Abstract
PURPOSE
To determine the long-term effect of sub-threshold diode laser treatment for drusen in patients with non-exudative age-related macular degeneration (AMD) with spectral domain optical coherence tomography combined with simultaneous scanning laser ophthalmoscope (SD-OCT/SLO).
METHODS
8 eyes of 4 consecutive AMD patients with bilateral drusen previously treated with sub-threshold diode laser were imaged with SD-OCT/SLO. Abnormalities in the outer retina layers reflectivity as seen with SD-OCT/SLO were retrospectively analyzed and compared with color fundus pictures and autofluorescence images (AF) acquired immediately before and after the laser treatment.
RESULTS
A focal discrete disruptions in the reflectivity of the outer retinal layers was noted in 29% of the laser lesions. The junction in between the inner and outer segment of the photoreceptor was more frequently affected, with associated focal damage of the outer nuclear layer. Defects of the RPE were occasionally detected. These changes did not correspond to threshold burns on color fundus photography, but corresponded to focal areas of increased AF in the majority of the cases.
CONCLUSIONS
Sub-threshold diode laser treatment causes long-term disruption of the retinal photoreceptor layer as analyzed by SD-OCT/SLO. The concept that sub-threshold laser treatment can achieve a selected RPE effect without damage to rods and cones may be flawed.
Keywords: Spectral-domain OCT-SLO, Sub-threshold laser, Autofluorescence, Photoreceptors, Age-related macular degeneration
INTRODUCTION
Laser photocoagulation is used to treat many retinal conditions. Beyond the direct damage induced to the peripheral retina in pathologic proliferative lesions, thermal burns induced by laser energy appear also to be effective in macular edema secondary to retinovascular diseases. Different mechanisms have been postulated to explain the decrease of retinal edema secondary to threshold laser photocoagulation. They include modulation of chemical factors, such as heat shock proteins, vascular endothelial growth factor, pigment epithelium derived factor, and alteration of inner-outer blood-retinal barrier.1, 2,3, 4 It has been suggested that neuroretinal damage may be unnecessary for therapeutic effect, and that the same outcome can be achieved with sub-threshold treatments.5,6,7,8,9,10
In the past years several clinical studies also reported evidence that a variety of laser treatment result in reduction of drusen in patients with non-exudative macular degeneration.11 We have previously shown that sub-threshold diode laser treatment in non-exudative AMD resulted in significant reduction in drusen.12 However, results from two different multicenter randomized clinical trials suggested that this treatment, which did reduce drusen number, did not demonstrate a clinically significant benefit for vision and was unlikely to decrease the incidence of severe vision loss in such patients from choroidal neovascularization (CNV).13,14
The initial rationale for the above studies, as well as ongoing studies of sub-threshold laser treatment, was the hypothesis that the use of sub-threshold laser would localize the treatment effect to the RPE. The RPE would be stimulated to phagocytize drusen without photoreceptor damage and thus minimal visual consequences.15,16
Despite all the interesting hypotheses suggesting a beneficial effect of sub-threshold laser, it has been shown that it is difficult to assess the effects of sub-threshold laser because the end-point is an invisible reaction and because laser treatment with the same parameters can produce variable effects on RPE even in the same eye.5 This is likely because of variable pigment density and variation in the optical media in a given eye. Such variation in optical transmission and pigment density may cause the same power laser to generate a true photocoagulation lesion with retinal destruction in one area and an invisible lesion in another. Interestingly, although sub-threshold lesions are by definition ophthalmoscopically invisible, they can be seen on fluorescein angiography and perhaps most sensitively by fundus autofluorescence in some cases.17
In the present study we wished to determine the long-term effects of sub-threshold diode laser on the outer retina (RPE and photoreceptors), using high-resolution spectral domain optical coherence tomography (SD-OCT) combined with simultaneous scanning laser ophthalmoscope (SLO). The effect of such treatment cannot be seen ophthalmoscopically, so an objective indicator of the treatment would be useful. SD-OCT has improved axial resolution and enables better visualization of intraretinal layers.18 It is especially advantageous in assessing the photoreceptor morphology and can identify fine details of RPE.19 SD-OCT imaging of the outer retina can reveal multiple landmarks including the RPE, external limiting membrane, and junction of inner/outer segments of photoreceptors (IS/OS). We reviewed SD-OCT scans of the patients who received prophylactic sub-threshold diode laser for drusen to determine the nature of focal changes that occurred. We also used autofluorescence (AF) and color fundus photos acquired at the time of the treatment and at follow-up. We analyzed them using overlay techniques to look at the correspondence between the lesions and to allow the localization of OCT changes in the AF and color fundus photos. We wished to determine if there was an anatomic correlate to the sub-threshold burn. This is important because, although sub-threshold diode laser treatment of drusen has been discontinued since the negative results of the most recent study, there is ongoing interest in this kind of therapy for macular edema secondary to retinovascular disorders.
METHODS
Subjects
Eight eyes of 4 consecutive female patients (mean age ± SD 80 ± 8.09 years) from the Jacobs Retina Center at the University of California, San Diego, in La Jolla, California, were imaged. Three-hundred-eighty-four laser applications were studied. Both eyes of the 4 enrolled patients underwent laser treatment at the time of the study, in a period of time from August to December 2002 in all cases. These were all patients who had sub-threshold laser for drusen 6 years prior to OCT scans. 14 At the time of treatment patients were ≥50 years of age and had at least 10 soft drusen (≥ 63 microns in diameter) without CNV. Eyes received a single-session of sub-threshold treatment using an 810-nm wavelength infrared diode laser (IRIS Medical OcuLight SLx, IRIDEX Corporation, Mountainview, CA). An anular grid of 48 diode laser lesions of 125 microns in diameters was performed, extending from 0.5 (750 microns) to 2.0 (3000 microns) disc diameters from the center of the foveal avascular zone, without considering the location of drusen (Figure 1). The power used was one half the power needed to produce a minimally visible threshold burn.
Figure 1.
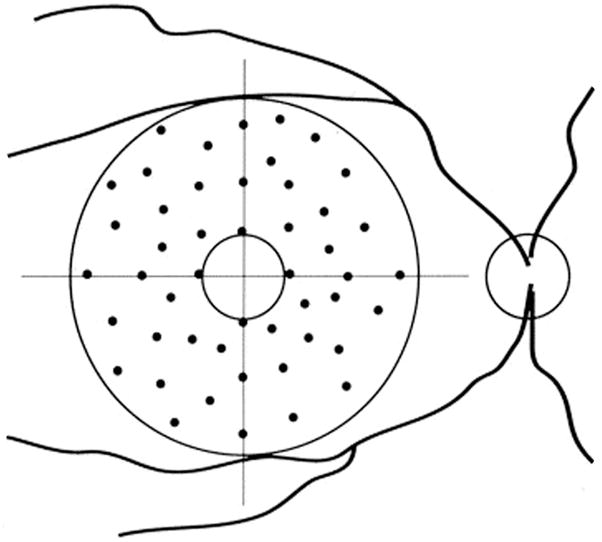
Scheme of sub-threshold laser application.
Color fundus photography and AF imaging were performed before, immediately after, and 3 months after the laser treatment. A conventional fundus camera (TRC-50VT, Topcon Corporation, Tokyo, Japan) was used for color fundus photography on Ektachrome 100 film. The HRA confocal scanning laser ophthalmoscope (HRA Running Viewer Software version 2.1.0.0, Heidelberg Engineering, Vista, CA) was used for AF imaging. (Kenichiro B. et al, Retina 2005;25(8):981–988) All imaging was performed through dilated pupils using phenylephrine (2.5%) and tropicamide (1%) eye drops.
As part of clinical follow-up, we examined the eyes using a SD-OCT (OCT SLO, Software Version 1.66, Opko Instrumentation/OTI, a division of OPKO Health, Miami, Florida). All the OCT scans were acquired six years after the laser treatment, from April to September 2008. We excluded eyes that developed, in the window time between laser and the Spectral OCT exam, CNV, GA or other retinal pathologies potentially responsible for damage to the normal anatomy of the posterior pole. Eyes with media opacities, preventing good quality of imaging, were also excluded. This was a retrospective case series study. All patients provided informed consent and approval for the study was obtained from the Institutional Review Board.
Spectral OCT imaging technique
The light source of the SD-OCT is a modified superluminescent diode, with a bandwidth of 40 nm centered at an 830nm wavelength. The axial resolution is equal to 5 microns, and the transverse resolution 16 microns. The acquisition speed is 28,000 A-Scan/sec. Every B-scan is composed of 512 A-Scans and is acquired in 0.18 seconds. The OCT is coupled to a scanning laser ophthalmoscope (SLO). In the sensing arm of the OCT, a beam splitter redirects part of the light returned from the eye toward a confocal optical receiver. Both OCT and confocal signals carrying the information about the reflectivity of the target are collected by a dual-input, which displays two images on a screen under computer control. A strict topographic correspondence of the OCT cross-sectional image and the SLO image allows the operator to sweep the cursor all over the posterior pole, focusing on specific notable areas. Each series of B-Scans contains 128 images.20 The software provides two main different acquisition modes, manual longitudinal scans and automatic raster scans. Since the manual acquisition has the highest resolution possible, our scanning protocol consisted of at least two high quality series of manually acquired longitudinal scans. Both the series were horizontal, and carefully swept the upper and lower half macula, covering an area of 8mm × 8mm throughout the posterior pole. All the retinal layers were evaluated for the analysis.
Image Analysis
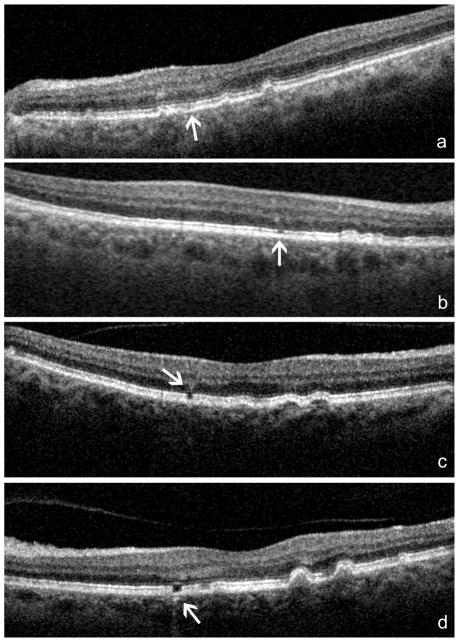
The SD-OCT B-scans were carefully and independently reviewed by 2 experienced retina specialists. In case of discrepancy in the analysis, a consensus was reached by the two operators. A grading system was use to score structural changes in the outer retinal layers: 0, no changes; 1, mild discrete disruption along the junction of the inner segments/outer segments (IS/OS) of the photoreceptors; 2, clear focal disruption of the IS/OS; 3 clear focal disruption of the IS/OS and abrupt thinning and increased reflectivity of the corresponding outer nuclear layer (ONL). Areas of increased transmission of the signal to the choroid, expression of RPE thinning or atrophy, were also analyzed (Figure 2). The location of the laser lesions was identified on the corresponding SLO image. The best SLO scan was used as a fundus reference image. On it, all of the above-described lesions were carefully drawn using photo-retouching software (Adobe Photoshop 7.0, Adobe Systems, Inc., San Jose, CA). Particular care was taken while evaluating the presence of retinal vessels. Seen as roundish hyper-reflective structures in the inner retina layers, vessels cause narrow shadows in the deeper structures, which were not considered outer retinal defects for the purpose of the study. Also, areas with clear presence of drusen were excluded from the analysis, since focal changes in situ may be related to the disease more than to laser treatment.
Figure 2. Grading system for diode-laser induced lesions as detected by SD-OCT/SLO.
a: mild discrete disruption along the junction of the junction of the inner segment/outer segment (IS/OS) of the photoreceptors (score = 1); b: clear focal disruption of the IS/OS (score = 2); c: clear focal disruption of the IS/OS and abrupt thinning and increased reflectivity of the corresponding outer nuclear layer (ONL) (score = 3); d: discrete increased transmission of the signal in the choroids, expression of RPE thinning or atrophy.
The color fundus photographs taken before and immediately after laser treatment were digitally scanned and separately analyzed using the above-mentioned software. On separate electronic transparency layers, the vessels were outlined as a reference tool for a correct alignment. It was possible in this way to precisely locate and diagram the threshold lesions. With a similar technique, the laser-related lesions were outlined on the AF taken three months after laser treatment.
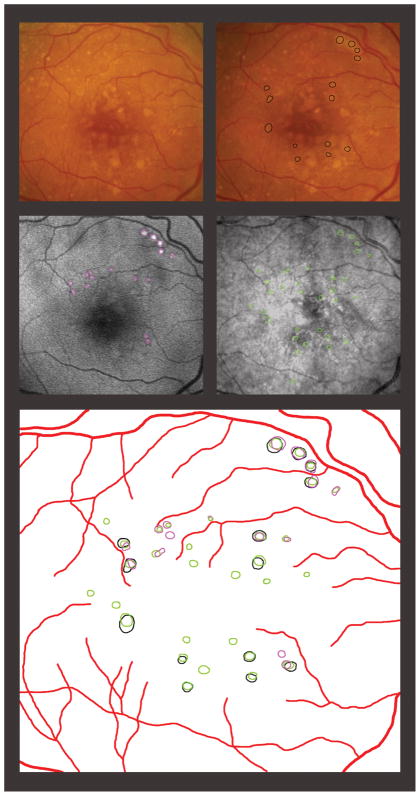
The final step of the analysis consisted in the overlapping of the SD-OCT results (mapped on the reference SLO image) to the lesions detected on the AF and at the color fundus photography (Figure 3). The diode laser-related lesions detected with the three techniques were compared afterward, and recorded. Only the true sub-threshold lesions, i.e. lesions not seen in the immediate post-treatment on color fundus photos, were considered for the statistical analysis.
Figure 3.
Detection of laser application. Top left: color fundus photo before the laser treatment. Top right: color fundus photo acquired immediately after the laser applications; black circles highlight the threshold lesions. Middle left: autofluorescence 3 months after laser treatment; purple circles correspond to visible laser effects. Middle right: Scanning Laser Ophthalmoscope image coupled with Spectral Optical Coherence Tomography (SD-OCT): the green circles correspond to the lesions detected by the SD-OCT. Bottom: Graphic reproduction of the lesions as detected by the three different imaging techniques.
Statistical methods
The detection rates of sub-threshold laser burns with AF and SD-OCT were compared using Generalized Estimating Equations (GEE). In addition, the photoreceptor disturbance grades and RPE disturbance on SD-OCT of all laser lesions were compared between visible laser burns and sub-threshold laser burns using GEE. All analysis were conducted using SAS software version 9.2 (SAS, Inc., Cary, NC).
RESULTS
Twenty-eight eyes of 16 patients affected by non-exudative AMD had subthreshold-laser treatment. Each eye had a single laser session, in which 48 spots were applied around the macula, for a total of 384 applications. At the follow-up, only 8 eyes of 4 patients could be included in the study. Four patients for a total of 6 eyes were not available for follow-up, 5 eyes had media opacities that did not allow a good interpretation of scans, 7 eyes developed geographic atrophy and 2 developed CNV.
The initial statistical analysis was determined for the presumed sub-threshold treatments. Among all the laser applications, those that were followed by immediate whitening of the retina were therefore excluded (N=51). Among the 333 sub-threshold lesions that were delivered, retinal changes were detected more often by SD-OCT than by AF (29% vs 20% burns), and this difference was statistically significant (p=0.01, Table 1, Figure 3).
Table 1.
Detection rate of real sub-threshold lesions by autofluorescence and SD-OCT/SLO.
| Autofluorescence detection | SD-OCT detection |
|---|---|
| 68/333 (20.42%) | 96/333 (28.83%) |
|
* p = 0.0143 † p = 0.0002 | |
Generalized Estimating Equations Analysis, (GEE).
McNemar’s Test.
In a further analysis, lesions imaged by SD-OCT were graded by changes in the reflectivity of the photoreceptor and detection of RPE disruption (Table 2). When we compared the threshold lesions versus the sub-threshold, the threshold had a higher grade of disruption of the photoreceptors (p<0.001). Seventy-one percent of the sub-threshold applications resulted in no visible OCT changes. The majority of the SD-OCT lesions seen after sub-threshold applications were grade 2 or 3 (10% and 15%, respectively). After threshold applications, the majority of the lesions graded 3 (82%). The incidence of focal RPE disruptions was minimal in sub-threshold lesions (4%) as compared to the threshold lesion (43%, p < 0.001).
Table 2.
SD-OCT/SLO findings of threshold and sub-threshold.
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | No RPE disruption | RPE disruption | |
|---|---|---|---|---|---|---|
| Threshold lesions | 1/51 (2%) | 3/51 (6%) | 5/51 (10%) | 42/51 (82%) | 29/51 (57%) | 22/51 (43%) |
| Subthreshold lesions | 236/333 (71%) | 15/333 (4%) | 33/333 (10%) | 49/333 (15%) | 321/333 (96%) | 12/333 (4%) |
| * p < 0.0001 | * p < 0.0001 | |||||
Grade 0: no changes. Grade 1: mild, discrete disruption along the junction of the inner segments/outer segments (IS/OS) of the photoreceptors. Grade 2: clear focal disruption of the IS/OS. Grade 3: clear focal disruption of the IS/OS and abrupt thinning and increased reflectivity of the corresponding outer nuclear layer. RPE: retinal pigment epithelium.
Generalized Estimating Equations Analysis, (GEE).
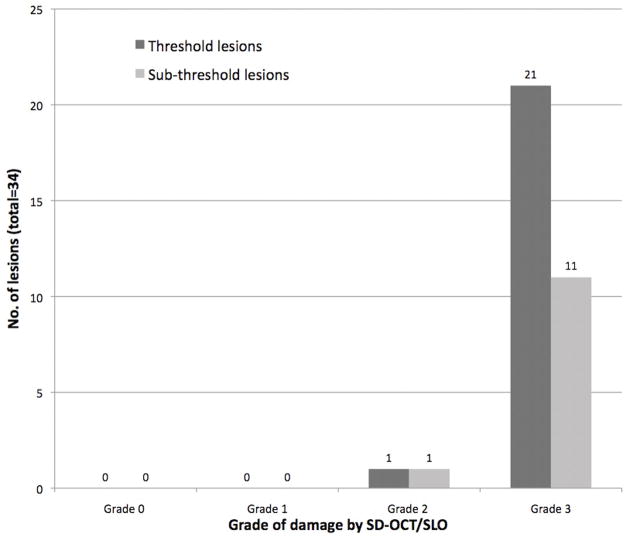
For the overall lesions, photoreceptor disruption grade on SD-OCT was significantly greater in the lesions that also manifested RPE changes on SD-OCT than in lesions that did not show RPE disruption (p<0.001, GEE). Similar result was shown also for the sub-analysis that considered only sub-threshold lesions: a higher grade of photoreceptor disruption was associated with RPE changes (p<0.0001). Figure 4 shows the distribution of photoreceptors disruption grade in all lesions with RPE changes on SD-OCT.
Figure 4.
Photoreceptors damage in lesions with RPE disruption.
DISCUSSION
In the current study we had the opportunity to evaluate the long-term effects of sub-threshold laser treatment to the RPE in AMD eyes with drusen. Our goal was to determine the long-term anatomic effect on the RPE and neurosensory retina using SD-OCT.
Sub-threshold laser is a treatment technique that has been of interest as a potential treatment of AMD, macular edema secondary to diabetes, branch retinal vein occlusion and possibly other retinal diseases.9,10,21,22,23–25 The theoretical advantage of this technique is the selective coagulation or stimulation of the RPE in the absence of photoreceptor damage and therefore the prevention of photoreceptor damage and vision loss. It has been hypothesized that new RPE growth stimulated by sub-threshold laser may be beneficial. In rabbits treated with sub-threshold micropulse laser, Roider and colleagues showed reversible photoreceptor damage with effects limited to the RPE; the outer segments of the photoreceptors recovered after 2 weeks in their rabbits exposed at light doses of laser.15,16 In another animal model, Pollack and co-workers, using both continuous-wave and micropulsed laser treatments produced variable effects on the RPE and damage also included permanent changes in the overlying neurosensory retina. They concluded that, near the minimum effective dose to give a visible lesion, individual RPE cell heterogeneity results in variable burn intensity and neuroretinal damage even when attempting sub-threshold therapy in a given eye. Their study suggested that uniform sub-threshold treatment may not be possible because of pigment variability and other factors.5
Because the outcome of sub-threshold laser application is not clinically visible, FA was initially used to detect in vivo RPE changes, and subsequently, we showed that AF was more sensitive than FA to identify and quantify the RPE changes.12 With these imaging techniques, however, it is not possible to resolve damage induced at the level of the photoreceptors, which are anatomically and functionally highly inter-connected to the RPE. OCT is the most sensitive in vivo tool to detect fine structural characteristics of all retinal layers, and is widely used to analyze retinal abnormalities at all depths in a wide range of pathologies. In a prior study, Lanzetta and colleagues demonstrated OCT changes 10 minutes after sub-threshold laser treatment at the lever of nerve fiber layers and outer retinal layers, but did not report follow-up on these anatomic changes.26 In the current study we showed the long term effect of sub-threshold infrared diode laser causes more prominent SD-OCT changes in the photoreceptor integrity than the RPE. The reason for this is not completely understood, however RPE may be more resistant to damage or the RPE may regenerate and or migrate to restore the OCT appearance of continuity of its monolayer.5,27 While sub-threshold laser is by definition not visible ophthalmoscopically immediately after treatment, it is possible that after several hours there could be visible damage or that damage is never visible by ophthalmoscopy because it is confined to the outer retina. Our study does not permit differentiation of these two possibilities. In our study, we showed that sub-threshold applications resulted in what appears to be permanent loss of the photoreceptors in 29% of the lesions, while only 4% of the applications resulted in abnormal RPE reflectivity. These percentages were calculated based on the closely spaced SD-OCT scans. The disruption of the photoreceptor as detected by SD-OCT has to be considered a permanent change. In prior studies we found that AF changes 3 months after sub-threshold treatment, but SD-OCT technology was unavailable to assess anatomic changes at that time.12 We note that our study treatment was performed using pulsed but not micropulsed 810 nm diode laser. Other studies have suggested that, at least histologically, similar effects may be seeing using micropulsing and it would be interesting to do such a long term study on eyes that had undergone micropulse diode laser treatment.
We also note that it was not possible to perform this study on all of our patient who had diode laser treatment with the PTAMD protocol because in the 6 years of follow-up many were unavailable for re-examination or had developed geographic atrophy or CNV, or media opacities, which would not allow appropriate imaging studies to be performed. A limit of our study is therefore the very small group of patients included in the study, who had a relatively favorable outcome from the treatment. Yet, our findings are important when we approach any kind of retinal disease with sub-threshold laser, because of the large number of lesions we analyzed and the long follow up. Since we studied AMD, which primarily affects the outer retinal layers, we excluded all disruptions clearly associated with drusen. We might therefore have underestimated the total amount of damage induced by sub-threshold laser.
Our data allows us to conclude that consistent sub-threshold diode laser treatment of the RPE without photoreceptor damage is not possible, at least with the instrument that we used. Ours is the first study to evaluate long-term structural changes secondary to this treatment. It might be possible to apply laser energy consistently to RPE without photoreceptor damage, but this would require titration of laser power-based on reflectometry performed at each location on the retina to be treated. The fact that the effect of sub-threshold laser on photoreceptors and on the RPE is variable may explain why also the therapeutic effect of sub-threshold treatment is variable. In order to scientifically study the effect of the sub-threshold treatment the therapy should be delivered most consistently. Evaluation of focal absorption could be performed with reflectometry or densitometry. 28,29 We advocate the use of SD-OCT technology to asses the structural damage in any future study of sub-threshold laser treatment.
SUMMARY STATEMENT.
Spectral OCT of patients treated with sub-threshold laser demonstrates disruption of the photoreceptors visible for years after treatment. The concept that sub-threshold laser treatment can achieve a selected RPE effect may be flawed.
Acknowledgments
FUNDING SUPPORT:
Research to Prevent Blindness (RPB inc), New York; Dr. Freeman is the recipient of an RPB Physician Scientist award, and support to Department National Eye Institute NIH-NEI grant #EY16323 (Bartsch D-U.G.) NIH grant #EY07366 (Freeman W.R.),
Footnotes
FINANCIAL DISCLOSURE:
Dr. Bartsch has received discounted products and specialized software from OPKO Instrumentation/OTI, Inc. Dr. Freeman has been consultant for OPKO. The other authors declare that they have no financial interest.
References
- 1.Mainster MA, Reichel E. Transpupillary thermotherapy for age-related macular degeneration: long-pulse photocoagulation, apoptosis, and heat shock proteins. Ophthalmic Surg Lasers. 2000;31(5):359–73. [PubMed] [Google Scholar]
- 2.Bresnick GH. Diabetic maculopathy. A critical review highlighting diffuse macular edema. Ophthalmology. 1983;90(11):1301–17. doi: 10.1016/s0161-6420(83)34388-8. [DOI] [PubMed] [Google Scholar]
- 3.Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331(22):1480–7. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 4.Stefansson E. The therapeutic effects of retinal laser treatment and vitrectomy. A theory based on oxygen and vascular physiology. Acta Ophthalmol Scand. 2001;79(5):435–40. doi: 10.1034/j.1600-0420.2001.790502.x. [DOI] [PubMed] [Google Scholar]
- 5.Pollack JS, Kim JE, Pulido JS, Burke JM. Tissue effects of subclinical diode laser treatment of the retina. Arch Ophthalmol. 1998;116(12):1633–9. doi: 10.1001/archopht.116.12.1633. [DOI] [PubMed] [Google Scholar]
- 6.Mainster MA. Decreasing retinal photocoagulation damage: principles and techniques. Semin Ophthalmol. 1999;14(4):200–9. doi: 10.3109/08820539909069538. [DOI] [PubMed] [Google Scholar]
- 7.Luttrull JK, Musch DC, Spink CA. Subthreshold diode micropulse panretinal photocoagulation for proliferative diabetic retinopathy. Eye. 2008;22(5):607–12. doi: 10.1038/sj.eye.6702725. [DOI] [PubMed] [Google Scholar]
- 8.Lanzetta P, Dorin G, Pirracchio A, Bandello F. Theoretical bases of non-ophthalmoscopically visible endpoint photocoagulation. Semin Ophthalmol. 2001;16(1):8–11. doi: 10.1076/soph.16.1.8.4216. [DOI] [PubMed] [Google Scholar]
- 9.Parodi MB, Spasse S, Iacono P, et al. Subthreshold grid laser treatment of macular edema secondary to branch retinal vein occlusion with micropulse infrared (810 nanometer) diode laser. Ophthalmology. 2006;113(12):2237–42. doi: 10.1016/j.ophtha.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 10.Sivaprasad S, Sandhu R, Tandon A, et al. Subthreshold micropulse diode laser photocoagulation for clinically significant diabetic macular oedema: a three-year follow up. Clin Experiment Ophthalmol. 2007;35(7):640–4. doi: 10.1111/j.1442-9071.2007.01566.x. [DOI] [PubMed] [Google Scholar]
- 11.Olk RJ, Friberg TR, Stickney KL, et al. Therapeutic benefits of infrared (810-nm) diode laser macular grid photocoagulation in prophylactic treatment of nonexudative age-related macular degeneration: two-year results of a randomized pilot study. Ophthalmology. 1999;106(11):2082–90. doi: 10.1016/S0161-6420(99)90487-6. [DOI] [PubMed] [Google Scholar]
- 12.Bessho K, Rodanant N, Bartsch DU, et al. Effect of subthreshold infrared laser treatment for drusen regression on macular autofluorescence in patients with age-related macular degeneration. Retina. 2005;25(8):981–8. doi: 10.1097/00006982-200512000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Laser treatment in patients with bilateral large drusen: the complications of age-related macular degeneration prevention trial. Ophthalmology. 2006;113(11):1974–86. doi: 10.1016/j.ophtha.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Friberg TR, Musch DC, Lim JI, et al. Prophylactic treatment of age-related macular degeneration report number 1: 810-nanometer laser to eyes with drusen. Unilaterally eligible patients. Ophthalmology. 2006;113(4):622, e1. doi: 10.1016/j.ophtha.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 15.Roider J, Michaud NA, Flotte TJ, Birngruber R. Response of the retinal pigment epithelium to selective photocoagulation. Arch Ophthalmol. 1992;110(12):1786–92. doi: 10.1001/archopht.1992.01080240126045. [DOI] [PubMed] [Google Scholar]
- 16.Roider J, Brinkmann R, Wirbelauer C, et al. Subthreshold (retinal pigment epithelium) photocoagulation in macular diseases: a pilot study. Br J Ophthalmol. 2000;84(1):40–7. doi: 10.1136/bjo.84.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodanant N, Friberg TR, Cheng L, et al. Predictors of drusen reduction after subthreshold infrared (810 nm) diode laser macular grid photocoagulation for nonexudative age-related macular degeneration. Am J Ophthalmol. 2002;134(4):577–85. doi: 10.1016/s0002-9394(02)01691-4. [DOI] [PubMed] [Google Scholar]
- 18.Drexler W, Sattmann H, Hermann B, et al. Enhanced visualization of macular pathology with the use of ultrahigh-resolution optical coherence tomography. Arch Ophthalmol. 2003;121(5):695–706. doi: 10.1001/archopht.121.5.695. [DOI] [PubMed] [Google Scholar]
- 19.Ko TH, Fujimoto JG, Schuman JS, et al. Comparison of ultrahigh- and standard-resolution optical coherence tomography for imaging macular pathology. Ophthalmology. 2005;112(11):1922, e1–15. doi: 10.1016/j.ophtha.2005.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mojana F, Cheng L, Bartsch DU, et al. The role of abnormal vitreomacular adhesion in age-related macular degeneration: spectral optical coherence tomography and surgical results. Am J Ophthalmol. 2008;146(2):218–27. doi: 10.1016/j.ajo.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friberg TR. Infrared micropulsed laser treatment for diabetic macular edema--subthreshold versus threshold lesions. Semin Ophthalmol. 2001;16(1):19–24. doi: 10.1076/soph.16.1.19.4217. [DOI] [PubMed] [Google Scholar]
- 22.Squirrell DM, Stewart AW, Joondeph BC, et al. Large-spot subthreshold infrared laser to treat diabetic macular edema. Retina. 2008;28(4):615–21. doi: 10.1097/IAE.0b013e31815ee567. [DOI] [PubMed] [Google Scholar]
- 23.Lanzetta P, Furlan F, Morgante L, et al. Nonvisible subthreshold micropulse diode laser (810 nm) treatment of central serous chorioretinopathy. A pilot study. Eur J Ophthalmol. 2008;18(6):934–40. doi: 10.1177/112067210801800613. [DOI] [PubMed] [Google Scholar]
- 24.Luttrull JK, Spink CJ. Serial optical coherence tomography of subthreshold diode laser micropulse photocoagulation for diabetic macular edema. Ophthalmic Surg Lasers Imaging. 2006;37(5):370–7. doi: 10.3928/15428877-20060901-03. [DOI] [PubMed] [Google Scholar]
- 25.Chen SN, Hwang JF, Tseng LF, Lin CJ. Subthreshold diode micropulse photocoagulation for the treatment of chronic central serous chorioretinopathy with juxtafoveal leakage. Ophthalmology. 2008;115(12):2229–34. doi: 10.1016/j.ophtha.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 26.Lanzetta P, Polito A, Veritti D. Subthreshold laser. Ophthalmology. 2008;115(1):216– e1. doi: 10.1016/j.ophtha.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Colome J, Ruiz-Moreno JM, Montero JA, Fernandez E. Diode laser-induced mitosis in the rabbit retinal pigment epithelium. Ophthalmic Surg Lasers Imaging. 2007;38(6):484–90. doi: 10.3928/15428877-20071101-07. [DOI] [PubMed] [Google Scholar]
- 28.Liem AT, Keunen JE, Van Norren D. Clinical applications of fundus reflection densitometry. Surv Ophthalmol. 1996;41(1):37–50. doi: 10.1016/s0039-6257(97)81994-7. [DOI] [PubMed] [Google Scholar]
- 29.Gellermann W, Bernstein PS. Noninvasive detection of macular pigments in the human eye. J Biomed Opt. 2004;9(1):75–85. doi: 10.1117/1.1628240. [DOI] [PubMed] [Google Scholar]