Abstract
Bone morphogenetic protein-2 (BMP-2) is known to induce both osteogenic and chondrogenic commitment of human mesenchymal stem cells (hMSCs). However, factors influencing BMP-2-dependent chondrogenic and osteogenic differentiation have not been investigated. In this study, we demonstrated that extracellular microenvironments, in the form of cell-derived matrices, play important roles in determining the specific lineage commitment of hMSCs in the presence of BMP-2. Extracellular matrices (ECMs) derived from osteoblasts and chondrocytes were utilized to regulate cell differentiation. Osteogenic and chondrogenic differentiation of hMSCs cultured on the two different cell-derived ECMs were assessed by quantitative real-time–polymerase chain reaction, immunocytochemistry, and western blot analysis. To minimize the effects of the cell-adhesion proteins contained in serum on the ECMs, hMSCs were cultured in serum-free osteogenic or chondrogenic differentiation medium. Fibronectin-, collagen type I-, or collagen type II-coated substrates were utilized as ECM controls. The ECM specific to each cell type promoted lineage-specific commitment of hMSCs in the presence of BMP-2, that is, osteoblast- and chondrocyte-derived ECM promoted osteogenic and chondrogenic commitment, respectively. Therefore, cell-specific ECMs are capable of modulating the BMP-2-induced osteogenic and chondrogenic differentiation of hMSCs.
Introduction
Human mesenchymal stem cells (hMSCs) are multipotent cells that are capable of differentiating into various cell types, such as osteoblasts, chondrocytes, and adipocytes. To a large extent, the therapeutic potential of hMSCs in tissue engineering applications is attributed to their ability to differentiate. The commitment of hMSCs to specific lineages is regulated by various signals from their microenvironment; it is very important to identify key factors that induce these signals for well-controlled in vitro differentiation of hMSCs for clinical use.1 In addition to the soluble signals, the insoluble factors that comprise the extracellular matrix (ECM) components play important roles in driving hMSCs to differentiate into specific cell types.2–10 Cell adhesion receptors transmit biological information from the ECM to the cells, modulate intracellular signaling cascades, and consequently, regulate key genes involved in the lineage-specific commitment of hMSCs.9,11,12
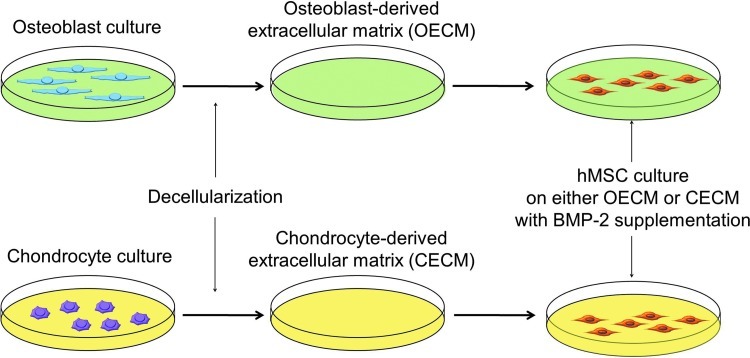
Bone morphogenetic protein-2 (BMP-2), a member of the transforming growth factor super family, is known to induce chondrogenic or osteogenic differentiation of MSCs.13,14 However, mechanisms for the BMP-2-mediated hMSC commitment to chondrocyte or osteoblast phenotypes have yet to be elucidated. The cell-derived ECM is a complex network of structural and functional macromolecules that provide cellular support and biochemical cues for physiological and phenotypic regulations.15,16 The aim of this research was to identify the ECM factors that influence the chondrogenic and osteogenic commitment of hMSCs in the presence of BMP-2. We hypothesized that BMP-2-mediated chondrogenic and osteogenic differentiation of hMSCs could be modulated by the type of cells from which the ECM was derived (Fig. 1). To test this hypothesis, we characterized an osteoblast-derived extracellular matrix (OECM) and a chondrocyte-derived extracellular matrix (CECM), and then evaluated their contributions to the osteogenic and chondrogenic differentiation of hMSCs. Further, the effects of OECM and CECM on hMSC differentiation were compared with the effects of fibronectin, collagen type I, and collagen type II.
FIG. 1.
A schematic diagram of the experimental design. Color images available online at www.liebertpub.com/tea
Materials and Methods
Osteoblast and chondrocyte isolation
Rat osteoblasts were isolated as previously described with minor modifications.17 Briefly, osteoblasts were isolated by an enzymatic digestive process from the calvaria of neonatal (less than 1 day old) Sprague-Dawley rats (SLC, Tokyo, Japan). The calvariae were isolated, and the connective tissues were carefully removed. The parietal bones were minced into pieces using sterile surgical scissors. The osteoblasts were isolated by an enzyme solution containing 1.37 mg/mL collagenase type I (Sigma, St. Louise, MO) and 0.5 mg/mL trypsin (Sigma). Following a 30 min incubation period, the released cells were discarded to prevent contamination with other cell types. The minced bones were re-digested with the enzyme solution for 15 min, and the supernatant was transferred to Dulbecco's modified Eagle's medium (DMEM; Gibco-BRL, Gaithersburg, MD) containing 10% (v/v) fetal bovine serum (FBS; Gibco-BRL) and 1% (v/v) penicillin–streptomycin (PS; Gibco-BRL). This process was repeated thrice. Finally, the collected solution was centrifuged for 10 min at 400 g. The cells were plated into tissue culture plates and cultured in a humidified incubator at 37°C under 5% (v/v) CO2.18
Articular chondrocytes were obtained from 6-week-old New Zealand white rabbits (Orient, Seoul, South Korea) as previously described.19 In brief, the cartilage fragments were minced, washed thrice in phosphate-buffered saline (PBS; Sigma), and digested with 0.05% (w/v) collagenase type II (Sigma) in DMEM/F-12 (Gibco-BRL) containing 10% (v/v) FBS and 1% (v/v) PS for 10 h. The recovered cells were washed in PBS and cultured in DMEM/F12 medium containing 10% (v/v) FBS and 1% (v/v) PS. The medium was changed every other day.
Preparation of OECM and CECM by decellularization
OECM and CECM were generated as previously described.20–22 In brief, primary rat osteoblasts and rabbit chondrocytes from passage 1 were expanded to confluency for 2 weeks and rinsed in PBS. Next, the cells were treated with PBS containing Triton X-100 (0.5%) and 20 mM NH4OH for 5 min at 37°C. The matrices were treated with DNase I (Sigma; 100 μg/mL) 1 h at 37°C, and then rinsed thrice with PBS. The obtained matrices were stored at 4°C in PBS.
Scanning electron microscopy
Osteoblasts+OECM and chondrocytes+CECM prior to decellularization, OECM, and CECM were fixed in 1% (v/v) buffered glutaraldehyde and 0.1% (v/v) buffered formaldehyde for 1 and 24 h, respectively, dehydrated with a graded ethanol series, and dried. The dried samples were mounted on an aluminum stub, coated with gold using a Sputter Coater (Cressington 108; Cressington Scientific Instruments, Cranberry Twp, PA), and examined via SEM (JSM-6701F; Jeol, Tokyo, Japan).
Confirmation of decellularization by immunocytochemistry
Osteoblasts+OECM and chondrocytes+CECM prior to decellularization, OECM, and CECM were fixed with 4% (w/v) paraformaldehyde (PFA) for 10 min at room temperature and rinsed with PBS. The slides were mounted with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA) to confirm the decellularization of osteoblasts and chondrocytes. The slides were photographed with a fluorescent microscope (IX71; Olympus, Tokyo, Japan).
Characterization of OECM and CECM by histochemistry
OECM and CECM were fixed with 4% (w/v) PFA for 10 min at room temperature and rinsed with PBS. For alcian blue staining, OECM and CECM were immersed in 0.5% (w/v) alcian blue in 0.1 M HCl for 30 min and counterstained with nuclear fast red. For von Kossa staining, OECM and CECM were incubated in a 5% (w/v) silver nitrate (Sigma) solution and exposed to a bright lamp for 30 min.
Immunofluorescent staining of OECM and CECM
OECM and CECM were fixed with 4% (w/v) PFA for 10 min at room temperature and rinsed with PBS. Antibodies against rat and rabbit fibronectin (rabbit-polyclonal, 1:100; Abcam, Cambridge, United Kingdom), laminin (rabbit-polyclonal, 1:100; Abcam), collagen type I (mouse-monoclonal, 1:100; Abcam), and collagen type II (rabbit-polyclonal, 1:40; Abcam) were used for immunofluorescent staining. Fibronectin and laminin were visualized with rhodamine-conjugated antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). Collagen type I and collagen type II were visualized with Fluorescein isothiocyanate-conjugated antibodies (Jackson ImmunoResearch Laboratories). The slides were mounted with DAPI and photographed with a fluorescent microscope.
Osteogenic and chondrogenic differentiation of hMSCs
hMSCs from the fourth passage were used for differentiation studies. hMSCs were plated onto OECM and CECM substrates at a density of 500 cells/cm2. For the ECM control plates, hMSCs were seeded on to plates coated with fibronectin (1 μg/cm2; Sigma), collagen type I (6 μg/cm2; Sigma), or collagen type II (6 μg/cm2; Sigma) following manufacturer's instructions. These substrates were coated and incubated overnight at room temperature. The ECM control plates were washed twice with PBS. To reduce the effects from FBS, hMSCs were initially maintained in a serum-free medium supplemented with ITS+1 (Sigma) for the first 24 h. Culture medium was then replaced by a slightly modified differentiation medium containing 1% (v/v) ITS+1, 100 nM dexamethasone, 50 μg/mL ascorbate-2-phosphate, and 100 ng/mL BMP-2.23
Quantitative real-time–polymerase chain reaction
hMSCs cultured on fibronectin, collagen type I, collagen type II, OECM, or CECM (n=3) for 3 weeks were rinsed with sterile PBS, and the total RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The concentration of extracted RNA was measured with a ND-2000c UV spectrophotometer (Nanodrop, Wilmington, DE), and 1 μg of each mRNA sample was used to synthesize the corresponding cDNA. For the relative quantification of mRNA expression, quantitative real-time–polymerase chain reaction (qRT-PCR) was performed with SYBR Green I (Takara, Shiga, Japan) with the Light Cycler 480 (Roche, Basel, Switzerland). After 5 min of preincubation, 35 amplification cycles were performed; each cycle consisted of the following three steps: 30 s at 94°C, 45 sc at 60°C, and 45 s at 72°C. The mRNA expression levels of the target genes were normalized by dividing their value by the value of the GAPDH mRNA level, and all data were analyzed by the 2−ΔΔCt method. The sequences of the primers were as follows: GAPDH (150 bp): forward primer, 5′-CGA CCA CTT TGT CAA GCT CA-3′, and reverse primer, 5′-GAG GGT CTC TCT CTT CCT CT-3′; aggrecan (157 bp): forward primer, 5′-GTC TCA CTG CCC AAC TAC-3′, and reverse primer, 5′-GGA ACA CGA TGC CTT TCA C-3′; Sox9 (151 bp): forward primer, 5′-GGA GCT CGA AAC TGA CTG GAA-3′, and reverse primer, 5′-GAG GCG AAT TGG AGA GGA GGA-3′; osteocalcin (129 bp): forward primer, 5′-GTG ACG AGT TGG CTG ACC-3′, and reverse primer, 5′-CAA GGG GAA GAG GAA AGA AGG-3′; Runx2 (125 bp): forward primer, 5′-AGA TGA TGA CAC TGC CAC CTC TG-3′, and reverse primer, 5′-GGG ATG AAA TGC TTG GGA ACT GC-3′.
Analysis of hMSC differentiation using immunocytochemistry
hMSCs were cultured for 3 weeks on different ECMs, fixed with 4% PFA for 10 min at room temperature, and rinsed with PBS. Antibodies against human Runx2 (mouse-monoclonal, 1:100; Abcam) and SOX9 (mouse-monoclonal, 1:100; Abcam) were used for immunofluorescent staining of the hMSCs to analyze cell differentiation. The staining signals were visualized with rhodamine-conjugated antibodies (Jackson ImmunoResearch Laboratories). The slides were mounted with DAPI to stain the cell nuclei and were photographed with a fluorescent microscope.
Western blot analysis
hMSCs were cultured for 3 weeks on OECM or CECM substrates with BMP-2 (n=3) and were lysed with sodium dodecyl-sulfate (SDS) sample buffer (62.5 mM Tris–HCl [pH 6.8], 2% [w/v] SDS, 10% [v/v] glycerol, 50 mM dithiothreitol, and 0.1% [w/v] Bromophenol Blue). The total protein concentration was determined by a bicinchoninic acid protein assay (Pierce Biotechnology, Rockford, IL). Western blot analysis was performed by 10% (w/v) SDS-polyacrylamide gel electrophoresis. After the proteins were transferred to an Immobilon-P membrane (Millipore Corp., Billerica, MA), they were probed with antibodies against human phosphate focal adhesion kinase (pFAK; Abcam), focal adhesion kinase (FAK; Abcam), RhoA (Abcam), and Rock1 (Abcam). The proteins were then incubated with a horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h at room temperature. The blots were developed by an enhanced chemiluminescence detection system (Amersham Bioscience, Piscataway, NJ).
Statistical analyses
Quantitative data were expressed as mean±standard deviation. Statistical analyses were performed using an analysis of variance. A p-value less than 0.05 was considered statistically significant.
Results
Characterization of the cell-derived ECM
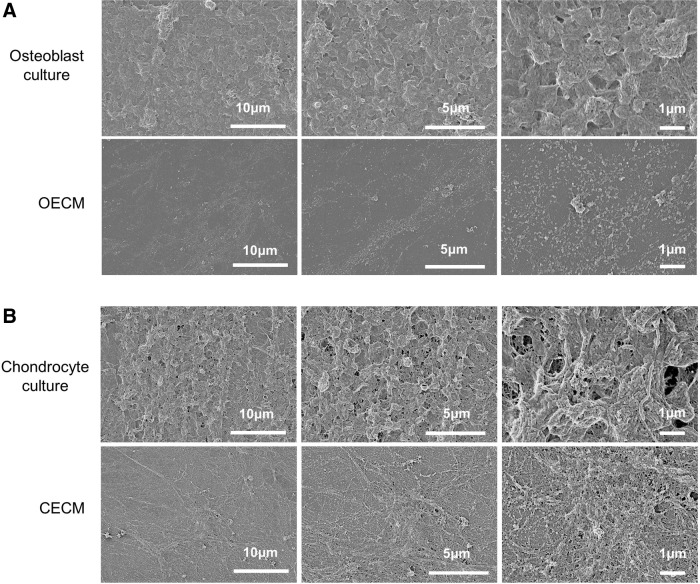
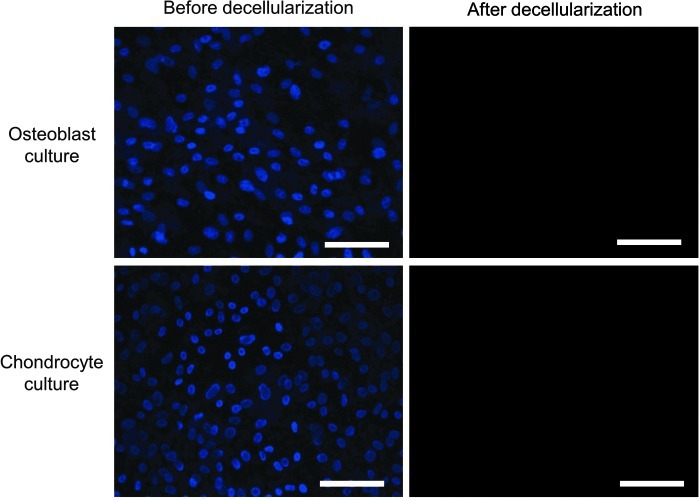
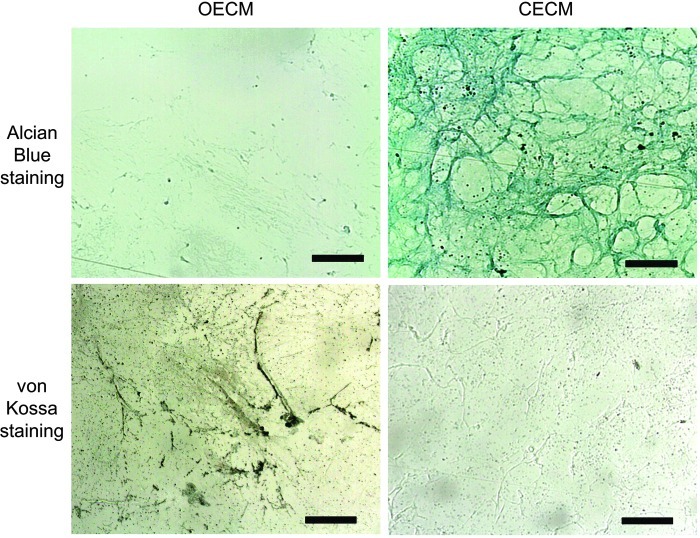
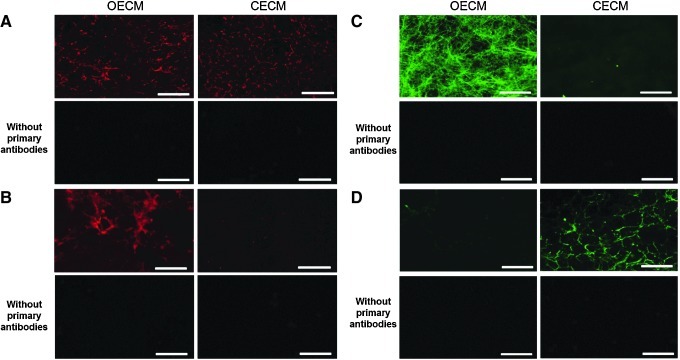
Following the decellularization of osteoblasts+OECM and chondrocytes+CECM, the SEM analysis revealed the presence of ECM and confirmed the removal of the cells (Fig. 2). The complete decellularization of the matrix was further confirmed by staining nuclei with DAPI (Fig. 3). Histochemistry was performed to evaluate the components of OECM and CECM. Alcian blue staining indicated that CECM contained a greater proportion of glycosaminoglycans than OECM (Fig. 4), whereas von Kossa staining indicated that OECM contained a larger proportion of calcium. The ECM components of OECM and CECM were further examined by immunofluorescent staining of fibronectin, laminin, collagen type I, and collagen type II (Fig. 5). Fibronectin and laminin were observed in both OECM and CECM (Fig. 5A, B), whereas collagen type I and collagen type II were found primarily in OECM and CECM, respectively (Fig. 5C, D).
FIG. 2.
SEM images of confluent osteoblast and chondrocyte cultures, and their decellularized counterparts. (A) osteoblast-derived extracellular matrix (OECM) and (B) chondrocyte-derived extracellular matrix (CECM) substrates were prepared by decellularization of confluent rat osteoblast and rabbit chondrocyte cultures, respectively.
FIG. 3.
Confirmation of cell-derived ECM substrate decellularization. Representative pictures of 4′,6-diamidino-2-phenylindole (DAPI) (blue)-stained rat osteoblast and rabbit chondrocyte monolayer cultures prior to and after decellularization. The scale bars represent 100 μm. Color images available online at www.liebertpub.com/tea
FIG. 4.
Characterization of cell-derived ECM substrates. Alcian blue staining of OECM and CECM for glycosaminoglycan. von Kossa staining of OECM and CECM for calcium deposition. The scale bars represent 100 μm. Color images available online at www.liebertpub.com/tea
FIG. 5.
Main components of OECM and CECM substrates visualized by immunocytochemistry. Osteoblasts and chondrocytes were cultured for 2 weeks prior to decellularization. Acellular matrices were immunofluorescently stained for (A) fibronectin (red), (B) laminin (red), (C) collagen type I (green), (D) and collagen type II (green). The scale bars represent 100 μm. Color images available online at www.liebertpub.com/tea
Osteogenic and chondrogenic differentiation
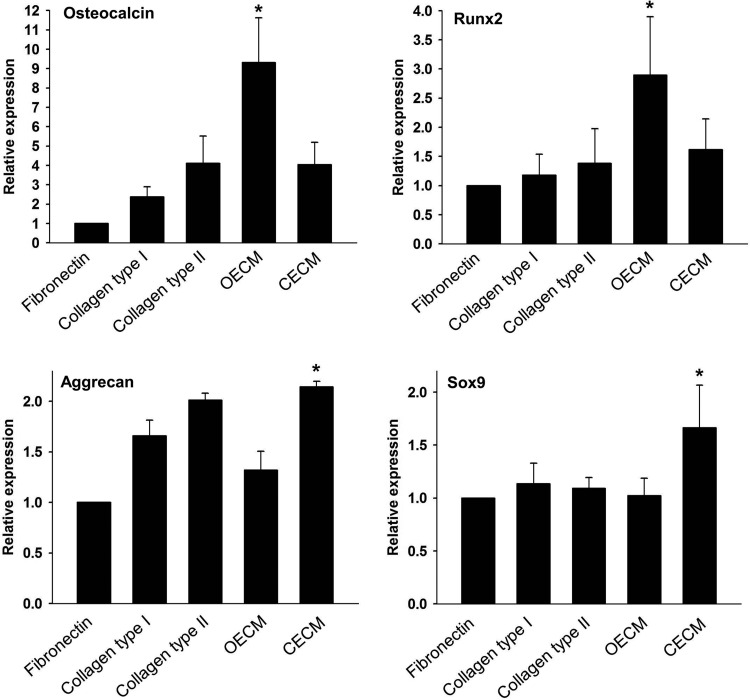
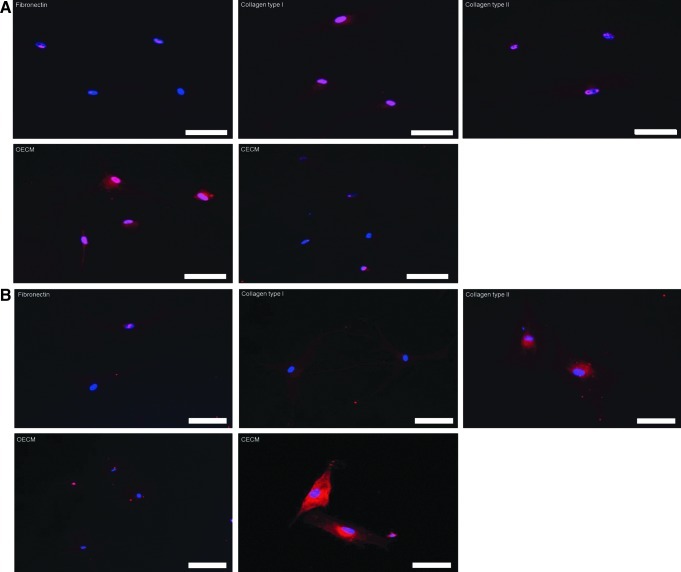
qRT-PCR analyses showed that hMSCs cultured on fibronectin, collagen type I, collagen type II, and CECM expressed low levels of osteogenic marker genes (i.e., osteocalcin and Runx2) and that hMSCs cultured on OECM expressed high levels of the osteogenic markers (Fig. 6). The hMSC expression of aggrecan and Sox9 was significantly enhanced on CECM substrate compared with the hMSCs cultured with other ECM substrates. Immunocytochemistry demonstrated a similar tendency for Runx2 and Sox9 3 weeks after cell seeding. The OECM group displayed high Runx2 expression (Fig. 7A), whereas the CECM group showed high Sox9 expression (Fig. 7B).
FIG. 6.
Effect of various ECM components (i.e., fibronectin, collagen type I, collagen type II, OECM, and CECM) on osteogenic and chondrogenic differentiation of human mesenchymal stem cells (hMSCs). Gene expression of osteogenic (osteocalcin and Runx2) and chondrogenic (aggrecan and Sox9) markers was evaluated using quantitative real-time–polymerase chain reaction. The expression levels were normalized with respect to fibronectin. *p<0.05 compared with other groups.
FIG. 7.
Assessment of osteogenic and chondrogenic differentiation of hMSCs by immunocytochemistry 3 weeks after cell plating on various ECM components (i.e., fibronectin, collagen type I, collagen type II, OECM, and CECM). (A) Runx2 (red) and DAPI (blue). (B) Sox9 (red) and DAPI (blue). The scale bars represent 100 μm. Color images available online at www.liebertpub.com/tea
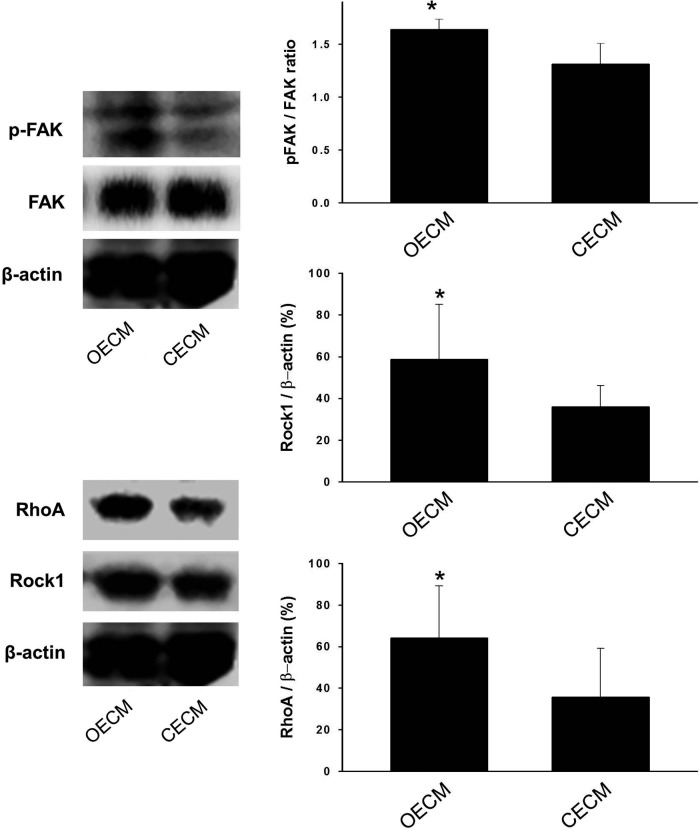
A previous study has shown that osteogenic differentiation is modulated by interlinked signaling cascades of FAK and RhoA.24 We thus investigated whether the decellularized matrix components influence the FAK-, RhoA-, or Rock1-mediated signaling cascades. Three weeks after cell seeding, we observed that the ratio of pFAK to FAK was higher in cells exposed to the OECM than cells exposed to the other substrates (Fig. 8). It was also found that Rock1 expression and RhoA expression were enhanced by the OECM substrate.
FIG. 8.
Mechanisms involved in the enhanced osteogenic differentiation of hMSCs. The expressions of specific proteins (phosphate focal adhesion kinase [pFAK], FAK, RhoA, and Rock1) involved in the signaling pathway for osteogenic commitment was examined by western blotting. *p<0.05 compared with other group.
Discussion
Decellularized matrices have been utilized for variety of biomedical applications.25 For instance, decellularized lung and liver have been successfully transplanted in vivo following recellularization.26,27 However, the biological effect on cells of decellularized ECMs in combination with exogenous growth factors has not been thoroughly investigated. By analyzing the cellular expression of genes and proteins, we have demonstrated that cell-derived ECM cultures supplemented with BMP-2 can induce the osteogenic and chondrogenic differentiation of hMSCs.
Previous publications utilized the geometry of the scaffolds to control the phenotypic expression in BMP-2 induced osteogenesis and chondrogenesis.28 Other studies have shown that changes in cell geometry are characterized by the differential expression of receptors and intracellular proteins that are involved in signal transductions pathways, including those associated with ECM interacting membrane proteins.29 In this study, we hypothesized that the ECM microenvironments secreted by osteoblasts and chondrocytes, which resembled the native ECM niches of bone and cartilage, respectively, modulate BMP-2-dependent differentiation. Indeed, our experiments confirmed that in the presence of BMP-2, the OECM microenvironment promoted efficient hMSC osteogenic commitment, whereas the CECM microenvironment promoted efficient hMSC chondrogenic commitment. An analysis of OECM and CECM components indicated that OECM consisted primarily of collagen type I (Fig. 5C), and that CECM consisted primarily of collagen type II (Fig. 5D). Differences in the ECM components may have contributed to the modulation of BMP-2-dependent differentiation. Interestingly, when the same experiments were performed on cell culture substrates supplemented with different proteins in isolation, these beneficial differentiation effects were not observed (Figs. 6 and 7). This finding suggests that using complex ECMs with physiologically relevant compositions would be more beneficial.
Herein, we have demonstrated that BMP-2-dependent osteogenic and chondrogenic differentiation can be controlled by the ECM microenvironment. Our data indicate that the extracellular proteins secreted by osteoblasts promoted the BMP-2-dependent osteogenic commitment of hMSCs, whereas the extracellular proteins secreted by chondrocytes promoted BMP-2-dependent chondrogenic commitment. Although the cell-secreted matrices may contain biochemical cues, physical interactions may have contributed to the osteogenic and chondrogenic commitments by varying the focal adhesions of hMSCs.30,31 To evaluate this hypothesis, we analyzed the general osteogenesis pathway by western blotting. FAK, pFAK, Rock1, and RhoA are the key factors necessary to trigger the osteogenic pathway.32 The ratio of pFAK/FAK was significantly enhanced by the OECM substrate compared with the CECM substrate (Fig. 8). The detection of significant levels of pFAK and the increased pFAK/FAK ratio indicated that the OECM microenvironment resulted in an increase in FAK activity. Rock1 and RhoA, which are down-stream signaling molecules of FAK, were consequently expressed at higher levels by the activated FAK (Fig. 8). The enhanced activities of Rock1 and RhoA have been observed during the osteogenic commitment of hMSCs.24 FAK is both affected and regulated by the physical interactions mediated by integrin. In contrast, the CECM substrate resulted in a low level of FAK. The RhoA/Rock1 signaling pathway was also not activated by the CECM substrate. These varying effects of BMP-2 on RhoA/Rock1 signaling and chondrogenic commitment are likely due to changes in the CECM-dependent cell phenotype.33 The chondrogenic commitment of hMSCs is dependent on the cellular context. Recently, three-dimensional culture systems have been praised as systems that closely mimic the in vivo environment. However, the results from this study illustrate the potential of CECM substrates to promote the chondrogenic commitment of hMSCs in vitro even when limited to two dimensions.
Several studies have emphasized the importance of the ECM in the field of tissue engineering.34,35 In this study, we confirmed that the cell-specific ECM, when supplemented with BMP-2, influences the differentiation of hMSCs. Further, our studies demonstrate that cell-specific ECMs provide microenvironments with key intracellular signals that are modulated to mediate osteogenic and chondrogenic differentiation. Therefore, differentiation of cell-derived ECM-based hMSCs may be a simple and effective strategy to regulated hMSC osteogenesis and chondrogenesis.
Conclusion
OECM and CECM promoted osteogenic and chondrogenic commitment of hMSCs, respectively, in the presence of BMP-2. Therefore, cell-specific ECMs are capable of modulating the BMP-2-induced osteogenic versus chondrogenic differentiation of hMSCs. ECMs with physiologically relevant, complex compositions may contribute to the modulation of BMP-2-dependent differentiation, as the beneficial differentiation effects were not observed when hMSCs were cultured on either collagen type I, a main component of OECM, or collagen type II, a main component of CECM.
Acknowledgments
This study was supported by the grants (2009-0092213 and 2010-0020352) from the National Research Foundation of Korea and the grant (A100443) from the Korean Health 21 R&D project, Ministry of Health and Welfare, Republic of Korea.
Disclosure Statement
No competing financial interests exist.
References
- 1.Phillips J.E. Petrie T.A. Creighton F.P. Garcia A.J. Human mesenchymal stem cell differentiation on self-assembled monolayers presenting different surface chemistries. Acta Biomater. 2010;6:12. doi: 10.1016/j.actbio.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salasznyk R.M. Klees R.F. Williams W.A. Boskey A. Plopper G.E. Focal adhesion kinase signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells. Exp Cell Res. 2007;313:22. doi: 10.1016/j.yexcr.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salasznyk R.M. Klees R.F. Hughlock M.K. Plopper G.E. ERK signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells on collagen I and vitronectin. Cell Commun Adhes. 2004;11:137. doi: 10.1080/15419060500242836. [DOI] [PubMed] [Google Scholar]
- 4.Caplan A.I. Mesenchymal stem cells. J Orthop Res. 1991;9:641. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 5.Le Blanc K. Pittenger M. Mesenchymal stem cells: progress toward promise. Cytotherapy. 2005;7:36. doi: 10.1080/14653240510018118. [DOI] [PubMed] [Google Scholar]
- 6.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 7.Huang C.H. Chen M.H. Young T.H. Jeng J.H. Chen Y.J. Interactive effects of mechanical stretching and extracellular matrix proteins on initiating osteogenic differentiation of human mesenchymal stem cells. J Cell Biochem. 2009;108:1263. doi: 10.1002/jcb.22356. [DOI] [PubMed] [Google Scholar]
- 8.Alsberg E. von Recum H.A. Mahoney M.J. Environmental cues to guide stem cell fate decision for tissue engineering applications. Expert Opin Biol Ther. 2006;6:847. doi: 10.1517/14712598.6.9.847. [DOI] [PubMed] [Google Scholar]
- 9.Bosnakovski D. Mizuno M. Kim G. Takagi S. Okumura M. Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng. 2006;93:1152. doi: 10.1002/bit.20828. [DOI] [PubMed] [Google Scholar]
- 10.Ayala R. Zhang C. Yang D. Hwang Y. Aung A. Shroff S.S., et al. Engineering the cell-material interface for controlling stem cell adhesion, migration, and differentiation. Biomaterials. 2011;32:3700. doi: 10.1016/j.biomaterials.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Kundu A.K. Putnam A.J. Vitronectin and collagen I differentially regulate osteogenesis in mesenchymal stem cells. Biochem Biophys Res Commun. 2006;347:347. doi: 10.1016/j.bbrc.2006.06.110. [DOI] [PubMed] [Google Scholar]
- 12.Klees R.F. Salasznyk R.M. Vandenberg S. Bennett K. Plopper G.E. Laminin-5 activates extracellular matrix production and osteogenic gene focusing in human mesenchymal stem cells. Matrix Biol. 2007;26:106. doi: 10.1016/j.matbio.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang A.H. Motlekar N.A. Stein A. Diamond S.L. Shore E.M. Mauck R.L. High-throughput screening for modulators of mesenchymal stem cell chondrogenesis. Ann Biomed Eng. 2008;36:1909. doi: 10.1007/s10439-008-9562-4. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz Z. Simon B.J. Duran M.A. Barabino G. Chaudhri R. Boyan B.D. Pulsed electromagnetic fields enhance BMP-2 dependent osteoblastic differentiation of human mesenchymal stem cells. J Orthop Res. 2008;26:1250. doi: 10.1002/jor.20591. [DOI] [PubMed] [Google Scholar]
- 15.Rosso F. Giordano A. Barbarisi M. Barbarisi A. From cell-ECM interactions to tissue engineering. J Cell Physiol. 2004;199:174. doi: 10.1002/jcp.10471. [DOI] [PubMed] [Google Scholar]
- 16.Streuli C. Extracellular matrix remodelling and cellular differentiation. Curr Opin Cell Biol. 1999;11:634. doi: 10.1016/s0955-0674(99)00026-5. [DOI] [PubMed] [Google Scholar]
- 17.Whitson S.W. Whitson M.A. Bowers D.E., Jr. Falk M.C. Factors influencing synthesis and mineralization of bone matrix from fetal bovine bone cells grown in vitro. J Bone Miner Res. 1992;7:727. doi: 10.1002/jbmr.5650070703. [DOI] [PubMed] [Google Scholar]
- 18.Park M.S. Kim S.S. Cho S.W. Choi C.Y. Kim B.S. Enhancement of the osteogenic efficacy of osteoblast transplantation by the sustained delivery of basic fibroblast growth factor. J Biomed Mater Res B Appl Biomater. 2006;79:353. doi: 10.1002/jbm.b.30549. [DOI] [PubMed] [Google Scholar]
- 19.Kang S.W. La W.G. Kim B.S. Open macroporous poly(lactic-co-glycolic Acid) microspheres as an injectable scaffold for cartilage tissue engineering. J Biomater Sci Polym Ed. 2009;20:399. doi: 10.1163/156856209X412236. [DOI] [PubMed] [Google Scholar]
- 20.Grunert M. Dombrowski C. Sadasivam M. Manton K. Cool S.M. Nurcombe V. Isolation of a native osteoblast matrix with a specific affinity for BMP2. J Mol Histol. 2007;38:393. doi: 10.1007/s10735-007-9119-0. [DOI] [PubMed] [Google Scholar]
- 21.Hoshiba T. Kawazoe N. Tateishi T. Chen G. Development of extracellular matrices mimicking stepwise adipogenesis of mesenchymal stem cells. Adv Mater. 2010;22:3042. doi: 10.1002/adma.201000038. [DOI] [PubMed] [Google Scholar]
- 22.Decaris M.L. Mojadedi A. Bhat A. Leach J.K. Transferable cell-secreted extracellular matrices enhance osteogenic differentiation. Acta Biomater. 2012;8:744. doi: 10.1016/j.actbio.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 23.Vater C. Kasten P. Stiehler M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biomater. 2011;7:463. doi: 10.1016/j.actbio.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 24.McBeath R. Pirone D.M. Nelson C.M. Bhadriraju K. Chen C.S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 25.DeQuach J.A. Mezzano V. Miglani A. Lange S. Keller G.M. Sheikh F., et al. Simple and high yielding method for preparing tissue specific extracellular matrix coatings for cell culture. PLoS One. 2010;5:e13039. doi: 10.1371/journal.pone.0013039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ott H.C. Clippinger B. Conrad C. Schuetz C. Pomerantseva I. Ikonomou L., et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 27.Uygun B.E. Soto-Gutierrez A. Yagi H. Izamis M.L. Guzzardi M.A. Shulman C., et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshinori K. Qiming J. Hiroko T. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. J Bone Joint Surg Am. 2001;83:S105. [PubMed] [Google Scholar]
- 29.Lehnert D. Wehrle-Haller B. David C. Weiland U. Ballestrem C. Imhof B.A., et al. Cell behaviour on micropatterned substrata: limits of extracellular matrix geometry for spreading and adhesion. J Cell Sci. 2004;117:41. doi: 10.1242/jcs.00836. [DOI] [PubMed] [Google Scholar]
- 30.Grassel S. Ahmed N. Influence of cellular microenvironment and paracrine signals on chondrogenic differentiation. Front Biosci. 2007;12:4946. doi: 10.2741/2440. [DOI] [PubMed] [Google Scholar]
- 31.Salasznyk R.M. Williams W.A. Boskey A. Batorsky A. Plopper G.E. Adhesion to vitronectin and collagen I promotes osteogenic differentiation of human mesenchymal stem cells. J Biomed Biotechnol. 2004;2004:24. doi: 10.1155/S1110724304306017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khatiwala C.B. Kim P.D. Peyton S.R. Putnam A.J. ECM compliance regulates osteogenesis by influencing MAPK signaling downstream of RhoA and ROCK. J Bone Miner Res. 2009;24:886. doi: 10.1359/JBMR.081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woods A. Wang G. Beier F. RhoA/ROCK signaling regulates Sox9 expression and actin organization during chondrogenesis. J Biol Chem. 2005;280:11626. doi: 10.1074/jbc.M409158200. [DOI] [PubMed] [Google Scholar]
- 34.Pei M. Li J.T. Shoukry M. Zhang Y. A review of decellularized stem cell matrix: a novel cell expansion system for cartilage tissue engineering. Eur Cells Mater. 2011;22:333. doi: 10.22203/ecm.v022a25. [DOI] [PubMed] [Google Scholar]
- 35.Lu H. Hoshiba T. Kawazoe N. Koda I. Song M. Chen G. Cultured cell-derived extracellular matrix scaffolds for tissue engineering. Biomaterials. 2011;32:9658. doi: 10.1016/j.biomaterials.2011.08.091. [DOI] [PubMed] [Google Scholar]