Abstract
Regenerative therapy using stem cells is a promising approach for the treatment of stroke. Recently, we reported that dental pulp stem cells (DPSC) ameliorated ischemic tissue injury in the rat brain and accelerated functional recovery after middle cerebral artery occlusion (MCAO). In this study, we investigated the effects of stem cells from human exfoliated deciduous tooth (SHED)-derived conditioned medium (SHED-CM) on permanent MCAO (pMCAO). Adult male Sprague-Dawley rats were subjected to pMCAO. SHED-CM were then administered intranasally, and the motor function and infarct volume were evaluated. Neurogenesis and vasculogenesis were determined using immunochemical markers. The SHED-CM group had more positive signals than the Dulbecco's modified Eagle's medium group, with doublecortin (DCX), neurofilament H, neuronal nuclei, and rat endothelial cell antigen observed in the peri-infarct area. Migration of neuronal progenitor cells (NPC) with DCX from the subventricular zone to the peri-infarct area was observed on days 6 and 16, with migration on day 6 being the most prominent. In conclusion, SHED-CM promoted the migration and differentiation of endogenous NPC, induced vasculogenesis, and ameliorated ischemic brain injury after pMCAO as well as transplantation of DPSC.
Introduction
Stroke is the third leading cause of death worldwide and the most frequent cause of long-term disability in humans.1 Recently, transplantation of bone marrow mononuclear cells was shown to achieve clinical efficacy by inducing angiogenesis in patients with cerebral ischemia.2,3 However, bone marrow aspiration to acquire bone marrow mesenchymal stem cells (BMMSCs) is an invasive and painful procedure for the donor. In addition, the number, proliferation, and differentiation potential of BMMSCs decline with increasing age.4 There are two potential sources of human dental pulp stem cells (DPSC), the last molar teeth and deciduous teeth. Above all, deciduous teeth can be obtained noninvasively from extracted teeth discarded as medical waste without raising any ethics issues. The proliferation rate of stem cells from human exfoliated deciduous teeth (SHED) is significantly higher than that of BMMSCs. SHED have the added advantages of being simple to harvest and their expression of several growth factors.5 Furthermore, if stem cells are banked from deciduous teeth, they may be used as the donor in future. Banking stem cells from deciduous teeth is a reasonable and simple alternative to harvesting stem cells from BMMSCs.6 Recently, we reported that transplantation of DPSC in rats promoted neurogenesis and vasculogenesis in an induced peri-infarct area and enhanced recovery after middle cerebral artery occlusion (MCAO). These cells also released vascular endothelial growth factor (VEGF) and promoted the migration and differentiation of endogenous neuronal progenitor cells (NPC) in the subventricular zone (SVZ).7 Recently, we reported that SHED possessed high proliferation ability and were enriched with the extracellular matrix, suggesting that they may be a useful source for stem cell-based therapy compared with DPSC and BMMSCs.5 SHED are therefore considered a potential source for cell therapy in stroke patients.
Recent studies have reported that growth factors derived from transplanted stem cells accelerate the recovery of several diseases.7,8 However, the methods used to administer stem cells or growth factors in clinical applications require revision. Intranasal administration provides a method of bypassing the blood–brain barrier (BBB) and delivering therapeutic agents directly to the central nervous system (CNS).9,10 There are some reports that intranasal administration of VEGF provides an effective way of treating CNS diseases.11 In the present study, we investigated the effects of a SHED-derived condition medium (SHED-CM) in a rat model of cerebral ischemia.
Materials and Methods
SHED harvest from deciduous teeth
Human dental pulp tissues were obtained from clinically healthy, extracted deciduous teeth from eight patients. The ethics committee of the Nagoya University approved the experimental protocols. SHED were isolated and cultured as previously described.12,13 Briefly, the pulp was removed gently and digested for 1 h at 37°C in a solution containing 3 mg/mL collagenase type I and 4 mg/mL dispase. After filtration through 70-μm cell strainers (Falcon; BD Labware), the cells were cultured at 37°C under 5% CO2 in the Dulbecco's modified Eagle's medium (DMEM; Gibco) containing 20% mesenchymal cell growth supplement (Lonza, Inc.) and antibiotics (100 U/mL penicillin, 100 mg/mL streptomycin, and 0.25 mg/mL amphotericin B; Gibco). After primary culture, the cells were subcultured at ∼1×104 cells/cm2 and used in the experiments after three to five passages.
Preparation of SHED-CM and BMMSC-CM
SHED and BMMSCs (Lonza, Inc.) (4×105 cells) were cultured in a serum-free DMEM. Conditioned media of SHED and BMMSCs were collected after 48 h of culture and centrifuged at 1500 rpm for 5 min. The supernatants were recentrifuged at 3000 rpm for 3 min followed by collection of the second supernatants, named SHED-CM and BMMSC-CM, respectively.
Cerebral ischemia model
All the animal experiments were approved by the Institutional Animal Care and Use Committee (Nagoya University). Adult male Sprague-Dawley rats (Japan SLC, Inc.) weighing 350–400 g were used in the experiments. Animals were anesthetized initially with 5% isoflurane (Abbott Laboratories) and were maintained under anesthesia with 1.5% isoflurane in a mixture of 70% N2O and 30% O2. The rectal temperature was maintained at 37°C±0.5°C on a heating pad. Focal cerebral ischemia was induced by permanent MCAO (pMCAO) (Fig. 1A).14 A 4-0 monofilament nylon suture (Shirakawa) with the tip rounded by flame heating and coated with silicone (KE-200; Shin-Etsu Chemical) was advanced from the external carotid artery into the internal carotid artery until it blocked the origin of the MCA. Regional cerebral blood flow in the MCA territory was measured after occlusion using a laser Doppler flowmeter (Omega FLO-N1; Omega Wave, Inc.). The response was considered positive and included only if the reduction in the regional cerebral blood flow was >70%.
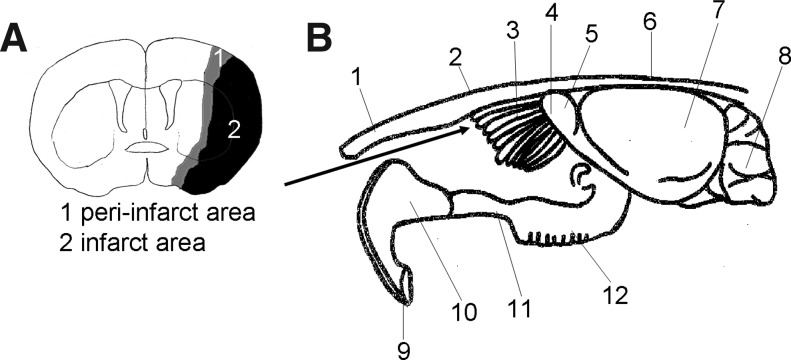
FIG. 1.
(A) The peri-infarct area. Peri-infarct area (gray) and infarct core (black). (B) Anatomy around the olfactory bulb. 1: nasal bone. 2: forehead bone. 3: ethmoid bone. 4: olfactory nerve area. 5: olfactory bulb. 6: forehead bone. 7: cerebrum. 8: cerebellum. 9: incisor teeth. 10: incisive bone. 11: maxilla bone. 12: molar teeth. The arrow displays the passage of nasal administration.
Intranasal administration of the conditioned media and DMEM
Seventy-two hours after pMCAO (day 3), the rats were anesthetized again with 1.5% isoflurane in a mixture of 70% N2O and 30% O2. The animals were divided randomly into three groups: the SHED-CM group (n=7, three sacrificed on day 6 and four on day 16), the BMMSC-CM group (n=3, three sacrificed on day 16), and the DMEM group (n=7, three sacrificed on day 6 and four on day 16). The rats were placed on their backs with their necks elevated by a 4×4-cm roll of gauze. A total of 100 μL of SHED-CM, BMMSC-CM, and DMEM was administered to each rat via the olfactory pathway using a Hamilton microsyringe (Fig. 1B). The preparations were administered in aliquots of 10 μL at a time and alternated nostril, with an interval of 2 min between each administration. During these procedures, the mouth and opposite nostril were closed. Intranasal administration was performed everyday from days 3 to 15.
Evaluation of motor disability
The rats were blindly examined on days 1, 3, 6, 9, 12, and 15 using a standardized motor disability scale with slight modifications.15 They scored 1 point for each of the following parameters: flexion of the forelimb contralateral to the stroke side when hung instantly by the tail, extension of the hind limb contralateral to the stroke side when pulled from a table, and rotation to the paretic side against resistance. In addition, 1 point was scored for circling motions to the paretic side when trying to walk, 1 point for failure to walk out of a 50-cm-diameter circle within 10 s, 2 points for failure to leave the circle within 20 s, and 3 points for inability to exit the circle within 60 s. In addition, 1 point each was scored for inability of the rat to extend the paretic forepaw when pushed against the table from above, laterally, and sideways. The motor disability scale was performed 3 times in each animal at each time point.
Assessment of infarct volume
The cryosections obtained from samples on day 16 were stained with hematoxylin and eosin.16 ImageJ (National Institutes of Health) was used to determine each infarct area in 12 coronal sections (20-μm thick) at 1.00-mm intervals. The entire infarction area was covered by these 12 coronal sections. Regional infarct volumes were calculated by summing the infarct areas and multiplying them by the distance between sections (1.00 mm), followed by remediation for brain edema.17
Immunohistochemistry
On day 16 after the injection, the rats were perfused transcardially with a 4% paraformaldehyde solution (Nakarai Tesque). Their brains were removed, postfixed in paraformaldehyde, and immersed in 30% sucrose solution the following day. Coronal sections (20-μm thick) were then prepared using a cryostat. For immunohistochemistry, the sections were preincubated for 2 h at room temperature (RT) in a blocking solution (Dulbecco's phosphate-buffered saline [PBS] containing 5% normal serum of the species in which the secondary antibody was raised) and incubated for 1 h at RT with diluted primary antibodies. The primary antibodies were as follows: marker of NPC, rabbit anti-doublecortin (anti-DCX; 1:50; Abcam, Inc.), neuronal markers, rabbit anti-neurofilament H (anti-NF; 1:200; Chemicon), mouse anti-neuronal nuclei (anti-NeuN; 1:500; Chemicon), endothelial cell marker, and mouse anti-rat endothelial cell antigen (anti-RECA1; 1:50; Monosan). After washing, the sections were incubated for 1 h at RT with secondary antibodies (NF/DCX, donkey anti-rabbit IgG FITC [1:400; Jackson ImmunoResearch], and NeuN/RECA1, goat anti-mouse IgG FITC [1:200; MP Biomedicals, LLC]) and counterstained with 4’, 6-diamidino-2-phenylindole (DAPI; Cell Biolabs, Inc.) for sections of DCX, NeuN and RECA1.
The adjacent sections were used as negative controls. In the control sections, all the procedures were performed in a similar manner, with the exception that the primary antibodies were omitted. To identify migration of NPC from the SVZ, we examined anti-DCX-stained cryosections in the SHED-CM and DMEM groups on days 6 and 16 by fluorescence microscopy (BZ-9000; Keyence).
Statistical analyses of cell density
The density of NPC, neurons, and endothelial cells in the peri-infarct (Fig. 1A) area of the SHED-CM and DMEM groups was determined. In these two groups (n=3), five sections cut at 100-μm intervals were stained with DCX, NeuN, or RECA1. The microscopic images were scanned, and five typical frames (0.49 mm2) were measured for each section. Therefore, on an average, 75 frames were determined for each group. The positive-stained area relative to the total area (7.41 mm2) was statistically analyzed using the software Dynamic cell count (Keyence) and ImageJ.
Statistical analyses
Data are expressed as means±SD. p values were calculated using the unpaired Student's t test.
Results
Evaluation of motor function
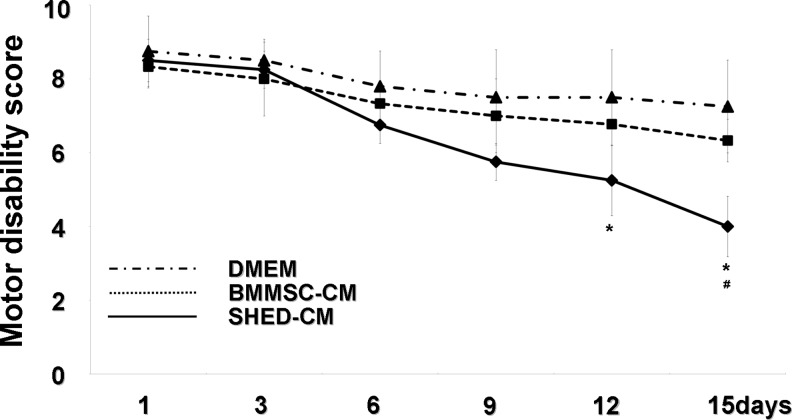
The three groups (SHED-CM, BMMSC-CM, and DMEM) displayed almost the same high score for motor function in the early stages, as shown in Figure 2 (day 1: 8.5±0.58 and 8.33±0.58; 8.75±0.96; day 3: 8.25±0.5, 8.00±1.0, and 8.50±0.6). Differences in the score appeared gradually between the three groups during the middle stage (day 6: 6.75±0.5, 7.33±0.58, 7.80±1.0; day 9: 5.75±0.5, 7.00±1.0, 7.50±1.29). On days 12 and 15, progressive improvement in motor disability became significant in the SHED-CM group compared with that in the DMEM group (day 12: 5.25±0.96, 6.77±0.58, 7.50±1.29; day 15: 4.0±0.81, 6.33±0.58, 7.25±1.26). On day 15, progressive improvement in motor disability became significant in the SHED-CM group compared with that in the BMMSC-CM group.
FIG. 2.
Motor disability test following intranasal administration of stem cells from human exfoliated deciduous tooth (SHED)-derived conditioned medium (SHED-CM), bone marrow mesenchymal stem cell (BMMSC)-derived conditioned medium (BMMSC-CM), and Dulbecco's modified Eagle's medium (DMEM) on days 1, 3, 6, 9, 12, and 15. Data are expressed as means±SD of 3 determinations. *p<0.05, versus DMEM. #p<0.05 versus BMMSC-CM. Student's t-test.
Reduction in infarct volume
As shown in Figure 3, there was a significant decrease in the infarct volume on day 16 in the SHED-CM group (day 16, 52.20±14.7 mm3, n=3) compared with the BMMSC-CM (day 16, 113.62±10.77 mm3, n=3) and DMEM groups (day 16, 147.43±5.50 mm3, n=3). These results suggest that SHED-CM promote regeneration.
FIG. 3.
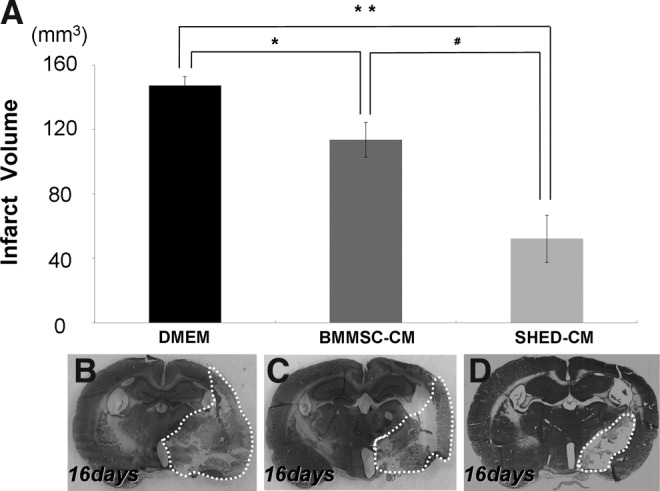
Reduction in the infarct volume 16 days after the injection of SHED-CM, BMMSC-CM, and DMEM (A). Infarct area on day 16 after nasal injection (B:DMEM, C:BMMSC-CM, and D:SHED-CM). Data are expressed as means±SD of 3 determinations (A). *p<0.05, **p<0.01, versus DMEM, #p<0.05, versus BMMSC-CM. Student's t-test.
SHED-CM outcome
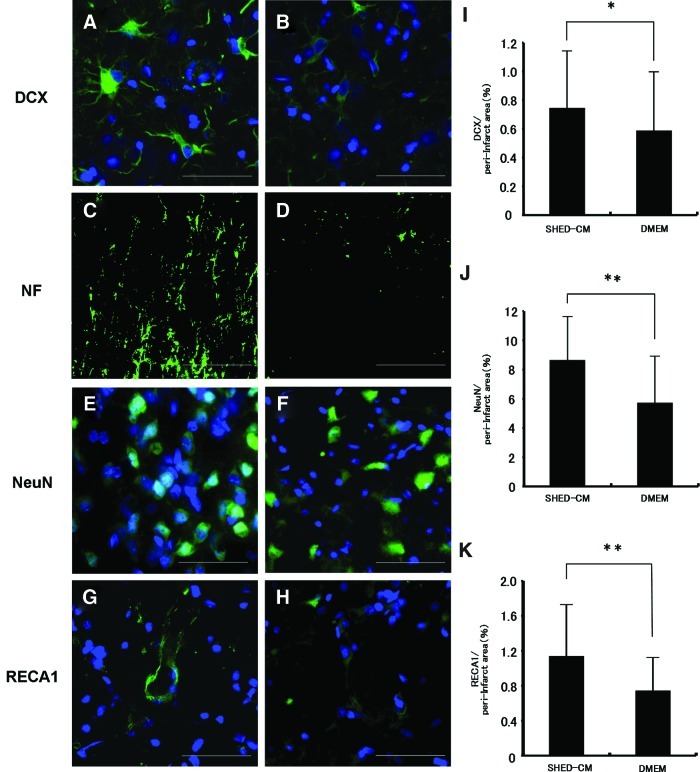
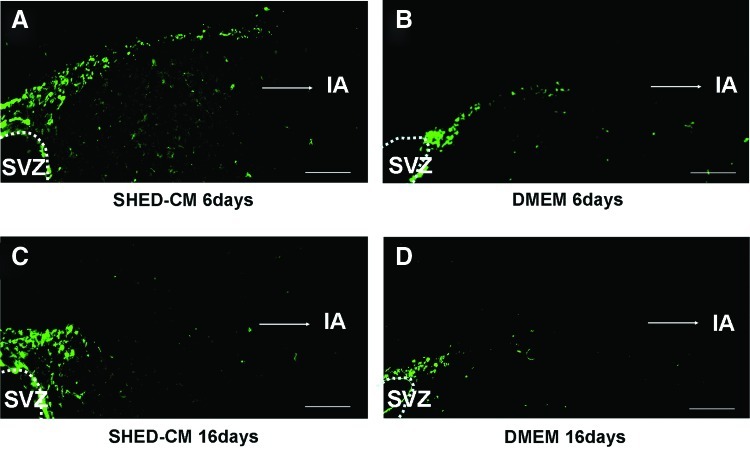
In the immunohistochemistry experiments, the SHED-CM group had more positive signals for DCX (Fig. 4A), NF (Fig. 4C), NeuN (Fig. 4E), and RECA1 (Fig. 4G) in the peri-infarct area compared with the DMEM group (Fig. 4B, D, F, H). Cell nuclei were labeled with DAPI (blue). There was a 1.27-fold increase in DCX-positive cells (Fig. 4I), 1.52-fold increase in NeuN-positive cells (Fig. 4J), and 1.53-fold increase in RECA1-positive cells (Fig. 4K) on day 16 in the SHED-CM group than in the DMEM group. The migration of NPC with DCX from the SVZ to the peri-infarct area was observed on days 6 (Fig. 5A) and 16 (Fig. 5C). The migration on day 6 was most prominent (Fig. 5A). These results suggest that SHED-CM also promote neurogenesis and angiogenesis after cerebral ischemia.
FIG. 4.
Doublecortin (DCX)-positive cells (A, B), neurofilament H (NF)-positive cells (C, D), neuronal nucleus (NeuN)-positive cells (E, F), and rat endothelial cell antigen (RECA1)-positive cells (G, H). SHED-CM group (A, C, E, G) on day 16. DMEM group (B, D, F, H) on day 16. Bar=50 μm (A–H). Cell nuclei were counterstained with DAPI. Statistical analyses of density of DCX (I), NeuN (J), and RECA1 (K) on day 16. *p<0.05, **p<0.001, Student's t-test. Each point is expressed as the mean±SD of 75 determinations. Color images available online at www.liebertpub.com/tea
FIG. 5.
Migration of neuronal progenitor cells (NPC) from the SVZ to the peri-infarct area on days 6 (A, B) and 16 (C, D). SHED-CM intranasal group (A, C). DMEM intranasal group (B, D). Bar=100 μm (A–D). IA=infarct area. Color images available online at www.liebertpub.com/tea
Discussion
We reported the characteristics of SHED compared with those of DPSC and BMMSCs. The results indicated that SHED possessed high proliferation ability and were enriched with the extracellular matrix, suggesting that they may be a useful source for stem cell-based therapy. In addition, using microarray analysis, we showed that SHED had higher expression levels of several growth factors, such as fibroblast growth factor, transforming growth factor, connective tissue growth factor, nerve growth factor, and bone morphogenetic protein.5 Taken together, these findings indicate that SHED are a more potentially useful source of stem cells for cell therapy than DPSC and BMMSCs.
However, cell therapy is always associated with problems, such as canceration,18 immune rejection, and ethics issues. Therefore, it is necessary to find alternative treatments. Studies in recent years have resulted in the recognition of a paracrine function in factors, and have suggested that stem cell transplantation may also be regarded as cell-based cytokine therapy.7,8 Accordingly, we investigated two steps for developing a new treatment for cerebral ischemia.
In the first step, we used SHED-CM as a new source for the treatment of cerebral ischemia. We have previously reported that needle administration of DPSC induced the recovery of motor disability and reduction in the infarct volume, proliferation of presumptive progeny in the SVZ, migration to the infarct, and differentiation into the appropriate neurons in a rat model of stroke.7 As several growth factors involved in neural regeneration are secreted from DPSC, we hypothesized that SHED-CM may improve the recovery of motor disability and reduce the infarct volume in the present study.
Moreover, we showed that the SHED-CM group significantly improved motor function and infarct volume compared with the BMMSC group (Figs. 2 and 3A).
Furthermore, the SHED-CM group had more positive signals for DCX, NF, NeuN, and RECA1 in the peri-infarct area (Fig. 1A) compared with the DMEM group (Fig. 4A–K). Migration of NPC with DCX from the SVZ to the peri-infarct area was observed on days 6 and 16, with the migration being the most prominent on day 6 in the SHED-CM group (Fig. 5A–D).
Brain injury is known to upregulate nestin expression.19 Nestin-positive, pluripotent NPC mature into neuroblasts that are positive for DCX.20 We found an increase in the DCX-positive population in pMCAO-injured animals treated with SHED-CM. Our results suggested that SHED-CM may trigger proliferation of NPC, and given the significant improvement in behavioral response, it is likely that these progenitor cells participate in the generation of new neurons.
In the second step, we investigated the intranasal administration of SHED-CM. Using this administration, therapeutic molecules traverse the BBB through the olfactory pathway and the less-studied trigeminal neural pathway.21 An important advantage of intranasal administration is that it is less invasive, with the factors being delivered directly to the brain.
Recently, it was reported that VEGF as a growth factor can bypass the BBB to reach multiple sites within the brain ∼30 min after the start of intranasal administration, and that intranasal administration may result in decreased systemic side effects because of decreased concentration in blood and peripheral organs.11 Our results suggested that SHED-CM, including some growth factors, may produce effects similar to those seen with VEGF in a stroke model.
The success of the two investigated steps suggests the possibility of using a shortcut in a clinical setting. Administration of SHED-CM resolves the ethics issues involved with cell therapies, because SHED-CM are not a cell, but a conjugate of many growth factors. As SHED-CM can be stocked, it is possible to use it for the acute stages of stroke, either alone or with readily available treatments, such as recombinant tissue plasminogen activator, anticoagulation, and antiplatelet therapy.
This study suggests that intranasal administration of SHED-CM may help in the recovery of acute stroke patients in future. In conclusion, regeneration therapy using SHED-CM is very safe with no associated problems; therefore, it is a potential candidate for the innovative treatment of cerebral ischemia.
Acknowledgments
We thank Mrs. Mami Naruse for her assistance. This work was supported by funds from the Collaborative Development of Innovative Seeds, Potentiality Verification Stage from Japan Science and Technology Agency, and a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture, Japan, #22890082 (M.S.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Donnan G.A. Fisher M. Macleod M. Davis S.M. Stroke. Lancet. 2008;371:1612. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 2.Honmou O. Houkin K. Matsunaga T. Niitsu Y. Ishiai S. Onodera R. Waxman S.G. Kocsis J.D. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011;134:1790. doi: 10.1093/brain/awr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bang O.Y. Lee J.S. Lee P.H. Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 4.Kern S. Eichler H. Stoeve J. Klüter H. Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura S. Yamada Y. Katagiri W. Sugito T. Ito K. Ueda M. Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. J Endod. 2009;35:1536. doi: 10.1016/j.joen.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 6.Pierdomenico L. Bonsi L. Calvitti M. Rondelli D. Arpinati M. Chirumbolo G. Becchetti E. Marchionni C. Alviano F. Fossati V. Staffolani N. Franchina M. Grossi A. Bagnara G.P. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation. 2005;80:836. doi: 10.1097/01.tp.0000173794.72151.88. [DOI] [PubMed] [Google Scholar]
- 7.Sugiyama M. Iohara K. Wakita H. Hattori H. Ueda M. Matsushita K. Nakashima M. Dental pulp-derived CD31{/CD146{ side population stem/progenitor cells enhance recovery of focal cerebral ischemia in rats. Tissue Eng Part A. 2011;17:1303. doi: 10.1089/ten.TEA.2010.0306. [DOI] [PubMed] [Google Scholar]
- 8.Nicaise C. Mitrecic D. Pochet R. Brain and spinal cord affected by amyotrophic lateral sclerosis induce differential growth factors expression in rat mesenchymal and neural stem cells. Neuropathol Appl Neurobiol. 2011;37:179. doi: 10.1111/j.1365-2990.2010.01124.x. [DOI] [PubMed] [Google Scholar]
- 9.Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci. 2000;11:1. doi: 10.1016/s0928-0987(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 10.Thorne R.G. Frey W.H., 2nd Delivery of neurotrophic factors to the central nervous system: pharmacokinetic considerations. Clin Pharmacokinet. 2001;40:907. doi: 10.2165/00003088-200140120-00003. [DOI] [PubMed] [Google Scholar]
- 11.Yang J.P. Liu H.J. Wang Z.L. Cheng S.M. Cheng X. Xu G.L. Liu X.F. The dose-effectiveness of intranasal VEGF in treatment of experimental stroke. Neurosci Lett. 2009;461:212. doi: 10.1016/j.neulet.2009.06.060. [DOI] [PubMed] [Google Scholar]
- 12.Gronthos S. Mankai M. Brahim J. Robey P.G. Shi S. Postnatal human dental pulp stem cells (DPSC) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miura M. Gronthos S. Zhao M. Lu B. Fisher L.W. Robey P.G. Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Longa E.Z. Weinstein P.R. Carlson S. Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 15.Leker R.R. Gai N. Mechoulam R. Ovadia H. Drug-induced hypothermia reduces ischemic damage: effects of the cannabinoid HU-210. Stroke. 2003;34:2000. doi: 10.1161/01.STR.0000079817.68944.1E. [DOI] [PubMed] [Google Scholar]
- 16.Ginsberg M.D. Adventures in the pathophysiology of brain ischemia: penumbra, gene expression, neuroprotection: the 2002 Thomas Willis Lecture. Stroke. 2003;34:214. doi: 10.1161/01.str.0000048846.09677.62. [DOI] [PubMed] [Google Scholar]
- 17.Leach M.J. Swan J.H. Eisenthal D. Dopson M. Nobbs M. BW619C89, a glutamate release inhibitor, protects against focal cerebral ischemic damage. Stroke. 1993;24:1063. doi: 10.1161/01.str.24.7.1063. [DOI] [PubMed] [Google Scholar]
- 18.Ooi A.T. Mah V. Nickerson D.W. Gilbert J.L. Ha V.L. Hegab A.E. Horvath S. Alavi M. Maresh E.L. Chia D. Gower A.C. Lenburg M.E. Spira A. Solis L.M. Wistuba I.I. Walser T.C. Wallace W.D. Dubinett S.M. Goodglick L. Gomperts B.N. Presence of a putative tumor-initiating progenitor cell population predicts poor prognosis in smokers with non-small cell lung cancer. Cancer Res. 2010;70:6639. doi: 10.1158/0008-5472.CAN-10-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frisén J. Johansson C.B. Török C. Risling M. Lendahl U. Rapid, widespread, and longlasting induction of nestin contributes to the generation of glial scar tissue after CNS injury. J Cell Biol. 1995;131:453. doi: 10.1083/jcb.131.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Itoh T. Satou T. Takemori K. Hashimoto S. Ito H. Neural stem cells and new neurons in the cerebral cortex of stroke-prone spontaneously hypertensive rats after stroke. J Mol Neurosci. 2010;41:55. doi: 10.1007/s12031-009-9279-3. [DOI] [PubMed] [Google Scholar]
- 21.Thorne R.G. Pronk G.J. Padmanabhan V. Frey W.H., 2nd Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127:481. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]