Abstract
Specific niches may affect how cells from different regions contribute to tendon biology, particularly in regard to the healing of certain tendinopathies. The objectives of this study are to determine whether distinct subpopulations of stem/progenitor cells are found within the tendon proper and the epi- and paratenon, the peritenon, as well as to characterize these stem/progenitor cell populations. In this study, we hypothesized that tendon stem/progenitor cells exist in each region, that these populations possess distinct features, and that these populations while multipotent could have differing potentials. To test this hypothesis, stem/progenitor cells were isolated and characterized from the peritenon and tendon proper of mouse Achilles tendons. Colony-forming unit and multipotency assays, as well as flow cytometry, and real-time quantitative polymerase chain reaction analyses of stem cell markers were performed. Significantly, more stem/progenitor cell colonies were observed from cells derived from the tendon proper relative to the peritenon. Analysis of surface markers for stem/progenitor cells from both regions indicated that they were Sca1+ (stem cell marker), Cd90+ and Cd44+ (fibroblast markers), Cd18− (leukocyte marker), Cd34− (hematopoietic and vascular marker), and Cd133− (perivascular marker). Tendon proper stem/progenitor cells had increased expression levels for tenomodulin (Tnmd) and scleraxis (Scx), indicative of enrichment of stem/progenitor cells of a tendon origin. In contrast, cells of the peritenon demonstrated relative increases in the vascular (endomucin) and pericyte (Cd133) markers relative to cells from the tendon proper. Stem/progenitor cells from both regions were multipotent (adipogenic, chondrogenic, osteogenic, and tenogenic). These findings demonstrated that different progenitor populations exist within discrete niches of the Achilles tendon—tendon proper versus peritenon. Overall, these data support the hypothesis that the progenitor pools from both regions have distinct properties and contain enriched progenitor subpopulations of different origins. Moreover, in considering their roles in tendon healing more broadly, they are potential cell sources that may differentially contribute to intrinsic and extrinsic tendon repair mechanisms. That is, intrinsic repair may require a progenitor class with predominant tendon marker expression, while extrinsic repair may involve a progenitor class recruited from perivascular cells of the peritenon.
Introduction
After an injury, tendon healing is characterized by inflammation, cell migration, and proliferation as well as matrix synthesis and tissue reorganization.1,2 There has been a great deal of debate as to which cell populations are involved in tendon healing. It is plausible that both intrinsic and extrinsic cell sources are involved, either separately or synergistically.1–3 That is, cells from within the tendon, such as tenocytes, may migrate and proliferate within the lesion. Along with intrinsic repair responses, extrinsic circulating cells, including inflammatory cells and cells from the surrounding connective and vascular tissues, may migrate into the wound to initiate the repair response.1–4 Cells migrating into the wound during repair could be serving trophic roles by releasing growth factors, stimulatory, or inhibitory molecules.5 In addition, they could be playing a catabolic role by secreting enzymes that break down and remodel structural molecules.6,7 Moreover, cells could be directing the synthesis and assembly of collagen molecules.2,8,9 The origin of these cells remains to be elucidated, and the level of contribution of each proliferating pool of cells is still unknown.
Mature tendons harbor small populations of stem/progenitor cells (TSPCs).10,11 In many tissues, this class of cells is responsible for growth, tissue homeostasis, and repair.12 These cells are clonogenic, multipotent, and self-renewing.10 Like many stem cells, the ability of TSPCs to self-renew and differentiate into tendon has been shown to be niche-dependent.10,12 That is, TSPC development and function depend upon the molecular surroundings. For example, when the small leucine-rich proteoglycans (SLRPs), fibromodulin (Fmod), and biglycan (Bgn), were removed from the tendon niche in Bgn−/0 Fmod−/− mice, TSPCs committed to bone formation along the patellar tendon at the tendon–patellar junction.10 In addition, in these mice, fibrils within the tendon proper were thinner, more progenitor cells were present, yet there was decreased expression of collagen I and scleraxis and tendon differentiation was impaired.10 In vitro, these TSPCs have been shown to be affected by inflammatory mediators and biomechanical stress, driving them down paths of adipogenic and osteogenic differentiation that might be related to clinical findings seen in tendinopathies.13–15 That is, tendinopathic sites generally demonstrate adipogenic, osteogenic, and chondrogenic changes.16 Therefore, TSPCs demonstrate the potential to be affected by niche, physiology, and pathology. When rat TSPCs were harvested, and then implanted as an allogeneic fibrin glue construct graft, TSPCs promoted earlier and more complete repair, yet were not retained within the healing injury site.17 Thus, while these TSPCs demonstrate a niche-dependent multipotency and possible trophic properties in healing, their native role in tendon repair is still unknown.
Past studies have examined the TSPCs found within one niche, the tendon proper, or, more explicitly, the intrinsic TSPC population existing within what is termed the endotenon.10,11,13–15,18 Yet, tendon-healing theories include both intrinsic and extrinsic repair responses, and healing resulting from cells originating from multiple niches. Therefore, the peritenon, that represents cell populations present along the surface of the tendon (epitenon) and its loose areolar connective tissue sleeve (paratenon), should be considered in the context of an extrinsic response.2,19 Stem/progenitor cells within this region could provide the origin of repair cells for extrinsic healing in certain tendinopathies. To this end, our general hypothesis is that progenitors endogenous to the tendon are involved in microinjury repair, while different progenitor cell populations within the tendon, as well as in the surrounding peritenon, have roles in cell recruitment following acute or traumatic injuries. In this study, the specific hypothesis addressed is that stem/progenitor cell populations exist within each region and there will be distinct regional properties, that is, numbers of progenitor cells, origins of progenitors, and differentiation potentials. We isolated cells from the tendon proper, including all cell types within the endotenon. We also isolated cells of the peritenon, representing cell populations within the epitenon and paratenon. Stem/progenitor cells were isolated from each location of the mouse Achilles tendon that were clonogenic and multipotent. In addition, stem/progenitor cells isolated from each location demonstrated an enrichment of cells with distinct characteristics. These data support our hypothesis.
Methods
Animals
Male C57BL/6 mice at P30 age were used in this study for stem/progenitor cell isolation. Tissues from male C57Bl/6 mice at P3-P4 were used as positive controls to compare to chondrogenic and tenogenic culture constructs. The utilization of animals and all protocols described within this study involving their use were approved by the Institutional Animal Care and Use Committee at the University of South Florida.
Progenitor cell isolation
Progenitor cells were isolated from the Achilles tendon proper as well as from the associated tissues, termed the peritenon (paratenon and epitenon), utilizing a series of enzyme digestions.10,20 Male C57Bl/6 mice at postnatal day 30 were used. Mice were euthanized and Achilles tendons with the surrounding paratenons were dissected using sterile technique, and kept on ice in Dulbecco's phosphate-buffered saline (D-PBS, Life Technologies, cat no 14190) with antibiotics/antimycotics (100 U/mL penicillin, 100 μg/mL streptomycin, and 250 ng/mL amphotericin B, Life Technologies, cat no 15240). Each biological replicate represents eight Achilles tendons from four mice. Tendons were first treated with 0.5% type I collagenase (CLS-1, Worthington) and 0.25% trypsin (Life Technologies, cat no 15090) in alpha-Minimum Essential Medium (MEM) for 10 min at 37°C. Then, the surfaces of the tendons were scraped carefully with a rubber policeman to strip away the cells of the peritenon. The peritenon cells were collected in ice-cold alpha-MEM. The remaining tendon tissue was rinsed in the Hank's Balanced Salt Solution with antibiotics/antimycotics, then cut into 1-mm3 pieces, and transferred into a solution of 3 mg/mL CLS-1 and 4 mg/mL Dispase II (Roche). The digestion was undertaken at 90 oscillations per minute in a 37°C water bath for 20 min. Digested tendon proper was transferred to a new 50-mL conical tube containing 20 mL of culture media kept on ice. A fresh collagenase/dispase solution was transferred into the tube with the remaining tendon pieces for 10-min incubations until all the tissue was digested, each time adding the digest to the media (alpha-MEM, 2 mM L-glutamine, antibiotics/antimycotics, 100 μM 2-mercaptoethanol, and 20% fetal bovine serum). From both digests (peritenon and tendon), cells were strained with a 70-μm cell strainer, and cells were collected by centrifugation for 5 min at 500 g. Cells were resuspended in media and counted using a hemocytometer with Trypan blue staining.
Characterization of consistency of regional enrichment after enzymatic dissociation
The stem/progenitor cells from the tendon proper or the peritenon were isolated to enrich populations from each region. To assess the consistency of these isolations, individual samples were evaluated for: stem/progenitor cell number; origin; and multipotency. First, to confirm technique consistency, numbers of stem/progenitor cell (adherent colonies) isolated from the different regions in individual isolations were investigated (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea). Sequential enzymatic isolation consistently resulted in differences in abundance of stem/progenitors by region. In each isolation, technical replicates were analyzed by the t-test; for all eight isolations, the difference was significant with no overlap between values intra- and interisolation for all eight isolations (Supplementary Fig. S1A). This consistency also is clearly depicted in a plot of the means and standard deviations of colony counts by isolation (Supplementary Fig. S1B). Second, the consistency of enzymatic dissociation also should be associated with constant representation of enriched subpopulations of stem/progenitor cells in the different isolations. Gene expression for tendon markers, Tenomodulin (Tnmd) and Scleraxis (Scx), for the vascular marker, Endomucin (Emcn), and for the pericyte marker (Cd133), was evaluated (Supplementary Table S1). Consistent differences in tendon markers as well as vascular and pericyte markers were observed in the different regions in all seven isolations. Finally, a specific difference in multigenic potential was observed between tendon proper- and peritenon-derived stem/progenitor cells, in all individual isolations. Tendon proper-derived stem/progenitor cells underwent osteogenic differentiation with deposition of calcium nodules in vitro. In contrast, peritenon-derived stem/progenitor cells were incapable of deposition of calcified matrix in vitro (Supplementary Fig. S2). Based on the findings detailed in the Results, no conspicuous contamination of tendon proper-derived stem/progenitor cells are noted within the peritenon-derived stem/progenitor cells. These findings were consistent for all four isolations examined.
Colony-forming unit assay
Tendon and peritenon cells were plated at 1000 cells and 5000 cells per 25 cm2 flask, respectively. Ten days into the culture, progenitor cell colonies were counted and characterized as previously described.10 Briefly, cells were rinsed with D-PBS, fixed in 4% paraformaldehyde, stained with 0.05% crystal violet for 30 min, and rinsed twice with water. Colonies were counted for 3–4 flasks each from both regions for eight biological replicates.
Flow cytometry
After 10 days in culture, flasks were rinsed with D-PBS, treated with 0.25% trypsin-EDTA at 37°C for 1–2 min, placed into media, and centrifuged for 5 min at 500 g to form a pellet. Cells were then resuspended in media, counted using a hemocytometer, and screened for surface markers. Cells were incubated with a dye-conjugated primary antibody on ice in D-PBS with 1% bovine serum albumin for 30 min, washed with D-PBS, and held on ice according to the manufacturer's instructions: FITC-Sca1/Ly-6A/E (BD, #557405), PE-Cd90.2 (BD, #553006), PE-Cd44 (BD, #553134), PE-Cd18 (BD, #553293), PE-Cd34 (BD, #551387), and FITC-Cd133/Prominin-1 (Millipore, #MAB4310×). The negative control had no conjugated antibody. Cells were examined for six pair-matched (tendon/peritenon) replicates with a FACSaria sorter (BD) for markers characteristic of different cell types (Table 1).21–51
Table 1.
| Marker | Gene Symbol | Marker Specification | Assay Technique | References |
|---|---|---|---|---|
| Cd18 antigen (Integrin beta-2) | Cd18 | Leukocyte adhesion molecule | FCA | 21, 22 |
| Hematopoietic progenitor cell antigen Cd34 | Cd34 | Hematopoietic and vascular stem cell marker | FCA | 23–25 |
| Cd44 antigen (Indian blood group) | Cd44 | Hyaluronic acid receptor; T lymphocyte and mesenchymal stem cell marker | FCA, RT-qPCR | 26–28 |
| Cd90.2 antigen (Thy-1 cell surface antigen, allele 2) | Cd90.2 | Fibroblast, epithelial cell, thymocyte, epithelial T lymphocyte, mesenchymal stem cell, and neuron marker in mice | FCA, RT-qPCR | 29–33 |
| Cd133 antigen (Prominin-1) | Cd133 | Neural, endothelial, epithelial, and pericyte stem cell marker | FCA, RT-qPCR | 34–36 |
| Endomucin | Emcn | Vascular endothelial marker | RT-qPCR | 37–40 |
| Guanine nucleotide-binding protein-like 3 (Nucleostemin) | Gnl3 | General stem cell marker; detected in bone marrow stroma, thyroid side population, and tendon stem cells | RT-qPCR | 41–43 |
| Musashi-1 | Msi1 | Muscle, neural, and pericyte stem cell marker | RT-qPCR | 36, 43–45 |
| Stem cell antigen-1 | Sca1 | General stem cell marker | FCA | 46–49 |
| Scleraxis | Scx | Tendon marker | RT-qPCR | 50, 51 |
| Tenomodulin | Tnmd | Tendon marker | RT-qPCR | 37, 51 |
FCA, flow cytometric analysis; RT-qPCR, real-time quantitative polymerase chain reaction.
Gene expression profiling
Total RNA was extracted from seven pairs of flasks, each pair representing separate isolations, using the QIAGEN RNeasy Plus Micro Kit according to the manufacturer's instructions. Total RNA (250 nanograms) was reverse transcribed into cDNA using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Life Technologies) to be used as a template for quantitative real-time polymerase chain reactions (qRT-PCR) with Fast-SYBR Green Master Mix (Applied Biosystems). Assays were performed using the StepONEPlus Real-Time PCR System (Applied Biosystems) (Table 2). Primers were designed using Primer Express software (Applied Biosystems) for markers that define the cell type (Table 1). The results were adjusted for efficiency as measured by LinRegPCR using the default fit option that measures the slope of a line containing 4–6 data points and the highest R2 correlation value.52,53 Real-time PCR data were normalized relative to the endogenous control gene, Actb. A nonparametric Mann–Whitney–Wilcoxon Rank Sum Test was used to assess statistical significance of qPCR results.
Table 2.
Primers Used for Expression Analysis of Tendon Stem/Progenitor Cells
| Marker | Gene Symbol | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|---|
| Beta-actin | Actb | 5-AGATGACCCAGATCATGTTTGAGA-3 | 5-CACAGCCTGGATGGCTACGT-3 |
| Cd44 antigen | Cd44 | 5- GAGAAGGTGTGGGCAGAAGAAA-3 | 5-CTTCCACCGTCCCATTGC-3 |
| Cd90.2 antigen | Cd90.2 | 5-GGATGAGGGCGACTACTTTTGT-3 | 5-TTGGAGCTCATGGGATTCG-3 |
| Cd133 antigen | Cd133 | 5- TGCCTCTACCCTGGAAGCAA-3 | 5-AAGGCCTGTTTCAGCTTTCCT-3 |
| Endomucin | Emcn | 5-CCAACAGTCTCTGCCACAGTGA-3 | 5-ACAGAGGCTTTTGTTGTGGAAGTT-3 |
| Nucleostemin | Gnl3 | 5-GGGAGCTGTCACCTGAGCAA-3 | 5-GGCAGACGACCCGTCAGA-3 |
| Musashi-1 | Msi1 | 5-CGAGGACATCGTAGAGAAAGTTTGT-3 | 5-GCATTCCACCATTTTGTTGTTG-3 |
| Scleraxis | Scx | 5-AAGTTGAGCAAAGACCGTGACA-3 | 5-TGTGGACCCTCCTCCTTCTAAC-3 |
| Tenomodulin | Tnmd | 5-CGCCACACCAGACAAGCA-3 | 5-CCAGCATTGGGTCAAATTCA-3 |
Exploring multipotency—adipogenesis, chondrogenesis, osteogenesis
After 13 days in culture, the tendon and epitenon cells were trypsinized, and then seeded into four-well slides or twelve-well plates (25,000 cells/cm2) in basal media of alpha-MEM with 10% fetal bovine serum (FBS) and antibiotics/antimycotics overnight. To assess multipotency, cells were incubated in specific differentiation media: adipogenesis in basal media with 1 μM dexamethasone, 10 μg/mL insulin, 100 μM indomethacin, and 0.5 mM isobutylmethylxanthine for 21 days10,11; chondrogenesis in basal media with 40 μg/mL proline, 39 ng/mL dexamethasone, 10 ng/mL recombinant human transforming growth factor-β 3 (R&D), 50 μg/mL ascorbic-2-phosphate, 100 μg/mL sodium pyruvate, and 50 mg/mL insulin-transferrin-selenious (ITS) acid mix (BD) for 28 days10,11; osteogenesis in basal media with 200 ng/mL recombinant human BMP-2 (R&D), 0.1 μM dexamethasone, 0.2 mM ascorbic-2-phosphate, and 10 mM glycerol 2-phosphate for 28 days.10,11,54,55 Control cells were cultured in progenitor cell media (alpha-MEM, 20% FBS, 2 mM L-glutamine, antibiotics/antimycotics, 100 μM 2-mercaptoethanol). The media were changed three times weekly.
Histological staining to evaluate multipotency
After culturing cells in adipogenesis and osteogenesis media, differentiation was assessed histocytochemically. Cells were rinsed with PBS, fixed in 4% paraformaldehyde in PBS for 30 min at 4°C. Adipogenesis was evaluated after Oil Red O staining using 0.18% Oil Red O in 60% isopropanol (Thermo-Fisher). Osteogenesis was assessed with Alizarin Red S staining using 2% Alizarin Red, S., pH 4.1 (Sigma).54 Imaging was with an Olympus IMT2 Inverted Fluorescent Microscope and with an Insight version 18.2 Color Mosaic Camera and SPOT Advanced software (Diagnostic Instruments, Inc.).
Immunohistochemistry to evaluate multipotency
The differentiation of the progenitor cell populations was further analyzed by investigating markers used to characterize specific tissues. Cells (adipogenesis and osteogenesis cultures) and 4-μm tissue sections (chondrogenesis and tendon construct cultures) were incubated with immunochemical reagents as previously described.56 Briefly, they were fixed in 4% paraformaldehyde in PBS for 20 min, and permeabilized with 0.3% Triton X-100 with 1% bovine serum albumin (BSA) in PBS for 30 min all at room temperature. Cells were rinsed in PBS and blocked with 5% BSA in PBS as recommended by the antibody manufacturer (R&D). Then, cells were incubated with primary antibodies in a blocking buffer for 2 h at room temperature or overnight at 2°C–8°C. Primary antibodies included: 10 μg/mL goat anti-mouse osteopontin (Opn) polyclonal (R&D) for osteogenesis, 10 μg/mL goat anti-mouse fatty acid-binding protein-4 (Fabp4) polyclonal (R&D) for adipogenesis, 10 μg/mL sheep anti-mouse collagen II polyclonal (R&D) for chondrogenesis, and 2 μg/mL goat anti-human/mouse tenomodulin (Tnmd) polyclonal (Santa Cruz Biotechnology) and 10 μg/mL rabbit anti-mouse collagen I polyclonal (Millipore) for tenogenesis. Cells were then rinsed for 5 min three times with PBS containing 0.05% Tween-20 followed by incubation with fluorescent dye-conjugated secondary antibodies (Alexa Fluor® dyes, Invitrogen) for 1 h at room temperature in 5% BSA diluted in PBS containing 0.05% Tween-20, and then cells were rinsed for 5 min twice with PBS containing 0.05% Tween-20 and once in PBS before being mounted with a VECTASHIELD with DAPI (Vector Labs) and a cover slip. Slides were imaged using an Olympus BX61 microscope with a Hamamatsu Orca-R2 camera and Metamorph for Olympus Premier software or with a Leica DM5500B microscope with a DFC340 FX camera and Leica Application Suite Advanced Fluorescence software.
Exploring multipotency—tendon constructs
Tendon and peritenon progenitor cells were analyzed for their ability to form tendons in vitro in a manner similar to that described previously.57,58 Briefly, each well of a six-well plate was coated with SYLGARD polymer (type 184 silicone elastomer, Dow Chemical). Within each well, two segments of size 0 silk were each pinned with a pair of minutiens insect pins (0.1-mm diameter, Fine Science Tools GmbH) into two size 0 silk suture segments (Fine Science Tools GmbH) positioned 10 mm apart. The contents of each well were sterilized by treatment with 100% ethanol, exposure to ultraviolet irradiation for 60 min, and then rinsed in PBS. Within each well, 6.15×105 tendon and peritenon cells in 400 μL media, 83 μL of 20 mg/mL fibrinogen, and 10 μL of 200 U/mL thrombin (Sigma) were combined and quickly spread over the polymer surface between the two suture segments. Plates were incubated at 37°C in alpha-MEM supplemented with 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, 250 ng/mL amphotericin B, 2 mM L-glutamine, and 200 mM ascorbic-2-phosphate. Three times per week the plates were scored to release the fibrin gel as it contracted and the culture medium was changed. Cultures were maintained for 45 days.
Electron microscopic examination of tenogenic differentiation
Tendon constructs were prepared as described previously.59 Briefly, tendon constructs were processed for transmission electron microscopy as previously described.60 Samples were sectioned using a Leica Ultracut UCT ultramicrotome, and stained with 2% aqueous uranyl acetate and 1% phosphotungstic acid, pH 3.2. Microscopy was undertaken using a JEOL 1400 Transmission Electron Microscope. Images were digitally captured using an Orius widefield side mount CCD camera (Gatan Inc., Pleasanton, CA).
Results
Isolation of stem/progenitors with clonogenic properties from tendon proper versus peritenon
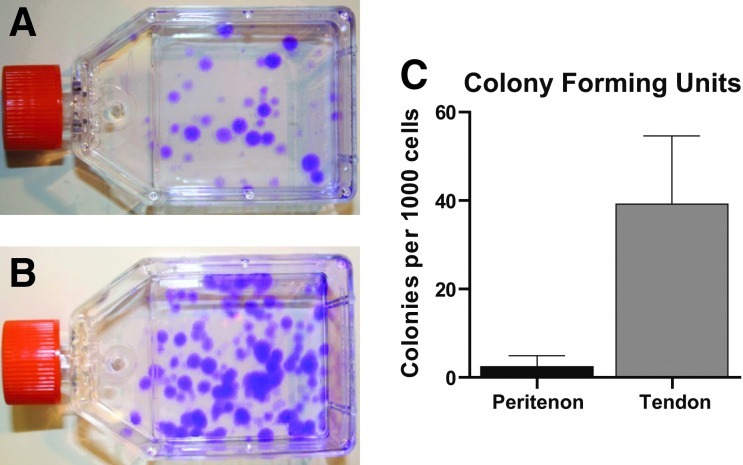
Clonogenicity is a property of stem/progenitor cells. Thus, upon adhering to tissue culture-treated flasks in stem cell media, individual cells form colonies as they proliferate. In this study, stem/progenitors were isolated from the tendon proper and the peritenon and the clonogenic properties were characterized. Stem/progenitor cells isolated from both regions formed adherent colonies (Fig. 1A, B). There was colony size heterogeneity related to the region from which the cells were isolated. Cell morphology was also heterogeneous among stem/progenitor cell colonies isolated from both regions (data not shown).
FIG. 1.
Isolation of stem/progenitor cells from tendon proper and peritenon. Isolated cells form colonies that adhered to the culture flask, a characteristic of progenitor cells. Flasks representative for cells of the peritenon (A) and tendon proper (B) are shown with more numerous colonies associated with cells isolated from the tendon proper. Roughly 16-fold more adherent progenitor colonies were observed from the tendon proper compared to peritenon (C) (p<0.01, n=8). Assuming each colony formed from a single progenitor cell, this indicated more progenitors were isolated from the Achilles tendon proper. Color images available online at www.liebertpub.com/tea
The relative number of progenitor cells present in each region was determined by counting adherent colonies after seeding isolated cells from the two regions. A significantly higher percentage of cells isolated from the tendon proper formed colonies compared to cells isolated from the peritenon (Fig. 1C). When normalized to the total number of cells seeded, the difference was 16-fold greater for cells isolated from within the tendon proper, relative to cells isolated from the peritenon (3.92%±1.55% vs. 0.24%±0.26%, respectively; p<0.01). The differences between the tendon proper and peritenon were observed in eight independent isolations (Supplementary Fig. S1). Based on the assumption that each colony formed from a single isolated progenitor cell, these data indicate significantly larger numbers of progenitor cells in the tendon proper relative to the peritenon. However, a substantial percentage of progenitor/stem cells were isolated from both regions. Based on their clonogenic properties, these cells have stem/progenitor cell properties.
Isolated cells were identifiable as progenitors via surface markers
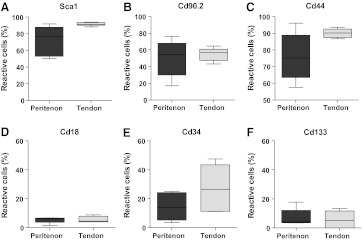
To further evaluate the stem/progenitor status of the clonogenic cells and to characterize their cellular origin, a cell surface marker analysis was preformed. Detection of the stem cell marker, stem cell antigen-1(Sca-1) expression, using flow cytometry supported the stem/progenitor cell properties of the cloned cells from both the tendon proper and the peritenon (Fig. 2A). Approximately, 91.5% of the clonogenic cells from the tendon proper were Sca-1-positive, while about 72.5% of the clonogenic cells from the peritenon were Sca-1-positive. Thus, a large percentage of progenitors can be classified as stem cells by expression of a general stem cell marker. More progenitors from the tendon proper were positive for the general stem cell marker Sca-1 than from the peritenon (paired t-test, p=0.055) (Fig. 2A).
FIG. 2.
General stem cell and fibroblastic surface markers for stem/progenitor cells of the tendon proper and peritenon. Progenitor cells from both tendon proper and peritenon were treated with fluorescent dye-conjugated antibodies for (A) Sca1, (B) Cd90.2, (C) Cd44, (D) Cd18, (E) Cd34, and (F) Cd133, and analyzed via flow cytometry (n=5). Progenitor cells from both regions demonstrated positive reactivity for the general stem cell marker, Sca1 (A), with greater reactivity demonstrated for cells of the tendon proper (p=0.055). Stem/progenitors were also positive for fibroblast markers, Cd90.2 (B) and Cd44 (C), yet only minimally positive for leukocyte Cd18 (D), hematopoietic Cd34 (E), and pericyte Cd133 (F) markers. Greater variation was observed in peritenon cells for some markers (A–C), indicative of heterogeneity of cell origin.
We hypothesized that the stem/progenitor cells from different niches would have niche-specific properties. To address this, surface antigens on stem/progenitors from both within the tendon and the peritenon were analyzed for well-characterized surface markers (Table 1) using flow cytometry. Distinct surface marker profiles were demonstrated for stem/progenitors from each region (Fig. 2). As was seen for Sca-1, a large percentage of clonable cells isolated from the tendon and peritenon demonstrated reactivity for the fibroblast markers, Cd90.2 and Cd44 (Fig. 2B, C). In addition to this similarity in marker expression, the analyses of the progenitors of the tendon proper and the peritenon demonstrated regional differences. The mean number of positive cells for Sca-1 was greater in cells isolated from the tendon proper versus the peritenon. There was also greater variability in marker expression of clonogenic cells from the peritenon. The mean values±sd for positive cells from the tendon versus peritenon were 91.5%±2.0% versus 72.5%±17.9% for Sca-1; 55.0%±7.8% versus 50.4%±21.4% for Cd90.2; and 90.1%±2.7% versus 76.0%±15.0% for Cd44, respectively.
In contrast, progenitors from both the tendon and the peritenon demonstrated a low percentage of cells positive for leukocytic, hematopoietic, and perivascular markers (5%–27%) by flow cytometric analysis. The percentages for these markers were leukocyte adhesion marker, Cd18 (5.6%±2.1% vs. 5.5%±2.2%); hematopoietic and vascular stem cell marker, Cd34 (27.6%±16.2% vs. 14.6%±9.6%); and pericyte stem cell marker (5.8%±5.5% vs. 7.1%±6.1%) (Fig. 2D, E). This indicates that the isolated cells from the tendon proper and the peritenon both demonstrated a profile of cell surface markers indicative of subpopulations of progenitor cells with stem cell properties, fibroblast features, and little contribution from leukocytic, hematopoietic, or perivascular sources.
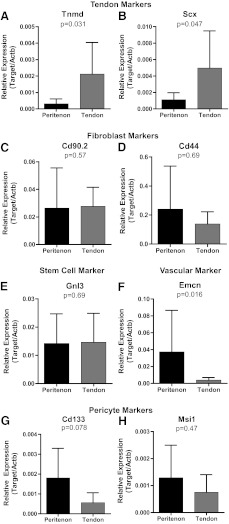
Gene expression profiles reveal distinct stem/progenitor cell populations
To further elucidate the phenotypes of stem/progenitor cells isolated from the tendon proper and the peritenon, marker characteristic of different cellular origins were analyzed using RT-qPCR (Table 1). The stem/progenitor cells isolated from the different regions demonstrate different marker profiles suggesting discrete cell populations. Transcripts for tendon markers, scleraxis (Scx) and tenomodulin (Tnmd), were analyzed (Fig. 3A, B). Both tendon markers demonstrated significantly increased expression in stem/progenitor cells from the tendon compared to the peritenon. The tendon compared to the peritenon progenitors demonstrated a 6.8-fold (p=0.047) and 4.5-fold (p=0.031) increase in Scx and Tnmd, respectively. Stem/progenitor cells from both regions demonstrated nearly equal transcript levels for the fibroblast markers, Cd90.2 and Cd44, and the stem cell marker, nucleostemin (Gnl3) (Fig. 3C–E). In contrast, expression of vascular and pericyte markers was increased in the stem/progenitors from the peritenon compared to the tendon (Fig. 3F–H). Transcripts of the vascular marker, endomucin (Emcn), demonstrated greater abundance in stem/progenitors of the peritenon, relative to tendon stem/progenitors, by 9.5-fold (p=0.016). A similar trend was seen for the pericyte marker, musashi-1 (Msi-1), where expression was 1.7-fold greater in peritenon progenitors (p=0.47). This was also the case for the pericyte marker Cd133 transcripts that were 3.2-fold more abundant in stem/progenitors from the peritenon, relative to tendon proper progenitors (p=0.078). Thus, gene expression profiles indicated that isolated stem/progenitor cells from within the tendon express greater levels of tendon marker transcripts, while peritenon progenitors express greater levels of pericyte and vascular marker transcripts.
FIG. 3.
Distinct real-time quantitative polymerase chain reaction expression profiles for stem/progenitors of the peritenon and tendon proper. Tendon compared to peritenon progenitors demonstrated a 4.5-fold (p=0.031) and a 6.8-fold (p=0.047) increase in Tnmd (A) and Scx (B), respectively, indicating that stem/progenitors of the endotenon are more tendon-like. Fibroblast markers, Cd90 (C) and Cd44 (D), and stemness marker, Gnl3 (E), levels were roughly equivalent. Vascular marker Emcn (F) transcripts were 9.5-fold more abundant in the peritenon (p=0.016), as was the trend for pericyte cell markers, Cd133 (G) and Msi1 (H). Thus, stem/progenitors from the peritenon were more vascular cell or perciyte in origin (n=7).
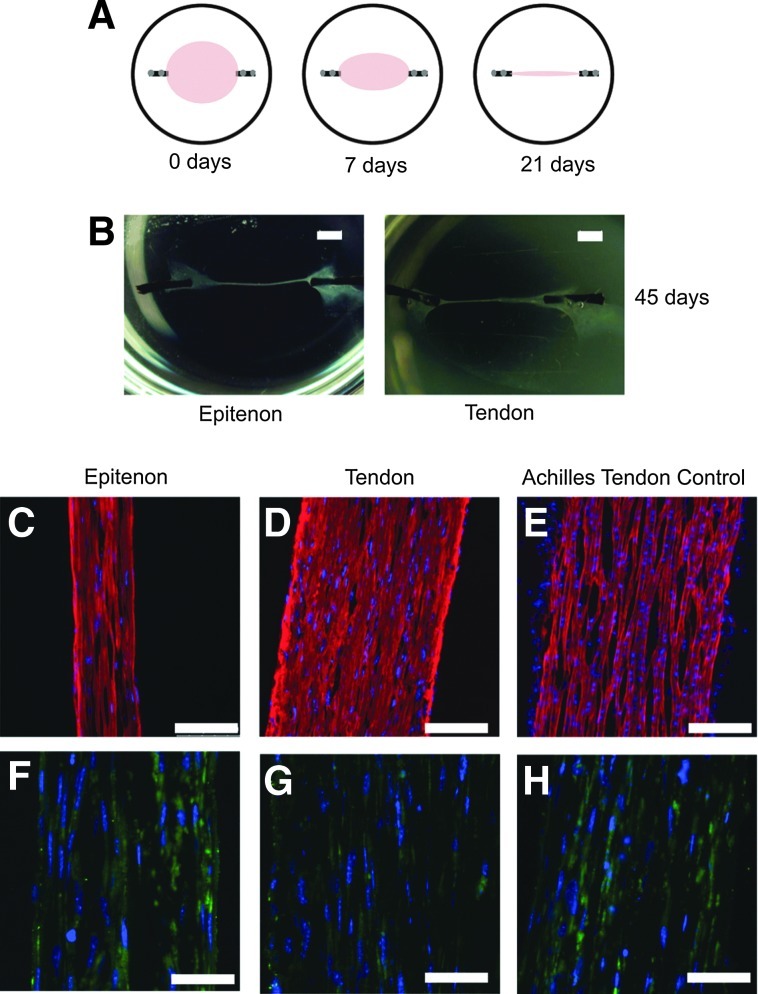
Stem/progenitor cells from the endotenon and peritenon form primitive tendons in vitro
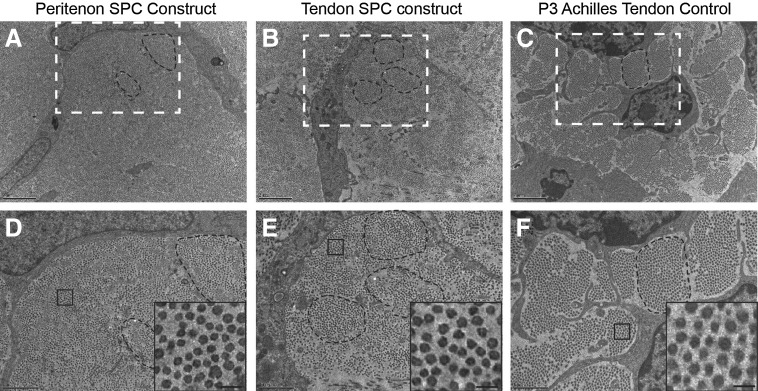
The data indicate that at least two distinct populations of stem cells can be isolated from the tendon and peritenon niches. The potential of these stem/progenitor cells to be driven toward tendon differentiation was analyzed. Isolated progenitor cells were seeded within an anchored fibrinogen/thrombin gel (Fig. 4A). Over time, the cells contracted the gels and established uniaxial tension across the construct. Both cells from the peritenon and the tendon proper contracted to form a three-dimensional tendon construct (Fig. 4B). Cells in constructs were aligned parallel to the strain axis as with the control tendon. Reactivity for collagen I was observed in tendon constructs created using progenitors from both regions (Fig. 4C, D), comparable to the P3 Achilles tendon control (Fig. 4E). Reactivity for tenomodulin (Tnmd) also was observed within the cells of the tendon constructs created with progenitors from both regions (Fig. 4F, G), again comparable to the Achilles tendon control (Fig. 4H). Stem/progenitor cell-derived tendon constructs exhibited morphological features, as well as tenomodulin and collagen I expression comparable to that observed in young Achilles tendons. The ultrastructure of the tendon constructs was compared to an Achilles tendon control analyzed using transmission electron microscopy. Cells within the constructs had attenuated processes that partitioned the developing fibers in a manner consistent with embryonic tenocytes (Fig. 5A–C). Collagen fibrils were assembled and organized along the axis of stress in constructs of progenitor cells derived from both the peritenon and the tendon proper comparable to that seen in the developing Achilles tendon (Fig. 5A–C). The fibrils coalesced to form bundles, fibers comparable to the developing tendon, but fiber organization was not as well organized as is seen in the P3 Achilles tendon (Fig. 5D–F). In addition, relatively small diameter fibrils with a homogeneous distribution of diameters were assembled in both constructs. The fibril structure was similar to that of the developing tendon (Fig. 5D–F, insets). Thus, in vitro contracting fibrin/stem cell constructs underwent cell morphology changes and fibril assembly events that typically define early tendon development. This demonstrates that stem/progenitor cells isolated from both regions are capable of forming tendon-like structures in vitro.
FIG. 4.
In vitro progenitor-derived tendon constructs. Tendon progenitors from both regions formed a tendon construct with tension in culture. Isolated progenitor cells were seeded within a fibrinogen/thrombin gel, fixed to suture silk anchored in place with insect pins; over 3 weeks, the constructs contracted, and uniaxial tension was applied to the cells (A). Both cells from the peritenon and the tendon proper contracted to form a tendon construct (B). Reactivity for collagen I was seen with tendon constructs created with progenitors from both regions (C, D), comparable to P4 Achilles tendon controls (E). Reactivity for tenomodulin (Tnmd) was seen with tendon constructs created with progenitors from both regions (F, G), similar to the P4 Achilles tendon control (H). [Bar: 200 μm (B), 100 μm (C–E), 50 μm (F–H); red, anti-collagen I; green, anti-TNMD; blue, DAPI].
FIG. 5.
Tendon constructs demonstrate features of embryonic tendon. Ultrastructural analysis of progenitor-derived tendon constructs. Tendon constructs assembled collagen fibrils and fibers (A, B), similar to those in a developing P3 Achilles tendon (C). Dashed white boxes (A, B, C) represent regions further magnified below (D, E, F, respectively). The dashed black line encircles a region exemplifying fibrils packing to form fibers. The insets in panels D–F magnify the fibril structure within the black boxes of the same panels. The insets show fibril structure in the constructs relative to the developing tendon. Relatively small diameter fibrils with a homogeneous distribution of diameters are observed in all cases. [Bar: 2 μm (A, B, C); 1 μm (D, E, F), 100 nm (Insets in D, E, F)].
Progenitor cells were multipotent (adipogenic, osteogenic, and chondrogenic)
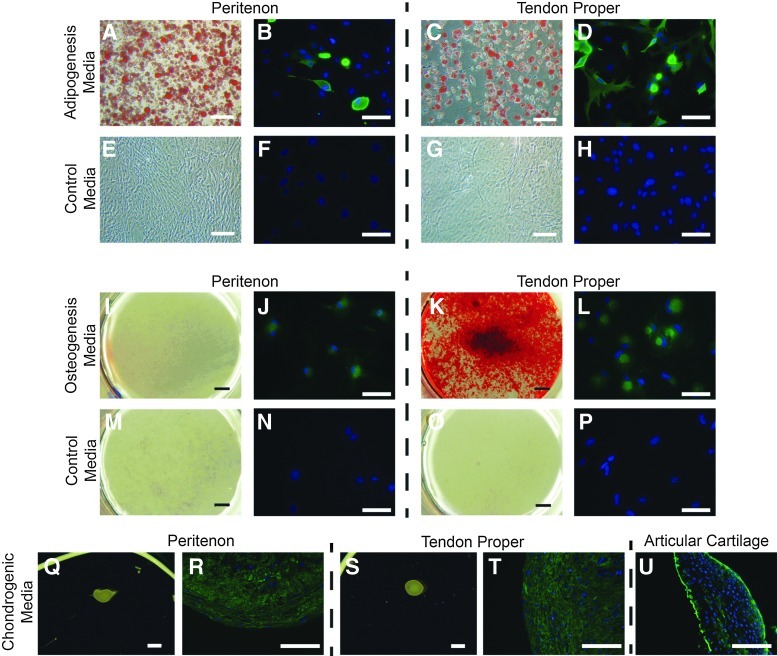
Multipotency of the cells isolated from the two regions was analyzed to further elucidate the stem/progenitor cell properties for these populations. Progenitor cells in culture were driven toward adipogenic, osteogenic, and chondrogenic differentiation. Thus, in addition to the tenogenic potential, the cells isolated from both the tendon proper and peritenon were multipotent (Fig. 6). When stem/progenitor cells were treated with the adipogenic culture medium, adipogenesis was evident by day 14 with the appearance of oil droplets. By day 21, oil droplets were abundant throughout the culture. Oil Red O staining of adipogenic cultures of TSPCs from both regions demonstrated significant amounts of newly differentiated adipocytes (Fig. 6A, C) relative to control cultures in the basal medium (Fig. 6E, G). Stem/progenitor cells driven into adipogenesis also demonstrated reactivity for the adipocyte marker, Fabp4, by immunohistochemistry relative to control cultures (Fig. 6B, D, F, H). Progenitors from both regions also were driven into osteogenesis by media containing rhBMP-2, dexamethasone, ascorbic-2-phosphate, and glycerol-2-phosphate. After 28 days in culture, stem/progenitor cells isolated from both regions demonstrated reactivity for the osteogenic marker, Opn, relative to progenitors in control media (Fig. 6J, L, N, P). However, only progenitors from the tendon proper deposited calcium within the cell layer when analyzed using Alizarin Red staining (Fig. 6I, K, M, O). When stem/progenitor cells from both regions were treated with chondrogenic media, cells condensed to form a pellet (Fig. 6Q, S). The cell pellets formed by stem/progenitors from both regions reacted positively for collagen II comparable to articular cartilage (Fig. 6R, T, U). Thus, in addition to the ability to form tendon-like tissue, the stem/progenitor cells isolated from both the tendon proper and the peritenon exhibited multipotency indicative of a stem cell population.
FIG. 6.
Multipotency of progenitors from both the tendon proper and the peritenon. Stem/progenitors from both the peritenon and the tendon proper were multipotent. Progenitors driven into adipogenic differentiation were positive for Oil Red O (A, C) and for fatty acid-binding protein-4 (Fabp4) (B, D), relative to cells in basal media (E–H). Progenitors from both regions were driven into osteogenic differentiation and had positive reactivity for osteopontin (Opn) (J, L), relative to cells in basal media (N, P). However, only progenitors from the tendon proper were able to produce a calcified matrix (K), relative to those from the peritenon (I). Progenitors from both regions underwent chondrogenic differentiation (Q, S); cell pellets were reactive for collagen II (R, T) as was the articular cartilage control (U). [Bar: 100 μm (A, C, E, G), 200 μm (B, D, F, H, J, L, N, P, R, T, U), 2 mm (I, K, M, O), 1 mm (Q, S); Oil Red O (A, C, E, G); Alizarin Red (I, K, M, O); blue, DAPI (B, D, F, H, J, L, N, P, R, T, U); green, anti-Fabp4 (B, D, F, H); green, anti-Opn (J, L, N, P); green, anti-collagen II (R, T, U)].
In summary, we demonstrated that distinct populations of stem/progenitor cells are found within both the tendon proper and the peritenon of the murine Achilles tendon. These cells are clonogenic and the stem/progenitors of both regions are positive for stem cell and fibroblast markers. Progenitors of the tendon proper express greater levels of tendon markers, scleraxis and tenomodulin, while progenitors of the peritenon express greater levels of vascular markers. Stem/progenitor cells from both regions are capable of forming tendons in vitro. In addition to their tenogenic potential, cells from both regions are multipotent.
Discussion
In this study, we demonstrated that distinct stem/progenitor cell populations exist in and around the mouse Achilles tendon, more specifically the tendon proper and the peritenon. The stem/progenitor cells selectively grew in vitro in stem cell media and were observed as adherent colonies.10,61–63 The observed heterogeneity in colony size and cell morphology for stem/progenitors isolated from the different regions suggested mixed progenitor cell populations both within the peritenon and the tendon proper. Heterogeneity in colony size for stem/progenitors was indicative of different proliferative rates that are also a feature of a mixed cell population. Flow cytometric and gene expression data supported the status of the isolated stem/progenitor cells. Progenitors from both regions were positive for a general stem cell marker, stem cell antigen-1 (Sca1), for surface antigens of fibroblast and mesenchymal stromal cell marker, Cd90.2, as well as for the fibroblast marker and hyaluronic acid receptor, Cd44, while being relatively negative for surface antigens of leukocyte adhesion molecule, Cd18, hematopoietic stem cell marker, Cd34, and endothelial/pericyte marker, Cd133. The marker profile of stem/progenitor cells isolated from the tendon proper is consistent with that described by Bi et al.10 However, we demonstrated for the first time that stem/progenitor cells within the peritenon also exhibited a tendon/progenitor cell surface profile.
Stem/progenitor cells from both regions were capable of forming primitive tendon constructs in vitro when seeded within a fibrin gel that contracted, allowing the cells to align parallel to the uniaxial force. Tendon-like characteristics observed for the tendon constructs included positive immunohistochemical reactivity for collagen I and tenomodulin as well as transmission electron microscopy imaging of collagen fibril and fiber formation aligned along the long axis for tendon constructs from stem/progenitor cells of both regions. However, tendon fibroblasts and tenocytes have also demonstrated tenogenic capabilities, so it was necessary to better define these stem/progenitor cells from both regions by demonstrating multipotency when driven toward adipogenesis, osteogenesis, and chondrogenesis. Stem/progenitor cells from both regions were multipotent. Taken together, these results indicate that progenitor/stem cell populations exist within the tendon proper and the peritenon.
Some findings underscored distinctions between the progenitor pools. For example, more progenitor cells were Sca-1-positive within the tendon proper, relative to the peritenon. Thus, more progenitors are found within the tendon and these cells are generally more stem-like. This suggests that a greater reserve population of stem cells exists within the tendon proper, though stemness is not an indicator of activity in homeostasis or repair and this study did not spatially localize exactly where within the tendon proper these cells are located. Moreover, greater variation was seen for Sca-1 for peritenon progenitors, further indicating that while cells within the paratenon and epitenon are progenitors, they may not necessarily be stem cells. Furthermore, greater variation was seen for peritenon progenitors for antigens, Cd90.2 and Cd44, which is evidence of greater heterogeneity in origin for these cells. Within the paratenon of the Achilles tendon are adipocytes, vascular endothelium, and pericytes, besides tenocytes; thus, not every stem or progenitor cell is necessarily fibroblastic in nature. Indeed, the variation in the markers throughout this study is an indication that there may be one or more subpopulations of stem/progenitor cells within each region. While this study did not sort out subpopulations within each region, the data indicate that distinct subpopulations of progenitors with diverse origins exist within the tendon proper and the paratenon and epitenon. Moreover, conclusions regarding progenitor origin can be made. Stem/progenitors of the tendon proper expressed greater levels of scleraxis and tenomodulin when compared to those of the peritenon. Thus, stem/progenitors of the tendon proper could be considered more tendon-like or to be enriched with populations of progenitors that are of tendon origin. In contrast, levels of the vascular marker, endomucin (Emcn), are greater in peritenon stem/progenitor cells, and pericyte markers, Cd133 and Musashi-1 (Msi1) expression levels, trend higher in peritenon stem/progenitors. The increased vascular and pericyte marker expression levels in the peritenon reflected other possible sources of enrichment for these progenitors besides cells of the endotendon.
One particular distinction observed between stem/progenitor cells of the tendon proper and those of the peritenon was noted when progenitors were supplemented with osteogenic media. Progenitors from both the tendon proper and the peritenon were driven to express osteopontin (Opn) in the rhBMP-2-supplemented media. However, only progenitors from the tendon proper deposited calcium within the cell layer. This unique feature provides an explanation for the calcification and ossification that occur within the tendon proper with tendinopathy64–66 or in the absence of SLRPs.10,67 This finding could make peritenon stem/progenitor cells a better choice for therapeutic strategies if they are less likely to result in a calcified or ossified repair tissue.
Our data demonstrate that distinct populations of stem/progenitor cells exist within the tendon proper and peritenon niches. Stem/progenitor cells characterized in this study were multipotent. More importantly, cells from both regions were capable of forming a primitive tendon. Results from this study address which progenitor cell populations are enriched in and around the Achilles tendon. The data lend support to the theory that stem/progenitor cells from both regions are plausible sources for tendon healing. That is, tendon repair could involve both intrinsic (tendon proper) and extrinsic (epitenon and paratenon) components. Based on this study, we can conclude that stem/progenitor cells exist in both regions and are capable of tenogenesis. We cannot yet say which cell populations are most active in healing, which populations repair microlesions from wear and tear, and which cells mount the repair response in larger tendon tears. However, this study does offer another resource for cell harvesting and potential therapeutic options, the cells of the paratenon and epitenon. Cells of the epitenon have been shown to migrate to the endotenon with treadmill training in mice, and thus are already considered essential to tendon maintenance.68 Much speculation is already ongoing with regard to the cells of the peritenon and sheath (for those with sheaths) being a cell source for repair.1,2 Our findings lend support to our combined intrinsic and extrinsic tendon repair model by highlighting the distinctions and potential of cells from both regions. Further studies are required to localize these populations spatially within tissue. Furthermore, it is equally important to determine each population's level of activity as well as their role in the repair process for the several classes of tendinopathies. Still, this study is the first such study to demonstrate that stem/progenitor cells exist in both sources—from the tendon proper and the paratenon and epitenon—and that they are capable of tenogenesis in vitro.
Conclusions
In this study, stem/progenitor cells were isolated from the tendon proper and the peritenon of mature murine Achilles tendons. Progenitors from the tendon proper exhibited more tendon-like marker expression, while progenitors of the paratenon and epitenon demonstrated greater vascular endothelial and pericyte marker expression. Stem/progenitor cells from both regions were similarly multipotent. Furthermore, stem/progenitor cells from both regions could be driven toward tenogenesis and the formation of a primitive tendon. These results emphasize the potential utility of stem/progenitor cells from both regions. Thus, both the tendon proper and the peritenon possess plausible cell sources for the dual intrinsic and extrinsic involvement of cell populations during tendon repair.
Supplementary Material
Acknowledgments
Funding was provided by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, Department of Health and Human Services (AR058027, MJM; AR044745, DEB). We thank Dr. Karoly Szekeres (Fred Wright Flow Cytometry Core Facility, USF) for assistance with flow cytometric analysis and Dr. Michael J. Shereff (Department of Orthopaedics & Sports Medicine, USF) for many helpful discussions.
Disclosure Statement
There are no competing financial interests.
References
- 1.Ingraham J.M. Hauck R.M. Ehrlich H.P. Is the tendon embryogenesis process resurrected during tendon healing? Plast Reconstr Surg. 2003;112:844. doi: 10.1097/01.PRS.0000070180.62037.FC. [DOI] [PubMed] [Google Scholar]
- 2.Sharma P. Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87:187. doi: 10.2106/JBJS.D.01850. [DOI] [PubMed] [Google Scholar]
- 3.Manske P.R. Gelberman R.H. Vande Berg J.S. Lesker P.A. Intrinsic flexor-tendon repair. A morphological study in vitro. J Bone Joint Surg Am. 1984;66:385. [PubMed] [Google Scholar]
- 4.Crisan M. Yap S. Casteilla L. Chen C.W. Corselli M. Park T.S. Andriolo G. Sun B. Zheng B. Zhang L. Norotte C. Teng P.N. Traas J. Schugar R. Deasy B.M. Badylak S. Buhring H.J. Giacobino J.P. Lazzari L. Huard J. Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Caplan A.I. Dennis J.E. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 6.Choi H.R. Kondo S. Hirose K. Ishiguro N. Hasegawa Y. Iwata H. Expression and enzymatic activity of MMP-2 during healing process of the acute supraspinatus tendon tear in rabbits. J Orthop Res. 2002;20:927. doi: 10.1016/S0736-0266(02)00016-5. [DOI] [PubMed] [Google Scholar]
- 7.Oshiro W. Lou J. Xing X. Tu Y. Manske P.R. Flexor tendon healing in the rat: a histologic and gene expression study. J Hand Surg Am. 2003;28:814. doi: 10.1016/s0363-5023(03)00366-6. [DOI] [PubMed] [Google Scholar]
- 8.Hazard S.W. Myers R.L. Ehrlich H.P. Demonstrating collagen tendon fibril segments involvement in intrinsic tendon repair. Exp Mol Pathol. 2011;91:660. doi: 10.1016/j.yexmp.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingraham J.M. Weber R.A. Childs E.W. Intrinsic tendon healing requires the recycling of tendon collagen fibril segments. J Hand Surg Eur Vol. 2011;36:154. doi: 10.1177/1753193410382959. [DOI] [PubMed] [Google Scholar]
- 10.Bi Y. Ehirchiou D. Kilts T.M. Inkson C.A. Embree M.C. Sonoyama W. Li L. Leet A.I. Seo B.M. Zhang L. Shi S. Young M.F. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J. Wang J.H. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet Disord. 2010;11:10. doi: 10.1186/1471-2474-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones D.L. Wagers A.J. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol. 2008;9:11. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J. Wang J.H. Production of PGE(2) increases in tendons subjected to repetitive mechanical loading and induces differentiation of tendon stem cells into non-tenocytes. J Orthop Res. 2010;28:198. doi: 10.1002/jor.20962. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J. Wang J.H. BMP-2 mediates PGE(2) -induced reduction of proliferation and osteogenic differentiation of human tendon stem cells. J Orthop Res. 2012;30:47. doi: 10.1002/jor.21485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J. Wang J.H. Mechanobiological response of tendon stem cells: implications of tendon homeostasis and pathogenesis of tendinopathy. J Orthop Res. 2010;28:639. doi: 10.1002/jor.21046. [DOI] [PubMed] [Google Scholar]
- 16.Khan K.M. Cook J.L. Bonar F. Harcourt P. Astrom M. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999;27:393. doi: 10.2165/00007256-199927060-00004. [DOI] [PubMed] [Google Scholar]
- 17.Ni M. Lui P.P. Rui Y.F. Lee Y.W. Tan Q. Wong Y.M. Kong S.K. Lau P.M. Li G. Chan K.M. Tendon-derived stem cells (TDSCs) promote tendon repair in a rat patellar tendon window defect model. J Orthop Res. 2012;30:613. doi: 10.1002/jor.21559. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J. Pan T. Liu Y. Wang J.H. Mouse treadmill running enhances tendons by expanding the pool of tendon stem cells (TSCs) and TSC-related cellular production of collagen. J Orthop Res. 2010;28:1178. doi: 10.1002/jor.21123. [DOI] [PubMed] [Google Scholar]
- 19.Benjmain M. Theobald P. Suzuki D. Toumi H. Ch. 2: The Anatomy of the Achilles Tendon. In: Maffulli N., editor; Almekinders L., editor. The Achilles Tendon. London: Springer-Verlag; 2007. pp. 5–16. [Google Scholar]
- 20.Jelinsky S.A. Archambault J. Li L. Seeherman H. Tendon-selective genes identified from rat and human musculoskeletal tissues. J Orthop Res. 2010;28:289. doi: 10.1002/jor.20999. [DOI] [PubMed] [Google Scholar]
- 21.Arnaout M.A. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 1990;75:1037. [PubMed] [Google Scholar]
- 22.Gahmberg C.G. Leukocyte adhesion: CD11/CD18 integrins and intercellular adhesion molecules. Curr Opin Cell Biol. 1997;9:643. doi: 10.1016/s0955-0674(97)80117-2. [DOI] [PubMed] [Google Scholar]
- 23.Furness S.G. McNagny K. Beyond mere markers: functions for CD34 family of sialomucins in hematopoiesis. Immunol Res. 2006;34:13. doi: 10.1385/IR:34:1:13. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen J.S. McNagny K.M. Novel functions of the CD34 family. J Cell Sci. 2008;121:3683. doi: 10.1242/jcs.037507. [DOI] [PubMed] [Google Scholar]
- 25.Sutherland D.R. Watt S.M. Dowden G. Karhi K. Baker M.A. Greaves M.F. Smart J.E. Structural and partial amino acid sequence analysis of the human hemopoietic progenitor cell antigen CD34. Leukemia. 1988;2:793. [PubMed] [Google Scholar]
- 26.Johnson P. Ruffell B. CD44 and its role in inflammation and inflammatory diseases. Inflamm Allergy Drug Targets. 2009;8:208. doi: 10.2174/187152809788680994. [DOI] [PubMed] [Google Scholar]
- 27.Pure E. Assoian R.K. Rheostatic signaling by CD44 and hyaluronan. Cell Signal. 2009;21:651. doi: 10.1016/j.cellsig.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu H. Mitsuhashi N. Klein A. Barsky L.W. Weinberg K. Barr M.L. Demetriou A. Wu G.D. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells. 2006;24:928. doi: 10.1634/stemcells.2005-0186. [DOI] [PubMed] [Google Scholar]
- 29.Halfon S. Abramov N. Grinblat B. Ginis I. Markers distinguishing mesenchymal stem cells from fibroblasts are downregulated with passaging. Stem Cells Dev. 2011;20:53. doi: 10.1089/scd.2010.0040. [DOI] [PubMed] [Google Scholar]
- 30.Jones E.A. Kinsey S.E. English A. Jones R.A. Straszynski L. Meredith D.M. Markham A.F. Jack A. Emery P. McGonagle D. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002;46:3349. doi: 10.1002/art.10696. [DOI] [PubMed] [Google Scholar]
- 31.Saalbach A. Klein C. Sleeman J. Sack U. Kauer F. Gebhardt C. Averbeck M. Anderegg U. Simon J.C. Dermal fibroblasts induce maturation of dendritic cells. J Immunol. 2007;178:4966. doi: 10.4049/jimmunol.178.8.4966. [DOI] [PubMed] [Google Scholar]
- 32.Sorrell J.M. Caplan A.I. Fibroblasts-a diverse population at the center of it all. Int Rev Cell Mol Biol. 2009;276:161. doi: 10.1016/S1937-6448(09)76004-6. [DOI] [PubMed] [Google Scholar]
- 33.Young H.E. Steele T.A. Bray R.A. Hudson J. Floyd J.A. Hawkins K. Thomas K. Austin T. Edwards C. Cuzzourt J. Duenzl M. Lucas P.A. Black A.C., Jr Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat Rec. 2001;264:51. doi: 10.1002/ar.1128. [DOI] [PubMed] [Google Scholar]
- 34.Corbeil D. Roper K. Hellwig A. Tavian M. Miraglia S. Watt S.M. Simmons P.J. Peault B. Buck D.W. Huttner W.B. The human AC133 hematopoietic stem cell antigen is also expressed in epithelial cells and targeted to plasma membrane protrusions. J Biol Chem. 2000;275:5512. doi: 10.1074/jbc.275.8.5512. [DOI] [PubMed] [Google Scholar]
- 35.Mizrak D. Brittan M. Alison M.R. CD133: molecule of the moment. J Pathol. 2008;214:3. doi: 10.1002/path.2283. [DOI] [PubMed] [Google Scholar]
- 36.Tempfer H. Wagner A. Gehwolf R. Lehner C. Tauber M. Resch H. Bauer H.C. Perivascular cells of the supraspinatus tendon express both tendon- and stem cell-related markers. Histochem Cell Biol. 2009;131:733. doi: 10.1007/s00418-009-0581-5. [DOI] [PubMed] [Google Scholar]
- 37.Docheva D. Hunziker E.B. Fassler R. Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol. 2005;25:699. doi: 10.1128/MCB.25.2.699-705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanda H. Tanaka T. Matsumoto M. Umemoto E. Ebisuno Y. Kinoshita M. Noda M. Kannagi R. Hirata T. Murai T. Fukuda M. Miyasaka M. Endomucin, a sialomucin expressed in high endothelial venules, supports L-selectin-mediated rolling. Int Immunol. 2004;16:1265. doi: 10.1093/intimm/dxh128. [DOI] [PubMed] [Google Scholar]
- 39.Liu C. Shao Z.M. Zhang L. Beatty P. Sartippour M. Lane T. Livingston E. Nguyen M. Human endomucin is an endothelial marker. Biochem Biophys Res Commun. 2001;288:129. doi: 10.1006/bbrc.2001.5737. [DOI] [PubMed] [Google Scholar]
- 40.Matsubara A. Iwama A. Yamazaki S. Furuta C. Hirasawa R. Morita Y. Osawa M. Motohashi T. Eto K. Ema H. Kitamura T. Vestweber D. Nakauchi H. Endomucin, a CD34-like sialomucin, marks hematopoietic stem cells throughout development. J Exp Med. 2005;202:1483. doi: 10.1084/jem.20051325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoshi N. Kusakabe T. Taylor B.J. Kimura S. Side population cells in the mouse thyroid exhibit stem/progenitor cell-like characteristics. Endocrinology. 2007;148:4251. doi: 10.1210/en.2006-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jafarnejad S.M. Mowla S.J. Matin M.M. Knocking-down the expression of nucleostemin significantly decreases rate of proliferation of rat bone marrow stromal stem cells in an apparently p53-independent manner. Cell Prolif. 2008;41:28. doi: 10.1111/j.1365-2184.2007.00505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamaki T. Okada Y. Uchiyama Y. Tono K. Masuda M. Wada M. Hoshi A. Ishikawa T. Akatsuka A. Clonal multipotency of skeletal muscle-derived stem cells between mesodermal and ectodermal lineage. Stem Cells. 2007;25:2283. doi: 10.1634/stemcells.2006-0746. [DOI] [PubMed] [Google Scholar]
- 44.Ferraro F. Celso C.L. Scadden D. Adult stem cells and their niches. Adv Exp Med Biol. 2010;695:155. doi: 10.1007/978-1-4419-7037-4_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okano H. Kawahara H. Toriya M. Nakao K. Shibata S. Imai T. Function of RNA-binding protein Musashi-1 in stem cells. Exp Cell Res. 2005;306:349. doi: 10.1016/j.yexcr.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 46.Gussoni E. Soneoka Y. Strickland C.D. Buzney E.A. Khan M.K. Flint A.F. Kunkel L.M. Mulligan R.C. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 47.Spangrude G.J. Heimfeld S. Weissman I.L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 48.Tamaki T. Akatsuka A. Ando K. Nakamura Y. Matsuzawa H. Hotta T. Roy R.R. Edgerton V.R. Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle. J Cell Biol. 2002;157:571. doi: 10.1083/jcb.200112106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Vlasselaer P. Falla N. Snoeck H. Mathieu E. Characterization and purification of osteogenic cells from murine bone marrow by two-color cell sorting using anti-Sca-1 monoclonal antibody and wheat germ agglutinin. Blood. 1994;84:753. [PubMed] [Google Scholar]
- 50.Schweitzer R. Chyung J.H. Murtaugh L.C. Brent A.E. Rosen V. Olson E.N. Lassar A. Tabin C.J. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- 51.Shukunami C. Takimoto A. Oro M. Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev Biol. 2006;298:234. doi: 10.1016/j.ydbio.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 52.Ramakers C. Ruijter J.M. Deprez R.H. Moorman A.F. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett. 2003;339:62. doi: 10.1016/s0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- 53.Schefe J.H. Lehmann K.E. Buschmann I.R. Unger T. Funke-Kaiser H. Quantitative real-time RT-PCR data analysis: current concepts and the novel “gene expression's CT difference” formula. J Mol Med (Berl) 2006;84:901. doi: 10.1007/s00109-006-0097-6. [DOI] [PubMed] [Google Scholar]
- 54.Bi Y. Stuelten C.H. Kilts T. Wadhwa S. Iozzo R.V. Robey P.G. Chen X.D. Young M.F. Extracellular matrix proteoglycans control the fate of bone marrow stromal cells. J Biol Chem. 2005;280:30481. doi: 10.1074/jbc.M500573200. [DOI] [PubMed] [Google Scholar]
- 55.Kostenuik P.J. Halloran B.P. Morey-Holton E.R. Bikle D.D. Skeletal unloading inhibits the in vitro proliferation and differentiation of rat osteoprogenitor cells. Am J Physiol. 1997;273:E1133. doi: 10.1152/ajpendo.1997.273.6.e1133. [DOI] [PubMed] [Google Scholar]
- 56.Zhang G. Chen S. Goldoni S. Calder B.W. Simpson H.C. Owens R.T. McQuillan D.J. Young M.F. Iozzo R.V. Birk D.E. Genetic evidence for the coordinated regulation of collagen fibrillogenesis in the cornea by decorin and biglycan. J Biol Chem. 2009;284:8888. doi: 10.1074/jbc.M806590200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kapacee Z. Richardson S.H. Lu Y. Starborg T. Holmes D.F. Baar K. Kadler K.E. Tension is required for fibripositor formation. Matrix Biol. 2008;27:371. doi: 10.1016/j.matbio.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 58.Kapacee Z. Yeung C.Y. Lu Y. Crabtree D. Holmes D.F. Kadler K.E. Synthesis of embryonic tendon-like tissue by human marrow stromal/mesenchymal stem cells requires a three-dimensional environment and transforming growth factor beta3. Matrix Biol. 2010;29:668. doi: 10.1016/j.matbio.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Birk D.E. Trelstad R.L. Extracellular compartments in tendon morphogenesis: collagen fibril, bundle, and macroaggregate formation. J Cell Biol. 1986;103:231. doi: 10.1083/jcb.103.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Birk D.E. Trelstad R.L. Extracellular compartments in matrix morphogenesis: collagen fibril, bundle, and lamellar formation by corneal fibroblasts. J Cell Biol. 1984;99:2024. doi: 10.1083/jcb.99.6.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hewlett G. Opitz H.G. Schlumberger H.D. Lemke H. Growth regulation of a murine lymphoma cell line by a 2-mercaptoethanol or macrophage-activated serum factor. Eur J Immunol. 1977;7:781. doi: 10.1002/eji.1830071107. [DOI] [PubMed] [Google Scholar]
- 62.Inui K. Oreffo R.O. Triffitt J.T. Effects of beta mercaptoethanol on the proliferation and differentiation of human osteoprogenitor cells. Cell Biol Int. 1997;21:419. doi: 10.1006/cbir.1997.0165. [DOI] [PubMed] [Google Scholar]
- 63.Pereira R.F. Halford K.W. O'Hara M.D. Leeper D.B. Sokolov B.P. Pollard M.D. Bagasra O. Prockop D.J. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci U S A. 1995;92:4857. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin L. Shen Q. Xue T. Yu C. Heterotopic ossification induced by Achilles tenotomy via endochondral bone formation: expression of bone and cartilage related genes. Bone. 2010;46:425. doi: 10.1016/j.bone.2009.08.057. [DOI] [PubMed] [Google Scholar]
- 65.Lui P.P. Cheuk Y.C. Lee Y.W. Chan K.M. Ectopic chondro-ossification and erroneous extracellular matrix deposition in a tendon window injury model. J Orthop Res. 2012;30:37. doi: 10.1002/jor.21495. [DOI] [PubMed] [Google Scholar]
- 66.Pierre-Jerome C. Moncayo V. Terk M.R. MRI of the Achilles tendon: a comprehensive review of the anatomy, biomechanics, and imaging of overuse tendinopathies. Acta Radiol. 2010;51:438. doi: 10.3109/02841851003627809. [DOI] [PubMed] [Google Scholar]
- 67.Ameye L. Aria D. Jepsen K. Oldberg A. Xu T. Young M.F. Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. FASEB J. 2002;16:673. doi: 10.1096/fj.01-0848com. [DOI] [PubMed] [Google Scholar]
- 68.Mendias C.L. Gumucio J.P. Bakhurin K.I. Lynch E.B. Brooks S.V. Physiological loading of tendons induces scleraxis expression in epitenon fibroblasts. J Orthop Res. 2012;30:606. doi: 10.1002/jor.21550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.