter diagnostic pacemaker study. J Am Coll Cardiol. 2002;40:1645–1652. doi: 10.1016/s0735-1097(02)02339-2. doi:10.1016/S0735-1097(02)02339-2. [DOI] [PubMed] [Google Scholar]
- 94.Wahbi K, Meune C, Porcher R, Becane HM, Lazarus A, Laforet P, Stojkovic T, Behin A, Radvanyi-Hoffmann H, Eymard B, Duboc D. Electrophysiological study with prophylactic pacing and survival in adults with myotonic dystrophy and conduction system disease. JAMA. 2012;307:1292–1301. doi: 10.1001/jama.2012.346. doi:10.1001/jama.2012.346. [DOI] [PubMed] [Google Scholar]
- 95.Blomstrom-Lundqvist C, Scheinman MM, Aliot EM, Alpert JS, Calkins H, Camm AJ, Campbell WB, Haines DE, Kuck KH, Lerman BB, Miller DD, Shaeffer CW, Stevenson WG, Tomaselli GF, Antman EM, Smith SC, Jr, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Hiratzka LF, Hunt SA, Jacobs AK, Russell RO, Jr, Priori SG, Blanc JJ, Budaj A, Burgos EF, Cowie M, Deckers JW, Garcia MA, Klein WW, Lekakis J, Lindahl B, Mazzotta G, Morais JC, Oto A, Smiseth O, Trappe HJ. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias—executive summary. a report of the American college of cardiology/American heart association task force on practice guidelines and the European society of cardiology committee for practice guidelines (writing committee to develop guidelines for the management of patients with supraventricular arrhythmias) developed in collaboration with NASPE-Heart Rhythm Society. Eur Heart J. 2003;24:1857–1897. doi: 10.1016/j.jacc.2003.08.013. doi:10.1016/j.ehj.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 96.Cohen MI, Triedman JK, Cannon BC, Davis AM, Drago F, Janousek J, Klein GJ, Law IH, Morady FJ, Paul T, Perry JC, Sanatani S, Tanel RE. PACES/HRS Expert Consensus Statement on the Management of the Asymptomatic Young Patient with a Wolff-Parkinson-White (WPW, Ventricular Preexcitation) Electrocardiographic Pattern: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology Foundation (ACCF), the American Heart Association (AHA), the American Academy of Pediatrics (AAP), and the Canadian Heart Rhythm Society (CHRS) Heart Rhythm. 2012;9:1006–1024. doi: 10.1016/j.hrthm.2012.03.050. doi:10.1016/j.hrthm.2012.03.050. [DOI] [PubMed] [Google Scholar]