Abstract
Introduction
Local application of bone morphogenetic proteins (BMPs) at the fracture site is known to stimulate bone regeneration. However, recent studies illustrate that the BMP-initiated mineralization may be enhanced by additional mechanical stimulation. Therefore, bone healing was monitored in vivo in order to investigate the effect of mechanical loading on the initiation and maturation of mineralization after cytokine treatment. We hypothesized that the mechanical stimulation would further enhance the efficacy of BMP2 treatment.
Method
: Female Sprague-Dawley rats underwent a 5-mm defect, stabilized with an external fixator. Type I collagen scaffolds containing 50 μg of BMP2 diluted in a solvent or solvent only were placed into the defects. The BMP2-treated specimens and control specimens were then each divided into two groups: one that underwent mechanical loading and a nonloaded group. In vivo loading began immediately after surgery and continued once per week for the entire 6-week experimental period. For all groups, the newly formed callus tissue was quantitatively evaluated first by in vivo microcomputed tomography at 2, 4, and 6 weeks and further by histologic or histomorphometric analysis at 6 weeks postoperation.
Results
Mechanical stimulation with BMP2 treatment significantly enhanced mineralized tissue volume and mineral content at 2 weeks. Histological analysis demonstrated a significantly greater area of fibrous connective tissue including bone marrow in the stimulated group, suggesting reconstitution of the endosteal canal and more advanced bone remodeling present in the mechanical loaded group. Both groups receiving BMP2 underwent massive bone formation, achieving bony bridging after only 2 weeks, while both control groups, receiving solvent only, revealed a persisting nonunion, filled with fibrous connective tissue, prolapsed muscle tissue, and a sealed medullary canal at week 6.
Conclusion
Mechanical loading further enhanced the efficacy of BMP2 application evidenced by increased mineralized tissue volume and mineralization at the stage of bony callus bridging. These data suggest that already a minimal amount of mechanical stimulation through load bearing or exercise may be a promising adjunct stimulus to enhance the efficacy of cytokine treatment in segmental defects. Further studies are required to elucidate the mechanistic interplay between mechanical and biological stimuli.
Introduction
Segmental bone defects resulting from high-energy trauma, bone tumors, and revision surgery1,2 represent a challenge for regeneration and current surgical and grafting techniques. Complications such as delayed healing, nonunions, or resulting limb length differences can lead to a significant reduction in the patient's quality of life.3,4 Due to limitations associated with current treatment strategies, alternative approaches have been investigated, including the development of bone graft substitutes or osteobiologics,5 which incorporate osteoconductive matrices and osteoinductive proteins. Bone morphogenetic proteins (BMPs), members of the transforming growth factor-beta superfamily that are well known to be osteo- and chondroinductive,6 are commercially available for clinical use, but their application is restricted to limited applications.7 Local application of BMPs has been extensively studied in animal models of bone healing to examine their regenerative capacity. However, promising experimental results have only been partly recapitulated in human patients.8 Furthermore, supraphysiological dosages9,10 of BMP2 and BMP7 are frequently required to reach effective bone formation and still with inconsistent results.11
It is clear that endogenous BMP2 plays a significant role during bone healing, as several studies have demonstrated the expression of BMPs and their inhibitors during normal and compromised fracture healing in animal models.12–16 As well, it is widely accepted that mechanical loading through controlled in vivo axial compressive external loading17–19 or dynamization through reduced fixation stiffness to allow more interfragmentary movement during locomotion20,21 can influence the healing process. Studies on small bone defects in rats that heal uneventfully have shown that early dynamization, reducing external fixation stiffness at 1 week postosteotomy, impairs healing, whereas late dynamization 3 or 4 weeks postosteotomy enhances healing.20,22 Additionally, in vitro23–25 and in vivo26–30 studies have shown that endogenous BMP2 expression is influenced by mechanical loading.
Only few studies have investigated the role of mechanical loading in combination with exogenous BMP2 application on critical-sized segmental defect healing,31–34 but with varying results. Boerckel et al.31 recently demonstrated that early dynamization through reduction in fixation plate stiffness significantly inhibited vascular invasion into the defect and reduced bone formation in comparison to a constant stiff fixation plate with a low BMP2 dose. In contrast, Glatt et al.34 suggested that early loading of the mechanical environment by varying external fixation stiffness after local application of BMP2 enhanced structural parameters of rat femoral critical-sized bone defects.
The use of mechanical loading in terms of weight bearing or exercise may be a promising therapy to augment BMP2 treatment of segmental bone defects. However, the combined effect of both mechanical and biological stimuli requires further study, and dosages of biologics such as BMP could eventually be minimized. Therefore, the aim of the present study was to investigate if mechanical loading would improve the efficacy of an osteoinductive growth factor locally applied in a rat femoral large segmental bone defect model over a 6-week time course. We hypothesized that weekly controlled in vivo axial compressive mechanical loading would further enhance the efficacy of BMP2 treatment.
Materials and Methods
Operative procedure and experimental design
Thirty-two female 12-week-old Sprague-Dawley rats (weight 250–300 g; Charles River) underwent diaphyseal femoral osteotomies of the left limb, resulting in a 5-mm critical defect. The operative procedure has been previously reported35; in brief, rats were administered ketamine hydrochloride (60 mg/kg, Ketamin 50 mg, Actavis®; München-Riem) and medetomidine (0.3 mg/kg; Domitor®; Pfizer), and also the antibiotic clindamycin-2-dihydrogenphosphate (45 mg/kg; Ratiopharm). An incision was made across the lateral aspect of the thigh, through the fascia, exposing the femur by separating the gluteus superficialis and biceps femoris muscles. The external fixator was attached, allowing a 7.5-mm offset. A 5-mm defect was created in the diaphysis of the femur using an oscillating saw by performing a double-transverse osteotomy. Rats were locally treated at the osteotomy site with either 50 μL of recombinant human BMP2 (rhBMP2) (1 mg/mL; Prof. Sebald) diluted in an aqueous low-concentrated hydrochloric acid (10 mM) or 50 μL aqueous low-concentrated hydrochloric acid (10 mM) only, with a collagen sponge (Lyostypt®; B. Braun) serving as a carrier. Animals tolerated the experimental procedure well; however, some animals developed pin infections and were excluded from the study, leaving the following groups: (1) collagen sponge with solvent (Control, n=4), (2) collagen sponge with solvent + mechanical loading (Control-load, n=4), (3) collagen sponge with rhBMP2 (BMP2, n=7), and (4) collagen sponge with rhBMP2 + mechanical loading (BMP2-load, n=8). Following surgery, the rats were housed two per cage and allowed to resume normal activity and given unrestricted access to food and water. All animals were sacrificed 6 weeks after surgery. All animal experiments were carried out according to the policies and procedures established by the NIH Guide for Care and Use of Laboratory Animals. The study was approved by the local legal representative (LAGeSo Berlin, G0071/07 and G0210/08).
Fixator design and characterization
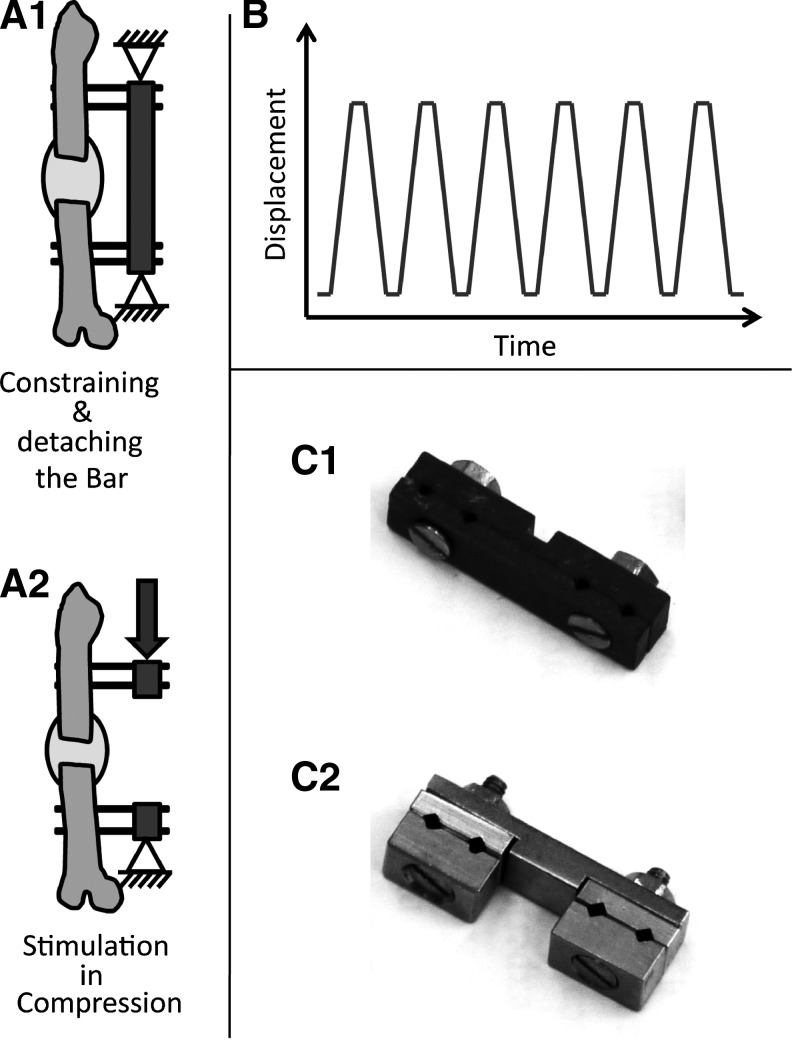
Two designs of external unilateral fixators were used to stabilize the defect, depending on whether the rats would undergo in vivo mechanical stimulation or not. In nonmechanically loaded groups, the defect fragments were stabilized with a fixator that contained two carbon fiber-reinforced polysulfon plates bound by two countersunk screws and additionally secured by two locking nuts (Fig. 1C1).35 A second fixator design allowed axial loading of the healing zone in groups intended for mechanical stimulation (Fig. 1C2).36 The fixator design allowed disassembly such that the fracture pins remained within the frame, but the defect bridging bar could be detached to allow mechanical loading. In all fixators four titanium threaded (0.65-mm core diameter/1.2-mm outer diameter) pinsfixed to the crossbar by thread tightening through compression of the fixator ensured fragment fixation. The offset distance, the free length of the pins between the rat's lateral femoral surface and the inner side of the fixator bar, was always 7.5 mm. To ensure comparability of fixator stiffness values for the two designs, the mechanical competence of all fixators was determined nondestructively in vitro. Both fixator designs were mounted to harvested cadaveric femurs of female 12-week-old Sprague Dawley rats (n=6/fixator design) to undergo axial compression and torsional testing using published protocols.36 The fixator design of the mechanically stimulated group resulted in an axial stiffness of 62.02±13.52 N/mm (mean±standard deviation) and a torsional stiffness of 15.35±2.69 N/mm, and the fixator design of the unstimulated group produced an axial stiffness of 59.05±14.45 N/mm and a torsional stiffness of 14.57±2.03 N/mm.
FIG. 1.
Schematic representation of (A1) constraining the external fixator within the custom-made setup and after the (A2) detachment of the fixator bar before the loading was started. (B) The trapezoidal waveform used for mechanical loading. (C1) Photo of the fixator used on the nonmechanically loaded groups and (C2) on the groups that underwent mechanical loading.
In vivo mechanical loading
After surgery and at weekly intervals thereafter, animals from the Control-load and BMP2-load groups were subjected to an in vivo biomechanical loading. All animals received intraperitoneal injection of anesthesia, regardless if they were subscribed to the loaded or nonloaded groups. For the mechanical loading, the external fixator was constrained to a custom-made setup, containing a precision linear actuator (M-230; Physik Instrumente) controlled by a Labview script (LabVIEW 8.5; National Instruments).36 While the proximal fixator site was clamped, the axial opposed fixator site was attached to the linear actuator. The load-bearing fixator bar was then detached, thereby permitting only an axial deformation. The loading protocol consisted of six compression cycles, where each compression included 500-μm displacement at a constant rate of 10 μm/s, followed by a dwell (resting phase) of 40 s, where the compression was kept constant. Then the actuator was removed back to the initial position, where it rested again for another 40 s.
Micro-computed tomography
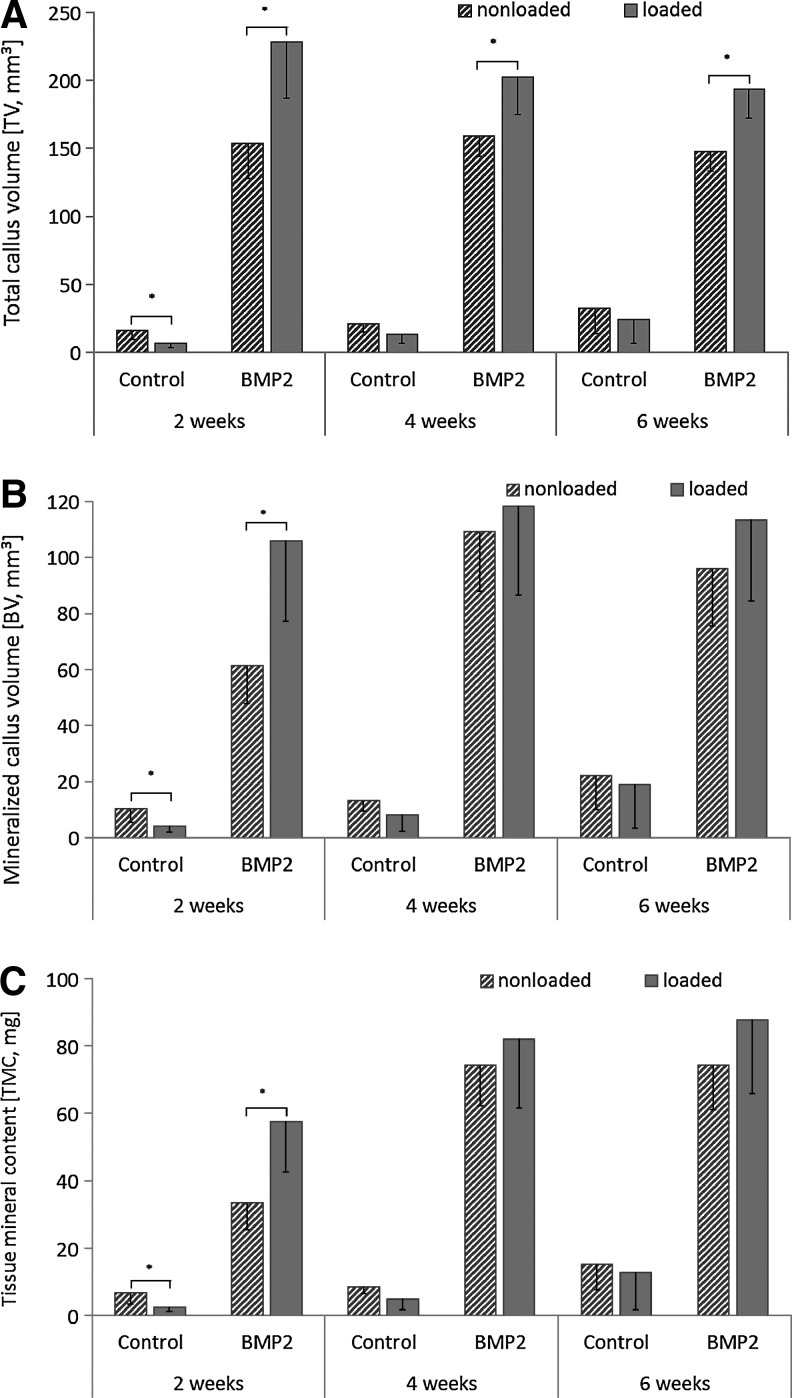
At 2, 4, and 6 weeks postoperation, bone healing was assessed by in vivo microcomputed tomography (microCT) (vivaCT 40; Scanco Medical; 55 kVp, 145 μA, 150 ms integration time) at an isotropic resolution of 35 μm. Analysis was performed using a semi-automated segmentation of cross-sectional tomograms to derive the volume of interest (VOI), defined by the periosteal callus as the outer boundary and the endosteal callus as the inner boundary, excluding the cortical bone. The total callus VOI included the 5-mm defect region and 0.5 mm in the proximal and distal directions from the borders of the original osteotomy. A global threshold of 50% of the mineral density of the intact limb, equivalent to 351 mg HA/ccm, was used to distinguish mineralized tissue (bone and calcified cartilage [BV]) from poorly mineralized and unmineralized tissue. Outcome measures included mineralized callus volume, which includes BV (mm3), total callus volume (TV, mm3), tissue mineral density (TMD, mg HA/ccm), and tissue mineral content (TMC, mg HA), defined as BV multiplied by TMD, with TMD measured using only voxels whose intensity exceeded the threshold. In vivo microCT-derived results for the BMP2-load and Control-load groups were previously published to show establishment of the in vivo mechanical loading system,36 whereas in the current study the data are used to demonstrate the influence of loading on tissue formation compared to novel nonloaded BMP2-treated and nontreated groups after a healing time course of 6 weeks.
Histology and histomorphometry
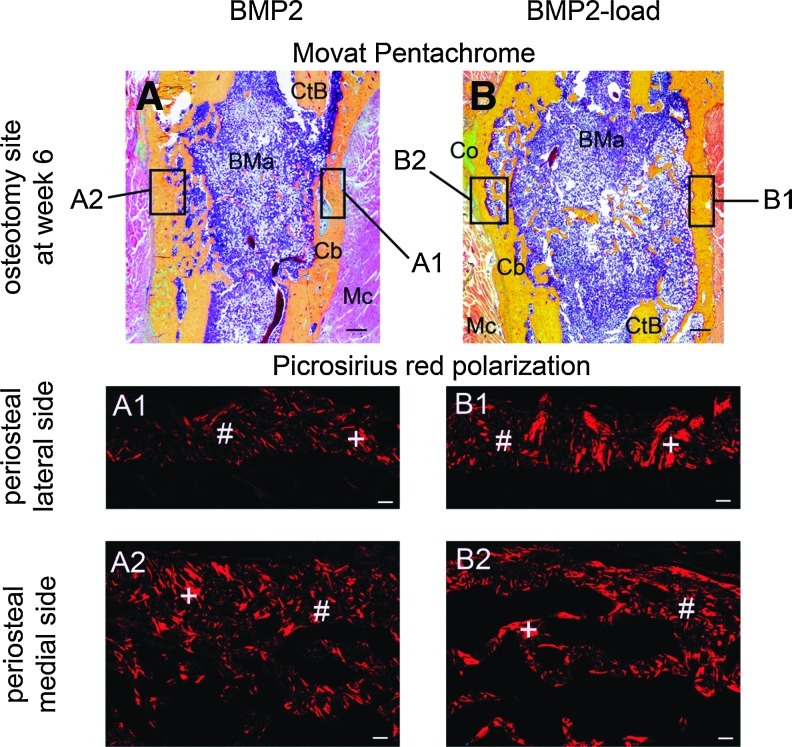
After 6 weeks postsurgery, rats were anaesthetized and sacrificed by an intracardial potassium-chloride injection. Femora were harvested and fixed directly in formaldehyde for 48 h and subsequently decalcified in ethylenediaminetetraacetic acid for approximately 4 weeks at 37°C. Fixed and decalcified tissues were dehydrated in graded ethanol up to 100%, transferred to xylene, and embedded in paraffin. Four-micrometer-thick longitudinal sections were prepared on a customary microtome (Leica RM 2125) and placed on glass slides. Quantitative histomorphometry was performed to analyze tissue differentiation for a single fixed region of interest (ROI), using semiautomated software (KS400 3.0 software; Carl Zeiss MicroImaging GmbH). The composition of the callus tissue was quantified after staining with Movat Pentachrome by measuring the area occupied within the osteotomy gap by bone (yellow), cartilage (blue to green), and fibrous connective tissue formation (pink to purple), with the fibrous connective tissue also including bone marrow elements. Tissue areas (mm2) were measured and tissue fractions (%) calculated based on the ROI. Qualitative analysis of remodeling of the newly formed bone was also performed by staining sections with Picrosirius red and analyzed with polarized light microscopy. Additionally, the callus width was calculated by ImageJ (Rasband, W.S., ImageJ, U. S. National Institutes of Health; http://imagej.nih.gov/ij/, 1997–2011).
Statistical analysis
Differences in the amount of bone formation and mineralization, cartilage formation, and fibrous tissue formation between the mechanically loaded and nonloaded groups were determined using either an independent-group t-test or a Mann–Whitney U-test, depending on normality, determined by a Shapiro–Wilk test. Analyses were performed using standard statistical software (SAS® 9.1; SAS Institute, Inc.). A p-value of<0.05 was considered as significant.
Results
Mechanical loading modulated the efficacy of BMP2 treatment
Bony bridging was achieved by 2 weeks in calluses of all animals treated with BMP2 (Fig. 2). In contrast, no animals from the solvent-only-treated Control group achieved bony bridging over the 6 weeks experimental time period. At 2 weeks, the BV (p=0.003), TMC (p=0.002), and TV (p=0.002) of the BMP2-load group was significantly greater compared to the BMP2 group (Fig. 3). The TV was also significantly greater in the BMP2-load than in the BMP2 group at 4 (p=0.003), and 6 (p<0.001) weeks postosteotomy. Similar to the microCT data, histomorphometric analysis demonstrated that the BMP2-load group had a significantly greater total callus area at 6 weeks postoperation than the BMP2 group (p=0.013) (Table 1). An increase in callus width also occurred in the mechanically loaded BMP2 group (BMP2 6±0.9 mm; BMP2-load 6.9±0.9 mm). Both the bone and fibrous connective tissue in the callus contributed to the increased total callus area and width, although only the fibrous connective tissue (mainly bone marrow) was significantly greater in the BMP2-load group compared to the BMP2 group (p=0.002) (Fig. 4A, B). At 6 weeks postosteotomy, both the BMP2-load and BMP2 groups had a similar limited amount of cartilage, primarily present in the periosteal callus bone tissue. The osteotomized ends of the cortical bone were mainly surrounded by newly formed woven bone. Picrosirius red staining under polarized light microscopy demonstrated that periosteal calluses from the BMP2 and the BMP2-load groups consisted of a mixture of woven and lamellar bone (Fig. 4A1–B2). However, qualitative assessment suggested that the BMP2-load group appeared to have more advanced remodeling with greater amounts of lamellar bone compared to woven bone present in the periosteal bridged callus tissue.
FIG. 2.
MicroCT images with (A) 3D renderings of calluses from the Control and Control-load groups at weeks 2, 4, and 6 postoperation; (B) 3D renderings and transversal cut images of calluses from the BMP2 and BMP2-load groups at weeks 2, 4, and 6 postoperation. Scale bars=1 mm. MicroCT, micro computed tomography; 3D, three dimensional; BMP, bone morphogenetic protein.
FIG. 3.
In vivo microCT data (A) TV, (B) BV, and (C) TMC for each nonloaded and mechanically loaded Control and BMP2 group at 2, 4, and 6 weeks postoperation (mean±standard deviation, *p<0.05). TV, total callus volume; BV, bone and calcified cartilage; TMC, tissue mineral content.
Table 1.
Absolute Data of the Histomorphometric Analysis and Callus Width Measurements for All Four Groups (Mean±Standard Deviation)
| Parameter/group | Control n=4 | Control-load n=4 | BMP2 n=7 | BMP2-load n=8 |
|---|---|---|---|---|
| Total area (mm2) | 18.9±9.3 | 17.6±1.0 | 24.3±4.5a | 32.1±5.7a |
| Bone area (mm2) | 3.2±2.3 | 3.1±1.4 | 8.6±2.2 | 9.2±4.1 |
| Cartilage area (mm2) | 0.1±0.1 | 0.0±0.0 | 0.1±0.1 | 0.1±0.1 |
| Fibrous connetive tissue area (mm2) | 13.2±8.3 | 12.8±1.6 | 15.7±3.8a | 22.8±3.1a |
| Muscle area (mm2) | 2.4±2.3 | 1.7±1.3 | 0.0±0.0 | 0.0±0.0 |
| Callus width (mm) | 5.0±0.8 | 4.8±0.7 | 6.0±0.9 | 6.9±0.9 |
BMP2 different from BMP2-load.
p<0.05; t test.
BMP, bone morphogenetic protein.
FIG. 4.
Periosteal bony bridging of the osteotomy gap 6 weeks after surgery (A, B). Note the bony bridging independent from the cortical bone, and the remodeling of the periosteal bony callus 6 weeks after surgery (A1–B2) on the lateral side (A1, B1) and the medial side (A2, B2) with predominant lamellar bone in the BMP2-load group (B1). Woven bone (#) is recognizable on the unorganized mesh pattern, whereas lamellar bone (+) shows organized thick bands. Cortical bone (CtB), callus bone (Cb), bone marrow (BMa), fibrous connective tissue (Co), and muscle tissue (Mc). Movat Pentachrome staining, scale bars=500 μm. Picrosirius red polarization method, scale bars=100 μm. Color images available online at www.liebertpub.com/tea
Mechanical loading alone did not enhance bone defect healing
At 2 weeks postoperation, the mechanically loaded control group (Control-load) had significantly less BV (p=0.032), TMC (p=0.029), and TV (p=0.019) than did the nonloaded Control group (Fig. 3). After 4 and 6 weeks of healing, mechanical loading in the control animals had no influence on bone healing parameters. Histomorphometric analysis showed similar total callus area as well as similar amounts of bone, cartilage, fibrous connective tissue, and muscle tissue within the callus of both mechanically loaded and nonloaded control specimens (Table 1). Only moderate bone formation (Control 3.2±2.3 mm2, Control-load 3.1±1.4 mm2) was measured over the 6-week period, and no bony bridging was achieved in both control groups. Furthermore, the control groups showed a similar defect repair result with a fibrous connective tissue and prolapsed muscle tissue filled gap and rounded cortical bone ends, without newly formed bone tissue. Using the Picrosirius red polarization method, a permanent bony sealing of the medullary canal by lamellar bone was obvious, covered by a layer of collagen fibers.
Discussion
To understand the combined effect of mechanical loading and exogenous BMP2 stimulation, we treated nonhealing critical-sized defects in rats with BMP2 or only a solvent in collagen scaffolds and compared healing outcomes at three different time points with and without weekly controlled in vivo axial compressive mechanical loading.
Local treatment of 50 μg BMP2 enhanced bone formation at 2 weeks postosteotomy with bony bridging achived at this time point in all specimens. In contrast, no animals from the solvent-only-treated control group achieved bony bridging after 6 weeks postosteotomy. We assume that there was a homogenous distribution of the BMP2 within the collagen sponge and thus throughout the osteotomy site based on our histological analysis that showed bony bridging of the BMP2-treated defects. Local application of BMP2 at the fracture site has been demonstrated in a number of preclinical37–39 and clinical40 studies to stimulate bone healing. Similar to mechanical loading, the timing of BMP2 treatment seems to have an effect on bone healing. Murnaghan et al.41 reported that locally applied BMP2 at days 0 and 4 has an enhanced effect compared to application at day 8 in mice. In our study, the application of exogenous BMP2 at day 0 revealed bony bridging of a critical-sized defect in the BMP2-treated specimens and thus an effective time point for administration of this growth factor.
Without BMP2, mechanical loading did not enhance bone defect healing, and led to lower callus volume, mineralized callus volume, and callus mineralization after 2 weeks, although there was no influence of loading after 4 and 6 weeks. Although many studies have examined the influence of mechanical loading during normal healing in rats,17–22 no studies have thus far examined the influence of mechanical loading alone on critical-sized defect healing. Claes et al.20 demonstrated that early loading through dynamization (reduced fixation stiffness) after osteotomy surgery (1 mm defect in rat) delays uneventful bone healing. They suggested that any improved bone healing found after early dynamization in previous studies42,43 was due to closure of the osteotomy gap rather than increasing the interfragmentary movement, which is known to influence healing.44 Our results support these findings, in that early loading decreased bone formation in our nonunion model when BMP2 treatment was absent.
In the present study, once per week mechanical loading enhanced healing in bone defects treated locally with 50 μg rhBMP2 at 2 weeks postosteotomy. The total callus formation, bone formation and bone mineralization was greater after 2 weeks of healing, at which time bony bridging had occurred in all loaded and nonloaded group specimens. As bony bridging was already achieved by 2 weeks after local application of BMP2, the loading may have enhanced the bone formation by stimulating vascular remodeling, as seen in uneventful osteotomy healing.45 Mechanical loading is known to enhance migration and proliferation of mesenchymal stem cells (MSCs)46 and their regulation of angiogenesis,47 and BMP2 stimulates osteogenic differentiation of MSCs.48–50 Thus, the combination may have led to mechanical loading-induced MSC migration and proliferation and exogenous BMP2-induced MSC differentiation. Additionally, a stimulatory effect of mechanical loading on osteoblast-like cells51 and an osteoblastic differentiation induced by BMP2 under loading in a three-dimensional bioreactor system has been documented52 in vitro. Kopf et al.52 have shown that BMP2 and mechanical loading cooperatively regulate the early signaling in the BMP pathway, indicating the mechanical environment as a trigger for bone metabolism.53 Wang et al.54 could show that after axial strain BMP2 was increased in osteoblasts and culture medium 4–12 h after mechanical stimulation and decreased at 24 h. Currently, there remains a paucity of in vivo data examining the molecular mechanism between BMP2 stimulation and mechanical load, which needs to be addressed in the future, but the findings could explain our enhanced bone formation by combined treatment of both mechanical loading and exogenous BMP2. Our data are consistent with a previous study in a rat critical-sized defect model that showed BMP2 in combination with early (day 0) dynamization, performed through reduction in fixation plate stiffness31 led to enhanced defect healing.
In our study at later time points, 4 and 6 weeks measured by microCT and at 6 weeks measured by histomorphometry, BV in the loaded BMP2 group was maintained at levels similar to those observed at 2 weeks. At 4 and 6 weeks, BV in the BMP2 group increased to similar BV levels measured in the BMP2-load group. However, TV slightly declined over time but continued to be significantly greater in the BMP2-load compared to the BMP2 group. Histomorphometric analysis at 6 weeks also demonstrated that although there was a nonsignificant increase in bone tissue with loading the major contributor to the increased total callus area was by fibrous connective tissue. These data suggest that once bony bridging was achieved the loading regime no longer enhanced bone formation and may have actually slowed or hindered the reduction in TV callus size required to regain the architecture of the original bone.
In contrast to our study, Boerckel et al.31 showed that late (week 4) compared to early (day 0) loading in combination with exogenous BMP2, led to an increased bone formation. However, a higher BMP2 dosage was applied in the late dynamization compared to the early dynamization groups in their study, which could explain the enhanced defect repair they observed independent of loading. Additonally, it is difficult to compare the influence of loading between these studies because we used once a week controlled loading while their loading was by dynamization through reduction in fixation plate stiffness. Although not directly transferable, our treatment strategy of BMP2 in combination with mechanical stimulation could be in principle be adapted in humans during the healing process as the inter-fragmentary strain allowed in our loading model is slightly lower than that allowed by an external fixator that has already been used clinically.55,56 Our data show that a very small amount of interfragmentary movement sufficiently stimulated bone formation in combination with BMP2 in a rat segmental bone defect model. However, the optimal timing and magnitude of loading in combination with BMP2 application requires further investigation.
In conclusion, we demonstrated that the combined effect of early controlled in vivo axial compressive mechanical loading administered once weekly combined with locally applied exogenous 50 μg BMP2 significantly enhanced bone defect healing in rats at 2 weeks. However, continued loading after bony bridging was achieved, which led to an increased amount of fibrous connective tissue, mainly bone marrow, resulting in a larger total callus at week 6. Exogenous BMP2 application alone led to bony bridging of all calluses after 2 weeks of healing and a smaller callus width. Mechanical loading alone without BMP2 application did not enhance the formation of bone; in fact, mechanical loading alone led to lower callus volume, mineralized callus volume, and callus mineralization after 2 weeks, although there was no influence of loading after 4 and 6 weeks. Biophysical therapies, whereby growth factor treatment is augmented with load bearing or exercise, could be an effective strategy to achieve bony bridging and successful clinical outcomes. Lower doses of growth factors could potentially be used in combination with mechanical loading administered before bony bridging and should be investigated in the future, as this strategy may attenuate complications associated with ectopic bone formation as well as reduce costs.
Acknowledgments
The authors thank Camilla Bergmann for her help with histological preparation, and Mario Thiele for technical assistance regarding both the histological and microCT measurements, and Prof. Dr. Petra Seemann for fruitful discussions and critical reading of the manuscript. We are grateful to Prof. Dr. Walter Sebald for kindly providing BMP2. This study was supported by a grant from the German Research Foundation DFG (partially by SFB 760 and Du 298/15-1). Carolin Schwarz is member of the DFG funded Berlin-Brandenburg School for Regenerative Therapies GSC 203.
Disclosure Statement
The authors state that no competing financial interests exist.
References
- 1.Cancedda R. Giannoni P. Mastrogiacomo M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials. 2007;28:4240. doi: 10.1016/j.biomaterials.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs B. Ossendorf C. Leerapun T. Sim F.H. Intercalary segmental reconstruction after bone tumor resection. Eur J Surg Oncol. 2008;34:1271. doi: 10.1016/j.ejso.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 3.DeCoster T.A. Gehlert R.J. Mikola E.A. Pirela-Cruz M.A. Management of posttraumatic segmental bone defects. J Am Acad Orthop Surg. 2004;12:28. doi: 10.5435/00124635-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Clements J.R. Carpenter B.B. Pourciau J.K. Treating segmental bone defects: a new technique. J Foot Ankle Surg. 2008;47:350. doi: 10.1053/j.jfas.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Cornell C.N. Osteobiologics. Bull Hosp Jt Dis. 2004;62:13. [PubMed] [Google Scholar]
- 6.Wozney J.M. Rosen V. Celeste A.J. Mitsock L.M. Whitters M.J. Kriz R.W., et al. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 7.Schmidmaier G. Schwabe P. Wildemann B. Haas N.P. Use of bone morphogenetic proteins for treatment of non-unions and future perspectives. Injury. 2007;38(Suppl 4):S35. doi: 10.1016/s0020-1383(08)70007-x. [DOI] [PubMed] [Google Scholar]
- 8.Kwong F.N. Harris M.B. Recent developments in the biology of fracture repair. J Am Acad Orthop Surg. 2008;16:619. doi: 10.5435/00124635-200811000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Han D. Liu W. Ao Q. Wang G. Optimal delivery systems for bone morphogenetic proteins in orthopedic applications should model initial tissue repair structures by using a heparin-incorporated fibrin-fibronectin matrix. Med Hypotheses. 2008;71:374. doi: 10.1016/j.mehy.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 10.Haidar Z.S. Hamdy R.C. Tabrizian M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part A: current challenges in BMP delivery. Biotechnol Lett. 2009;31:1817. doi: 10.1007/s10529-009-0099-x. [DOI] [PubMed] [Google Scholar]
- 11.Garrison K.R. Shemilt I. Donell S. Ryder J.J. Mugford M. Harvey I., et al. Bone morphogenetic protein (BMP) for fracture healing in adults. Cochrane Database Syst Rev. 2010;6:CD006950. doi: 10.1002/14651858.CD006950.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niikura T. Hak D.J. Reddi A.H. Global gene profiling reveals a downregulation of BMP gene expression in experimental atrophic nonunions compared to standard healing fractures. J Orthop Res. 2006;24:1463. doi: 10.1002/jor.20182. [DOI] [PubMed] [Google Scholar]
- 13.Lienau J. Schmidt-Bleek K. Peters A. Weber H. Bail H.J. Duda G., et al. Insight into the molecular patho-physiology of delayed bone healing in a sheep model. Tissue Eng Part A. 2010;16:191. doi: 10.1089/ten.TEA.2009.0187. [DOI] [PubMed] [Google Scholar]
- 14.Cho T.J. Gerstenfeld L.C. Einhorn T.A. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res. 2002;17:513. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- 15.Bostrom M.P. Expression of bone morphogenetic proteins in fracture healing. Clin Orthop Relat Res. 1998;355:S116. doi: 10.1097/00003086-199810001-00013. [DOI] [PubMed] [Google Scholar]
- 16.Bostrom M.P. Lane J.M. Berberian W.S. Missri A.A. Tomin E. Weiland A., et al. Immunolocalization and expression of bone morphogenetic proteins 2 and 4 in fracture healing. J Orthop Res. 1995;13:357. doi: 10.1002/jor.1100130309. [DOI] [PubMed] [Google Scholar]
- 17.Gardner M.J. van der Meulen M.C. Demetrakopoulos D. Wright T.M. Myers E.R. Bostrom M.P. In vivo cyclic axial compression affects bone healing in the mouse tibia. J Orthop Res. 2006;24:1679. doi: 10.1002/jor.20230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weaver A.S. Su Y.P. Begun D.L. Miller J.D. Alford A.I. Goldstein S.A. The effects of axial displacement on fracture callus morphology and MSC homing depend on the timing of application. Bone. 2010;47:41. doi: 10.1016/j.bone.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith-Adaline E.A. Volkman S.K. Ignelzi M.A., Jr. Slade J. Platte S. Goldstein S.A. Mechanical environment alters tissue formation patterns during fracture repair. J Orthop Res. 2004;22:1079. doi: 10.1016/j.orthres.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Claes L. Blakytny R. Gockelmann M. Schoen M. Ignatius A. Willie B. Early dynamization by reduced fixation stiffness does not improve fracture healing in a rat femoral osteotomy model. J Orthop Res. 2009;27:22. doi: 10.1002/jor.20712. [DOI] [PubMed] [Google Scholar]
- 21.Utvag S.E. Korsnes L. Rindal D.B. Reikeras O. Influence of flexible nailing in the later phase of fracture healing: strength and mineralization in rat femora. J Orthop Sci. 2001;6:576. doi: 10.1007/s007760100015. [DOI] [PubMed] [Google Scholar]
- 22.Claes L. Blakytny R. Besse J. Bausewein C. Ignatius A. Willie B. Late dynamization by reduced fixation stiffness enhances fracture healing in a rat femoral osteotomy model. J Orthop Trauma. 2011;25:169. doi: 10.1097/BOT.0b013e3181e3d994. [DOI] [PubMed] [Google Scholar]
- 23.Siddhivarn C. Banes A. Champagne C. Riche E.L. Weerapradist W. Offenbacher S. Mechanical loading and delta12prostaglandin J2 induce bone morphogenetic protein-2, peroxisome proliferator-activated receptor gamma-1, and bone nodule formation in an osteoblastic cell line. J Periodontal Res. 2007;42:383. doi: 10.1111/j.1600-0765.2006.00965.x. [DOI] [PubMed] [Google Scholar]
- 24.Sumanasinghe R.D. Bernacki S.H. Loboa E.G. Osteogenic differentiation of human mesenchymal stem cells in collagen matrices: effect of uniaxial cyclic tensile strain on bone morphogenetic protein (BMP-2) mRNA expression. Tissue Eng. 2006;12:3459. doi: 10.1089/ten.2006.12.3459. [DOI] [PubMed] [Google Scholar]
- 25.Rui Y.F. Lui P.P. Ni M. Chan L.S. Lee Y.W. Chan K.M. Mechanical loading increased BMP-2 expression which promoted osteogenic differentiation of tendon-derived stem cells. J Orthop Res. 2011;29:390. doi: 10.1002/jor.21218. [DOI] [PubMed] [Google Scholar]
- 26.Rauch F. Lauzier D. Croteau S. Travers R. Glorieux F.H. Hamdy R. Temporal and spatial expression of bone morphogenetic protein-2, −4, and −7 during distraction osteogenesis in rabbits. Bone. 2000;27:453. doi: 10.1016/s8756-3282(00)00337-9. [DOI] [PubMed] [Google Scholar]
- 27.Radomisli T.E. Moore D.C. Barrach H.J. Keeping H.S. Ehrlich M.G. Weight-bearing alters the expression of collagen types I and II, BMP 2/4 and osteocalcin in the early stages of distraction osteogenesis. J Orthop Res. 2001;19:1049. doi: 10.1016/S0736-0266(01)00044-4. [DOI] [PubMed] [Google Scholar]
- 28.Sato M. Ochi T. Nakase T. Hirota S. Kitamura Y. Nomura S., et al. Mechanical tension-stress induces expression of bone morphogenetic protein (BMP)-2 and BMP-4, but not BMP-6, BMP-7, and GDF-5 mRNA, during distraction osteogenesis. J Bone Miner Res. 1999;14:1084. doi: 10.1359/jbmr.1999.14.7.1084. [DOI] [PubMed] [Google Scholar]
- 29.Sato M. Yasui N. Nakase T. Kawahata H. Sugimoto M. Hirota S., et al. Expression of bone matrix proteins mRNA during distraction osteogenesis. J Bone Miner Res. 1998;13:1221. doi: 10.1359/jbmr.1998.13.8.1221. [DOI] [PubMed] [Google Scholar]
- 30.Aspenberg P. Basic N. Tagil M. Vukicevic S. Reduced expression of BMP-3 due to mechanical loading: a link between mechanical stimuli and tissue differentiation. Acta Orthop Scand. 2000;71:558. doi: 10.1080/000164700317362172. [DOI] [PubMed] [Google Scholar]
- 31.Boerckel J.D. Uhrig B.A. Willett N.J. Huebsch N. Guldberg R.E. Mechanical regulation of vascular growth and tissue regeneration in vivo. Proc Natl Acad Sci U S A. 2011;108:E674. doi: 10.1073/pnas.1107019108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boerckel J.D. Dupont K.M. Kolambkar Y.M. Lin A.S. Guldberg R.E. In vivo model for evaluating the effects of mechanical stimulation on tissue-engineered bone repair. J Biomech Eng. 2009;131:084502. doi: 10.1115/1.3148472. [DOI] [PubMed] [Google Scholar]
- 33.Boerckel J.D. Kolambkar Y.M. Stevens H.Y. Lin A.S. Dupont K.M. Guldberg R.E. Effects of in vivo mechanical loading on large bone defect regeneration. J Orthop Res. 2012;30:1067. doi: 10.1002/jor.22042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glatt V. Miller M. Matthys R. Evans C. Development of a modulated mechanical environment for enhancing bone healing in a rat segmental defect model. Presented at the 56th Annual Meeting of the Orthopaedic Research Society; New Orleans. 2010. [Google Scholar]
- 35.Mehta M. Schell H. Schwarz C. Peters A. Schmidt-Bleek K. Ellinghaus A., et al. A 5-mm femoral defect in female but not in male rats leads to a reproducible atrophic non-union. Arch Orthop Trauma Surg. 2011;131:121. doi: 10.1007/s00402-010-1155-7. [DOI] [PubMed] [Google Scholar]
- 36.Wulsten D. Glatt V. Ellinghaus A. Schmidt-Bleek K. Petersen A. Schell H., et al. Time kinetics of bone defect healing in response to BMP-2 and GDF-5 characterised by in vivo biomechanics. Eur Cell Mater. 2011;21:177. doi: 10.22203/ecm.v021a14. [DOI] [PubMed] [Google Scholar]
- 37.Barnes G.L. Kostenuik P.J. Gerstenfeld L.C. Einhorn T.A. Growth factor regulation of fracture repair. J Bone Miner Res. 1999;14:1805. doi: 10.1359/jbmr.1999.14.11.1805. [DOI] [PubMed] [Google Scholar]
- 38.Wildemann B. Lange K. Strobel C. Fassbender M. Willie B. Schmidmaier G. Local BMP-2 application can rescue the delayed osteotomy healing in a rat model. Injury. 2011;42:746. doi: 10.1016/j.injury.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 39.Einhorn T.A. Majeska R.J. Mohaideen A. Kagel E.M. Bouxsein M.L. Turek T.J., et al. A single percutaneous injection of recombinant human bone morphogenetic protein-2 accelerates fracture repair. J Bone Joint Surg Am. 2003;85-A:1425. doi: 10.2106/00004623-200308000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Jones A.L. Bucholz R.W. Bosse M.J. Mirza S.K. Lyon T.R. Webb L.X., et al. Recombinant human BMP-2, allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88:1431. doi: 10.2106/JBJS.E.00381. [DOI] [PubMed] [Google Scholar]
- 41.Murnaghan M. McIlmurray L. Mushipe M.T. Li G. Time for treating bone fracture using rhBMP-2: a randomised placebo controlled mouse fracture trial. J Orthop Res. 2005;23:625. doi: 10.1016/j.orthres.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 42.Egger E.L. Gottsauner-Wolf F. Palmer J. Aro H.T. Chao E.Y. Effects of axial dynamization on bone healing. J Trauma. 1993;34:185. doi: 10.1097/00005373-199302000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Larsson S. Kim W. Caja V.L. Egger E.L. Inoue N. Chao E.Y. Effect of early axial dynamization on tibial bone healing: a study in dogs. Clin Orthop Relat Res. 2001;240 doi: 10.1097/00003086-200107000-00033. [DOI] [PubMed] [Google Scholar]
- 44.Claes L. Augat P. Suger G. Wilke H.J. Influence of size and stability of the osteotomy gap on the success of fracture healing. J Orthop Res. 1997;15:577. doi: 10.1002/jor.1100150414. [DOI] [PubMed] [Google Scholar]
- 45.Lienau J. Schell H. Duda G.N. Seebeck P. Muchow S. Bail H.J. Initial vascularization and tissue differentiation are influenced by fixation stability. J Orthop Res. 2005;23:639. doi: 10.1016/j.orthres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Forslund C. Aspenberg P. CDMP-2 induces bone or tendon-like tissue depending on mechanical stimulation. J Orthop Res. 2002;20:1170. doi: 10.1016/S0736-0266(02)00078-5. [DOI] [PubMed] [Google Scholar]
- 47.Kasper G. Dankert N. Tuischer J. Hoeft M. Gaber T. Glaeser J.D., et al. Mesenchymal stem cells regulate angiogenesis according to their mechanical environment. Stem Cells. 2007;25:903. doi: 10.1634/stemcells.2006-0432. [DOI] [PubMed] [Google Scholar]
- 48.Luu H.H. Song W.X. Luo X. Manning D. Luo J. Deng Z.L., et al. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res. 2007;25:665. doi: 10.1002/jor.20359. [DOI] [PubMed] [Google Scholar]
- 49.Yamaguchi A. Regulation of osteoblast differentiation mediated by BMP, Notch, and CCN3/NOV. Jpn Dent Sci Rev. 2008;44:48. [Google Scholar]
- 50.Ryoo H.-M. Critical molecular switches involved in BMP-2-induced osteogenic differentiation of mesenchymal cells. Gene. 2006;366:51. doi: 10.1016/j.gene.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 51.Kadow-Romacker A. Hoffmann J.E. Duda G. Wildemann B. Schmidmaier G. Effect of mechanical stimulation on osteoblast- and osteoclast-like cells in vitro. Cells Tissues Organs. 2009;190:61. doi: 10.1159/000178022. [DOI] [PubMed] [Google Scholar]
- 52.Kopf J. Petersen A. Duda G.N. Knaus P. BMP2 and mechanical loading cooperatively regulate immediate early signalling events in the BMP pathway. BMC Biol. 2012;10:37. doi: 10.1186/1741-7007-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ozcivici E. Luu Y.K. Adler B. Qin Y.-X. Rubin J. Judex S., et al. Mechanical signals as anabolic agents in bone. Nat Rev Rheumatol. 2010;6:50. doi: 10.1038/nrrheum.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang L. Zhang X. Guo Y. Chen X. Li R. Liu L., et al. Involvement of BMPs/Smad signaling pathway in mechanical response in osteoblasts. Cell Physiol Biochem. 2010;26:1093. doi: 10.1159/000323987. [DOI] [PubMed] [Google Scholar]
- 55.Goodship A.E. Watkins P.E. Rigby H.S. Kenwright J. The role of fixator frame stiffness in the control of fracture healing. An experimental study. J Biomech. 1993;26:1027. doi: 10.1016/s0021-9290(05)80002-8. [DOI] [PubMed] [Google Scholar]
- 56.Goodship A.E. Kenwright J. The influence of induced micromovement upon the healing of experimental tibial fractures. J Bone Joint Surg Br. 1985;67:650. doi: 10.1302/0301-620X.67B4.4030869. [DOI] [PubMed] [Google Scholar]