SUMMARY
Purpose
Interictal increase of 11C-alpha-methyl-L-tryptophan (AMT) on PET can be seen in cortical epileptic foci, and is particularly common in cortical developmental malformations. Therefore, in the present study, we evaluated the clinical and histopathological correlates of AMT-PET abnormalities in children with intractable epilepsy undergoing resective surgery.
Methods
Thirty children (mean age: 6.7 ± 3.2 years) were included in this study. All patients received AMT PET as part of their presurgical evaluation and subsequently underwent epilepsy surgery. MRI scans were normal in 15, showed non-specific changes in 8, and suggested malformations of cortical development (MCD) in 9 children. Asymmetry indices (AIs) were calculated to determine increased AMT uptake.
Key findings
Histopathology revealed MCD in 16 (53%) children, including 12 with cortical dysplasia (CD) [mild MCD=3; CD type IA=2; CD type IIA=2 and CD type IIB (severe CD with balloon cells)=5]. Polymicrogyria and heterotopias (P&H) were seen in 3 cases and subependymal heterotopias (SEH) in 1 child. The remaining 14 cases showed normal histopathology with varying degrees of gliosis. Increased AMT uptake was found in all 5 with CD type IIB, and all 3 with P&H, but in none with mild MCD and types IA-IIA CD or SEH. Whereas all 5 children with CD IIB and 2 with P&H had excellent surgical outcome (class-I), children with milder CD or SEH had variable surgical outcome. The 14 patients with normal histopathology included 7 patients with focally increased and 7 with normal AMT uptake. While patients with normal pathology and normal AMT-PET had better surgical outcome (class I=5; II=2), those with normal pathology, normal MRI but abnormal AMT-PET had poor surgical outcome (class III=4; IV=3).
Significance
Increased AMT uptake in children with CD may predict type IIB dysplasia (with balloon cells) and good surgical outcome Histopathological similarities between CD type IIB and epileptogenic cortical tubers may imply a common role of the inflammatory kynurenine pathway of tryptophan metabolism in these lesions. In children with normal histopathology, there is a subgroup with increased AMT uptake and poor surgical outcome.
Keywords: alpha-methyl tryptophan, cortical dysplasia, epilepsy surgery, malformation of cortical development (MCD), positron emission tomography (FDG PET)
INTRODUCTION
11C-alpha-methyl-L-tryptophan (AMT) was initially developed as a positron emission tomography (PET) ligand to measure brain serotonin synthesis (Diksic, et al. 1991). However, we found that AMT can identify epileptogenic tubers interictally in patients with tuberous sclerosis complex (TSC) by showing increased uptake in epileptogenic tubers compared to non-epileptogenic ones, which show low uptake (Chugani, et al. 1998a, Asano, et al. 2000, Kagawa, et al. 2005). Studies of resected epileptogenic tubers showed high concentration of quinolinic acid, a neurotoxic and convulsant metabolite of the kynurenine pathway, suggesting activation of kynurenine pathway to be the key mechanism for increased AMT uptake in epileptogenic tubers (Chugani & Muzik 2000, Juhasz, et al. 2004). Subsequently, it was realized that increased AMT uptake in epileptic tissue is not limited to patients with TSC only, but may also be seen in patients with developmental brain malformations, particularly those with focal cortical dysplasia(Fedi, et al. 2001, Juhasz, et al. 2003, Wakamoto, et al. 2008). Since not all malformations of cortical development (MCD) causing epilepsy are associated with increased AMT uptake on PET, we asked whether increased AMT uptake in epileptogenic cortex is specific for particular types of MCD. Therefore, in the present study, we evaluated the clinical and histopathological correlates of AMT-PET abnormalities in non-TSC, non-tumor children undergoing resective epilepsy surgery. We hypothesized that the potential ability of AMT PET to predict specific CD subtypes prior to surgery would have high clinical significance, as histopathological differences of epileptogenic CDs often translate into important electro-clinical differences and have significant implication for epilepsy surgery outcome (Tassi, et al. 2002, Fauser, et al. 2004, Tassi, et al. 2010). Further, this information may contribute to a better understanding of the underlying mechanisms of epilepsy in MCDs.
METHODS
Subjects
The study group consisted of 30 children (mean age: 6.7 ± 3.2 years; 17 males), who underwent cortical resection for intractable epilepsy between 1998 and 2008 at the Children’s Hospital of Michigan/Wayne State University in Detroit. All patients had undergone pre-operative evaluation with interictal and ictal EEG recordings, MRI, FDG-PET and AMT-PET scans. Subsequently, they underwent surgical resection after extensive subdural electrode placement. Six children had one surgery prior to AMT-PET scan and underwent repeat surgery after the AMT-PET scan. Children with TSC or tumor were excluded from this study.
Complete demographic, clinical, neuroimaging and surgical data, including histopathology, were obtained. In the six children undergoing repeat surgery, neuroimaging, surgical and histopathological data after the first surgery were included in the present analysis. Seizure frequency was categorized into 4 groups, with a score of 1 to 4 (1 = ≤ 1 seizure per week, 2 = 2–7 seizures per week, 3 = 1–5 seizures per day, and 4 = > 5 seizures per day). Mean follow-up duration after surgery was 8.7 ± 1.9 years (3.6–11.3 years) and the latest surgical outcome was obtained for each child and categorized according to Engel’s classification (I-IV) (Engel, et al. 1993).
AMT-PET procedure and analysis
PET studies were performed using the CTI/Siemens EXACT/HR whole-body positron tomograph (Knoxville, TN) located in the PET Center of Children’s Hospital of Michigan. This scanner has a 15 cm field of view and generates 47 image planes with a slice thickness of 3.125 mm. The reconstructed image in-plane resolution obtained is 7.5 ± 0.4 mm at FWHM and 7.0 ±0.5 mm in the axial direction (reconstruction parameters: Hanning filter with 1.26 cycles/cm cutoff frequency). Twenty-five minutes after tracer injection, a dynamic emission brain scan (7 × 5 minutes) was acquired in three-dimensional mode. Measured attenuation and decay correction were applied to the PET images. Children were sedated intravenously with either nembutal (3 mg/kg) or midazolam (0.2 mg/kg), if necessary. Prior studies performed on five adults, scanned twice (with and without sedation using midazolam), have shown no significant difference in brain AMT uptake between the two testing conditions (Chugani, et al. 1998b, Muzik, et al. 1998). All AMT-PET studies were performed in compliance with the regulations of Wayne State University Human Investigation Committee, and written informed consent of the patient, parent or legal guardian was obtained.
Initially, all AMT-PET scans were evaluated visually for focal areas with increased uptake. Normally, there should not be any focally increased cortical uptake. Therefore, in this article, the words ‘increased’ AMT and ‘abnormal’ AMT uptake are used interchangeably. In addition, the degree of AMT increases were quantified in all cases where increased uptake was observed visually, using asymmetry indices (AIs) to calculate % increase of AMT uptake in epileptogenic cortex compared to contralateral homotopic cortex (Wakamoto, et al. 2008).
Surgical resection
All children underwent extensive subdural electrocorticography (ECoG) and cortical mapping prior to surgical resection. Although subdural electrode placement was guided by scalp EEG, ictal semiology as well as clinical neuroimaging (MRI, FDG PET) data, resection margins were ultimately decided by ECoG findings, including ictal and interictal epileptiform activity as well as the results of cortical stimulation data. Although surgical resection was not based on AMT PET findings, the area of increased AMT uptake was always included in the surgical resection in all children with AMT abnormality since they corresponded to the electrographically-defined epileptogenic zone. Similarly, the MRI-visible abnormality (MCD in 6 children) was also included in the surgical resection, with a variable extension to surrounding cortical areas based on the ECoG results.
Neuropathological evaluation
All resected specimens were subjected to detailed histopathological evaluation, as described previously (Juhasz, et al. 2003). Resected brain specimens were further re-reviewed by a neuropathologist (WJK). CD, where present, was classified according to Palmini et al. (Palmini, et al. 2004). CD was classified as i) mMCD if cortex was essentially normal with excess ectopic neurons in the molecular layer (layer I) or subcortical white matter, ii) type I if cortical disorganization and dyslamination was present without abnormal dysmorphic–cytomegalic neurons or balloon cells (type IA if only cortical disorganization without any other abnormalities, or type IB if cortical disorganization was present along with immature or hypertrophic but not dysmorphic neurons), or iii) type II if there was cortical disorganization and dyslamination along with abnormal dysmorphic–cytomegalic neurons and balloon cells (type IIA if only dysmorphic–cytomegalic neurons were present without balloon cells, or type IIB if balloon cells were also present).
Statistical analysis
Quantitative values are expressed as mean ± SD. Qualitative or categorical values are given as numbers or percentages. Chi-square test was used to evaluate the difference between the proportions of various categorical variables for two or more groups, and t-test or analysis of variance was performed to determine the difference in various continuous variables. Corresponding non-parametric tests were used wherever applicable. SPSS 18.0 (SPSS Inc, Chicago, IL) was used for the data analyses.
RESULTS
Complete clinical and demographic details are given inTable-1.
Table 1.
Demographic and clinical profile
| Characteristics | |
|---|---|
| Total number of children | 30 |
| Gender | 17 males; 13 females |
| Age at seizure onset* | 1.4 ± 1.1 years |
| Age at AMT scan* | 6.7 ± 4.3 years |
| Seizure duration* | 5 ± 3.5 years |
| Seizure frequency before AMT scan | ≤ 1/week (21%); 2–7/week (12.5%); 1–5/day (45.8%); > 5/day (20.8%) |
| Seizure Type | Past or present history of Infantile spasms (46.7%) Partial or complex partial seizure (30.1%) Secondary generalization (23.2%) |
| Histopathology | MCD- 16 (53%) [12 CD, 3 PMGH & 1 SHE] Normal-14 (47%) |
| Surgery outcome | Engel class I-13; II-4; III-8; IV-5 |
| Duration of post-surgical follow-up* | 8.7 ± 1.8 years |
values are given as mean ± SD; AMT:11C-alpha-methyl-L-tryptophan
MCD: malformation of cortical development; CD: cortical dysplasia; PMGH: polymicrogyria and heterotopias; SHE: sub-ependymal heterotopias
MRI and FDG PET findings
MRI scans were normal in 17 (56.7%) children, showed only non-specific changes from a previous surgery in 6 (20%), and suggested MCD in 7 (23.3%) children. Focal hypometabolism was seen in the hemisphere of epileptogenesis in 28 (93.3%) children including 9 (30%) with bilateral hypometabolism. Two children had hypometabolism in the hemisphere contralateral to the side of epileptogenesis.
Histopathological findings
Histopathology was reported as MCD in 16 (53%) children, including 12 with CD [mild MCD=3; CD type IA=2; CD type IIA=2 and CD type IIB (severe CD with balloon cells)=5]. Three children had polymicrogyria and heterotopias, and one child was reported to have small foci of subependymal heterotopias. Normal histopathology with varying degrees of gliosis was reported in the remaining 14 (47%) children.
Surgical Outcome
Seventeen children (43.3 %) had excellent surgical outcome (Engel’s class-I and II). Whereas all 5 children with CD IIB and 2 with polymicrogyria and heterotopias had excellent surgical outcome (Engel class-I), children with milder CD or subependymal heterotopias had variable surgical outcome (Engel class-I=1, II=2, III=4, IV=1). Similarly, children with normal histopathology also had variable surgical outcome (Engel class-I= 5, II=2, III=4 and IV=3).
Clinical and histopathological correlates of AMT-PET findings
Fifteen children (50%) showed focally increased AMT uptake, which was ipsilateral to the resection site in all cases. None of the clinical variables, such as age, age at seizure onset, seizure frequency scores or duration of epilepsy, was found to be significantly different between children with increased or normal AMT uptake. However, there was a highly significant difference in histopathological abnormality between children with increased or normal AMT uptake. While children with increased AMT uptake had either CD type IIB or polymicrogyria and heterotopias, those with normal AMT uptake had either mMCD or types IA-IIA CD (p=0.0001). Children with normal histopathology consisted of 2 groups based on AMT-PET findings: 7 children each with focally increased or normal AMT uptake (Table 2).
Table 2.
Histopathology, AMT PET findings and surgical outcome
| Histopathology | No. of Children | Mean Age (years) | AMT PET Scan | AI | Surgical Outcome* |
|---|---|---|---|---|---|
| mMCD + SEH | 3+1 | 7.4 | Normal | II-1, III-2+1 | |
| CD Type IA | 2 | Normal | I-1, III-1 | ||
| CD Type IIA | 2 | Normal | II-1, IV-1 | ||
| CD Type IIB | 5 | 9.2 | Abnormal | 10.1 ± 3.6 | I-5 |
| Polymicrogyria & Heterotopia | 3 | 7.9 | Abnormal | 12.3 ± 9.9 | I-2, IV-1 |
| Normal with some gliosis | 7 | 4.1 | Abnormal | 15.5 ± 8.3 | III-4, IV-3 |
| 7 | 5.9 | Normal | I-5, II-2 |
AMT: 11C-alpha-methyl-L-tryptophan; AI: asymmetry index in AMT PET scan;
Engel’s Classification; mMCD: mild malformation of cortical development; SHE: subependymal heterotopias; CD: Cortical Dysplasia (Cortical dysplasia was classified according to Palmini’s classification)
Although there was no overall difference in surgical outcome between children with increased or normal AMT uptake, there was an interesting relationship between surgical outcome and AMT uptake, depending upon the underlying histopathology. Among children with abnormal histopathology (n=16), those with increased AMT uptake, seen in children with CD type IIB or polymicrogyria and heterotopias, much more often had seizure-free outcome (7 of 8 children) as compared to those with normal AMT uptake (only 1 of 8 had seizure-free outcome), seen in children with mMCD or CD types IA-IIA, (p=0.01). On the contrary, among children with normal pathology, the surgical outcome was worse in children with increased AMT uptake compared to those with normal AMT PET findings (p=0.02). Indeed, while all seven children with normal pathology and normal AMT-PET had good surgical outcome (class I=5; II=2), all seven children with normal pathology but abnormal AMT-PET had normal MRI and poor surgical outcome (class III=4; IV=3) (Table 2). None of the clinical and seizure variables were found to be different between these two subgroups (p>0.05 in all comparisons).
DISCUSSION
Two major findings emerge from the present study. First, when increased AMT uptake is seen in children with CD and intractable epilepsy, the histopathology reveals type IIB (with balloon cells) and the surgical outcome is favorable (Figure 1). This is an important and unique finding in that it provides useful prognostic information. The concept that molecular neuroimaging can be so specific for a particular histopathological subtype is intriguing and opens up the possibility that other types of histopathology can be predicted noninvasively with preoperative neuroimaging.
Figure 1.
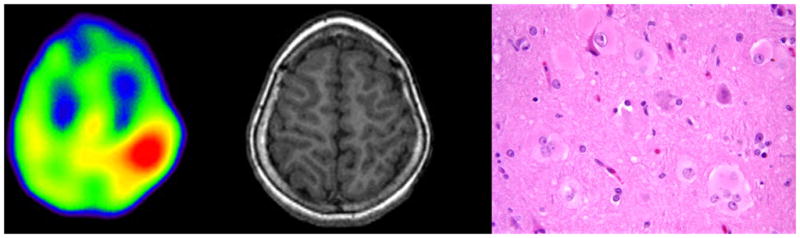
AMT PET scan (left) showing increased tracer uptake in left parietal lobe in a 8 years old girl with intractable seizures and normal MRI (middle). Post-surgical histopathology revealed cortical dysplasia Type-IIB with balloon cells (right: 40x, H&E). The child is seizure free for 6 years after surgery.
The second major finding of the present study is that among children with intractable epilepsy and normal histopathology, there is a subgroup showing increased focal AMT uptake but poor surgical outcome (Figure 2). This subgroup of patients needs further characterization.
Figure 2.
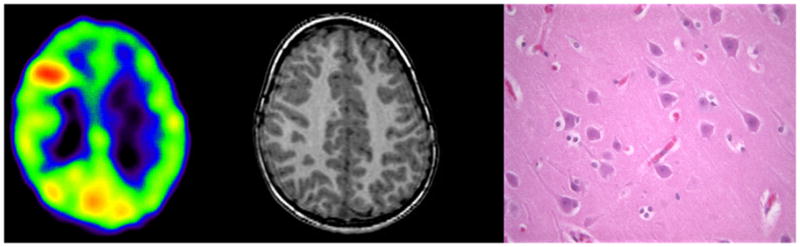
AMT PET scan (left) showing increased tracer uptake in right frontal lobe in a 3 years old boy with intractable seizures and normal MRI (middle). Post-surgical histopathology (right: 40x, H&E) was also normal. The child is having persistent seizures after the surgery.
In our present cohort, there were six children who had a resection prior to AMT-PET scan, followed by a repeat surgery. It may be argued that AMT uptake and overall surgical outcome might have been influenced by this fact. However, out of these six, only three children showed increased AMT uptake. Among these three AMT-positive children, AMT PET was performed 2 and 6 years after the first surgery in two children. We have previously reported that post-surgical inflammation can lead to short-term (within 2 months) increases of AMT uptake in the surgical bed (Juhasz, et al. 2004), but we have never seen this years after surgery. In the third child, AMT PET was done 2 months after the first surgery; however, the site of increased AMT uptake (medial frontal, resected during the second surgery) was located remotely from the site of the first surgery (when parts of the lateral frontal cortex were removed). Therefore, post-surgical inflammation was a very unlikely cause of increased AMT uptake in any of these three cases. Interestingly, five of these six children had good surgical outcome (Engel class I-4, II-1), suggesting that previous surgery or AMT status did not influence the results in this subgroup.
Tryptophan metabolism via kynurenine pathway in epilepsy
Increased tryptophan metabolism (AMT uptake) in the epileptic focus is not due to increased serotonin synthesis but due to an alternate pathway of tryptophan metabolism (Chugani & Muzik 2000, Chugani & Chugani 2005). It is known that, in addition to being converted into serotonin or incorporated into protein, under some circumstances (e.g., ischemia, immune activation and epilepsy), tryptophan may be metabolized by tryptophan 2,3-dioxygenase (Haber, et al. 1993) and indoleamine 2,3-dioxygenase (IDO) (Yamazaki, et al. 1985) via the kynurenine pathway. IDO is inducible by various inflammatory cytokines, particularly interferon-gamma (Taylor & Feng 1991). In brain, human microglia has been shown to express IDO upon interferon-gamma treatment (Heyes, et al. 1996). Recent studies have provided evidence for activation of microglia (Boer, et al. 2006) and markers of various inflammatory pathways in epileptogenic tubers as well as in type II dysplasia (Boer, et al. 2008, Iyer, et al. 2010, Shu, et al. 2010); interestingly, these two histologically similar malformations also show increased AMT uptake on PET. This raises the possibility that, like tubers, increased AMT uptake in type II CD may be related to abnormal tryptophan metabolism via the kynurenine pathway. Cytokines appear to have a robust proconvulsive effect in experimental seizure models (Ravizza, et al. 2008, Vezzani, et al. 2008). In addition, since several kynurenine pathway metabolites (e.g., quinolinic acid, kynurenine and 3-hydroxykynurenine) are convulsants acting as agonists at N-methyl-D-aspartate (NMDA) receptors (Lapin 1978, Perkins & Stone 1982, Vezzani, et al. 1985), accumulation of these metabolites may also contribute to the epileptogenicity of these lesions.
Indeed, the epileptogenic effect of some kynurenine metabolites has been well demonstrated in animal studies (Vezzani, et al. 1989). Increased quinolinic acid and kynurenine pathway enzymes have been reported in epilepsy-prone E1 mice (Nakano, et al. 1993). Lehrmann et al. showed that activation of microglia and astrocytes after innoculation with hamster neurotropic measles virus in weanling Balb/C mice was associated with increased levels of the neurotoxic kynurenine metabolites 3-hydroxykynurenine and quinolinic acid in hippocampus leading to seizures (Lehrmann, et al. 2008), thus supporting previous data that kynurenine pathway metabolites might play a role in human epileptogenesis (Feldblum et al., 1988; Heyes et al., 1990).
Under normal circumstances, tryptophan metabolites of the kynurenine pathway are 100–1000 times lower than tryptophan concentration in brain (Saito, et al. 1993). In comparison, the sum of the concentrations of serotonin and its metabolite 5-HIAA is approximately one-fifth the concentration of tryptophan in brain (Hery, et al. 1977). However, IDO induction can result in a 10-fold increase of quinolinic acid in brain (Saito, et al. 1993). We found much higher levels of quinolinic acid in brain tubers showing increased AMT uptake on PET compared to adjacent brain tissue or tubers not showing increased AMT uptake in children with tuberous sclerosis (Chugani & Muzik 2000, Juhasz, et al. 2004). These findings indicate that epileptogenic tubers are associated with IDO induction leading to the production of endogenous convulsants, and provide an intriguing perspective of epileptogenesis in TSC and possibly other epileptic disorders.
The similarities between CD type IIB and cortical tubers in TSC may imply a role of IDO for increased AMT uptake in CD also. In addition, a similar mechanism could be implicated in the 3 patients with polymicrogyria and heterotopias (P&H), all of whom showed focally increased AMT uptake. Therefore, these patients may share a similar mechanism of epileptogenesis to patients with tuberous sclerosis and increased AMT uptake in the epileptogenic tuber. In contrast, none of the patients with mild MCD and types IA-IIA CD or subependymal heterotopias (SEH) showed focal areas of increased AMT uptake, thus suggesting that different mechanisms of epileptogenesis may be accounting for their seizures.
Subgroup with poor outcome
An interesting finding of the present study is the identification of a group of seven patients who had several common features, including normal MRI scan and normal histopathology, but focally increased AMT uptake concordant with ictal EEG localization. These seven subjects all had poor surgical outcome (4 had class III and 3 had class IV outcome). Indeed, the outcomes in these patients were in sharp contrast to the good surgical outcome (class I=5; II=2) in those seven children who also had normal histopathology, but normal AMT-PET scans. The basis for the results in the poor surgical outcome group is not clear, as none of the evaluated clinical variables were different in this subgroup. One could speculate that perhaps these patients might have an underlying (or additional) diffuse epileptic process, such as a channelopathy. The notion that focal seizures can occur in epileptic channelopathies is exemplified in patients with SCN1A mutations, who often present with prolonged refractory partial seizures.
Limitations
This is a retrospective study with a relatively small sample size, and therefore suffers from the inherent limitations of a retrospective data analysis, such as inclusion of a biased study population. Perhaps only those children underwent AMT PET scan who were very intractable, had very difficult seizure localization or failed epilepsy surgery. Although extension of the present findings to a more general epilepsy population can be argued on these grounds, the extreme starkness of the findings suggest that these findings are robust and will be reproducible in a larger unselected epilepsy population.
Future directions
Clearly, PET scanning with the ligand AMT is a valuable tool not only in the surgical management of intractable epilepsy, but also in advancing our knowledge of the basic mechanisms of epileptic disorders. Unfortunately, although PET scanners are now widely available, few centers have access to AMT. One reason for this is that AMT is labeled with carbon-11, which has a half-life of only 20 minutes and therefore has to be synthesized on site using a cyclotron. It would be much more readily available if AMT could be labeled with fluoride-18, which has a half-life of 110 minutes, allowing it to be transported as in the case of FDG.
Our findings also highlight the possibility that molecular neuroimaging with PET using selected tracers may assist in the elucidation of various underlying mechanisms of epilepsy. Several pharmacological approaches to treating epilepsy with agents aimed at the kynurenine pathway in animal models have been reported (Chiarugi, et al. 1995, Wu, et al. 2002, Nemeth, et al. 2004, Zhang, et al. 2005). Based on these studies, pharmacological agents are currently under development targeting the kynurenine pathway as a new option for the treatment of epilepsy. If such pharmacological agents are applied, AMT PET scans may be an ideal method of selecting appropriate patients for treatment and monitoring treatment effects.
Acknowledgments
This work was supported by grants from the National Institutes of Health to HTC (R01-NS34488, R01-NS064989), EA (K23-047550, R01-064033), and CJ (R01-CA123451).
Footnotes
Disclosure
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. None of the authors have any conflict of interest to disclose.
References
- Asano E, Chugani DC, Muzik O, Shen C, Juhasz C, Janisse J, Ager J, Canady A, Shah JR, Shah AK, Watson C, Chugani HT. Multimodality imaging for improved detection of epileptogenic foci in tuberous sclerosis complex. Neurology. 2000;54:1976–1984. doi: 10.1212/wnl.54.10.1976. [DOI] [PubMed] [Google Scholar]
- Boer K, Jansen F, Nellist M, Redeker S, van den Ouweland AM, Spliet WG, van Nieuwenhuizen O, Troost D, Crino PB, Aronica E. Inflammatory processes in cortical tubers and subependymal giant cell tumors of tuberous sclerosis complex. Epilepsy Res. 2008;78:7–21. doi: 10.1016/j.eplepsyres.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Boer K, Spliet WG, van Rijen PC, Redeker S, Troost D, Aronica E. Evidence of activated microglia in focal cortical dysplasia. J Neuroimmunol. 2006;173:188–195. doi: 10.1016/j.jneuroim.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Chiarugi A, Carpenedo R, Molina MT, Mattoli L, Pellicciari R, Moroni F. Comparison of the neurochemical and behavioral effects resulting from the inhibition of kynurenine hydroxylase and/or kynureninase. J Neurochem. 1995;65:1176–1183. doi: 10.1046/j.1471-4159.1995.65031176.x. [DOI] [PubMed] [Google Scholar]
- Chugani DC, Chugani HT, Muzik O, Shah JR, Shah AK, Canady A, Mangner TJ, Chakraborty PK. Imaging epileptogenic tubers in children with tuberous sclerosis complex using alpha-[11C]methyl-L-tryptophan positron emission tomography. Ann Neurol. 1998a;44:858–866. doi: 10.1002/ana.410440603. [DOI] [PubMed] [Google Scholar]
- Chugani DC, Muzik O. Alpha[C-11]methyl-L-tryptophan PET maps brain serotonin synthesis and kynurenine pathway metabolism. J Cereb Blood Flow Metab. 2000;20:2–9. doi: 10.1097/00004647-200001000-00002. [DOI] [PubMed] [Google Scholar]
- Chugani DC, Muzik O, Chakraborty P, Mangner T, Chugani HT. Human brain serotonin synthesis capacity measured in vivo with alpha-[C-11]methyl-L-tryptophan. Synapse. 1998b;28:33–43. doi: 10.1002/(SICI)1098-2396(199801)28:1<33::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Chugani DC. Imaging of serotonin mechanisms in epilepsy. Epilepsy Curr. 2005;5:201–206. doi: 10.1111/j.1535-7511.2005.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diksic M, Nagahiro S, Chaly T, Sourkes TL, Yamamoto YL, Feindel W. Serotonin synthesis rate measured in living dog brain by positron emission tomography. J Neurochem. 1991;56:153–162. doi: 10.1111/j.1471-4159.1991.tb02575.x. [DOI] [PubMed] [Google Scholar]
- Engel JJ, Van Ness PC, Rasmussen TB, Ojemann LM. Outcome with respect to epileptic seizures. In: Engel JJ, editor. Surgical treatment of the epilepsies. Raven; New York: 1993. pp. 609–622. [Google Scholar]
- Fauser S, Schulze-Bonhage A, Honegger J, Carmona H, Huppertz HJ, Pantazis G, Rona S, Bast T, Strobl K, Steinhoff BJ, Korinthenberg R, Rating D, Volk B, Zentner J. Focal cortical dysplasias: surgical outcome in 67 patients in relation to histological subtypes and dual pathology. Brain. 2004;127:2406–2418. doi: 10.1093/brain/awh277. [DOI] [PubMed] [Google Scholar]
- Fedi M, Reutens D, Okazawa H, Andermann F, Boling W, Dubeau F, White C, Nakai A, Gross DW, Andermann E, Diksic M. Localizing value of alpha-methyl-L-tryptophan PET in intractable epilepsy of neocortical origin. Neurology. 2001;57:1629–1636. doi: 10.1212/wnl.57.9.1629. [DOI] [PubMed] [Google Scholar]
- Haber R, Bessette D, Hulihan-Giblin B, Durcan MJ, Goldman D. Identification of tryptophan 2,3-dioxygenase RNA in rodent brain. J Neurochem. 1993;60:1159–1162. doi: 10.1111/j.1471-4159.1993.tb03269.x. [DOI] [PubMed] [Google Scholar]
- Hery F, Chouvet G, Kan JP, Pujol JF, Glowinski J. Daily variations of various parameters of serotonin metabolism in the rat brain. II. Circadian variations in serum and cerebral tryptophan levels: lack of correlation with 5-HT turnover. Brain Res. 1977;123:137–145. doi: 10.1016/0006-8993(77)90648-5. [DOI] [PubMed] [Google Scholar]
- Heyes MP, Achim CL, Wiley CA, Major EO, Saito K, Markey SP. Human microglia convert l-tryptophan into the neurotoxin quinolinic acid. Biochem J. 1996;320 (Pt 2):595–597. doi: 10.1042/bj3200595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer A, Zurolo E, Spliet WG, van Rijen PC, Baayen JC, Gorter JA, Aronica E. Evaluation of the innate and adaptive immunity in type I and type II focal cortical dysplasias. Epilepsia. 2010;51:1763–1773. doi: 10.1111/j.1528-1167.2010.02547.x. [DOI] [PubMed] [Google Scholar]
- Juhasz C, Chugani DC, Muzik O, Shah A, Asano E, Mangner TJ, Chakraborty PK, Sood S, Chugani HT. Alpha-methyl-L-tryptophan PET detects epileptogenic cortex in children with intractable epilepsy. Neurology. 2003;60:960–968. doi: 10.1212/01.wnl.0000049468.05050.f2. [DOI] [PubMed] [Google Scholar]
- Juhasz C, Chugani DC, Padhye UN, Muzik O, Shah A, Asano E, Mangner TJ, Chakraborty PK, Sood S, Chugani HT. Evaluation with alpha-[11C]methyl-L-tryptophan positron emission tomography for reoperation after failed epilepsy surgery. Epilepsia. 2004;45:124–130. doi: 10.1111/j.0013-9580.2004.30303.x. [DOI] [PubMed] [Google Scholar]
- Kagawa K, Chugani DC, Asano E, Juhasz C, Muzik O, Shah A, Shah J, Sood S, Kupsky WJ, Mangner TJ, Chakraborty PK, Chugani HT. Epilepsy surgery outcome in children with tuberous sclerosis complex evaluated with alpha-[11C]methyl-L-tryptophan positron emission tomography (PET) J Child Neurol. 2005;20:429–438. doi: 10.1177/08830738050200050701. [DOI] [PubMed] [Google Scholar]
- Lapin IP. Stimulant and convulsive effects of kynurenines injected into brain ventricles in mice. J Neural Transm. 1978;42:37–43. doi: 10.1007/BF01262727. [DOI] [PubMed] [Google Scholar]
- Lehrmann E, Guidetti P, Love A, Williamson J, Bertram EH, Schwarcz R. Glial activation precedes seizures and hippocampal neurodegeneration in measles virus-infected mice. Epilepsia. 2008;49(Suppl 2):13–23. doi: 10.1111/j.1528-1167.2008.01489.x. [DOI] [PubMed] [Google Scholar]
- Muzik O, Chugani DC, Shen C, Chugani HT. Noninvasive imaging of serotonin synthesis rate using PET and α-methyltryptophan in autistic children. In: Carson RE, Daube-Witherspoon ME, Herscovitch P, editors. Quantitative functional brain imaging with positron emission tomography. Academic Press; San Diego: 1998. pp. 201–206. [Google Scholar]
- Nakano K, Takahashi S, Mizobuchi M, Kuroda T, Masuda K, Kitoh J. High levels of quinolinic acid in brain of epilepsy-prone E1 mice. Brain Res. 1993;619:195–198. doi: 10.1016/0006-8993(93)91612-v. [DOI] [PubMed] [Google Scholar]
- Nemeth H, Robotka H, Kis Z, Rozsa E, Janaky T, Somlai C, Marosi M, Farkas T, Toldi J, Vecsei L. Kynurenine administered together with probenecid markedly inhibits pentylenetetrazol-induced seizures. An electrophysiological and behavioural study. Neuropharmacology. 2004;47:916–925. doi: 10.1016/j.neuropharm.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Palmini A, Najm I, Avanzini G, Babb T, Guerrini R, Foldvary-Schaefer N, Jackson G, Luders HO, Prayson R, Spreafico R, Vinters HV. Terminology and classification of the cortical dysplasias. Neurology. 2004;62:S2–8. doi: 10.1212/01.wnl.0000114507.30388.7e. [DOI] [PubMed] [Google Scholar]
- Perkins MN, Stone TW. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982;247:184–187. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- Ravizza T, Gagliardi B, Noe F, Boer K, Aronica E, Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008;29:142–160. doi: 10.1016/j.nbd.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Saito K, Crowley JS, Markey SP, Heyes MP. A mechanism for increased quinolinic acid formation following acute systemic immune stimulation. J Biol Chem. 1993;268:15496–15503. [PubMed] [Google Scholar]
- Shu HF, Zhang CQ, Yin Q, An N, Liu SY, Yang H. Expression of the interleukin 6 system in cortical lesions from patients with tuberous sclerosis complex and focal cortical dysplasia type IIb. J Neuropathol Exp Neurol. 2010;69:838–849. doi: 10.1097/NEN.0b013e3181eaeae5. [DOI] [PubMed] [Google Scholar]
- Tassi L, Colombo N, Garbelli R, Francione S, Lo Russo G, Mai R, Cardinale F, Cossu M, Ferrario A, Galli C, Bramerio M, Citterio A, Spreafico R. Focal cortical dysplasia: neuropathological subtypes, EEG, neuroimaging and surgical outcome. Brain. 2002;125:1719–1732. doi: 10.1093/brain/awf175. [DOI] [PubMed] [Google Scholar]
- Tassi L, Garbelli R, Colombo N, Bramerio M, Lo Russo G, Deleo F, Milesi G, Spreafico R. Type I focal cortical dysplasia: surgical outcome is related to histopathology. Epileptic Disord. 2010;12:181–191. doi: 10.1684/epd.2010.0327. [DOI] [PubMed] [Google Scholar]
- Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5:2516–2522. [PubMed] [Google Scholar]
- Vezzani A, Balosso S, Ravizza T. The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun. 2008;22:797–803. doi: 10.1016/j.bbi.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Stasi MA, Wu HQ, Castiglioni M, Weckermann B, Samanin R. Studies on the potential neurotoxic and convulsant effects of increased blood levels of quinolinic acid in rats with altered blood-brain barrier permeability. Exp Neurol. 1989;106:90–98. doi: 10.1016/0014-4886(89)90149-0. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Ungerstedt U, French ED, Schwarcz R. In vivo brain dialysis of amino acids and simultaneous EEG measurements following intrahippocampal quinolinic acid injection: evidence for a dissociation between neurochemical changes and seizures. J Neurochem. 1985;45:335–344. doi: 10.1111/j.1471-4159.1985.tb03993.x. [DOI] [PubMed] [Google Scholar]
- Wakamoto H, Chugani DC, Juhasz C, Muzik O, Kupsky WJ, Chugani HT. Alpha-methyl-l-tryptophan positron emission tomography in epilepsy with cortical developmental malformations. Pediatr Neurol. 2008;39:181–188. doi: 10.1016/j.pediatrneurol.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Wu HQ, Lee SC, Scharfman HE, Schwarcz R. L-4-chlorokynurenine attenuates kainate-induced seizures and lesions in the rat. Exp Neurol. 2002;177:222–232. doi: 10.1006/exnr.2002.7971. [DOI] [PubMed] [Google Scholar]
- Yamazaki F, Kuroiwa T, Takikawa O, Kido R. Human indolylamine 2,3-dioxygenase. Its tissue distribution, and characterization of the placental enzyme. Biochem J. 1985;230:635–638. doi: 10.1042/bj2300635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DX, Williamson JM, Wu HQ, Schwarcz R, Bertram EH. In situ-produced 7-chlorokynurenate has different effects on evoked responses in rats with limbic epilepsy in comparison to naive controls. Epilepsia. 2005;46:1708–1715. doi: 10.1111/j.1528-1167.2005.00281.x. [DOI] [PubMed] [Google Scholar]


