Abstract
This study investigates the effects of cyclic hydrostatic pressure (CHP) on chondrogenic differentiation of human adipose-derived stem cells (hASCs) in three-dimensional (3-D) agarose constructs maintained in a complete growth medium without soluble chondrogenic inducing factors. hASCs were seeded in 2% agarose hydrogels and exposed to 7.5 MPa CHP for 4 h per day at a frequency of 1 Hz for up to 21 days. On days 0, 7, 14, and 21, the expression levels of collagen II, Sox9, aggrecan, and cartilage oligomeric matrix protein (COMP) were examined by real-time reverse transcriptase–polymerase chain reaction analysis. Gene expression analysis found collagen II mRNA expression in only the CHP-loaded construct at day 14 and at no other time during the study. CHP-loaded hASCs exhibited upregulated mRNA expression of Sox9, aggrecan, and COMP at day 7 relative to unloaded controls, suggesting that CHP initiated chondrogenic differentiation of hASCs in a manner similar to human bone marrow-derived mesenchymal stem cells (hMSC). By day 14, however, loaded hASC constructs exhibited significantly lower mRNA expression of the chondrogenic markers than unloaded controls. Additionally, by day 21, the samples exhibited little measurable mRNA expression at all, suggesting a decreased viability. Histological analysis validated the lack of mRNA expression at day 21 for both the loaded and unloaded control samples with a visible decrease in the cell number and change in morphology. A comparative study with hASCs and hMSCs further examined long-term cell viability in 3-D agarose constructs of both cell types. Decreased cell metabolic activity was observed throughout the 21-day experimental period in both the CHP-loaded and control constructs of both hMSCs and hASCs, suggesting a decrease in cell metabolic activity, alluding to a decrease in cell viability. This suggests that a 2% agarose hydrogel may not optimally support hASC or hMSC viability in a complete growth medium in the absence of soluble chondrogenic inducing factors over long culture durations. This is the first study to examine the ability of mechanical stimuli alone, in the absence of chondrogenic factors transforming growth factor beta (TGF-β)3, TGF-β1 and/or bone morphogenetic protein 6 (BMP6) to induce hASC chondrogenic differentiation. The findings of this study suggest that CHP initiates hASC chondrogenic differentiation, even in the absence of soluble chondrogenic inductive factors, confirming the importance of considering both mechanical stimuli and appropriate 3-D culture for cartilage tissue engineering using hASCs.
Introduction
Treatments for cartilage defects due to trauma, genetic predisposition, or metabolic conditions are often invasive and only serve to temporarily reduce joint pain. The challenges with these treatments have encouraged further investigations into new methods of cartilage repair. Stem cells from various sources have been shown to differentiate into multiple musculoskeletal tissues, including cartilage, making them a promising prospect for the treatment of cartilage defects.1–4 While human bone marrow-derived mesenchymal stem cells (hMSCs) have shown promise for cartilage regeneration, the relative population of these cells decreases with age and in cases of osteoporosis. Additionally, when the cells proliferate excessively in vitro, it can negatively affect the differentiation potential of the cells. These issues are key because many cell therapy techniques require high-density cell seeding for successful tissue engineering or regeneration.5,6 For this reason, interest has increased in the use of human-adipose derived stem cells (hASCs), a relatively more abundant and accessible population of multipotent cells.5,7–9 hASCs have the ability to differentiate down multiple lineages,5 similar to hMSCs, and the harvesting procedure may be more tolerable to many patients.
Previous investigations have shown successful hASC chondrogenic differentiation in the presence of appropriate soluble chondrogenic induction factors.5,6 However, some studies have reported that hMSCs have higher chondrogenic potential than hASCs under the same culture conditions.1–3,6,10–13 The expression profile and response to growth factors seen in these studies strongly indicate that hMSCs and hASCs are not identical cell populations, and thus require distinct growth factor supplementation,6 and possibly different external environmental cues, such as cyclic hydrostatic pressure (CHP), to optimally promote chondrogenesis.6
Although chemical stimuli have been found to induce chondrogenic differentiation in hMSCs and hASCs, the chemically differentiated cell constructs that are produced do not possess sufficient material properties to withstand the in vivo loading environment associated with their function.7 The mechanical environment is a critical factor in the differentiation of various stem cell types, suggesting that the in vivo loading environment may be just as critical in creating functional differentiated stem cell constructs.2,14–16 Theoretical models predict that different mechanical loading modalities can direct tissue differentiation down specific lineages. More specifically, these models predict cartilage formation under hydrostatic pressure-loading modalities.2,10,17,18 Previous studies have demonstrated that intermittent loading of hydrostatic pressure within the physiological range of 7–10 MPa results in increased matrix synthesis and improved chondrogenesis in chondrocytes.19,20 Further, we and others have empirically shown that hMSCs in three-dimensional (3-D) culture can be chondrogenically induced by CHP with and without chemical stimuli2,21,22; however, to our knowledge, no previous studies have been performed to determine whether hASCs can be induced to differentiate down a chondrogenic pathway with mechanical stimuli alone. A recent study by Ogawa et al.23 demonstrated chondrogenic differentiation of ASC in a 3-D collagen scaffold with the combined treatment of CHP and chemical stimuli23; however, the authors did not investigate the effect of CHP alone.23
The purpose of this study was to determine if, in the absence of a chondrogenic medium (i.e., a medium including transforming growth factor beta [TGF-β]-1, -3, and/or bone morphogenetic protein-6 [BMP6]), hASCs could be chondrogenically differentiated in response to CHP in a 3-D culture. We hypothesize that CHP without chemical stimulation will induce chondrogenesis of hASCs, as evidenced by upregulation of chondrogenic gene markers Sox9, aggrecan, and/or cartilage oligomeric matrix protein (COMP), consistent with results seen in previous hMSC studies.2
Further, we investigate the viability of hASCs as well as hMSCs in response to CHP. This component of the study was to determine the effect of our loading and culture parameters on the viability of either hASCs or hMSCs over time. The effects of the loading environment on inducing chondrogenesis may vary over time, and longer studies may provide better insight on how to best utilize this mechanical stimuli in tissue engineering. By observing the effects of mechanical loading and the agarose hydrogel construct on cell viability over time, we are better able to assess the suitability of agarose hydrogel constructs and long-term loading environments for hASC or hMSC cell culture in cartilage tissue engineering.
Materials and Methods
Cell culture and construct formation
Excess adipose tissue was obtained from two donors (50- and 51-year-old Caucasian women) in accordance with an approved IRB protocol at UNC Chapel Hill (IRB 04-1622). hASCs were isolated from the tissue using a method described by Zuk et al.5 as previously described by our laboratory.24–26 The cell lines were then examined for their differentiation potential as previously described26 using a 14-day monolayer differentiation assay where cells were assessed on their ability to differentiate down bone and adipose tissue pathways when cultured in osteogenic and adipogenic media. Further, these cell lines were cultured together with three other cell lines in a pellet culture for up to 21 days in a chondrogenic medium to assess chondrogenic potential.
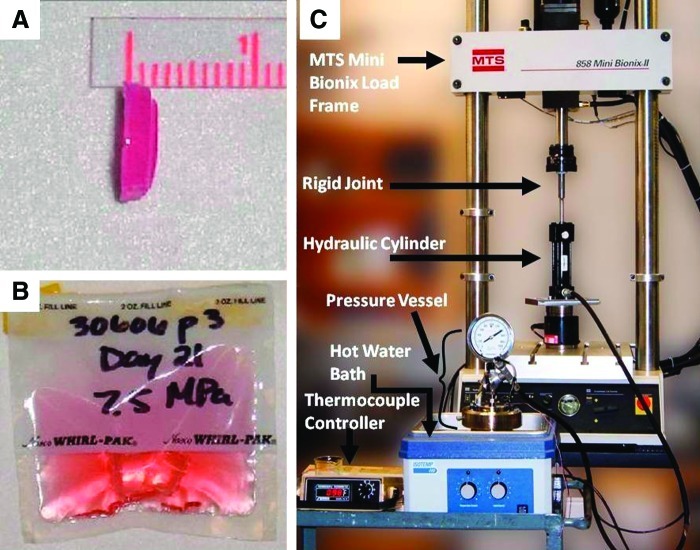
At passage 2, the cells were seeded in a complete growth medium (CGM) comprised of an alpha-modified minimal essential medium with l-glutamine (Invitrogen Life Technologies) supplemented with 10% fetal bovine serum (Premium Select; Atlanta Biologicals), 200 mM l-glutamine, and 100 IU penicillin/100 μg streptomycin per mL (Mediatech, Inc.). The cells were allowed to proliferate at 37°C in 5% carbon dioxide until reaching 90% confluency. They were then detached with trypsin and suspended in an equal volume mixture of growth medium and low-melt 4% type VII agarose (Sigma) to produce a 2% agarose solution with 9×106 hASCs/mL. The agarose-cell solution was molded into constructs in a 0.4-μm polycarbonate membrane, 10-mm tissue culture inserts (Nunc), and allowed to polymerize at room temperature for 15 min. The constructs' final dimensions were 10 (diameter)×2 mm (height) (Fig. 1A). Once the polymerization was complete, the constructs were placed in the CGM and incubated for 24 h, with a medium change after 12 h. After 24 h, the constructs were placed in heat-sealed polyethylene bags (two constructs/bag; NASCO) containing 15 mL of CGM (Fig. 1B). Bags and medium were changed every 3–4 days.
FIG. 1.
hASC-seeded 2% agarose gel construct removed from polycarbonate tissue culture insert (A), two constructs with 15 mL of growth medium in a heat-sealed bag to be mechanically loaded in hydrostatic pressure vessel (B), and labeled hydrostatic pressure vessel setup (C). hASC, human adipose-derived stem cell. Color images available online at www.liebertpub.com/tea
Application of hydrostatic pressure
CHP was applied to an hASC-seeded agarose construct using a custom-designed system consisting of a pressure vessel, hydraulic cylinder, and high-pressure tubing as described in our previous work (Fig. 1C).2 The hydraulic cylinder was mounted to an MTS 858 Mini Bionix II load frame. The actuator of the Mini Bionix provided the power for the system. The hydraulic cylinder was connected to a 1-L stainless steel pressure vessel (Parr Instruments) via high-pressure stainless steel tubing. The hydraulic cylinder, tubing, and pressure vessel were all filled with paraffin oil (STE Oil) to maintain the lubrication of the piston device within the hydraulic cylinder during loading cycles. The pressure vessel was stored in a water bath to maintain a temperature of 37°C inside the vessel as monitored by a thermocouple controller. The control program MTS TestStar (MTS System Corp.) was used to monitor and control the magnitude and frequency of CHP applied to the experimental groups. Validation testing was performed to ensure that the MTS load frame was able to exert the necessary forces required to pressurize the vessel to 7.5 MPa and that the system remained stable at a 1-Hz loading frequency.
Experimental groups consisted of a control group (unloaded) and a CHP group (loaded) for each of the two donors, with each group having four constructs (n=2 per donor per condition). The control group was placed in a static culture in an oil-filled container within an incubator while the other constructs were loaded. The CHP group was loaded at 7.5 MPa at 1 Hz for 4 h/day for up to 21 days. Previous studies by our laboratory and others have demonstrated that intermittent loading of hydrostatic pressure within the physiological range of 7–10 MPa results in increased matrix synthesis and improved chondrogenesis in both chondrocytes and hMSCs.2,19,20 Additionally, our previous work has shown that a steady cyclic loading protocol was more effective in inducing chondrogenesis in bone-marrow-derived stem cells than ramped cyclic loading.2
Real-time reverse transcriptase–polymerase chain reaction
Constructs from each condition were collected on days 0, 7, 14, and 21 of loading for quantitative reverse transcriptase–polymerase chain reaction (RT-PCR) analysis. Each construct was dissolved in an RNA lysis buffer, and total RNA was isolated using a Perfect RNA Eukaryotic Mini kit (Eppendorf). Complementary DNA was synthesized using a SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen Life Technologies) with oligo(dt)20 primers. Primers and probes for human collagen II (Assay HS00264051 M1), aggrecan (Assay HS00153936 M1), Sox9 (Assay HS00165814 M1), COMP (Assay HS00164359 M1), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Assay HS99999905 M1) were purchased from Assays-on-demand (Applied Biosystems). Real-time RT-PCR was performed using TaqMan PCR Master Mix (Applied Biosystems) in an ABI Prism 7000 system (Applied Biosystems). Signals were normalized to GAPDH expression levels.
Histological analysis
One construct from each condition was collected on days 0, 7, 14, and 21 for histological analysis. The constructs were fixed in 10% formalin and embedded into paraffin blocks. The paraffin blocks were sectioned and stained with Fast Green FCF (Fisher Chemical). The constructs were viewed at room temperature with a Leica DM LFSA microscope equipped with a 40× water immersion, high-resolution camera, and SimplePCI image capture and analysis software (Compix).
Experimental replication for analysis of viability of both hASCs and hMSCs in 3-D agarose culture in the presence and absence of CHP
hASCs and human bone marrow-derived cells (hMSCs) (Cambrex) were seeded at passages 4 and 5, respectively, at 5000 cells/cm2 in a growth medium and allowed to proliferate at 37°C in 5% CO2 until reaching 90% confluency. Cell-seeded agarose constructs were created as described above to create groups of four constructs per experimental group consisting of a control group (unloaded) and a steady CHP group (loaded) for each of the two donors (n=2 per donor per condition). CHP was applied as described above, and unloaded controls were maintained in a static culture as previously described.
AlamarBlue analysis
On days 0, 7, 14, and 21, both loaded and unloaded constructs of each cell type were placed in 10% alamarBlue (AbD Serotec Ltd.) and 90% growth medium. The constructs were kept in the incubator in static culture within heat-sealed bags for 12 h. After 12 h, the alamarBlue/growth medium mixture was collected from the heat-sealed bags and analyzed using Tecan GENios with Magellan 5 software (Tecan) to obtain absorbance readings at 570 and 600 nm for viability analyses. Percent reduction of the alamarBlue, indicating proliferative activity, was determined from the absorbance readings using the standard manufacturer protocols (AbD Serotec Ltd.).
Statistical analysis
Real time RT-PCR results were quantified and compared using the method described by Livak and Schmittgen.27 Student t-tests were used to determine significance with p-values<0.05. Data are presented as mean±standard errors.
Results
Differentiation potential
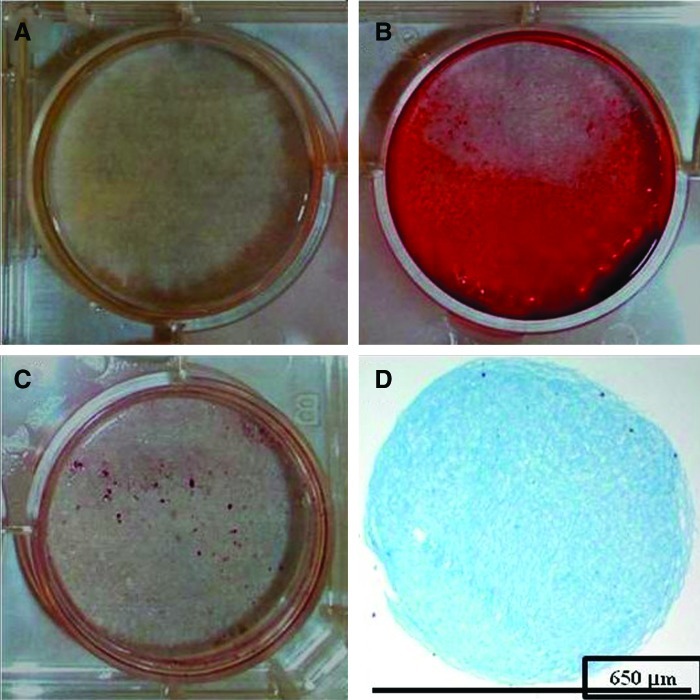
Both cell lines demonstrated at least 30–50% bone and adipose differentiation after 14 days in monolayer culture as observed by positive alizarin red and Oil Red O staining, respectively (Fig. 2A–C). In pellet culture, the cell lines demonstrated positive chondrogenic differentiation with a positive Alcian blue stain after 21 days in a chondrogenic medium (Fig. 2D).
FIG. 2.
hASCs demonstrate osteogenic, adipogenic, and chondrogenic differentiation potential. Monolayer culture of hASCs for 14 days in a growth medium or an osteogenic medium and stained with alizarin red for calcium deposition, respectively (A, B). Monolayer culture of hASCs for 14 days in an adipogenic medium stained with Oil Red O (C). Pellet culture of cell lines for 21 days in a chondrogenic medium stained with Alcian blue (D). Color images available online at www.liebertpub.com/tea
CHP system validation
This custom system for applying CHP has been previously tested and validated.2 Experimental validation was repeated here, confirming that the MTS was capable of pressurizing the vessel in excess of 7.5 MPa. Validation tests demonstrated a linear relationship between the generated force and the achieved pressure within the vessel. It should be noted that temperature increases within the vessel have been previously observed during CHP for loading frequencies over 3 Hz, though these temperature fluctuations did not occur in this study, as the frequency was limited to 1 Hz. Some variance was observed in the temperature of the water bath, rarely requiring modest adjustments to maintain a temperature of 37°C within the vessel. The temperature of the water bath before and during all loading was maintained at 37°C.2
CHP effects on gene expression
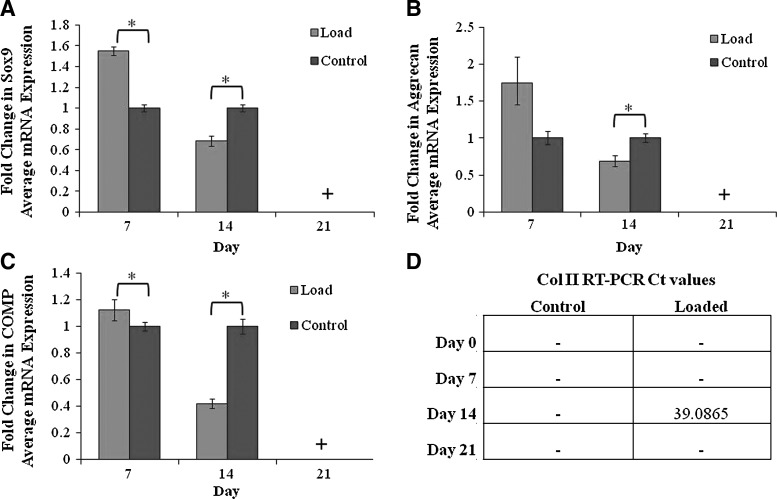
Messenger RNA expression levels of Sox9, aggrecan, collagen II, and COMP mRNA were normalized to GAPDH. Experimental constructs exposed to CHP of magnitude 7.5 MPa (4 h/day, f=1 Hz) were compared to control constructs maintained in static culture. Sox9, aggrecan, and COMP mRNA expression levels were upregulated relative to unloaded control constructs at day 7 (Fig. 3A–C). Sox9, aggrecan and COMP mRNA expression levels then decreased below those of the unloaded control constructs on day 14 (Fig. 3A–C). By day 21, there was no mRNA expression for Sox9, aggrecan, or COMP in the either loaded or unloaded control constructs. Additionally, the constructs demonstrated low levels of GAPDH expression; therefore, we have reported no mRNA expression for day 21 in both the loaded and unloaded control constructs. Quantitative RT-PCR analysis found very slight collagen II mRNA expression in only the CHP-loaded construct at day 14 with very high RT-PCR Ct values, whereas no Ct values were able to be determined in any other construct at any other time point (Fig. 3D).
FIG. 3.
hASC Sox9 messenger ribonucleic acid (mRNA) expression levels (A), hASC aggrecan mRNA expression levels and (B), COMP mRNA expression levels (C) of loaded and unloaded hASC constructs. All signals were normalized to GAPDH.+denotes data not reported for loaded and unloaded controls at day 21 due to low GAPDH expression (*p<0.05). hASC collagen II mRNA RT-PCR Ct values (- denotes no expression detected). COMP, cartilage oligomeric matrix protein; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; RT-PCR, reverse transcriptase–polymerase chain reaction.
AlamarBlue analysis
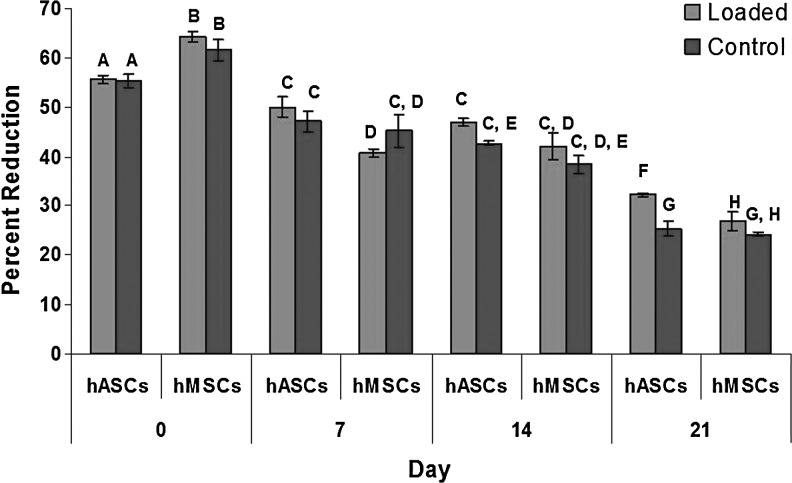
The relative percent reduction of alamarBlue decreased throughout the entire experiment with the highest reading on day 0 and the lowest on day 21 indicating a decrease in proliferation (Fig. 4). Between day 7 and 14, there was a little change between the percent reduction readings; however, between day 0 and 7, and day 14 and 21, there was a significant decrease of percent reduction in both the control and loaded hASC- and hMSC-seeded agarose constructs (Fig. 4).
FIG. 4.
Average percent reduction of alamarBlue for hASCs and hMSCs. Different letters represent statistical differences (p<0.05). Data presented as mean±standard error. hMSC, human bone marrow-derived mesenchymal stem cell.
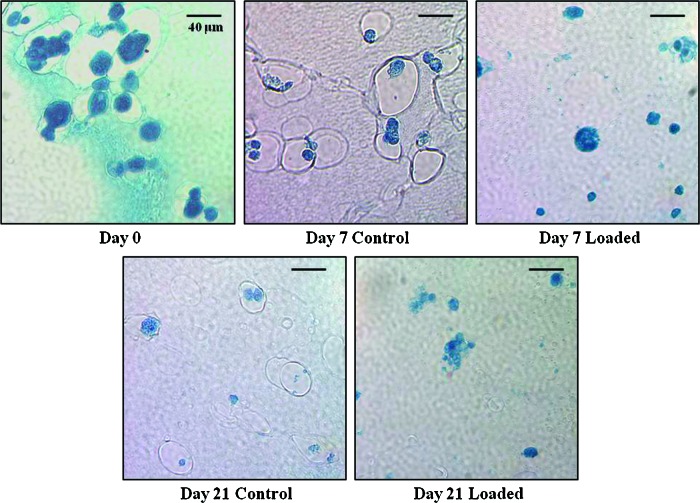
Histological analysis
The lack of cell proliferation over time was further investigated by histological analyses. One construct from each condition was stained with Fast Green FCF to visualize the cytoplasm of the cells and to provide data on the quantity and morphology of the cells for each condition. The staining revealed a smaller number of cells present on day 21 in both loaded and control constructs as compared to days 0, 7, and 14 (Fig. 5). Further, many of the cells had lost their round, spherical morphology by day 21 relative to their morphologies at days 0, 7, and 14 (Fig. 5).
FIG. 5.
Cytoplasm-staining Fast Green FCF results for days 0, 7, and 21 control and loaded. Color images available online at www.liebertpub.com/tea
Discussion
The early upregulation of Sox9, aggrecan, and COMP mRNA expression in the CHP-loaded hASC samples on day 7 suggests early chondrogenesis. The level of upregulation seen at day 7 is similar to the upregulation seen in an experiment performed previously in our laboratory with hMSCs.2 In that previous study with hMSCs, chondrogenic markers were highly upregulated by day 4, and decreased significantly by day 9. In this experiment, markers were upregulated significantly in loaded samples at day 7, with a similar decrease in expression by day 14. This suggests that hASCs might respond to CHP in a similar, but not identical, manner as hMSCs, with respect to CHP-induced chondrogenic differentiation potential.9 Both experiments demonstrate the influence of CHP on chondrogenic gene expression in the absence of known chondrogenic inducing soluble factors, such as TGF-β1, β3, and BMP-6, while identifying CHP sensitive gene markers. While this study investigated the long-term effects of CHP loading, focusing on days 7, 14, and 21, the results suggest that peak expression of some chondrogenic markers upregulated by CHP may occur between day 0 and 7. Future experiments should examine timepoints before day 7 to determine the day of maximal expression of these chondrogenic markers in hASCs.
GAPDH is typically strongly expressed in hASCs, and the lack of polymerase chain reaction signal in any day-21 samples, loaded or unloaded, suggested that hASC viability might have decreased as a result of the 3-D agarose culture used. This conclusion was supported with the results of the Fast Green staining. The lack of any mRNA expression at day 21 was investigated by Fast Green analysis. The decrease in hASCs present on day 21 suggests a decrease in cell viability due to the breakdown of cells, a lack of proliferation as previously demonstrated by Awad et al.,7 or a leaching of the cells out of the construct, commonly seen with long-term culture in agarose and alginate. Future studies concentrated on DNA quantification both in the scaffold and in the medium throughout culture are needed to truly understand the reason for the decrease in the cell number.
Additionally, the cells began to lose their characteristic spherical morphology by day 21. While some studies have linked mechanical loading with changes in cell morphology, these changes were observed in both loaded and unloaded samples.28 The spherical shape has been shown to be a key factor in successful chondrogenic differentiation,29 and the change in morphology suggests the decomposition of the chondrogenic phenotype in hASCs by day 21.
The experiment was replicated with both hASCs and hMSCs to verify that there was a lack of cell viability or decrease in the cell number in both cell types by day 21. Human MSCs were included in the study with hASCs, since previous hMSC CHP studies in our laboratory were only carried out to day 14.2 AlamarBlue is a measure of cell metabolism, and thus a decrease in alamarBlue reduction could indicate reduced metabolic activity or a decrease in viability or cell number. The decreased alamarBlue reduction in both cell lines, in addition to our histological analysis, suggests a decrease in the cell number and proliferation of hASCs and hMSCs in agarose by day 21. These results, observed in both loaded and control constructs, suggest that the agarose constructs were the primary factor in the decreased viability or number of the cells. The use of heat-sealed bags for culture of the loaded and control groups could additionally have an effect on the viability of the cells through oxygen limitations; however, heat-sealed bags are commonly used in hydrostatic pressure experiments without report of decreased viability.
These decreased viability and proliferation findings are consistent with those of Awad et al.,7 who found that hASCs seeded in agarose constructs, similar to those used in this experiment, both with and without soluble chondrogenic inducing factors in the medium, have been shown to only proliferate until day 7.7 The cells exhibited a decreasing DNA count starting at day 7, which they attributed to a lack of natural ligands for cell–matrix adhesion.7 Gelatins containing natural cell surface ligands allow hASC proliferation until day 14, resulting in a much higher DNA count than agarose constructs through day 28.7 Our study has demonstrated that CHP mechanical loading does not appear to negatively affect cell viability; however, agarose constructs, both loaded and unloaded, do not support long-term cell viability or maintenance of cell number. While agarose gel constructs have been shown to support chondrogenic differentiation in MSCs30,31 and the production of cartilaginous extracellular matrix by seeded chondrocytes,30,32 these constructs may not be suitable for long-term culture of hASCs or hMSCs for cartilage repair. Although proliferation is expected to decrease with chondrogenic differentiation, the additional time for proliferation that matrices containing ligands provide may improve cell viability and later chondrogenesis. Hydrogels and other biomaterials with extracellular matrix cell-binding ligands should be investigated to optimally analyze the effects of CHP on stem cells. Additionally, future studies using live/dead assays and DNA quantification in both the scaffold and medium should be performed to evaluate the contribution of cell death and cell leaching to the decrease in cell number with time in culture.
This study has demonstrated that applying CHP to hASCs in 3-D agarose culture upregulates some early chondrogenic gene markers, in the absence of known soluble chondrogenic induction factors. An early study of cyclic compression of rabbit BM-MSCs suggested that this method of mechanical loading was equivalent to TGF-β1 in its effectiveness of inducing chondrogenic differentiation, implying that the mechanical loading induced synthesis of TGF-β1 to complete this function.31 Future CHP studies observing mRNA expression at earlier time points and improved methods for 3-D cell culture are needed to build on these observations. While the exact mechanisms by which CHP induces chondrogenesis are still being explored, further studies must be conducted in this field to determine the optimum physical, chemical, and 3-D biomaterial environments to induce chondrogenic differentiation of multipotent cells, in particular hASCs, for the treatment of cartilage defects.
Acknowledgments
The authors would like to thank all the members of the Cell Mechanics Laboratory, especially Neil Shah and John Petitte. They also thank Drs. John van Aalst and Wolfgang Losken for the adipose samples, and Drs. Peter Mente and Chris Ashwell for their helpful advice. Support for this research was provided by an NC Biotechnology Center Institutional Development Grant (E.G.L.) and by an NIH/NIBIB grant R03EB008790 (E.G.L.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Afizah H. Yang Z. Hui J.H.P. Ouyang H.W. Lee E.H. A comparison between the chondrogenic potential of human bone marrow stem cells (BMSC) and adipose-derived stem cells (ADSCs) taken from the same donors. Tissue Eng. 2007;13:659. doi: 10.1089/ten.2006.0118. [DOI] [PubMed] [Google Scholar]
- 2.Finger A.R. Sargent C.Y. Katherine D.O. Bernacki S.H. Loboa E.G. Differential effects on messenger ribonucleic acid expression by bone marrow-derived human mesenchymal stem cells seeded in agarose constructs due to ramped and steady applications of cyclic hydrostatic pressure. Tissue Eng. 2007;13:1151. doi: 10.1089/ten.2006.0290. [DOI] [PubMed] [Google Scholar]
- 3.Huang J.I. Kazmi N. Durbhakula M.M. Hering T.M. Yoo J.U. Johnstone B. Chondrogenic potential of progenitor cells derived from human bone marrow and adipose tissue: a patient-matched comparison. J Orthop Res. 2005;23:1383. doi: 10.1016/j.orthres.2005.03.008.1100230621. [DOI] [PubMed] [Google Scholar]
- 4.Mackay A.M. Beck S.C. Murphy J.M. Barry F.P. Chichester C.O. Pittenger M.F. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 5.Zuk P.A. Zhu M. Mizuno H. Huang J. Futrell J.W. Katz A.J. Benhaim P. Lorenz H.P. Hedrick M.H. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 6.Puetzer J.L. Petitte J.N. Loboa E.G. Compartive review of growth factors for induction of three-dimensional in vitro chondrogenesis in human mesenchymal stem cells isolated from bone marrow and adipose tissue. Tissue Eng. 2010;4:435. doi: 10.1089/ten.TEB.2009.0705. [DOI] [PubMed] [Google Scholar]
- 7.Awad H.A. Wickham M.Q. Leddy H.A. Gimble J.M. Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25:3211. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 8.Gimble J.M. Katz A.J. Bunnell B.A. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J.I. Zuk P.A. Jones N.F. Zhu M. Lorenz H.P. Hedrick M.H. Benhaim P. Chondrogenic potential of multipotential cells from human adipose tissue. Plast Reconstr Surg. 2004;113:585. doi: 10.1097/01.PRS.0000101063.27008.E1. [DOI] [PubMed] [Google Scholar]
- 10.Carter D.R. Beaupré G.S. Giori N.J. Helms J.A. Mechanobiology of skeletal regeneration. Clin Orthop Relat Res. 1998;355:S41. doi: 10.1097/00003086-199810001-00006. [DOI] [PubMed] [Google Scholar]
- 11.Im G.I. Shin Y.W. Lee K.B. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthritis Cartilage. 2005;13:845. doi: 10.1016/j.joca.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Kim H.J. Im G.I. Chondrogenic differentiation of adipose tissue-derived mesenchymal stem cells: greater doses of growth factor are necessary. J Orthop Res. 2009;27:612. doi: 10.1002/jor.20766. [DOI] [PubMed] [Google Scholar]
- 13.Hennig T. Lorenz H. Thiel A. Goetzke K. Dickhut A. Geiger F. Richter W. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGF beta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol. 2007;211:682. doi: 10.1002/jcp.20977. [DOI] [PubMed] [Google Scholar]
- 14.Sharma B. Elisseeff J.H. Engineering structurally organized cartilage and bone tissues. Ann Biomed Eng. 2004;32:148. doi: 10.1023/b:abme.0000007799.60142.78. [DOI] [PubMed] [Google Scholar]
- 15.Mauck R.L. Soltz M.A. Wang C.C. Wong D.D. Chao P.H. Valhmu W.B. Hung C.T. Ateshian G.A. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 16.Caplan A.I. Tissue engineering designs for the future: new logics, old molecules. Tissue Eng. 2000;6:1. doi: 10.1089/107632700320838. [DOI] [PubMed] [Google Scholar]
- 17.Carter D.R. Blenman P.R. Beaupré G.S. Correlations between mechanical stress history and tissue differentiation in initial fracture healing. J Orthop Res. 1988;6:736. doi: 10.1002/jor.1100060517. [DOI] [PubMed] [Google Scholar]
- 18.Loboa E.G. Beaupré G.S. Carter D.R. Mechanobiology of initial pseudarthrosis formation with oblique fractures. J Orthop Res. 2001;19:1067. doi: 10.1016/S0736-0266(01)00028-6. [DOI] [PubMed] [Google Scholar]
- 19.Elder B.D. Athanasiou K.A. Hydrostatic pressure in articular cartilage tissue engineering: from chondrocytes to tissue regeneration. Tissue Eng. 2009;15:43. doi: 10.1089/ten.teb.2008.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grad S. Eglin D. Alini M. Stoddart M.J. Physical stimulation of chondrogenic cells in vitro: a review. Clin Ortho Relat Res. 2011;469:2764. doi: 10.1007/s11999-011-1819-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angele P. Yoo J.U. Smith C. Mansour J. Jepsen K.J. Nerlich M. Johnstone B. Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro. J Orthop Res. 2003;21:451. doi: 10.1016/S0736-0266(02)00230-9. [DOI] [PubMed] [Google Scholar]
- 22.Miyanishi K. Trindade M.C. Lindsey D.P. Beaupré G.S. Carter D.R. Goodman S.B. Schurman D.J. Smith R.L. Dose- and time-dependent effects of cyclic hydrostatic pressure on transforming growth factor-b3-induced chondrogenesis by adult human mesenchymal stem cells in vitro. Tissue Eng. 2006;12:2253. doi: 10.1089/ten.2006.12.2253. [DOI] [PubMed] [Google Scholar]
- 23.Ogawa R. Mizuno S. Murphy G.F. Orgill D.P. The effect of hydrostatic pressure on three-dimensional chondroinduction of human adipose-derived stem cells. Tissue Eng. 2009;15:2937. doi: 10.1089/ten.tea.2008.0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCullen S.D. Stevens D.R. Roberts W.A. Clarke L.I. Bernacki S.H. Gorga R.E. Loboa E.G. Characterization of electrospun nanocomposite scaffolds and biocompatibility with adipose-derived human mesenchymal stem cells. Int J Nanomed. 2007;2:253. [PMC free article] [PubMed] [Google Scholar]
- 25.Wall M.E. Rachlin A. Otey C.A. Loboa E.G. Human adipose-derived adult stem cells upregulate paladin during osteogenesis and in response to cyclic tensile strain. Am J Physiol Cell Physiol. 2007;293:C1532. doi: 10.1152/ajpcell.00065.2007. [DOI] [PubMed] [Google Scholar]
- 26.Bernacki S.H. Wall M.E. Loboa E.G. Isolation of human mesenchymal stem cells from bone and adipose tissue. Methods Cell Biol. 2008;86:257. doi: 10.1016/S0091-679X(08)00011-3. [DOI] [PubMed] [Google Scholar]
- 27.Livak K.J. Schmittgen T.D. Analysis of relative gene expression data using real time quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Cheng C. Steward R. LeDuc P. Probing cell structure by controlling the mechanical environment with cell-substrate interactions. J Biomech. 2009;42:187. doi: 10.1016/j.jbiomech.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Guilak F. Estes B.T. Diekman B.O. Moutos F.T. Gimble J.M. Nicolas Andry Award: multipotent adult stem cells from adipose tissue for musculoskeletal tissue engineering. Clin Orthopedics Relat Res. 2010;468:2530. doi: 10.1007/s11999-010-1410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang C.-YC. Reuben P.M. D'Ippolito G. Schiller P.C. Cheung H.S. Chondrogenesis of human bone marrow-derived mesenchymal stem cells in agarose culture. Anat Rec Part A Discov Mol Cell Evol Biol. 2004;278:428. doi: 10.1002/ar.a.20010. [DOI] [PubMed] [Google Scholar]
- 31.Huang C-YC. Hagar K.L. Frost L.E. Sun Y. Cheung H.S. Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem Cells. 2004;22:313. doi: 10.1634/stemcells.22-3-313. [DOI] [PubMed] [Google Scholar]
- 32.Buschmann M.D. Gluzband Y.A. Grodzinsky A.J. Kimura J.H. Hunziker E.B. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J Ortho Res. 1992;10:745. doi: 10.1002/jor.1100100602. [DOI] [PubMed] [Google Scholar]