Abstract
Postprostatectomy erectile dysfunction (ED) is the major problem for patients with clinically localized prostate cancer. Recently, gene and stem cell-based therapy of the corpus cavernosum has been attempted for postprostatectomy ED, but those therapies are limited by rapid blood flow and disruption of the normal architecture of the corpus cavernosum. In this study, we attempted to regenerate the damaged cavernous nerve (CN), which is the main cause of ED. We investigated the effectiveness of human adipose-derived stem cell (hADSC) and nerve growth factor-incorporated hyaluronic acid-based hydrogel (NGF-hydrogel) application on the CN in a rat model of bilateral cavernous nerve crush injury. Four weeks after the operation, erectile function was assessed by detecting the intracavernous pressure (ICP)/arterial pressure level by CN electrostimulation. The ICP was significantly increased by application of hADSC with NGF-hydrogel compared to the other experimental groups. CN and penile tissue were collected for histological examination. PKH-26 labeled hADSC colocalized with beta III tubulin were shown in CN tissue sections. hADSC/NGF-hydrogel treatment prevented smooth muscle atrophy in the corpus cavernosum. In addition, the hADSC/NGF-hydrogel group showed increased endothelial nitric oxide synthase protein expression. This study suggests that application of hADSCs with NGF-hydrogel on the CN might be a promising treatment for postprostatectomy ED.
Introduction
Prostate cancer is one of the most common cancers in males and affects more than 16% of men; the United States has the highest incidence in the world. In addition, with a Westernized lifestyle, prostate cancer has also increased rapidly in Asia.1,2 For curative treatment of localized prostate carcinoma, radical prostatectomy (RP) has been performed as the main technique.3 Recently, robot-assisted surgery has carried out sensitive removal of the prostate to prevent erectile dysfunction (ED). Although more surgical advances in prostatectomy, such as robot-assisted surgery, have been applied to prevent cavernous nerve (CN) injury, complications, such as ED and urinary incontinence, still frequently occur following RP.4 Postprostatectomy ED ultimately results in damage of the neurovascular system, such as the CN. CN injury is the main reason for postprostatectomy ED.5,6 Walsh et al. first developed nerve-sparing radical prostatectomy (NSRP) in 1982, which is conducted by removing the entire prostate, while preserving the autonomic nerves surrounding the gland to prevent ED.7 Despite the continuous development of NSRP technical innovation, the incidence of ED and urinary incontinence is still high for postprostatectomy patients. Oral phosphodiesterase type 5 (PDE5) inhibitors are one of the most common treatment options after surgery. However, it has had a poor response in postprostatectomy patients.8 Recently, researchers have been more focused on the prevention of CN injury and stimulating nerve generation. Consequently, adult stem cells and neurotrophic factors (including nerve growth factor [NGF] and the brain-derived neurotrophic factor [BDNF]) have been regarded as a prospective treatment for ED by remedying CN injury. Recently, intracavernous injection of nonhematopoietic bone marrow stem cells and adipose-derived stem cells (ADSCs) has been reported to improve erectile function after CN crush injury.1,9 However, conventional reports involved direct injection of cells into the corpus cavernosum. Furthermore, such studies have focused on smooth muscle atrophy in the corpus cavernosum. To prevent and cure ED from a different angle, we directly applied stem cells and the growth factor in injured CN as the ultimate cause of ED.
In this study, we selected human adipose-derived stem cells (hADSCs), which are increasingly garnering public interest in clinical trials10,11 and NGF, which contributes to the survival and neuronal differentiation of stem cells.12–14 Burgers et al. demonstrated the ability of NGF to enhance regeneration of the CN in rat.15 In this study, we introduced a tissue-engineered hydrogel system as a controlled delivery vector for NGF to facilitate a sustained and localized application into the CN. Among many current hydrogels, hyaluronic acid-poly(ethylene oxide) (HA-PEO) has various biological properties, including biocompatibility, biodegradation, and controlled release of bioactive molecules.16–18 In addition, HA specifically binds to proteins in the extracellular matrix, on the cell surface, and within the cellular cytosol; thus, it plays a role in target cell stabilization, angiogenesis, cell mobility, inflammation regulation, and growth factor actions.19,20
Therefore, we evaluated the effect of ADSCs and NGF-incorporated hydrogel on ED in a rat model of CN injury.
Materials and Methods
Fabrication of HA-PEO hydrogel containing NGF
To synthesize the HA derivative, adipic dihydrazide (ADH; Sigma-Aldrich) and acrylic acid (Sigma-Aldrich) were sequentially grafted to hyaluronic acid (HA; Hanmi Pharmaceutical Co.) as reported in previous reports.16 In brief, after dissolving the HA polymer (0.10 g) in distilled water (70 mL), ADH (0.04 g) and N-(3-diethylpropyl)-N′-ethylcarbodiimide hydrochloride (EDC; Fluka Chemie GmbH, 0.05 mL) were added to the HA solution at defined molar ratios. HA-ADH was obtained through an EDC-mediated coupling reaction between the carboxyl groups of HA and the primary amines of ADH by stirring the mixture solution at room temperature for 3 h. After addition of acrylic acid (0.04 mL) and EDC (0.09 mL) to the HA-ADH solution, the reaction was continued by stirring with a magnetic stirrer for another 3 h. Acrylated HA powders (HA-ADH-Ac) were obtained after lyophilizing for 2 days. The synthesized HA-ADH-Ac powders (0.04 g/mL) were dissolved in triethanolamine, leading to a formation of 4% (w/v) HA-ADH-Ac solution. After mixing the PEO-hexa thiols (MW=10 kDa; Sunbio, Inc.) and NGF (600 ng/mL) in the triethanolamine-buffered solution (200 μL), the HA-ADH-Ac solution was added to 200 μL PEO solution in a 1-mL disposable syringe. HA-PEO hydrogel was spontaneously synthesized via the Michael type addition reaction without any further treatment.21,22
Growth factor release study
Growth factor (NGF)-incorporated HA-PEO hydrogels (volume, 200 μL) were incubated in a 20 mL phosphate-buffered saline (PBS) supplemented with 1% bovine serum albumin (BSA; Sigma-Aldrich) at 37°C for a period of up to 5 weeks, while undergoing mild shaking (∼50 rpm) to perform the release study. The medium was changed at preset times and the collected medium was placed in a 50-mL conical tube (Falcon) and frozen in a deep-freezer until ELISA analysis. The amount of NGF released from the hydrogel was determined using a NGF ELISA Kit (Duoset®; R&D Systems). The optical density was determined at a wavelength of 450 nm with a reference wavelength of 570 nm.
Preparation of adipose-derived stem cells
Human adipose tissues were obtained by simple liposuction from abdominal subcutaneous fat. The subcutaneous adipose tissue was digested with collagenase I (1 mg/mL) under gentle agitation for 60 min at 37°C. The digested tissues were filtered through a 100-μm nylon sieve to remove cellular debris and were centrifuged at 470 g for 5 min to obtain a pellet. The pellet was resuspended in the Dulbecco's modified Eagle's medium (DMEM; Invitrogen)-based media containing 0.2 mM ascorbic acid and 10% fetal bovine serum (FBS). The cell suspension was recentrifuged at 470 g for 5 min. The supernatant was discarded, and the cell pellet was collected. The cell fraction was cultured overnight at 37°C/5% CO2 in DMEM-based media containing 0.2 mM ascorbic acid and 10% FBS. After 24 h, the cell adhesion was checked under an inverted microscope, and nonadherent cells were removed by washing with PBS. The cell medium was changed to keratinocyte-SFM (Invitrogen)-based media containing 0.2 mM ascorbic acid, 0.09 mM calcium, 5 ng/mL epidermal growth factor, and 5% FBS. The cells were maintained for 4–5 days until confluent (passage 0). When the cells reached 90% confluency, they were subculture-expanded in keratinocyte-SFM-based media until passage 3.23 The procedure for hADSC preparation was performed under good manufacturing practice (GMP) conditions in the Stem Cell Research Center of RNL BIO (Seoul, Korea). Immunophenotypic profiles of hADSC were demonstrated by a previous study.23
CN injury rat model and ADSC transplantation
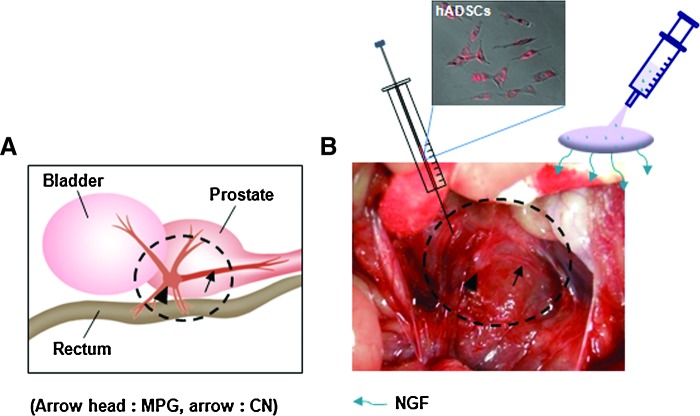
We used 8- to 10-week-old adult male rats (Sprague-Dawley; Orient Bio Co.), and assigned five rats to each group. All protocols were performed in accordance with the guidelines and regulations pertinent to animal experiments of the Institutional Animal Care and Use Committee, Catholic University Medical College. Normal rats were anesthetized with subcutaneous injection of ketamine (110 mg/kg). The urinary bladder and prostate were exposed by a lower abdominal incision. The bilateral CN was identified (Fig. 1). The CN exits the major pelvic ganglion in the groove between the urethra and rectum.24 The nerve was damaged by compressing it with a clamp for 30 s (nerve crush).25 Then, PKH-26-labeled hADSCs (PKH26 Red Fluorescent Cell Linker Kit; Sigma; cell dose: 1×106) were applied at the site of the damaged CN using a Hamilton syringe (25-gauge needle). NGF-hydrogel was then immediately applied to the site of the hADSCs. Experimental animals were divided into five groups (n=5 per each group): a normal group (Nr group), the saline application after bilateral cavernous nerve crush injury (BCNI) group (C group), the hADSCs application after the BCNI group (A group), the NGF-hydrogel applied after the BCNI group (N group), and the ADSCs covered with NGF-hydrogel after the BCNI group (AN group).
FIG. 1.
Diagrams showing application method of human adipose-derived stem cells (hADSCs) and nerve growth factor (NGF)-hydrogel above CN. (A) Schematic diagram shows anatomical location of CN. (B) PKH-26-labeled hADSCs were applied above injured CN using the Hamilton syringe, and then immediately covered with NGF-hydrogel. MPG, major pelvic ganglion; CN, cavernous nerve. Color images available online at www.liebertpub.com/tea
Erectile function measurement
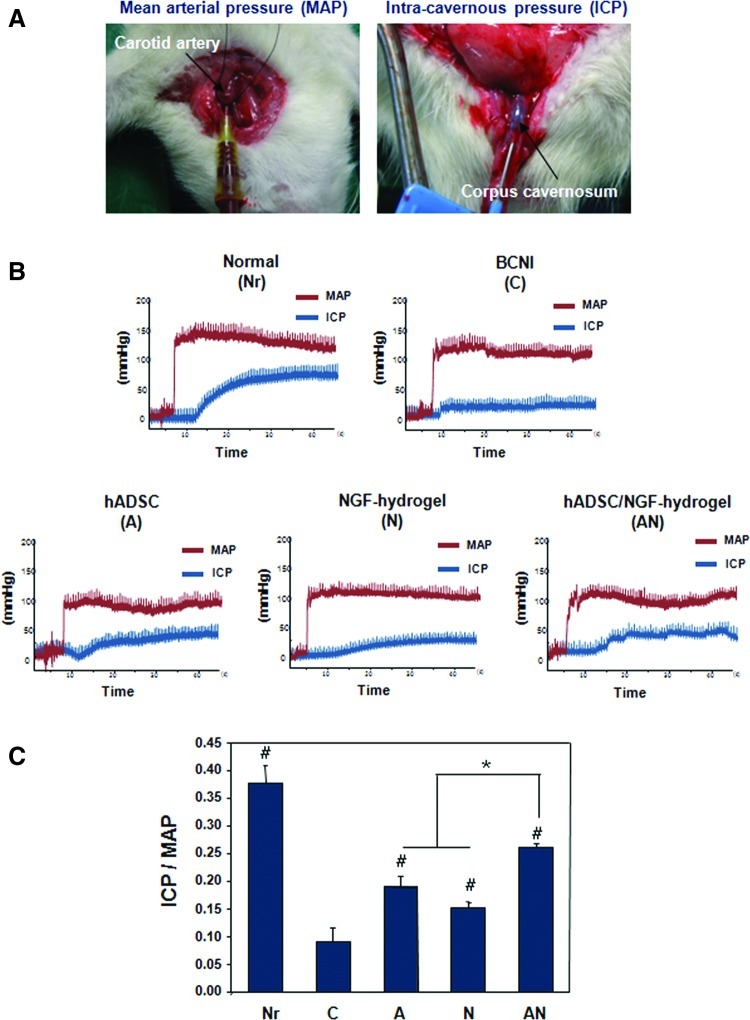
Four weeks after operation, the rats were anesthetized with subcutaneous injection of ketamine (110 mg/kg) and placed on the table in the supine position. The carotid artery and CN were exposed to detect the mean arterial pressure (MAP) and intracavernosal pressure (ICP). PE-50 tubing was inserted into the carotid artery to measure the MAP (Fig. 2A). At the same time, a 23-gauge butterfly needle filled with heparin was inserted in the corpus cavernosum, and then connected to a pressure transducer (Grass model S48K, Grass Instrument Division; Astro-Med, Inc.) to measure the ICP. Then, a bipolar stainless steel electrical stimulator was placed on the ganglion to stimulate the CN at 10 V for 50 s and 2.4 mA with a pulse width of 0.5 ms. CN stimulation was conducted at least three times, and the interval between stimulations was maintained for over 10 min. The peak ICP and MAP were measured through an isometric force transducer and were recorded on a computer with a commercial data acquisition system (PowerLab®; AD Instruments). The ratio of ICP to MAP was calculated to determine the erectile function.
FIG. 2.
Evaluating erectile function by electrical stimulation of the CN. (A) Macroscopic images show measurement of the mean arterial pressure (MAP) and intracavernous pressure (ICP). (B) Representative recordings of MAP and ICP in response to pelvic ganglion stimulation for each group. (C) Ratios of mean ICP/MAP were calculated for each group (*p<0.05, #p<0.05 compared to bilateral cavernous nerve crush injury [BCNI] group). Color images available online at www.liebertpub.com/tea
PKH-26/NGF costaining into CN
Immediately following the MAP and ICP measurements, the CN was carefully dissected. To detect PKH-26-labeled hADSCs around the CN, tissue samples from the CN were frozen using 2-methylbutane, then embedded in optimal cutting temperature compound (Tissue-Tek, Torrance), and sectioned at 5 μm on a microtome (Leica). the slides were washed with PBTx (0.1% Triton X-100 in PBS), blocked with 1% BSA (Amresco), and 1.5% normal goat serum (Vector laboratories) in PBTx at 37°C for 1 h. Then the sections were incubated at 4°C overnight with a primary antibody to neuron-specific beta-III tubulin (diluted 1:100; Abcam) for PKH-26/beta-III tubulin costaining. After washing with PBTx, the samples were then incubated with a secondary antibody (Alexa Fluor® 488 goat anti-rabbit IgG; Invitrogen) in 1% BSA and 1.5% normal goat serum in PBTx at room temperature for 1 h. Then, a coverslip was mounted on the slide using a mounting medium with 4,6-diamino-2-phenyl-indole (DAPI; Vector Labs Burlingame) to observe the cell nuclei. The slide was observed by a fluorescent microscope (Model BX51; Olympus) and the distribution of colocalization hADSC for PKH-26 was quantified in five fields per slide in triplicate by Image analysis software (i-solution; IMT).
Histological and Immunohistochemical analyses in the corpus cavernosum
For histological analysis of the corpus cavernosum, the corpus cavernosum specimens were fixed in 4% paraformaldehyde for 24 h at 4°C, embedded with paraffin, and cut into 5-μm sections with a microtome. The cross-sectioned tissues were mounted on positively charged slides. For the observation of the smooth muscle and collagen content, in the corpus cavernosum, the tissue sections were stained with Masson's trichrome staining, using the general procedures (n=5 per group). Histological images were obtained using a light microscope and the image capture were carried out in triplicate for each group.
To examine the endothelial nitric oxide synthase (eNOS) expression in the corpus cavernosum, The tissue sections were deparaffinized, rehydrated, treated with 3% hydrogen peroxidase to block endogenous peroxidase, rinsed, and then kept in 0.01 M PBS, which were then microwaved to retrieve the antigen, and then exposed to a 10% normal serum to block nonspecific reactions. Sections were subsequently incubated with the primary antibody, an anti-eNOS antibody (diluted 1:100; Abcam) overnight at 4°C. After washing with PBTx, the slides were incubated with horseradish peroxidase-conjugated antibodies (DAKO), and then visualized with a DAB kit (Invitrogen). Cell nuclei were counterstained with hematoxylin. Images were captured under a microscope, and then the positively stained vessels were counted in three separate high-power fields (×400) per slide in triplicate by a blinded observer.
For detection of venous smooth muscle, the paraffin sections were immunostained with anti-α-smooth muscle actin (SMA) (diluted 1:75; Abcam). The procedure used for α-SMA staining was identical to that described above for PKH-26/beta-III tubulin costaining. The Alexa Fluor® 488- secondary antibody was used for signal detection. Digital images were obtained with an Olympus BX51 fluorescence microscope, and the percentage of cells immune-positive for α-SMA was quantified in five fields per slide in triplicate by Image analysis software.
Statistical analysis
Statistical analyses were performed with the SPSS 15.0 software (SPSS, Inc.). Data are expressed as the mean±SEM. Differences between groups were evaluated by analysis of variance tests with the Tukey post-test. A two-group comparison was done using the unpaired t-test, and p<0.05 were considered statistically significant.
Results
NGF release behavior from HA-PEO hydrogels
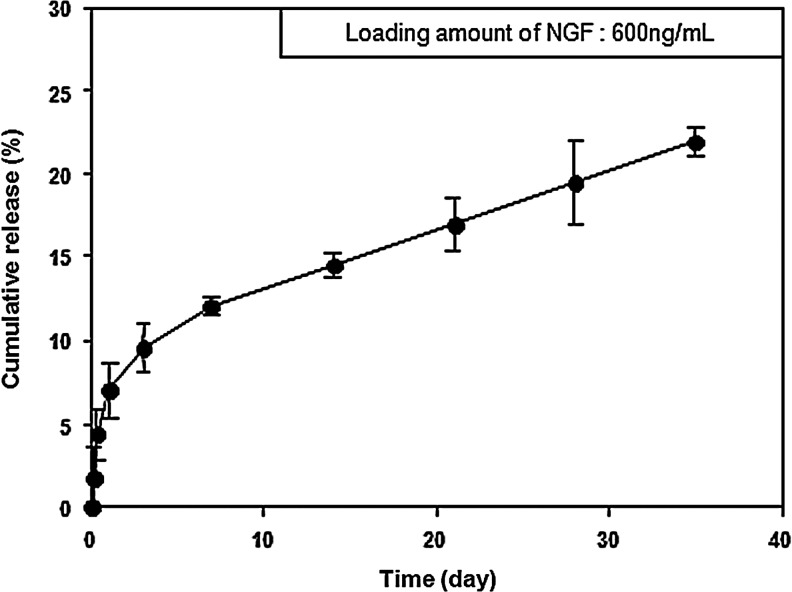
NGF was loaded into HA-PEO hydrogels to investigate whether these hydrogels have a positive effect on hADSC-mediated nerve regeneration in vivo. The release profiles of growth factors from HA-PEO hydrogels are shown in Figure 3. The growth factors were continuously released from the hydrogel for 4 weeks (up to 19.1%; 114.65±2.98 ng/mL). In addition, HA-PEO hydrogels maintained their gel-like state for 5 weeks.
FIG. 3.
Percent cumulative release of NGF from a hyaluronic acid-poly(ethylene oxide) hydrogel incubated at 37°C for 35 days. Error bars represent the standard deviation for n=3 samples.
Ratio of ICP and MAP
Representative recordings of the MAP and ICP in response to pelvic ganglion stimulation are shown in Figure 2B. There was little difference in the MAP among the animal groups. As a result, we checked the ratio of ICP/MAP in each group, and then calculated the mean ICP/MAP. ICP/MAP ratios were 0.38±0.03, 0.09±0.02, 0.19±0.02, 0.15±0.01, and 0.26±0.01 mmHg in the Nr, C, A, N, and AN groups, respectively. As shown in Figure 2C, the ICP/MAP ratio in the C group was decreased at 4 weeks after surgery, indicating that the ED of the rats was effectively induced by the crush injury of the CN. The ICP/MAP ratios in the A, N, and AN groups were increased compared to the C group. In particular, the ICP in the AN group was significantly higher than in the A and N groups.
Fluorescence-labeled hADSC localization in vivo
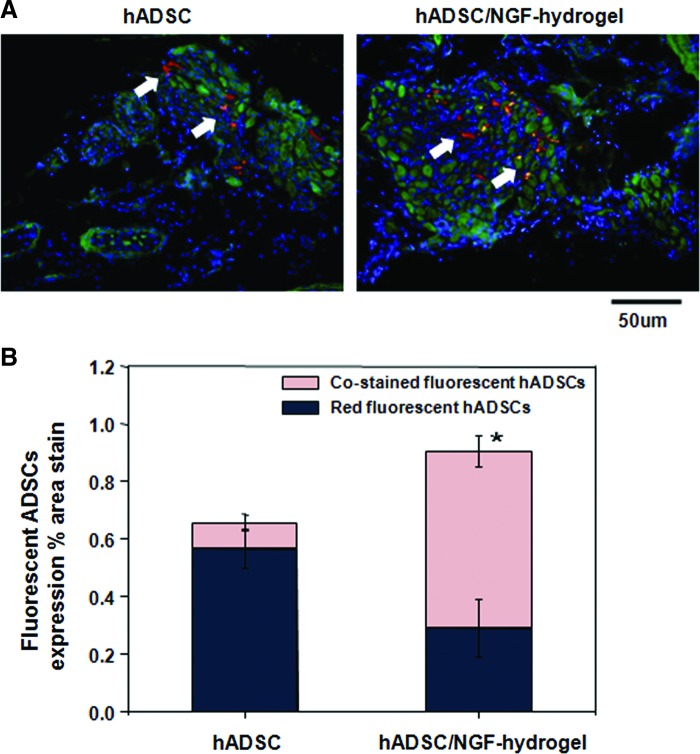
Four weeks after implanting red fluorescence-labeled ADSCs, frozen sections of CN tissue were stained with green-fluorescent beta-III tubulin. Representative images of the A and AN groups are shown in Figure 4. In the A group, a small amount of hADSCs were located with surrounding tissue aside the CN with a red color. However, in the AN group, red-fluorescent hADSCs costained with green fluorescent NGF, showing a yellow color, indicated that incorporating with NGF-hydrogel contributed to hADSCs engraftment into CN in vivo (Fig. 4A). The density of costained hADSCs with the CN was significantly increased in the hADSC/NGF-hydrogel group (0.61%±0.05%), compared with the value in the hADSC group (0.09%±0.03%) (Fig. 4B).
FIG. 4.
Double immunohistochemistry using beta-III tubulin and PKH-26 for tissue sections obtained from around the CN at 4 weeks. (A) Magnification is ×400. PKH26-labeled hADSC (red) in the adipose-derived stem cell (ADSC) group was shown around the CN. Merged ADSCs (yellow, arrow) in the ADSC/brain-derived neurotrophic factor-membrane group were shown in colocalization of PKH26-labeled ADSCs and CN by immunostaining of beta-III tubulin (green). (B) Quantitative analysis of fluorescent ADSCs expression was quantified as the relative percentage of ADSCs expression in area stain for the entire CN cross section. Each bar shows the mean values (±standard error) from n=5 rats per group. (*p<0.05, costained fluorescent hADSCs in the hADSC/NGF-hydrogel group compared with the hADSC group). Color images available online at www.liebertpub.com/tea
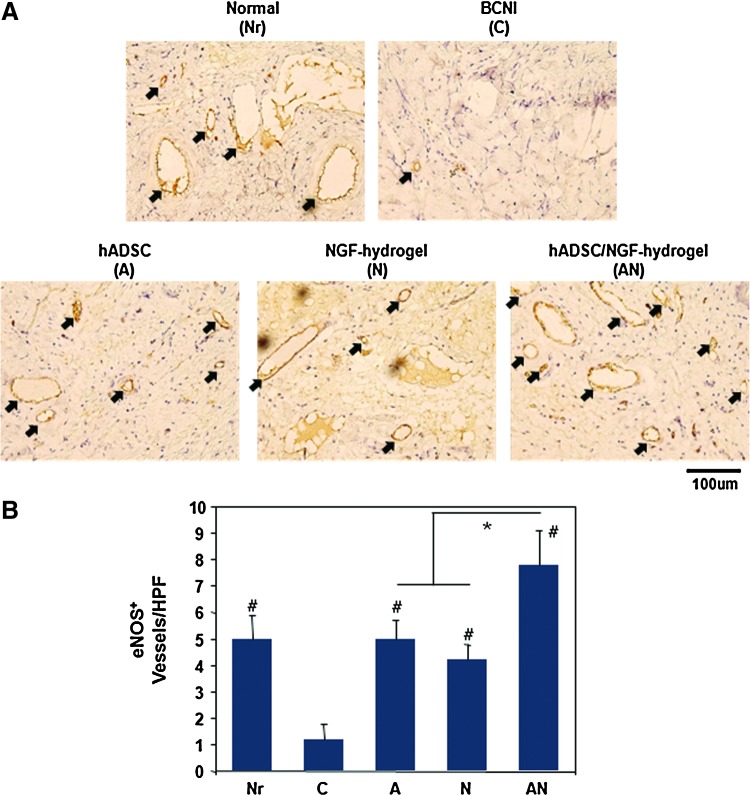
DAB staining of eNOS expression in the corpus cavernosum
We evaluated the content of eNOS in the corpus cavernosum by eNOS DAB staining (Fig. 5A). After DAB staining, eNOS-positive vessels were counted in the corpus cavernosum (Fig. 5B). The number of eNOS-positive vessels were 5.0±0.89, 1.2±0.58, 5.0±0.70, 4.2±0.58, and 7.8±1.28 in the Nr, C, A, N, and AN groups, respectively. The number of eNOS-positive vessels was decreased in the CN crush injury group (C group) when compared with that of the Nr group. The reduced eNOS-positive vessels were significantly increased in all treated groups. In particular, the AN group showed a significantly enhanced eNOS-positive vessel density when compared to that of the A and N groups (p<0.05).
FIG. 5.
(A) Representative images of endothelial nitric oxide synthase (eNOS) expression in the corpus cavernosum 4 weeks after surgery. Arrow indicates eNOS-positive vessels (brown). (B) Result of eNOS quantification expressed as the number of eNOS-positive vessels in the corpus cavernosum (Magnification is ×400, *p<0.05, #p<0.05 compared to BCNI group). Color images available online at www.liebertpub.com/tea
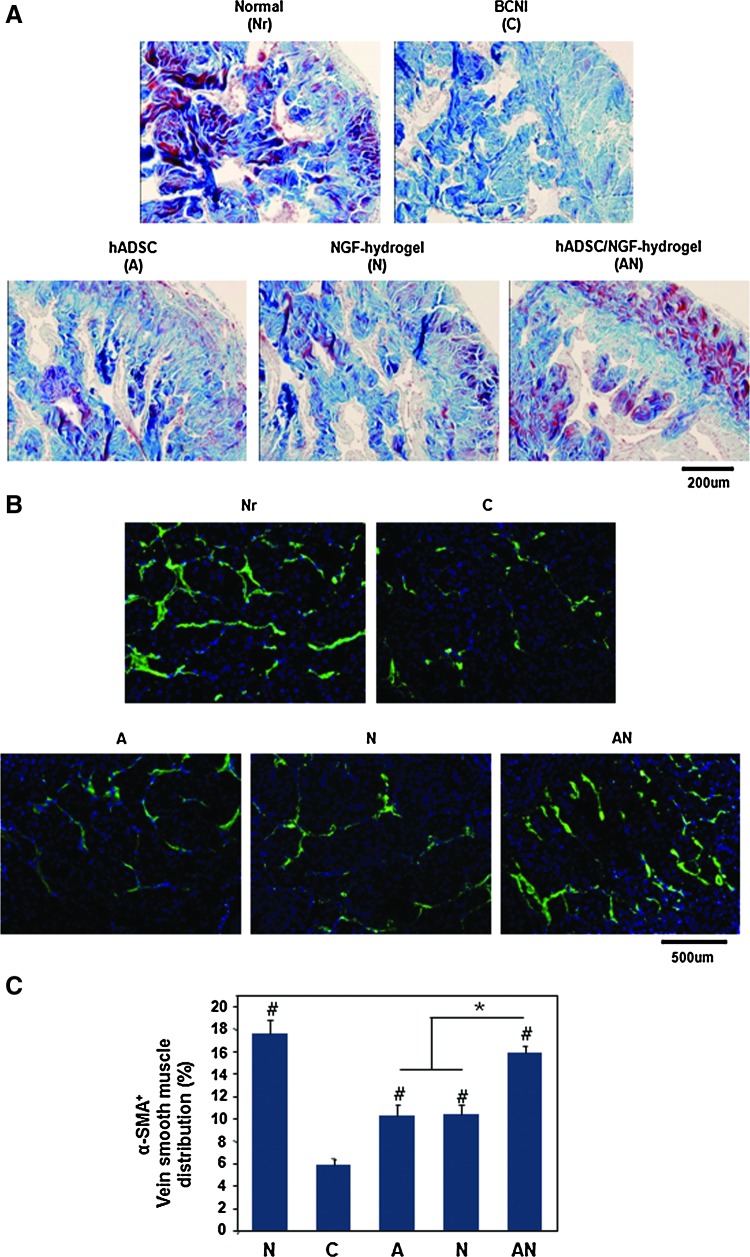
Masson's trichrome staining of corpus cavernosum
The content of collagenous connective tissue and smooth muscle in the corpus cavernosum tissue was observed by Masson's trichrome staining. In group C, cavernous tissue exhibited a much higher density of collagenous connective tissue compared with the normal group (Fig. 6A). This result indicated that smooth muscle atrophy in corpus cavernous caused by CN injury leads to a relative collagen deposition. In the A or N group, smooth muscle atrophy was moderate, and there was no difference between the A and N groups However, smooth muscle atrophy was minimal in the AN group. Those results showed that smooth muscle atrophy in the corpus cavernosum was effectively prevented by coapplication of hADSCs and NGF-hydrogel.
FIG. 6.
Histological analyses in the corpus cavernosum 4 weeks after surgery. (A) Masson's trichrome staining for smooth muscle and collagen in the corpus cavernosum. Smooth muscle was stained red, and collagen was stained blue (magnification is ×200). (B) Representative fluorescent images of α-smooth muscle actin (SMA)-positive area in a section of adult rat penile corpus cavernosum (smooth muscle, green color; magnification is ×200). (C) Result of α-SMA quantification expressed as the area of α-SMA-positive tissue in the corpus cavernosum (*p<0.05, #p<0.05 compared to BCNI group). Color images available online at www.liebertpub.com/tea
α-SMA staining of corpus cavernosum
Figure 6B shows representative images of α-SMA immunohistochemistry in the corpus cavernosum. The percentage of α-SMA-positive areas in the Nr, C, A, N, and AN groups were 17.56%±1.19%, 5.80%±0.62%, 10.21%±1.01%, 10.36%±0.87%, and 15.84%±0.67%, respectively (Fig. 6C). Group C exhibited a severe deficiency of α-SMA downregulated smooth muscle. This result indicated that the damage of CN induced significant apoptosis of smooth muscle cells in corpus cavernosum. However, reduced α-SMA-positive tissues were significantly increased in all experimental groups. In particular, α-SMA expression in the AN group was similar to that of the normal group.
Discussion
With advancements in the early detection of prostate cancer, RP is the most widely performed procedure for patients. However, ∼25%–75% of men experience postoperative ED.26 To find a cure for ED, numerous researchers in recent years have studied gene (molecular target) and stem cell-based therapies in preclinical studies. Stem cells hold great promise for regenerative medicine because of their ability to self-renew and to differentiate into various cell types. Kendirci et al. reported that transplantation of bone marrow stem cells into the corpus cavernosum ameliorated erectile function after BCNI.27 Albersen et al. showed that injection of ADSCs improved recovery of erectile function in a rat BCNI model.1 In particular, ADSCs are increasingly investigated for their potential in tissue repair and regeneration because of their many benefits, such as their abundance and ease of isolation.
The critical role of molecular targets, including the vascular endothelial growth factor (VEGF), NGF, and BDNF, has been reported in a number of studies on erectile function in ED. Henry et al. showed that intracavernous injections of VEGF seemed to protect penile corporal endothelium against the damaging effects of hypercholesterolemia in a rabbit model of ED.28 In addition, several investigators have examined the ability of neurotrophic factors, such as NGF and BDNF, using a gene transfer system to restore ED due to CN injury.15,29,30 To apply those neurotrophic factors around damaged CN, we used HA-PEO hydrogel as the delivery system of NGF in this study. Both HA and PEO have excellent biological properties, such as biodegradability, biocompatibility, and nonimmunogenicity.31,32 Hydrogelation of the HA-PEO hydrogel was spontaneously achieved via the Michael-type addition reaction between the methacrylate side groups in the HA and the six thiol groups in PEO.33 Hahn et al. demonstrated sustained release formulation of protein using HA-based hydrogels crosslinked by the Michael addition in the presence of proteins to be loaded.34 In this study, Figure 2 shows that the release of NGF from the HA-PEO hydrogel was sustained, even though, NGF in HA-PEO hydrogel was released by the diffusion of them through the pores in the hydrogel network in vitro. It may be much more released from HA-PEO hydrogel through enzymatic degradation in vivo. Furthermore, NGF-hydrogel provides matrices for hADSCs to anchor around the CN (Fig. 1) and prevents direct tissue adhesion by surrounding organs. As shown in Figure 4, hADSCs were detected in and around CN after 4 weeks. The survival of hADSCs in the hydrogel is affected by the interaction between the cells and surrounding environment. From a histological phenotype perspective, colocalization (yellow color) of hADSCs and CN could be evidence for neuronal differentiation of hADSCs (Fig. 4). Consequently, those results indicate that NGF-hydrogel provides a microenvironment for neuronal differentiation of hADSCs.
To analyze erectile function, we used a rat model of CN injury. As shown in Figure 1, the rat major pelvic ganglion lies on either side of the dorsolateral lobes of the prostate.24 The ED model was created by crush injury of the CN. In the BCNI group, there were no erections in any of the rats (Fig. 3). Even though this rat model was able to objectively evaluate erectile function, the present study has just one limitation. This limitation is the use of immunodeficient nude mice instead of out-bred rats. It is well known that xenografts lead to an immune-mediated response in vivo. However, the CN of nude mice was too small to be identified with the naked eye. As a result, it was difficult to perform physiological tests. However, the present study did not trigger an immune reaction, such as tumor formation (data not shown). Song et al. demonstrated that human-derived neural crest stem cells transplanted in a rat model reconstructed endothelial and smooth muscle cells in the corpus cavernosum.35
From physiological tests on erectile function, it was confirmed that the hADSC/NGF-hydrogel group was significantly higher than other groups (Fig. 2C). We performed the immunohistochemistry to elucidate evidence from those results. Neuronal NOS initiates cavernous tissue and vascular relaxation, whereas eNOS further facilitates blood flow into erectile tissue and maintains erection. Nitric oxide triggers the increase in cGMP, a crucial signal molecule for corporal smooth muscle relaxation within the penis.36 eNOS staining demonstrated that eNOS expression in the hADSC/NGF-hydrogel group was significantly higher than the other groups (Fig. 5B). Those results indicate that the veno-occlusive dysfunction was improved in the corpus cavernosum. This result is consistent with physiological tests.
The corpus cavernosum extracellular matrix is essential for normal penile erection and has been implicated in a number of types of ED.37 In that structure, there are numerous blood-filled venous spaces surrounded by connective tissue and slips of smooth muscle; this is erectile tissue.38 The apoptosis of smooth muscle cells in the corpus cavernosum was attributable to veno-occlusive dysfunction, which leads to postprostatectomy ED. As shown in Figure 6C, the number of α-SMA-positive cells in the BCNI group was significantly lower than in the normal group. In contrast, the hADSC/NGF-hydrogel group showed a significantly increased number of α-SMA-positive cells, similar to the normal group. These data suggest that hADSC/NGF-hydrogel could play a key role in preventing smooth muscle atrophy in the corpus cavernosum. These facts were reconfirmed with the collagen/muscle ratio in the corpus cavernosum by Masson's trichrome staining.
Pinheiro et al. stated that the collagen is a key structural protein in tissue subjected to stretching forces, as can be seen by the thick and frizzy bundles in the corpus cavernosum.39 In addition, Wespes et al. reported that, in impotent patients with corporeal veno-occlusive dysfunction and arterial lesions, the percentage of collagen fibers replacing the smooth muscle cells is increased.40 However, the exact function of these collagen is not yet clear. Figure 6A shows that the muscle content (red color) was decreased and the collagen content (blue color) was increased in the BCNI group. In the hADSC/NGF-hydrogel group, the muscle distribution was higher than in the other groups.
The present study shows that transplantation of hADSCs and NGF-hydrogel into damaged CN improved erectile function. However, these data cannot explain the mechanisms underlying CN regeneration of hADSCs. We can only speculate that hADSCs may affect CN recovery via the paracrine effect of cytokines and growth factor. Furthermore, NGF may act as an activator of CN regeneration by contributing to the neuronal differentiation of hADSCs.
Conclusion
In this study, we showed that application of hADSCs and NGF-hydrogel into the CN could restore erectile function in a rat model of CN crush injury. As a result, hADSC and NGF-hydrogel treatment showed a positive effect on eNOS expression and collagen/smooth muscle distribution in the corpus cavernosum. Therefore, combined transplantation of hADSCs and NGF-hydrogel may be used as a novel therapy for postprostatectomy ED.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2011-0030075).
Disclosure Statement
No competing financial interests exist
References
- 1.Albersen M. Fandel T.M. Lin G. Wang G. Banie L. Lin C.S. Lue T.F. Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med. 2010;7:3331. doi: 10.1111/j.1743-6109.2010.01875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanda M.G. Dunn R.L. Michalski J. Sandler H.M. Northouse L. Hembroff L. Lin X. Greenfield T.K. Litwin M.S. Saigal C.S. Mahadevan A. Klein E. Kibel A. Pisters L.L. Kuban D. Kaplan I. Wood D. Ciezki J. Shah N. Wei J.T. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 3.Yee D.S. Ahlering T.E. Radical prostatectomy: a current perspective. J Urol. 2007;178:376. doi: 10.1016/j.juro.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 4.penson D.F. 5-year urinary and sexual outcomes after radical prostatectomy: results from the prostate cancer outcomes study. J Urol. 2008;179:40. doi: 10.1016/j.juro.2008.03.136. [DOI] [PubMed] [Google Scholar]
- 5.Hatzimouratidis K. Amar E. Eardley I. Giuliano F. Hatzichristou D. Montorsi F. Vardi Y. Wespes E. Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur Urol. 2010;57:804. doi: 10.1016/j.eururo.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Albersen M. Joniau S. Claes H. Van Poppel H. Preclinical evidence for the benefits of penile rehabilitation therapy following nerve-sparing radical prostatectomy. Adv Urol. 2008:594868. doi: 10.1155/2008/594868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh P.C. Radical prostatectomy for localized prostate cancer provides durable cancer control with excellent quality of life: a structured debate. J Urol. 2000;163:1802. [PubMed] [Google Scholar]
- 8.Kendirci M. Bivalacqua T.J. Hellstrom W.J. Vardenafil: a novel type 5 phosphodiesterase inhibitor for the treatment of erectile dysfunction. Expert Opin Pharmacother. 2004;5:923. doi: 10.1517/14656566.5.4.923. [DOI] [PubMed] [Google Scholar]
- 9.Sariola H. The neurotrophic factors in non-neuronal tissues. Cell Mol Life Sci. 2001;58:1061. doi: 10.1007/PL00000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko M.S. Jung J.Y. Shin I.S. Choi E.W. Kim J.H. Kang S.K. Ra J.C. Effects of expanded human adipose tissue-derived mesenchymal stem cells on the viability of cryopreserved fat grafts in the nude mouse. Int J Med Sci. 2011;8:231. doi: 10.7150/ijms.8.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yarak S. Okamoto O.K. Human adipose-derived stem cells: current challenges and clinical perspectives. An Bras Dermatol. 2010;85:647. doi: 10.1590/s0365-05962010000500008. [DOI] [PubMed] [Google Scholar]
- 12.Dhar S. Yoon E.S. Kachgal S. Evans G.R. Long-term maintenance of neuronally differentiated human adipose tissue-derived stem cells. Tissue Eng. 2007;13:2625. doi: 10.1089/ten.2007.0017. [DOI] [PubMed] [Google Scholar]
- 13.Chang C.J. Effect of Pulse-released nerve growth factor from genipin-crosslinked gelatin in schwann cell–seeded polycaprolactone conduits on large-gap peripheral nerve regeneration. Tissue Eng Part A. 2009;15:547. doi: 10.1089/ten.tea.2007.0342. [DOI] [PubMed] [Google Scholar]
- 14.Bella A.J. Lin G. Lin C.S. Hickling D.R. Morash C. Lue T.F. Nerve growth factor modulation of the cavernous nerve response to injury. J Sex Med. 2009;6(Suppl 3):347. doi: 10.1111/j.1743-6109.2008.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burgers J.K. Nelson R.J. Quinlan D.M. Walsh P.C. Nerve growth factor, nerve grafts and amniotic membrane grafts restore erectile function in rats. J Urol. 1991;146:463. doi: 10.1016/s0022-5347(17)37825-4. [DOI] [PubMed] [Google Scholar]
- 16.Kim J. Kim I.S. Cho T.H. Lee K.B. Hwang S.J. Tae G. Noh I. Lee S.H. Park Y. Sun K. Bone regeneration using hyaluronic acid-based hydrogel with bone morphogenic protein-2 and human mesenchymal stem cells. Biomaterials. 2007;28:1830. doi: 10.1016/j.biomaterials.2006.11.050. [DOI] [PubMed] [Google Scholar]
- 17.Leach J.B. Schmidt C.E. Characterization of protein release from photocrosslinkable hyaluronic acid-polyethylene glycol hydrogel tissue engineering scaffolds. Biomaterials. 2005;26:125. doi: 10.1016/j.biomaterials.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 18.Fedorovich N.E. Alblas J. de Wijn J.R. Hennink W.E. Verbout A.J. Dhert W.J. Hydrogels as extracellular matrices for skeletal tissue engineering: state-of-the-art and novel application in organ printing. Tissue Eng. 2007;13:1905. doi: 10.1089/ten.2006.0175. [DOI] [PubMed] [Google Scholar]
- 19.Fraser J.R. Laurent T.C. Laurent U.B. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242:27. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 20.Ascher B. Cerceau M. Baspeyras M. Rossi B. Soft tissue filling with hyaluronic acid. Ann Chir Plast Esthet. 2004;49:465. doi: 10.1016/j.anplas.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Kim G.W. Choi Y.J. Kim M.S. Park Y.D. Lee K.B. Kim I.S. Hwang S.J. Noh I. Synthesis and evaluation of hyaluronic acid-poly (ethylene oxide) hydrogel via Michael-type addition reaction. Curr Appl Phys. 2007;7:28. [Google Scholar]
- 22.Noh I. Kim G.W. Choi Y.J. Kim M.S. Park Y. Lee K.B. Kim I.S. Hwang S.J. Tae G. Effects of cross-linking molecular weights in a hyaluronic acid-poly(ethylene oxide) hydrogel network on its properties. Biomed Mater. 2006;1:116. doi: 10.1088/1748-6041/1/3/004. [DOI] [PubMed] [Google Scholar]
- 23.Ra J.C. Shin I.S. Kim S.H. Kang S.K. Kang B.C. Lee H.Y. Kim Y.J. Jo J.Y. Yoon E.J. Choi H.J. Kwon E. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011;20:1297. doi: 10.1089/scd.2010.0466. [DOI] [PubMed] [Google Scholar]
- 24.Kaufmann O. Claro J. Cury J. Andrade E. Longo B. Aguiar W. Mello L. Srougi M. The development of a rat model of erectile dysfunction after radical prostatectomy: preliminary findings. BJU Int. 2008;102:1026. doi: 10.1111/j.1464-410X.2008.07760.x. [DOI] [PubMed] [Google Scholar]
- 25.Kim H. Sohn D.W. Kim S.D. Hong S.H. Suh H.J. Lee C.B. Kim S.W. The effect of mirodenafil on the penile erection and corpus cavernosum in the rat model of cavernosal nerve injury. Int J Impot Res. 2010;22:291. doi: 10.1038/ijir.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costa W.S. Carrerete F.B. Horta W.G. Sampaio F.J. Comparative analysis of the penis corpora cavernosa in controls and patients with erectile dysfunction. BJU Int. 2006;97:567. doi: 10.1111/j.1464-410X.2005.05917.x. [DOI] [PubMed] [Google Scholar]
- 27.Kendirci M. Trost L. Bakondi B. Whitney M.J. Hellstrom W.J. Spees J.L. Transplantation of nonhematopoietic adult bone marrow stem/progenitor cells isolated by p75 nerve growth factor receptor into the penis rescues erectile function in a rat model of cavernous nerve injury. J Urol. 2010;184:1560. doi: 10.1016/j.juro.2010.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henry G.D. Byrne R. Hunyh T.T. Abraham V. Annex B.H. Hagen P.O. Donatucci C.F. Intracavernosal injections of vascular endothelial growth factor protects endothelial dependent corpora cavernosal smooth muscle relaxation in the hypercholesterolemic rabbit: a preliminary study. Int J Impot Res. 2000;12:334. doi: 10.1038/sj.ijir.3900621. [DOI] [PubMed] [Google Scholar]
- 29.Hsieh P.S. Bochinski D.J. Lin G.T. Nunes L. Lin C.S. Lue T.F. The effect of vascular endothelial growth factor and brain-derived neurotrophic factor on cavernosal nerve regeneration in a nerve-crush rat model. BJU Int. 2003;92:470. doi: 10.1046/j.1464-410x.2003.04373.x. [DOI] [PubMed] [Google Scholar]
- 30.Bakircioglu M.E. Lin C.S. Fan P. Sievert K.D. Kan Y.W. Lue T.F. The effect of adeno-associated virus mediated brain derived neurotrophic factor in an animal model of neurogenic impotence. J Urol. 2001;165:2103. doi: 10.1097/00005392-200106000-00078. [DOI] [PubMed] [Google Scholar]
- 31.Chung C. Mesa J. Miller G.J. Randolph M.A. Gill T.J. Burdick J.A. Effects of auricular chondrocyte expansion on neocartilage formation in photocrosslinked hyaluronic acid networks. Tissue Eng. 2006;12:2665. doi: 10.1089/ten.2006.12.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelner A. Schacht E.H. Tailor-made polymers for local drug delivery: release of macromolecular model drugs from biodegradable hydrogels based on poly(ethylene oxide) J Control Release. 2005;101:13. doi: 10.1016/j.jconrel.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 33.Lutolf M.P. Tirelli N. Cerritelli S. Cavalli L. Hubbell J.A. Systematic modulation of Michael-type reactivity of thiols through the use of charged amino acids. Bioconjug Chem. 2001;12:1051. doi: 10.1021/bc015519e. [DOI] [PubMed] [Google Scholar]
- 34.Hahn S.K. Oh E.J. Miyamoto H. Shimobouji T. Sustained release formulation of erythropoietin using hyaluronic acid hydrogels crosslinked by Michael addition. Int J Pharm. 2006;322:44. doi: 10.1016/j.ijpharm.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 35.Song Y.S. Lee H.J. Park I.H. Lim I.S. Ku J.H. Kim S.U. Human neural crest stem cells transplanted in rat penile corpus cavernosum to repair erectile dysfunction. BJU Int. 2008;102:220. doi: 10.1111/j.1464-410X.2008.07469.x. [DOI] [PubMed] [Google Scholar]
- 36.Chiou W.F. Liu H.K. Juan C.W. Abnormal protein expression in the corpus cavernosum impairs erectile function in type 2 diabetes. BJU Int. 2010;105:674. doi: 10.1111/j.1464-410X.2009.08852.x. [DOI] [PubMed] [Google Scholar]
- 37.Maia R.S. Babinski M.A. Figueiredo M.A. Chagas M.A. Costa W.S. Sampaio F.J. Concentration of elastic system fibers in the corpus cavernosum, corpus spongiosum, and tunica albuginea in the rabbit penis. Int J Impot Res. 2006;18:121. doi: 10.1038/sj.ijir.3901404. [DOI] [PubMed] [Google Scholar]
- 38.Traish A. Kim N. The physiological role of androgens in penile erection: regulation of corpus cavernosum structure and function. J Sex Med. 2005;2:759. doi: 10.1111/j.1743-6109.2005.00094.x. [DOI] [PubMed] [Google Scholar]
- 39.Pinheiro A.C. Costa W.S. Cardoso L.E. Sampaio F.J. Organization and relative content of smooth muscle cells, collagen and elastic fibers in the corpus cavernosum of rat penis. J Urol. 2000;164:1802. [PubMed] [Google Scholar]
- 40.Wespes E. Goes P.M. Schiffmann S. Depierreux M. Vanderhaeghen J.J. Schulman C.C. Computerized analysis of smooth muscle fibers in potent and impotent patients. J Urol. 1991;146:1015. doi: 10.1016/s0022-5347(17)37990-9. [DOI] [PubMed] [Google Scholar]