Abstract
Data from the whole body hypothermia trial was analyzed to examine the effects of phenobarbital administration prior to cooling (+PB) on the esophageal temperature (Te) profile, during the induction phase of hypothermia. A total of 98 infants were analyzed. At enrollment, +PB infants had a higher rate of severe HIE and clinical seizures and lower Te and cord pH than infants that have not received PB (−PB). There was a significant effect of PB itself and an interaction between PB and time in the Te profile. Mean Te in the +PB group was lower than in the −PB group and the differences decreased over time. In +PB infants the time to surpass target Te of 33.5°C and to reach the minimum Te during overshoot were shorter. In conclusion, the administration of PB prior to cooling was associated with changes that may reflect a reduced thermogenic response associated with barbiturates.
Keywords: phenobarbital, hypoxic-ischemic encephalopathy, hypothermia, temperature control
Introduction
The use of therapeutic hypothermia is very likely increasing over the last 5 years, since publications of six randomized controlled trials and a meta-analysis showed that therapeutic hypothermia in newborns with hypoxic-ischemic encephalopathy is safe and significantly reduces both death and disability. 1–6 In these trials, hypothermia was induced and maintained by active cooling and the initial temperature applied to the infants was well below thermoneutrality. The average time to achieve the target temperature was between 1– 2 hours, which raises the possibility that some infants responded to cooling with an initial increase in heat production, or thermogenesis. In fact, a thermogenic response to cooling is characteristic of all homeotherms, is rapidly triggered after birth and may result in increases in oxygen consumption by about 34% in a healthy newborn exposed to an ambient temperature of 26°C. 7–9 Although asphyxiated infants have a decreased body temperature (Tb) during the first hours after birth, the thermogenic response of these infants to lower ambient temperatures has not been investigated. 10
After a hypoxic ischemic insult, clinical seizures have been reported in 43 to 59% of infants before initiation of hypothermia. 1–3 In the setting of neonatal encephalopathy secondary to hypoxia-ischemia, seizures are commonly treated with phenobarbital (PB). 11,12 Effective plasma concentrations of PB can be achieved within 10 min after a loading dose of 15mg/kg 13,14 and barbiturates can substantially reduce brain metabolic rates at doses that are 10 to 20% of the usual anesthetic doses and decrease thermogenesis. 15 Therefore, the administration of phenobarbital to infants undergoing therapeutic hypothermia may modify their core temperature profile during the induction phase due to its effect on brain metabolic rate (BMR) and thermogenesis.
In the NICHD whole body cooling trial the temperature profile of the neonates undergoing therapeutic hypothermia for hypoxic-ischemic encephalopathy has been characterized.16 The induction phase of cooling is defined as the time from initiation of cooling to equilibration of target core temperature. Since a significant number of infants undergoing body cooling received PB for seizures (either treatment or prophylaxis) as part of usual care in the participating centers, and cooling on transport was not part of the trial, it provided the opportunity to examine the effects of PB administration on the profile of temperature changes during this phase of the hypothermic treatment. This is important because careful management of Tb during induction, with the goal to prevent oscillations, overshoot and to achieve the target temperature safely and quickly may play a critical role in the effectiveness of this therapy. In this study, we hypothesized that there would be a more rapid and/or greater fall in esophageal temperature during the initiation of hypothermia among infants given PB prior to cooling when compared to infants that did not receive phenobarbital. This may reflect the effects of barbiturates on BMR and thermogenesis.
Methods
Study Infants
This was a secondary analysis of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network Whole Body Hypothermia Trial.2 The trial was performed after informed consent was obtained. Eligibility criteria for therapeutic hypothermia included gestational age of ≥ 36 weeks, postnatal age of ≤ 6 hours, and subsequent fulfillment of specific physiologic and/or clinical criteria (severe acidosis or an acute perinatal event, low Apgar scores, and need for ventilation), followed by demonstration of moderate/severe encephalopathy using the modified Sarnat criteria.
Infants included in this analysis were those who underwent hypothermia in the study and were grouped according to receipt of phenobarbital before initiation of cooling (+PB) or not (−PB). The information on lag time between PB administration and initiation of cooling was not available in the data bank. We used any dose of phenobarbital given before the initiation of the hypothermia to define +PB and −PB groups.
Hypothermic treatment
The methods used for therapeutic hypothermia was described in detail previously. 2 Briefly, infants in the hypothermia group had an esophageal probe inserted and were placed on an infant-size blanket (25 × 33 inches) that was precooled to 5°C (Blanketrol II Hyper-Hypothermia System, Cincinnati Sub-Zero®). The Blanketrol was used in the servo-controlled mode with a target esophageal temperature (Te) of 33.5°C. No external heat source was used during the cooling intervention. The cooling period lasted 72 hours and was followed by 6hr of rewarming. Abdominal-wall skin temperature was monitored with a skin probe. Esophageal and skin temperatures were monitored continuously and recorded every 15 minutes for the first 4 hours, every hour for the next 8 hours, and every 4 hours for the duration of cooling.
Outcomes
A thermogenic response is elicited immediately after the application of a cooling stimulus and any effect of barbiturates on this response would be expected to occur during the induction phase defined as from initiation of cooling to achievement of a stable target temperature. Thus, temperatures analyzed for this study were those evaluated during the induction phase. The primary outcome was the esophageal temperature profile during induction and the specific aim was to determine if the profile differed between +PB and −PB infants. Te profile was evaluated by comparing Te values at baseline and at 30, 45 and 60 minutes of cooling.
Secondary outcomes of interest were the time to first surpass 33.5°C (overshoot), time to reach minimum Te below 33.5°C (prior to equilibration), minimum Te below 33.5°C, and the time to stabilize Te at 33.5 °C (amount of time after overshoot required to bring Te within 0.1°C of the target, i.e., to the first Te ≥ 33.4°C after overshoot).
Group Characteristics
Baseline data used to characterize each group included gestational age, birth weight, Apgar score at 10 minutes, umbilical blood gas, and out-born status. Other variables collected at the time of randomization included clinical seizures, use of PB and other medications, stage of encephalopathy, and use of inotropes. EEG confirmation of seizures was not available as part of this trial.
Statistical Analysis
Demographic and descriptive characteristics of the two groups were compared using Wilcoxon Two-Sample tests for continuous variables and Fisher’s exact test for comparisons of categorical variables. Results are presented as mean ± SD, median and interquartile range or percentages as appropriate. A longitudinal mixed model was used to evaluate differences in the trajectory of temperature profiles over time between +PB and −PB groups during the induction phase of cooling. This model was adjusted for changes over time, and also included gestational age, level of encephalopathy at randomization, and an interaction term between PB and time. Secondary outcomes were not adjusted since they were considered exploratory analyses.
Results
Data from 98 out of 102 infants randomized to the hypothermia arm of the study were analyzed since in three patients data on the use of PB was missing and one patient was not submitted to hypothermia. Baseline characteristics are summarized in Table 1. A total of 44 infants were given PB before cooling. These infants were predominantly outborn and hypothermia was initiated at 5.2 ± 1.2 hours of life (−PB 4.9 ± 1.1 hrs; p=0.18). At study enrollment, +PB infants had a significantly higher proportion of severe HIE and clinical seizures and had lower cord pH values when compared to −PB infants. There were no differences between the two groups in the use of other anticonvulsants, analgesics or inotropes prior to the initiation of cooling,
Table 1.
Baseline characteristics
| No Phenobarbital (n = 54) | Phenobarbital (n = 44) | p value | |
|---|---|---|---|
| Outborn | 19 (35) | 28 (64) | <0.01 |
| Cord pH | 6.91 ± 0.18 | 6.80 ± 0.19 | <0.02 |
| Apgar 10 min (median, quartiles) | 4 (3–5) | 4 (1–5) | 0.35* |
| Cord Base deficit (mEq/L) | 17.4 ± 6.5 | 19.9 ± 6.9 | 0.07 |
| Birth weight (g) | 3416 ± 618 | 3358 ± 620 | 0.75 |
| Gestational age (wk) | 39.1 ± 1.7 | 39.0 ± 1.5 | 0.81 |
| Age of randomization (hrs) | 4.1 ± 1.3 | 4.5 ± 1.2 | 0.10 |
| Seizures | 8 (15) | 35 (80) | < 0.0001 |
| Moderate HIE# | 43 (80) | 23 (53) | 0.008 |
| Severe HIE# | 11 (20) | 20 (47) | |
| Use of medications prior to initiation of therapeutic hypothermia | |||
| Analgesics | 6 (11) | 6 (14) | 0.76 |
| Other anti-convulsants | 5 (9) | 10 (23) | 0.09 |
| Neuromuscular blocking agents | 3 (6) | 1 (2) | 0.63 |
| Vasopressor | 16 (30) | 17 (39) | 0.39 |
Median Two-Sample Test. All other p-values are Fisher’s Exact Test (categorical) or Wilcoxon Two-Sample Test (means).
One +PB infant did not have the neurological exam but was included in the hypothermia study due to seizures.
Primary Outcome
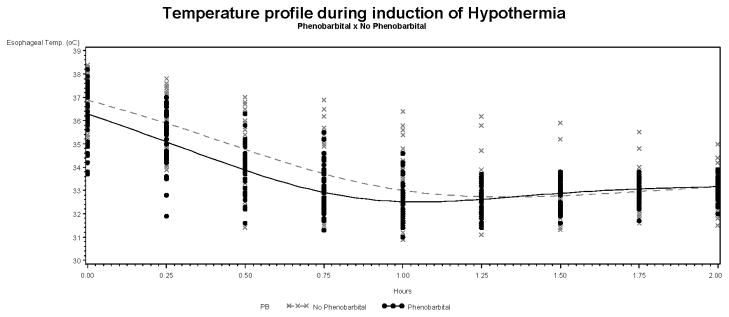
The temperature profile of infants in the +PB and −PB groups is illustrated in the Figure 1. The Te at the initiation of cooling were 36.3 ± 1.1 °C for +PB and 36.9 ± 0.8 °C for −PB (p=0.02). There was a significant effect of PB itself (p=0.0002) and a significant interaction between PB and time (p = 0.0033) in the temperature profile of the infants undergoing hypothermia. Specifically, the overall mean differences in temperature between the +PB and −PB groups were different with temperatures in the +PB group lower than those in the −PB group. These group adjusted mean differences decreased over time: baseline = 0.86 °C ± 0.22 (p=0.0002); 30 min = 0.58°C ± 0.23 (p = 0.009); 45 min = 0.45°C ± 0.24 (p = 0.06) and 60 min = 0.31°C ± 0.26 (p = 0.24).
Figure 1.
Esophageal temperature profile in +PB and −PB infants during the induction phase of hypothermia.
Secondary Outcomes
The unadjusted analysis of the esophageal temperature profile is summarized in Table 2. In +PB infants the time to surpass 33.5°C (0.77 ± 0.39hr vs 0.99 ± 0.51hr, p < 0.01)) and to reach the minimum Te below 33.5°C (1.24 ± 1.15hr vs 1.43 ± 0.93hr, p < 0.01) were shorter when compared to −PB infants. No significant differences were observed in the minimum temperature below the target or time to stabilize at target.
Table 2.
Effects of Phenobarbital administration prior to initiation of therapeutic hypothermia on the profile of the esophageal temperature (Te) during the induction phase.
| No Phenobarbital (N=54) | Phenobarbital (N=44) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Q1 | Median | Q3 | Mean | SD | Q1 | Median | Q3 | |
| Time to surpass 33.5°C (hours) † | 0.99 | 0.51 | 0.75 | 1.00 | 1.25 | 0.77 | 0.39 | 0.50 | 0.75 | 0.75 |
| Time to reach the minimum Te below 33.5°C (hours) † | 1.43 | 0.93 | 1.00 | 1.25 | 1.50 | 1.24 | 1.15 | 0.75 | 1.00 | 1.25 |
| Minimum temperature below the target (°C) | 32.2 | 0.5 | 31.8 | 32.2 | 32.5 | 32.1 | 0.7 | 31.7 | 32.1 | 32.4 |
| Depth of minimum Te below 33.5°C (°C) | 1.3 | 0.5 | 1.00 | 1.3 | 1.7 | 1.4 | 0.7 | 1.1 | 1.4 | 1.8 |
| Time to stabilize Te at 33.5°C after overshoot (hours) | 1.53 | 3.12 | 0.50 | 1.00 | 1.25 | 2.34 | 4.25 | 0.75 | 1.00 | 1.75 |
| Duration of induction, from initiation of cooling (hours) | 2.96 | 3.80 | 2.00 | 2.25 | 2.75 | 3.60 | 4.78 | 1.75 | 2.00 | 2.75 |
p≤0.01 (Wilcoxon two-sample test, t approximation).
Discussion
In this study we were able to demonstrate that phenobarbital administration prior to cooling was associated with an altered temperature profile during the induction phase of whole body hypothermia. The esophageal temperatures of +PB infants were significantly lower than −PB infants which may reflect a reduced metabolic rate and thermogenic response associated with the use of barbiturates. Even though targeted temperature reductions may be achieved more accurately with newer servo controlled devices than what was used in the current study, the induction of any cold stress even for therapeutic purposes may trigger counter-regulatory processes that may offset putative neuroprotective effects of hypothermia. 17–19
The differences in the baseline characteristics of the two groups such as extent of fetal acidemia, severity of encephalopathy, occurrence of clinical seizures and differences on esophageal temperature before initiation of cooling, may also have affected the control of Tb and thermogenesis and account for the differences found between +PB and −PB groups. In this section we will separately discuss these important variables.
Extent of fetal acidemia
Cold-induced thermogenesis is present in neonates immediately after birth.9 Several studies have demonstrated that thermogenesis is decreased during hypoxia and asphyxia 8,20,21 but there are few data describing this response after restoration of oxygenation. Animals exposed to antenatal or postnatal hypoxia had reduced brown adipose tissue mass and thermogenin concentration but normal thermogenic response when exposed to cold after discontinuation of the hypoxic insult. 22,23 Term newborn infants born at high altitude have decreased thermogenic response but were able to increase metabolic rate when ambient temperature (Ta) was further decreased.8 Therefore, we would expect a thermogenic response to cooling in both groups of infants in our study. However, +PB infants had lower cord pH when compared to the −PB infants, which may reflect a more severe perinatal insult. Whether the magnitude of the thermogenic response is modified by the severity of the perinatal insult has not been investigated and may have contributed to differences in the Tb trajectory of +PB and −PB infants.
Severity of encephalopathy and occurrence of clinical seizures
At study enrollment, the +PB group had a higher proportion of infants classified as severe HIE. Severity of HIE was based on neurological exam or the presence of clinical seizures, since EEG was not performed. Although there were no differences in Apgar scores at 10 min or base deficit the cord pH was lower in the +PB infants providing evidence of greater impairment of gas exchange and possibly a more severe perinatal event. Therefore, the +PB group of infants were presumably exposed to a more severe brain insult and although we adjusted our model for the level of encephalopathy, a direct effect of severe brain injury upon thermogenesis may have affected the Tb profile during the induction phase. Animal studies have showed that even severely asphyxiated piglets 23 or decorticated rats 24 were able to elicit a significant thermogenic response when exposed to moderate cold conditions during the first days of life. In human adults, a significant response to cold was present in patients that had suffered from severe stroke or traumatic brain injury, when submitted to hypothermia.25 Interestingly, several animal and human studies have reported an elevation in Tb following severe traumatic brain injury or global hypoxia ischemia in the absence of therapeutic hypothermia.26–28 The brain lesions reported in these animal experiments share interesting parallels with specific MRI patterns of injury in term neonates after moderate to severe HIE.29 Furthermore, as in our population, seizures are more common in more severely asphyxiated subjects, and are associated with an increase in neuronal activity and brain metabolic rate (BMR). 30 Indirect effects of a severe hypoxic ischemic insult could also have caused the faster drop in Tb of the +PB infants by an increased heat loss secondary to either hyperventilation or vasodilatation (secondary to hypoxia). Barbiturates administration can also cause vasodilatation.31 However, in our study, apart from the use of barbiturates, there were no differences in inotropes and other anti-convulsants between +PB and −PB infants. Therefore, differences in the thermogenic response of moderate and severely asphyxiated infants submitted to hypothermia with or without PB require further investigation.
Esophageal temperature before initiation of cooling
In this study we found that +PB infants had significantly lower Te before the initiation of cooling which may have been related to a direct effect of the barbiturate administration. Indeed, the inhibition of the brain metabolic activity is the primary explanation for the hypothermia often seen in patients who undergo general anesthesia with barbiturates. 32,33 In neonates, BMR is a major contributor to overall metabolic rate 34 and PB used as an anti-convulsant medication decreases BMR within 10–15 minutes of its administration when given at doses between 10–15mg/kg.35,36 Therefore, we speculate that administration of PB has affected BMR and thermogenesis, decreasing baseline values of Tb which in turn were associated with an earlier time to reach Tb during the induction phase of hypothermia. In this particular analysis the number of infants treated with other anti-convulsants such as diazepam or phenytoin is too small for any comparisons between these drugs and PB.
This study was not designed and did not have the objective to evaluate the use of PB alone or in combination with hypothermia as a neuroprotective therapy for infants with HIE. Due to the small sample size we did not investigate any differences in short- and long-term outcomes between +PB and −PB infants. Also, it is possible that the administration of PB at any point during hypothermia would affect thermogenesis and temperature control of these infants. This was not investigate since it would require data on the exact time of PB administration and close monitoring of Te and blanket temperature during the following hours.
In summary, +PB infants were more severely asphyxiated based on neurological exam and presence of clinical seizures, had lower Te at the initiation of cooling and a different trajectory of Te during the induction phase hypothermia. These differences in Te trajectory between the groups may reflect a decreased thermogenic capacity secondary to the use of barbiturates or a more severe brain injury. Further studies are needed to understand the effects of active cooling on thermogenesis in HIE infants with or without PB and potentially help optimize cooling regimens in future studies.
Acknowledgments
This study was presented at the Pediatric Academic Society meeting in Baltimore, 2009. We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators, in addition to those listed as authors, participated in this study:
NRN Steering Committee Chair: Alan H. Jobe, MD PhD, University of Cincinnati.
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (U10 HD27904) – William Oh, MD; Angelita M. Hensman, RN BSN.
Case Western Reserve University Rainbow Babies & Children’s Hospital (U10 HD21364, M01 RR80) – Avroy A. Fanaroff, MD; Nancy S. Newman, RN; Bonnie S. Siner, RN.
Cincinnati Children’s Hospital Medical Center, University of Cincinnati Hospital, and Good Samaritan Hospital (U10 HD27853, M01 RR8084) – Edward F. Donovan, MD; Kurt Schibler, MD; Barbara Alexander, RN; Cathy Grisby, BSN CCRC; Marcia Worley Mersmann, RN CCRC; Holly L. Mincey, RN BSN; Jody Hessling, RN.
Duke University School of Medicine University Hospital, Alamance Regional Medical Center, and Durham Regional Hospital (U10 HD40492, M01 RR30) – C. Michael Cotten, MD MHS; Kathy J. Auten, MSHS.
Emory University Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory Crawford Long Hospital (U10 HD27851, M01 RR39) – Barbara J. Stoll, MD; Lucky Jain, MD; Ellen C. Hale, RN BS CCRC; Ann M. Blackwelder, RNC BS MS.
Eunice Kennedy Shriver National Institute of Child Health and Human Development – Linda L. Wright, MD; Elizabeth M. McClure, MEd.
Indiana University Indiana University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (U10 HD27856, M01 RR750) – James A. Lemons, MD; Brenda B. Poindexter, MD MS; Lucy C. Miller, RN BSN CCRC.
RTI International (U01 HD36790) – W. Kenneth Poole, PhD; Betty K. Hastings; Jeanette O’Donnell Auman, BS; Carolyn Petrie Huitema, MS; Scott E. Schaefer, MS.
Stanford University Lucile Packard Children’s Hospital (U10 HD27880, M01 RR70) – David K. Stevenson, MD; M. Bethany Ball, BS CCRC.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (U10 HD34216, M01 RR32) – Waldemar A. Carlo, MD; Namasivayam Ambalavanan, MD; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN.
University of California – San Diego Medical Center and Sharp Mary Birch Hospital for Women (U10 HD40461) – Neil N. Finer, MD; Maynard R. Rasmussen, MD; David Kaegi, MD; Kathy Arnell, RNC; Clarence Demetrio, RN; Chris Henderson, RCP CRTT; Wade Rich, BSHS RRT.
University of Miami Holtz Children’s Hospital (U10 HD21397, M01 RR16587) – Shahnaz Duara, MD; Charles R. Bauer, MD; Ruth Everett-Thomas, RN MSN.
University of Rochester Golisano Children’s Hospital (U10 HD40521, M01 RR44) – Ronnie Guillet, MD PhD; Dale L. Phelps, MD; Linda J. Reubens, RN CCRC.
University of Texas Southwestern Medical Center at Dallas Parkland Health & Hospital System and Children’s Medical Center Dallas (U10 HD40689, M01 RR633) – Pablo J. Sánchez, MD; Walid A. Salhab, MD; Susie Madison, RN; Gaynelle Hensley, RN; Nancy A. Miller, RN; Alicia Guzman.
University of Texas Health Science Center at Houston Medical School, Children’s Memorial Hermann Hospital, and Lyndon Baines Johnson General Hospital/Harris County Hospital District (U10 HD21373, M01 RR2588) – Kathleen A. Kennedy, MD MPH; Esther G. Akpa, RN BSN; Patty A. Cluff, RN; Claudia Y. Franco, RN BSN; Anna E. Lis, RN, BSN; Georgia E. McDavid, RN; Patti L. Tate, RCP.
Wayne State University Hutzel Women’s Hospital and Children’s Hospital of Michigan (U10 HD21385) – Geraldine Muran, RN BSN.
Yale University Yale-New Haven Children’s Hospital (U10 HD27871, M01 RR6022) – Monica Konstantino, RN BSN; Patricia Gettner, RN; JoAnn Poulsen, RN
Footnotes
Conflict of Interest
Authors disclose no commercial, financial, or other associations that could pose a conflict of interest in connection with the submitted article.
Ethical approval
This was a secondary analysis of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network Whole Body Hypothermia Trial. The trial was performed after informed consent was obtained.
Author’s contribution and roles
- Guilherme Sant’Anna: conception and design, analysis and interpretation, writing the article, critical revision of the article, final approval of the article and overall responsibility
- Abbot R. Laptook: conception and design, data collection, analysis and interpretation, writing the article, critical revision, final approval of the article, overall responsibility, obtaining funding.
- Seetha Shankaran: analysis and interpretation, data collection, writing the article, critical revision, final approval of the article, overall responsibility, obtaining funding.
- Rebecca Bara: data collection, critical revision of the article and overall responsibility.
- Scott A. McDonald: data collection, critical revision of the article, statistical analysis
- Rosemary D. Higgins: data collection, critical revision of the article, overall responsibility, obtaining funing.
- Jon E. Tyson: data collection, critical revision of the article, overall responsibility, obtaining funding.
- Richard A. Ehrenkranz: data collection, critical revision of the article, overall responsibility, obtaining funding.
- Abhik Das: data collection critical revision of the article, overall responsibility, statistical analysis.
- Ronald N. Goldberg: data collection critical revision of the article, overall responsibility, obtaining funding.
- Michele C. Walsh: data collection critical revision of the article, overall responsibility, obtaining funding.
Financial disclosure/funding
The National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) provided grant support for the NICHD Neonatal Research Network’s Whole-Body Cooling for Hypoxic Ischemic Encephalopathy Study. Participating NRN sites collected data and transmitted it to RTI International, the data coordinating center (DCC) for the network, which stored, managed and analyzed the data for this study. On behalf of the NRN, Dr. Abhik Das (DCC Principal Investigator), Mr. Scott A. McDonald (DCC Statisticians) had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
References
- 1.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365(9460):663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 2.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 3.Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361(14):1349–1358. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 4.Zhou WH, Cheng GQ, Shao XM, et al. Selective head cooling with mild systemic hypothermia after neonatal hypoxic-ischemic encephalopathy: a multicenter randomized controlled trial in china. J Pediatr. 2010;157(3):367–372. 372. doi: 10.1016/j.jpeds.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 5.Simbruner G, Mittal RA, Rohlmann F, Muche R. Systemic Hypothermia After Neonatal Encephalopathy: Outcomes of neo. nEURO. network RCT. Pediatrics. 2010 doi: 10.1542/peds.2009-2441. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs SE, Morley CJ, Inder TE, et al. Whole-Body Hypothermia for Term and Near-Term Newborns With Hypoxic-Ischemic Encephalopathy: A Randomized Controlled Trial. Arch Pediatr Adolesc Med. 2011 Apr 4; doi: 10.1001/archpediatrics.2011.43. [DOI] [PubMed] [Google Scholar]
- 7.Hey EN. The relation between environmental temperature and oxygen consumption in the new-born baby. J Physiol. 1969;200(3):589–603. doi: 10.1113/jphysiol.1969.sp008710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frappell PB, Leon-Velarde F, Aguero L, Mortola JP. Response to cooling temperature in infants born at an altitude of 4,330 meters. Am J Respir Crit Care Med. 1998;158(6):1751–1756. doi: 10.1164/ajrccm.158.6.9803071. [DOI] [PubMed] [Google Scholar]
- 9.Sahni R, Schulze K. Neonatal thermal regulation. In: Polin R, Fox W, editors. Fetal and Neonatal Physiology. 3. Philadelphia: W.B. Saunders; 2004. pp. 676–702. [Google Scholar]
- 10.Burnard ED, Cross KW. Rectal temperature in the newborn after birth asphyxia. Br Med J. 1958;2(5106):1197–1199. doi: 10.1136/bmj.2.5106.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Painter MJ, Scher MS, Stein AD, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N Engl J Med. 1999;341(7):485–489. doi: 10.1056/NEJM199908123410704. [DOI] [PubMed] [Google Scholar]
- 12.Glass HC, Wirrell E. Controversies in neonatal seizure management. J Child Neurol. 2009;24(5):591–599. doi: 10.1177/0883073808327832. [DOI] [PubMed] [Google Scholar]
- 13.Buchthal F, Svensmark O, Simonsen H. Relation of eeg and seizures to phenobarbital in serum. Arch Neurol. 1968;19(6):567–572. doi: 10.1001/archneur.1968.00480060037004. [DOI] [PubMed] [Google Scholar]
- 14.Kokwaro GO, Ogutu BR, Muchohi SN, Otieno GO, Newton CR. Pharmacokinetics and clinical effect of phenobarbital in children with severe falciparum malaria and convulsions. Br J Clin Pharmacol. 2003;56(4):453–457. doi: 10.1046/j.1365-2125.2003.01951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belopavlovic M, Buchthal A. Barbiturate therapy in the management of cerebral ischaemia. Anaesthesia. 1980;35(3):271–278. doi: 10.1111/j.1365-2044.1980.tb05095.x. [DOI] [PubMed] [Google Scholar]
- 16.Shankaran S, Laptook AR, McDonald SA, et al. Temperature profile and outcomes of neonates undergoing whole body hypothermia for neonatal hypoxic-ischemic encephalopathy. Pediatr Crit Care Med. 2011 Apr 14; doi: 10.1097/PCC.0b013e31821926bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon CJ, McMahon B, Richelson E, Padnos B, Katz L. Neurotensin analog NT77 induces regulated hypothermia in the rat. Life Sci. 2003;73(20):2611–2623. doi: 10.1016/s0024-3205(03)00663-5. [DOI] [PubMed] [Google Scholar]
- 18.Gordon CJ. The therapeutic potential of regulated hypothermia. Emerg Med J. 2001;18(2):81–89. doi: 10.1136/emj.18.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz LM, Young AS, Frank JE, Wang Y, Park K. Regulated hypothermia reduces brain oxidative stress after hypoxic-ischemia. Brain Res. 2004;1017(1–2):85–91. doi: 10.1016/j.brainres.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 20.Mortola JP, Dotta A. Effects of hypoxia and ambient temperature on gaseous metabolism of newborn rats. Am J Physiol. 1992;263(2 Pt 2):R267–R272. doi: 10.1152/ajpregu.1992.263.2.R267. [DOI] [PubMed] [Google Scholar]
- 21.Mortola JP. How newborn mammals cope with hypoxia. Respir Physiol. 1999;116(2–3):95–103. doi: 10.1016/s0034-5687(99)00038-9. [DOI] [PubMed] [Google Scholar]
- 22.Mortola JP, Naso L. Thermogenesis in newborn rats after prenatal or postnatal hypoxia. J Appl Physiol. 1998;85(1):84–90. doi: 10.1152/jappl.1998.85.1.84. [DOI] [PubMed] [Google Scholar]
- 23.Herpin P, Wosiak F, Le DJ, Bertin R. Effects of acute asphyxia at birth on subsequent heat production capacity in newborn pigs. Res Vet Sci. 1999;66(1):45–49. doi: 10.1053/rvsc.1998.0238. [DOI] [PubMed] [Google Scholar]
- 24.Osaka T. Thermogenesis elicited by skin cooling in anaesthetized rats: lack of contribution of the cerebral cortex. J Physiol. 2004;555(Pt 2):503–513. doi: 10.1113/jphysiol.2003.053215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Crit Care Med. 2009;37(3):1101–1120. doi: 10.1097/CCM.0b013e3181962ad5. [DOI] [PubMed] [Google Scholar]
- 26.Thompson HJ, Tkacs NC, Saatman KE, Raghupathi R, McIntosh TK. Hyperthermia following traumatic brain injury: a critical evaluation. Neurobiology of Disease. 2003;12(3):163–173. doi: 10.1016/s0969-9961(02)00030-x. [DOI] [PubMed] [Google Scholar]
- 27.Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet. 2008;371(9628):1955–1969. doi: 10.1016/S0140-6736(08)60837-5. [DOI] [PubMed] [Google Scholar]
- 28.Laptook A, Tyson J, Shankaran S, et al. Elevated temperature after hypoxic-ischemic encephalopathy: risk factor for adverse outcomes. Pediatrics. 2008;122(3):491–499. doi: 10.1542/peds.2007-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller SP, Ramaswamy V, Michelson D, et al. Patterns of brain injury in term neonatal encephalopathy. J Pediatr. 2005;146(4):453–460. doi: 10.1016/j.jpeds.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 30.Grant PE, Roche-Labarbe N, Surova A, et al. Increased cerebral blood volume and oxygen consumption in neonatal brain injury. J Cereb Blood Flow Metab. 2009;29(10):1704–1713. doi: 10.1038/jcbfm.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charney DS, Mihic SJ, Harris RA. The pharmacological basis of therapeutics. 11. New York: McGraw-Hill; 2006. Hypnotics and sedatives; pp. 401–427. [Google Scholar]
- 32.Kiyatkin EA, Brown PL. Brain and body temperature homeostasis during sodium pentobarbital anesthesia with and without body warming in rats. Physiol Behav. 2005;84(4):563–570. doi: 10.1016/j.physbeh.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Erickson KM, Lanier WL. Anesthetic technique influences brain temperature, independently of core temperature, during craniotomy in cats. Anesth Analg. 2003;96(5):1460–1466. doi: 10.1213/01.ANE.0000061221.23197.CE. table. [DOI] [PubMed] [Google Scholar]
- 34.Sahni R, Schulze K. Temperature control in the newborn infants. In: Polin R, Fox W, Abman S, editors. Fetal and Neonatal Physiology. 3. Philadelphia: Saunders; 2004. pp. 548–569. [Google Scholar]
- 35.Michenfelder JD. Hypothermia plus barbiturates: apples plus oranges? Anesthesiology. 1978;49(3):157–158. doi: 10.1097/00000542-197809000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Steen PA, Michenfelder JD. Mechanisms of barbiturate protection. Anesthesiology. 1980;53(3):183–185. [PubMed] [Google Scholar]