Abstract
Human adipose tissue has been recently recognized as a potential source of stem cells for regenerative medicine applications, including bone tissue engineering (TE). Despite the gathered knowledge regarding the differentiation potential of human adipose tissue-derived stem cells (hASCs), in what concerns the endothelial lineage many uncertainties are still present. The existence of a cell subpopulation within the human adipose tissue that expresses a SSEA-4 marker, usually associated to pluripotency, raises expectations on the differentiation capacity of these cells (SSEA-4+hASCs). In the present study, the endothelial and osteogenic differentiation potential of the SSEA-4+hASCs was analyzed, aiming at proposing a single-cell source/subpopulation for the development of vascularized bone TE constructs. SSEA-4+hASCs were isolated using immunomagnetic sorting and cultured either in α-MEM, in EGM-2 MV (endothelial growth medium), or in osteogenic medium. SSEA-4+hASCs cultured in EGM-2 MV formed endothelial cell-like colonies characterized by a cobblestone morphology and expression of CD31, CD34, CD105, and von Willebrand factor as determined by quantitative reverse transcriptase (RT)-polymerase chain reaction, immunofluorescence, and flow cytometry. The endothelial phenotype was also confirmed by their ability to incorporate acetylated low-density lipoprotein and to form capillary-like structures when seeded on Matrigel. SSEA-4+hASCs cultured in α-MEM displayed fibroblastic-like morphology and exhibited a mesenchymal surface marker profile (>90% CD90+/CD73+/CD105+). After culture in osteogenic conditions, an overexpression of osteogenic-related markers (osteopontin and osteocalcin) was observed both at molecular and protein levels. Matrix mineralization detected by Alizarin Red staining confirmed SSEA-4+hASCs osteogenic differentiation. Herein, we demonstrate that from a single-cell source, human adipose tissue, and by selecting the appropriate subpopulation it is possible to obtain microvascular-like endothelial cells and osteoblasts, the most relevant cell types for the creation of vascularized bone tissue-engineered constructs.
Introduction
The concept of using adipose tissue as a source of adult stem cells for regenerative medicine applications is highly appealing mainly due to its abundance and accessibility for harvesting following minimally invasive procedures.1 Human adipose tissue-derived stem cells (hASCs) isolated from the stromal vascular fraction (SVF) of the adipose tissue bear resemblances to bone marrow-derived mesenchymal cells2,3 demonstrated by their similar morphology, and common surface markers and gene expression profiles.4–6 However, the SVF of the adipose tissue harbors more than 2% of cells featuring potential for multilineage differentiation compared to the 0.002% of the bone marrow.1,4,7 Additionally, a large number of studies have proven the hASCs differentiation potential towards multiple lineages, namely the osteogenic,8 chondrogenic,8 adipogenic,8 myogenic,8 and neurogenic.9 Also, the developmental plasticity of hASCs was demonstrated both in vitro10 and in vivo.11 Therefore, hASCs clearly hold a great promise in tissue regeneration therapies, including the creation of a wide range of autologous tissue-engineered substitutes.1 Although there has been extensive research effort to create functional-engineered tissues, the success of such approaches still relies on the construction of vascular networks capable of delivering oxygen and nutrients within the engineered constructs.12 Thus, in the context of bone engineering, the development of strategies that could effectively induce the microcirculation within the engineered constructs has become a major pursuit.13 Previous in vivo studies showed that vascularization within engineered constructs using mature endothelial cells (ECs) improved blood perfusion, cell viability, and their survival after implantation.14–16 However, the limited availability and proliferation capability of mature ECs hinder their use in tissue engineering (TE) approaches.17 Therefore, it became a priority to find a suitable source of ECs that do not present such constrains and that will be ready-to-use for therapeutic applications. A significant number of studies has been reported regarding the isolation of endothelial progenitor cells (EPCs)18–20 and the endothelial differentiation of both embryonic21,22 and adult stem cells from different origins.23–25 The distinction between adult mesenchymal stem cells (MSCs) and endothelial precursors based on cell surface markers is far from ideal as these cells share many common markers. However, the selection of specific subpopulations has gained increasing interest and has revealed significance for cell-based therapies as a way to overcome difficulties imposed by the heterogeneity of each tissue populations.
Considering adipose tissue as a pool of cells containing multipotent stem cells, Rada et al. demonstrated the osteogenic and chondrogenic differentiation potential of distinct subpopulations residing in the SVF.26,27 These results together with other studies28,29 underline the complexity of the SVF of adipose tissue composed by several subpopulations exhibiting distinct differentiation potentials. Other subpopulations, within SVF and hASCs fractions have been identified as possessing endothelial differentiation.30–33 Martínez-Estrada et al.34 expanded the subpopulation expressing Flk-1, a receptor for the vascular endothelial growth factor (VEGF) and one of the earliest markers of EPCs,31 residing within the adipose stroma, and were able to lead their maturation into endothelial-like cells. Miranville et al.32 and Sengenes et al.30,33 have demonstrated that the CD34+/CD31− subpopulation when cultured under appropriate conditions give rise to functional ECs as demonstrated after intravenous injection in a mouse ischemic hindlimb model. The hASCs-derived CD31−/CD45− subpopulation under shear stress and treatment with the endothelial cell growth supplement (ECGS) also acquired some endothelial characteristics, but not others, such as nitric oxide synthase, von Willebrand factor (vWF) expression, which suggests the need to further knowledge regarding this subpopulation before its use in cell-based therapies.35 So far, the definition of the appropriate surface marker(s) to isolate a specific subpopulation from human adipose tissue, relevant for vascularization purposes was restricted to endothelial or hematopoietic progenitor markers.
Stage-specific embryonic antigen (SSEA-4) has been widely used as one of the markers for monitoring the maintenance of an undifferentiated state of human embryonic stem cells.36,37 Moreover, SSEA-4+ cells retain features of pluripotency, characterized by nearly unlimited self-renewal and differentiation capacity into any of the three germ layers.37–39 Riekstina et al.36 examined the expression of embryonic stem cell markers within adult MSC populations derived from different cell sources and showed the presence of approximately 8% of SSEA-4+ cells within the adipose tissue.36 Based on these findings, we hypothesized that the SSEA-4+hASCs subpopulation might exhibit multipotent features relevant for bone TE applications. By triggering its differentiation toward the endothelial and osteogenic lineages, we were able to demonstrate the usefulness of the proposed strategy to obtain these two cell types from the same cell source, in opposition to using the entire SVF. Furthermore, this work comprises the first step to assemble a TE construct using a subpopulation whose differentiation level might be modulated toward the two most relevant lineages to achieve a successful in bone regeneration approach.
Materials and Methods
hASCs and human umbilical vein endothelial cells harvest
All the human samples were obtained after written protocols were established between the 3B's Research Group and the provider-involved institutions. The protocols were approved by the respective ethics committees to assure that the requirements defined by the Helsinki declaration guidelines regarding human rights, thus assuring the patient's informed consent as well as patient's anonymity, were followed.
The lipoaspirate samples were kindly provided by Hospital de Prelada, Porto, Portugal, and human umbilical vein endothelial cells (HUVECs) were obtained from the umbilical cord of healthy babies provided by Hospital de São Marcos, Braga, Portugal. In more detail, human abdominal subcutaneous fat tissue samples were obtained from healthy females (n=6), with an average age of 42 years old, undergoing the lipoaspiration procedure, after informed consent. The tissue samples were transported to the laboratory in phosphate-buffered saline (PBS) supplemented with 10% penicillin–streptomycin (Pen/Strep; Gibco) at a final concentration of 1000U/1000 μg/mL and processed within 24 h after surgery, according to a standard isolation protocol.40 Briefly, the lipoaspirates were digested with 0.075% collagenase II A (Sigma-Aldrich) in PBS, at pH 7.4 for 45 min and at 37°C in a shaking water bath, and finally filtered using a strain with 200-μm pore size. Mature adipocytes and connective tissue cells were separated by centrifugation at 1000 g, for 10 min at 4°C. The cell pellet was resuspended and incubated for 10 min at room temperature in a pH 7.4 erythrocyte lysis buffer of 155 mM NH4Cl (Merck), 5.7 mM K2HPO4 (Riedel-de-Häen), and 0.1 mM EDTA (Sigma-Aldrich) in distillated water. The cell suspension was centrifuged at 800 g for 10 min at 4°C. The pellet was resuspended in PBS and filtered with a 100-μm cell strainer to obtain the SVF.
Primary cultures of macrovascular HUVECs were used as a positive control for the endothelial phenotype. Cells were obtained according to a previously published method.41
Immunomagnetic beads cell separation
The immunomagnetic beads (Dynal M-450 Epoxy beads from Dynal Biotech) were first coated with the SSEA-4 antibody (Abcam) following the manufacturer's instruction. For this purpose, 50 μL of the immunomagnetic beads solution containing 2×107 beads, were washed in the coupling buffer (0.1 M sodium phosphate buffer: pH 7.4–8.0), resuspended with 10 μL of the SSEA-4 antibody at a concentration of 200 μg/mL, and then incubated, overnight, at room temperature, under gentle stirring. After this period, the SSEA-4-coupled beads were separated with the Dynal MPC® magnet (Dynal Biotech) and the supernatant discarded. The coupled beads were mixed and incubated for 5 min with a gentle rotation in 1 mL of a 0.2% (w/v) bovine serum albumin (BSA; Sigma-Aldrich) solution in PBS (0.2%BSA/PBS), at pH 7.4 and again separated using the magnet. This procedure was repeated three times. The antibody-coupled beads were resuspended in 0.2%BSA/PBS at a concentration of 4×108 Dynabeads/mL until further use.
To select the SVF subpopulation of interest for the study, (SSEA-4+hASCs), the SSEA-4 antibody-coated beads were mixed with the SVF and incubated for 30 min at 4°C under gentle stirring. Subsequently, the mixture was washed with 0.2% BSA/PBS and the cells bonded to the beads were separated from the rest of the cell suspension using the magnet as previously described.
Cell culture
The SVF and the SSEA-4+hASCs, were both cultured in Minimum Essential Medium Eagle-alpha modification (α-MEM, Sigma-Aldrich) with 10% fetal bovine serum (FBS, Gibco) and 1% Pen/Strep (100U/100 μg/mL), and in the Microvascular Endothelial Cell Growth Medium (EGM −2 MV bullet kit (Lonza) containing 5% FBS and the supplemental growth factors (provided in the culture medium kit) and maintained until confluence. Cells were detached from the culture flasks using trypsin (0.25% trypsin–EDTA solution; Sigma-Aldrich) and kept under the same conditions along the passages. All the subsequent experimental procedures/study groups are summarized in the Supplementary Figure S1 (Supplementary Data are available online at www.liebertpub.com/tea).
HUVECs were cultured in the M199 medium (Sigma-Aldrich) supplemented with 20% FBS, 1% Pen/Strep (100U/100 μg/mL), 2 mM glutamax I (Life Technologies), 25 mg/mL sodium heparin (Sigma-Aldrich), and 25 mg/mL ECGS (BD Biosciences)
Osteogenic differentiation
Confluent hASCs and SSEA-4+hASCs (passage 3), were removed from the culture flasks using trypsin and seeded at a density of 2,000 cells/cm2 in the α-MEM supplemented with 10% fetal bovine serum, 1% antibiotic/antimycotic, 10 mM β-glycerophosphate (Sigma-Aldrich), 10−8 M dexamethasone (Sigma-Aldrich), and 50 μg/mL ascorbic acid phosphate (Sigma-Aldrich). Cells were incubated in a humidified environment at 37°C with 5% CO2 for 7, 14, 21, and 28 days, with culture media replenishment every 3–4 days. Cells cultured in the same medium, but without the osteogenic factors were used as controls.
Alizarin red staining
Cells cultured under osteogenic differentiation conditions were fixed at the different time points with a 10% formalin solution and washed, first with PBS, and then with diH2O. The cells were then incubated for 10 min with a 2% (wt/v) Alizarin Red solution (Merck) in diH2O, at a pH of 4.1–4.3. After incubation, cells were washed again with diH2O and the staining observed under a stereo microscope Stemi 1000 (Zeiss).
Flow cytometry
The freshly isolated SVF, the hASCs, and SSEA-4+hASCs cultured in the α-MEM and EGM-2 MV were harvested with trypsin upon reaching 80% confluence, along 4 passages. About 5×105 cells from each one of the experimental conditions were incubated for 30 min on ice with the following primary antibodies: mouse anti-human CD31-APC (R&D Systems), mouse anti-human CD90-APC (eBioscience), CD73-PE (BD Biosciences), mouse anti-human CD105-FITC (AbD Serotec), mouse anti-human SSEA-4-Alexa Fluor 488 (eBioscience), mouse anti-human CD34-PE (BD Biosciences), and mouse anti-human CD45-FITC (BD Biosciences). After washing with PBS, the cells were resuspended in the acquisition buffer (PBS containing 1% formaldehyde and 0.1% sodium azide) until analysis. In each run, at least 20,000 events were acquired with the FACS-Calibur flow cytometer (BD Biosciences) and the results analyzed with the CellQuest software (BD Biosciences). The number of positive events for each cell-specific marker was expressed as a percentage of the total cell number within each condition.
Immunocytochemistry
SSEA-4+hASCs and hASCs cultured onto tissue culture polystyrene slides (Sarstedt) in α-MEM and EGM-2 MV, passage 0 to 4, and in osteogenic conditions at the selected time points, were washed twice with PBS, fixed with 2% formalin for 30 min, washed again with PBS, and stored at 4°C until use. Fixed cells were washed with PBS, permeabilized with 0.2% Triton 100×solution for 2 min, and nonspecific binding was blocked with a 3% BSA/PBS solution. Cells were incubated for 1 h at room temperature with the primary antibodies mouse anti-human CD31 (1:50; Dako), rabbit anti-human von Willebrand factor (vWF, 1:200; Dako), mouse anti-human SSEA-4 (1:50; Abcam), CD34-PE (1:50; BD Biosciences), and mouse anti-human CD105 (1:50; eBioscience), mouse anti-human osteocalcin (OCN, 1:50, AbD Serotec), and rabbit anti-human osteopontin (OPN, 1:50; Abcam). All antibody dilutions were performed in 1.5% BSA/PBS. After incubation, cells were washed three times with PBS for 5 min and incubated 60 min with the appropriate secondary antibody, either donkey anti-rabbit Alexa Fluor 488 (Invitrogen), or donkey anti-mouse Alexa Fluor 594 (Invitrogen) diluted 1:500 in 1.5% BSA/PBS. Cell nuclei were counterstained with 4, 6-Diamidino-2-phenyindole dilactate (DAPI), at a 1:10000 dilution in PBS, for 10 min, and then washed three times. Negative control samples were prepared by replacing the primary antibody incubation with PBS. Immunolabeling was qualitatively analyzed under the Axioplan Imager Z1 fluorescence microscope (Zeiss) and photographed using the Axio Cam MRm camera (Zeiss) and the AxioVision 4.8 software (Zeiss).
Real-time reverse transcriptase-polymerase chain reaction
mRNA extraction and cDNA synthesis
Total mRNA of hASCs and SSEA-4+hASCs cultures in α-MEM and EGM-2 MV from passage 1 to 3 was extracted using the TRIzol Reagent (Invitrogen) according to the manufacturer's instructions. Briefly, 800 μL of TRIzol Reagent was added to each sample and stored at −80°C until further analysis. Upon thawing, 160 μL of chloroform (Sigma Aldrich) was added to each sample, incubated for 15 min at 4°C, and centrifuged at the same temperature at 13,000 g for 15 min. After the centrifugation, the aqueous part was collected and an equal part of isopropanol (Sigma Aldrich) was added. After 2-h incubation at −20°C, the samples were washed in ethanol, centrifuged at 4°C and 9000 g for 5 min, and resuspended in 12 μL of water RNase/DNase free (Gibco). RNA quantity and purity were determined using the NanoDrop ND-1000 Spectrophotometer (Thermo Fischer Scientific). For the cDNA synthesis, only the samples with a 260/280 ratio between 1.7 and 2.0 were used. The cDNA synthesis was performed with the qScript cDNA Synthesis kit (Quanta Biosciences), and the Mastercycler ep realplex thermal cycler (Eppendorf), using an initial amount of total RNA of 2 μg in a total volume of 20 μL.
Quantitative polymerase chain reaction
The quantification of the transcripts of the genes of interest was carried out by quantitative polymerase chain reaction (qPCR) using 5 ng of cDNA and the PerfeCTA SYBR Green FastMix kit (Quanta Biosciences) following the procedure suggested by the manufacturer, in a Real-Time Mastercycler ep realplex thermal cycler (Eppendorf). The primers were previously designed using the Primer 3 online software (v0.4.0) (Supplementary Table S1) and synthesized by MGW Biotech, Germany. For each sample, GAPDH was used as the housekeeping gene. A concentration of 300 nM was used for all the primers, in a final volume of 20 μL. Samples with RNAse free water (Gibco), SYBR Green (Quanta Biosciences), and without cDNA, were used as blanks. The relative quantification of the gene expression was performed using the Pfaffl method to obtain the ΔΔCt method.42 All values were first normalized against GAPDH values, and then to the SSEA-4+cells separated from the freshly isolated SVF (for the expression of the endothelial markers: CD31 and vWF) or to the two cell types cultured in α-MEM (for the expression of osteogenic related markers: OCN and OPN).
Acetylated low-density lipoprotein uptake and lectin binding
Low-Density Lipoprotein (LDL) uptake was assessed by incubating the hASCs and SSEA-4+hASCs cultured in EGM-2 MV and α-MEM (passage 4) for 4 h at 37°C with 2 μg/mL of acetylated LDL labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine (Dil-ac-LDL; Invitrogen). Cells were incubated with FITC-conjugated lectin Ulex Europaeus-1 (Sigma Aldrich) 100 μg/mL for 1 h at 37°C along with the Dil-ac-LDL and protected from light. Cells were then washed twice with PBS, fixed for 15 min in 10% formalin solution, and analyzed by fluorescence microscopy as described for the immunocytochemistry.
Matrigel assay
To analyze the capacity of the SSEA-4+hASCs and hASCs cultured in EGM-2- MV and α-MEM to form tubular structures at different passages (1 to 4), a 96-well cell culture plate, chilled at 4°C, was loaded with 32 μL of Matrigel (BD Biosciences) and incubated at 37°C. Cells were suspended in the EGM-MV2 medium at a concentration of 2.1×105 cells/mL and 64 μL of this cell suspension was seeded in each well onto the surface of the solidified Matrigel. Cells were incubated at 37°C in a 5% CO2 humidified atmosphere for 4 h. HUVECs cells were used as a control and were seeded following the procedure mentioned above. Three representative images of each condition were recorded using an inverted microscope, Axiovert 40 (Zeiss), equipped with a digital image capture software.
Statistical analysis
The flow cytometry data collected from six independent experiments, and the qRT-PCR data, obtained from three independent experiments, with three replicates for each experiment are expressed as arithmetic means±standard deviation. The qRT-PCR results were analyzed with the ANOVA single-factor method (post-testing for pair-wise comparisons were performed using the Tukey's range test). The values were considered statistically significant for p≤0.05 (*).
Results
SVF and hASCs characterization
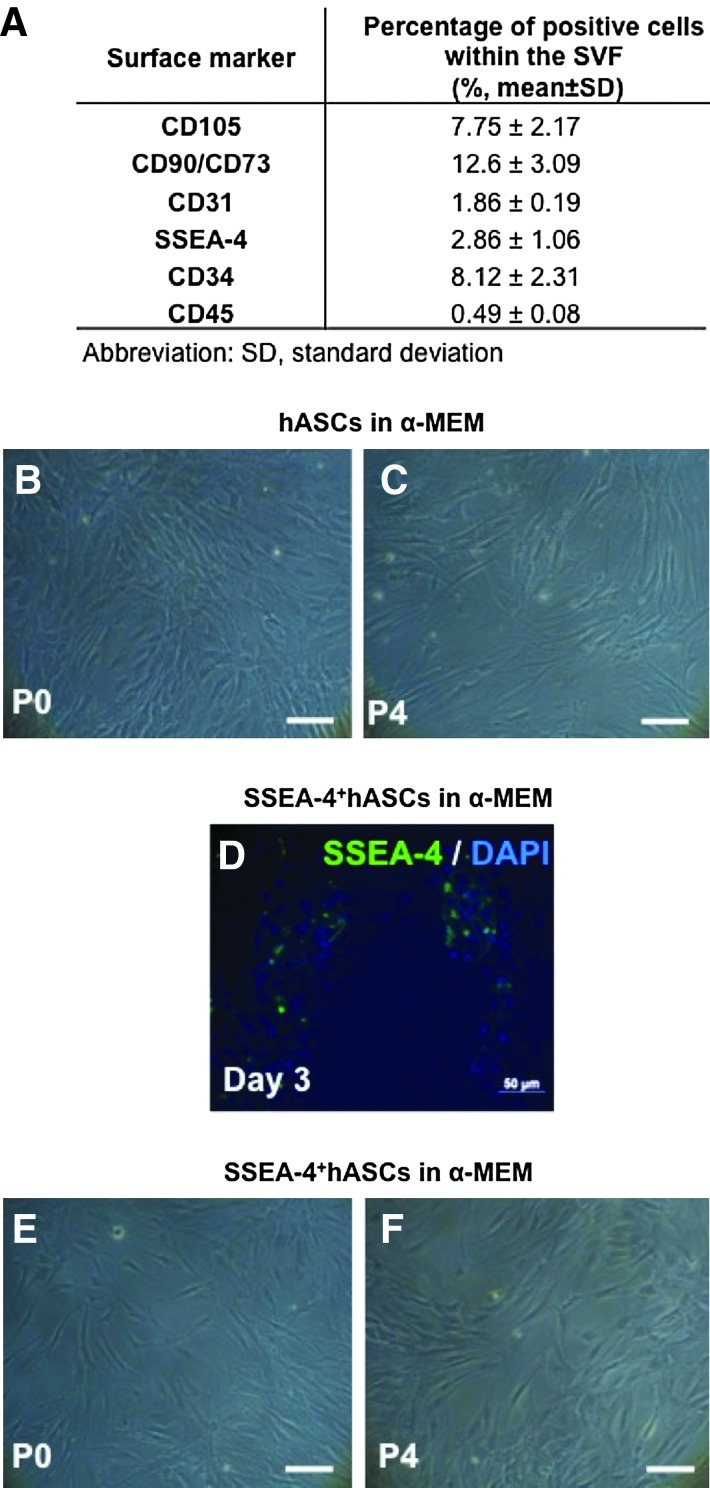
The percentage of cells expressing MSC markers, CD105, CD90, and CD73, the pluripotency marker, SSEA-4, the endothelial marker, CD31, and the hematopoietic markers, CD34 and CD45, were examined by flow cytometry. Within the SVF, 2.86% of the total isolated cell expressed the pluripotency marker, SSEA-4, while only about 1.86% were positive for the EC surface marker, CD31, and 8.12% of the total population expressed CD34 (Fig. 1A). After selection by plastic adherence and culture in α-MEM, the obtained hASCs displayed a homogeneous fibroblast-like morphology characteristic of mesenchymal cells, that was maintained up to passage 4 (Fig. 1B, C). The surface marker profile confirmed the hASCs mesenchymal phenotype by the coexpression of CD90, CD105, and CD73 in more than 90% of the cells, while lacking expression of CD45 and CD34 (Table 1). The absence of cells expressing CD31 after the selection by adherence was also verified. These characteristics were conserved along the passages, contrarily to the percentage of cells expressing SSEA-4 that diminished from 9.18% to 1.86%, respectively, from passages 1 to 4 (Table 1).
FIG. 1.
Morphology and surface marker profile characterization of human adipose tissue-derived stem cells (hASCs) and SSEA-4+hASCs. (A) Cell surface marker profile of stromal vascular fraction (SVF), obtained by flow cytometry. Optical micrographs showing the morphology of hASCs (B, C) and SSEA-4+hASCs (E, F) cultured in alpha-minimum essential medium (α-MEM) at Passage 0 (P0) and 4 (P4). Immunocytochemistry of selected SSEA-4+cells cultured for 3 days in α-MEM confirming the majority of the cells expressing SSEA-4 (D). Cell nuclei were counterstained with 4, 6-diamidino-2-phenyindole dilactate (DAPI). Scale bar represents 100 μm. Color images available online at www.liebertpub.com/tea
Table 1.
Cell Surface Marker Profile of Human Adipose Tissue-Derived Stem Cells Cultured in α-Minimum Essential Medium at Different Passages, Obtained by Flow Cytometry
| |
Percentage of positive cells within the considered population (%, means±SD) |
|||||
|---|---|---|---|---|---|---|
| Passage | CD105 | CD90/CD73 | CD31 | SSEA-4 | CD34 | CD45 |
| 1 | 87.9±9.05 | 89.7±8.98 | 0.17±0.04 | 9.18±3.21 | 5.52±0.09 | 0.25±0.02 |
| 2 | 88.3±11.2 | 91.2±11.1 | 0.11±0.03 | 7.40±3.23 | 0.23±0.07 | 0.01±0.01 |
| 3 | 89.5±9.10 | 93.7±8.45 | 0.01±0.00 | 4.60±2.45 | 0.13±0.03 | 0.01±0.00 |
| 4 | 94.2±3.56 | 96.3±7.67 | 0.02±0.00 | 1.86±0.23 | 0.34±0.01 | 0.05±0.01 |
SD, standard deviation.
SSEA-4+hASCs subpopulation characterization
SSEA-4+hASCs were obtained by immunomagnetic selection of the SSEA-4+ fraction (about 3%) of the adipose tissue SVF. The success of the method was confirmed by the immunocytochemistry results after 2 days of culture in α-MEM showing the majority of the adhered cells expressing SSEA-4 (Fig. 1D). SSEA-4+hASCs cultured in α-MEM displayed the same fibroblast-like morphology as the hASCs cultured under the same conditions, independently of the passage, and up to passage 4 (Fig. 1E, F). However, from passages 1 to 4, the flow cytometry analysis revealed that CD105+, CD90+, and CD73+ cells, comprised more than 90% of the total population, whereas SSEA-4+ cells dramatically decreased (1.98%±0.59%) by passage 4. The selected SSEA-4+hASCs subpopulations were also negative for CD31 and a low percentage of cells, from 3.34% at P1 to 1.40% at P4, were positive for CD45. The percentage of CD34+ cells (13.2% at P1) did not significantly vary with the passage (Table 2).
Table 2.
Cell Surface Marker Profile of SSEA-4+ Human Adipose Tissue-Derived Stem Cells Cultured in α-MEM at Different Passages, Obtained by Flow Cytometry
| |
Percentage of positive cells within the considered population (%, means±SD) |
|||||
|---|---|---|---|---|---|---|
| Passage | CD105 | CD90/CD73 | CD31 | SSEA-4 | CD34 | CD45 |
| 1 | 88.5±12.2 | 94.8±8.94 | 0.20±0.02 | 4.35±1.29 | 13.2±4.69 | 3.34±0.95 |
| 2 | 90.2±6.34 | 93.5±4.23 | 0.01±0.00 | 5.23±1.21 | 16.9±3.45 | 2.98±1.03 |
| 3 | 89.2±0.60 | 93.4±5.14 | 0.00±0.00 | 2.73±1.57 | 15.8±4.69 | 2.52±0.70 |
| 4 | 94.5±5.31 | 95.5±4.82 | 0.00±0.00 | 1.98±0.59 | 13.6±2.36 | 1.40±0.62 |
hASCs and SSEA-4+hASCs osteogenic differentiation
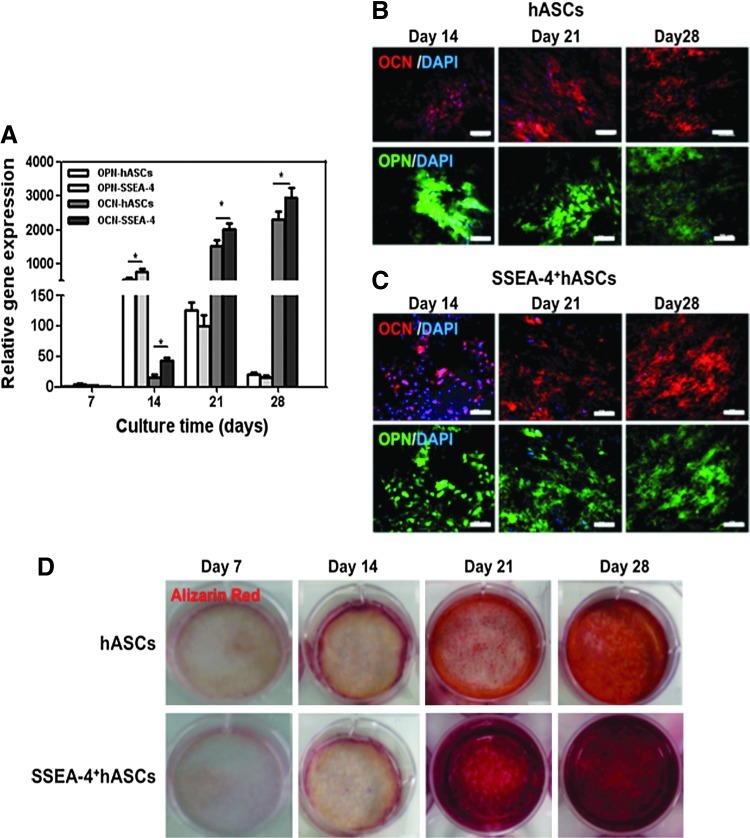
Induction of hASCs and SSEA-4+hASCs into the osteogenic lineage, was maintained up to 28 days of culture. Real-time RT-PCR analysis was performed to study the expression of the osteogenic-related markers, OPN and OCN. This analysis demonstrated that the osteogenic differentiation of both hASCs and SSEA-4+hASCs was triggered within the first 7 days of culture in the osteogenic medium and that by day 14 the expression of OPN reached a 600-fold increase (Fig. 2A). From day 21 to day 28, a significant downregulation of OPN transcripts occurred in both cell populations. In contrast, a significant upregulation was observed for OCN from day 7 up to day 28 at which reached a maximum 2750-fold. Immunocytochemistry confirmed, at the protein level, the molecular analysis; both hASCs and SSEA-4+hASCs cells cultured under osteogenic conditions started to deposit OPN and OCN (Fig. 2B, C). Furthermore, the Alizarin Red staining confirmed an intense matrix mineralization from day 14 onward, again for both hASCs and SSEA-4+hASCs cultured under osteogenic conditions, although it seems that mineralization occurred faster and in a more homogeneous mode with the SSEA-4+hASCs (Fig. 2D).
FIG. 2.
In vitro osteogenic differentiation of hASCs and SSEA-4+hASCs. (A) quantitative RT-polymerase chain reaction (qRT-PCR) results showing the overexpression of osteogenic-related genes, osteocalcin (OCN), and osteopontin (OPN) in both cell populations along the culture in osteogenic conditions (B, C) Immunostaining of OPN and OCN deposition by hASCs (B) and SSEA-4+hASCs (C) at days 14, 21, and 28 of culture under osteogenic conditions confirming the osteogenic differentiation. Cell nuclei were counterstained with DAPI. (D) Optical micrographs showing the gradual mineralization of the deposited matrix after Alizarin Red staining from day 7, 14, 21, and 28. Scale bar represent 50 μm. Color images available online at www.liebertpub.com/tea
Endothelial differentiation
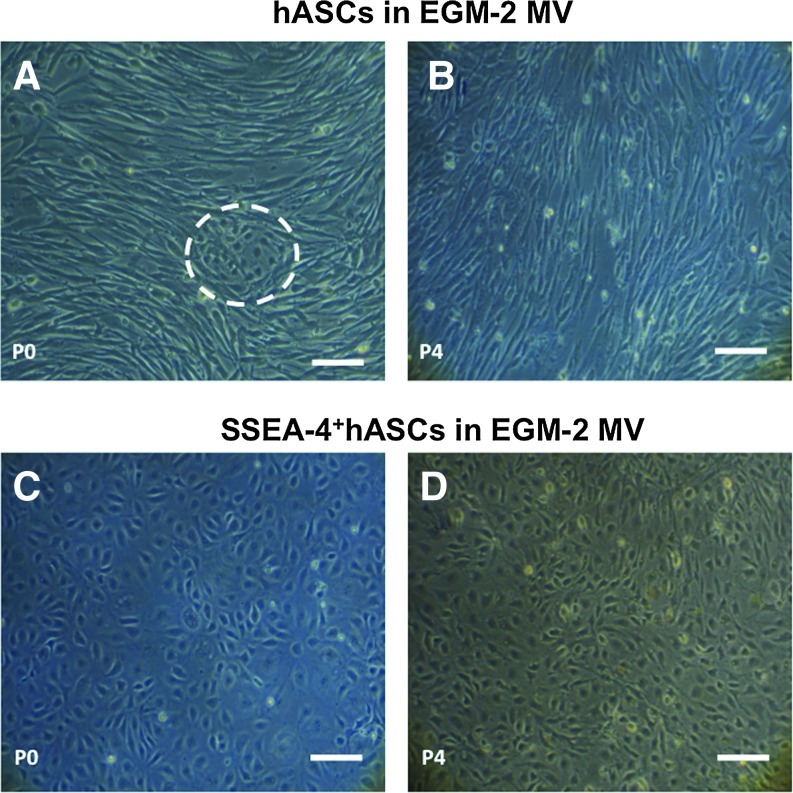
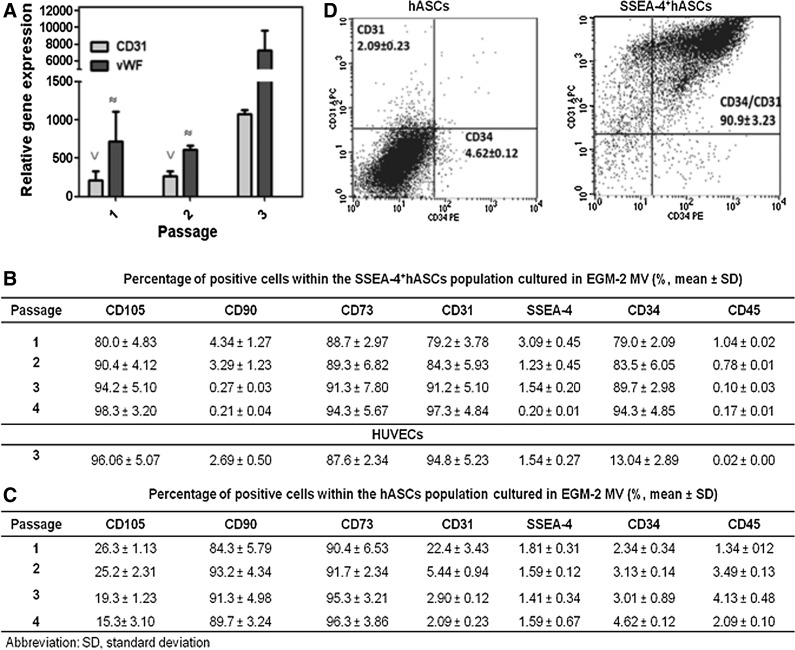
Culturing the SVF and the SSEA-4+hASCs subpopulation in EGM-2 MV pursued cells differentiation into the endothelial lineage. Despite the small endothelial-like colonies present in the SVF-derived culture at early time points, these were surrounded by fibroblast-like cells (Fig. 3A), which took over the culture along the passages (Fig. 3B). In contrast, when the SSEA-4+hASCs were cultured in the same conditions, small cobblestone-like colonies could be first observed between days 3 and 7. These colonies were able to grow until confluence (Fig. 3C) maintaining the endothelial-like cobblestone morphology up to passage 4 (Fig. 3D). The shifting of phenotype of the SSEA-4+hASCs subpopulation along the passages was followed by the analysis of the relative expression of CD31 and vWF genes, to confirm the endothelial phenotype. As it can be seen in Fig. 4A, SSEA-4+hASCs derived cells cultured in EGM-2 MV exhibited significantly increased levels of expression of the endothelial markers at different passages when compared with freshly selected SSEA-4+hASCs. The expression levels of CD31 and vWF reached, respectively, around 1000- and 7000-fold increase in comparison to the initial selected subpopulation. The gene expression study was complemented by flow cytometry analysis (Fig. 4B). The percentage of cells expressing CD31 and CD34 increased, respectively, from 79.2% and 79.0% (P1) to 97.3% and 94.3% (P4), respectively. Moreover, in comparison to the SSEA-4+hASCs cultured in α-MEM, this subpopulation grown in EGM-2 MV revealed a significant downregulation of CD90, maintaining similar percentages of the CD105+ and CD73+ fractions, around 90% of the total population. Concerning hASCs cultured in EGM-2 MV, only 22% of the cells were positive for CD31 at passage 1. The expression of this marker rapidly decreased along passages and at passage 4, only around 2% of the total population was expressing it. Moreover, while the percentage of cells expressing CD34, CD45, and SSEA-4 can be considered neglectable, CD90+ and the CD73+ cells comprise around 90% of the total population. These levels were maintained along passages. Contrarily, the percentage of CD105+cells decreased from 26.3% at passage 1 to 15.3% at passage 4 (Fig. 4C). By passage 4, the percentage of CD31+/CD34+ cells within the hASCs cultured in EGM-2 MV was neglectable, whereas in the SSEA-4+hASCs cultured under the same conditions reached 90.9% of the total population (Fig. 4D).
FIG. 3.
Optical micrographs showing the morphology of hASCs (A, B) and SSEA-4+hASCs (C, D) cultured in EGM-2 MV at passages 0 (A, C) and 4 (B, D). hASCs at passage 0 exhibit endothelial-like colonies (dashed circle) surrounded by fibroblast-like cells (A), but depict a homogeneous fibroblast-like morphology at passage 4 (B). SSEA-4+hASCs at passage 0 are characterized by presenting a cobblestone morphology consistent with the endothelial phenotype (C), which was kept up to passage 4 (D). Scale bar represents 100 μm. Color images available online at www.liebertpub.com/tea
FIG. 4.
Characterization of hASCs and SSEA-4+hASCs cultured in EGM-2 MV. (A) qRT-PCR results showing the overexpression of endothelial-related genes, CD31 and vWF, at different passages in hASCs and SSEA-4+hASCs in relation to freshly selected SSEA-4+hASCs (P0). ∨ and ≈ indicate statistical significance (p<0.05) when compared with P3 (B, C) Cell surface marker profile of SSEA-4+hASCs (B) and hASCs (C) at different passages, obtained by flow cytometry. (D) Representative dot plots of hASCs and SSEA-4+hASCs expressing CD34 and CD31 markers at passage 4 demonstrating the endothelial differentiation of SSEA-4+hASCs. Values in histogram plots indicate averages±SD from six independent experiments.
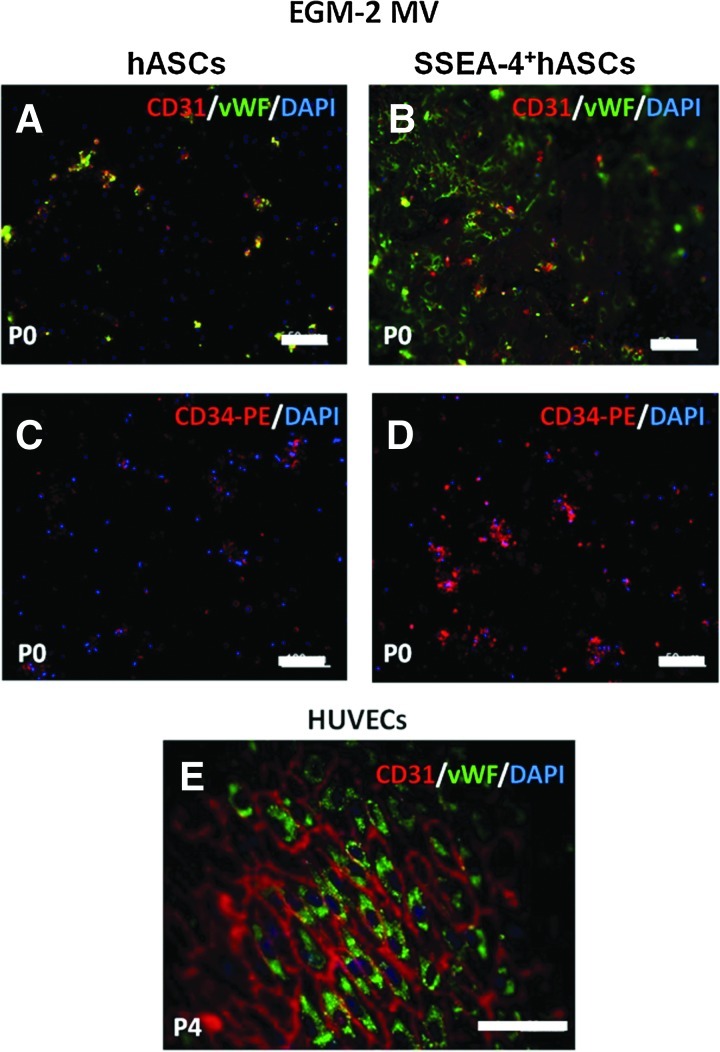
The flow cytometry results regarding the endothelial phenotype were confirmed by immunocytochemistry to monitor the expression of vWF and CD31. A low number of hASCs cultured in EGM-2 MV showed expression of vWF at P0 (Fig. 5A). This feature was lost at passage 1. Contrarily, for the SSEA-4+hASCs in EGM-2 MV, vWF was present in the cell cytoplasm as a small dotted pattern surrounding the nuclei representing the Weibel-Palade bodies and in the majority of the cells starting with P0 (Fig. 5B). In addition, the CD31 pattern of SSEA-4+hASCs derived cells, corresponding to a cell–cell contact, was found predominant for all the cells in passage 4 (Fig. 5C). Moreover, the CD34 marker was absent in hASCs cultured in EGM-2 MV (Fig. 5D), while the majority of SSEA-4+hASCs in EGM-2 MV were found positive for the same marker (Fig. 5E).
FIG. 5.
Immunostaining of hASCs and SSEA-4+hASCs cultured in EGM-2 MV. Immunostaining of vWF/CD31 (A, B, E) and CD34 (C, D) expressed by hASCs and SSEA-4+hASCs. At passage 0, only some hASCs expressed CD31 and vWF (A) and CD34 (C), while the majority of the SSEA-4+hASCs expressed these markers (B, D). The stability of the expression of CD31/vWF by differentiated SSEA-4+hASCs was confirmed at passage 4 (E). Cell nuclei were counterstained with DAPI. Color images available online at www.liebertpub.com/tea
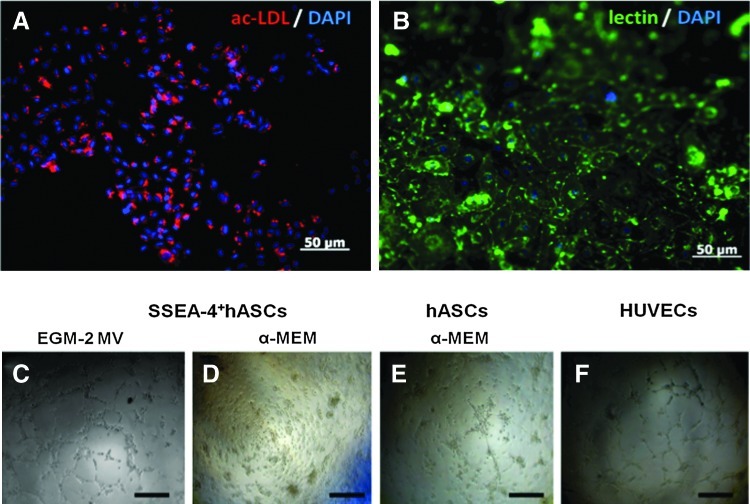
The endothelial phenotype of the SSEA-4+hASCs derived cells was also assessed by their ability to uptake the Dil-ac-LDL complex and to bind lectin Ulex europeus-1, as well as to form capillary-like network when seeded on Matrigel. In contrast to SSEA-4+hASCs cultured in α-MEM, SSEA-4+hASCs growing in EGM-2 MV were able to uptake the labeled acetylated lipoprotein into secondary lysosomes (Fig. 6A) and to bind the UEA-1 (Fig. 6B). Furthermore, SSEA-4+hASCs cultured in EGM-2 MV were capable of forming, similarly to HUVECs, tubular structures when seeded on Matrigel (Fig. 6C). When SSEA-4+hASCs derived cells and hASCs cultured in α-MEM were seeded on Matrigel, they remained spherical and formed cell aggregates without forming tubular structures (Fig. 6D). Nevertheless, hASCs cultured in EGM-2 MV exhibited a slow, but evident formation of tubes on the Matrigel substrate (Fig. 6E). Still, this ability was lost after passage 2.
FIG. 6.
Endothelial-like cells obtained by culturing SSEA-4+hASCs in EGM-2 MV. Cells were able to (A) uptake acetylated low-density lipoprotein (ac-LDL) and (B) bind lectin from Ulex europeus-1. Cell nuclei were counterstained with DAPI. When seeded on Matrigel at different passages, cells had the capacity to form capillary-like structures (C) similarly to HUVECS, used as control (F). Contrarily, SSEA-4+hASCs cultured in α-MEM, independently of the passage, aggregated in small clumps without any tubular-like structure appearance (D). hASCs cultured in EGM-2 MV were only able to form these structures on Matrigel at passage 1 (E). These are likely to result from the organization of the microvascular endothelial cells present in the stromal vascular fraction of the adipose tissue and proved to be present at this stage of culture as CD31+ cells. Color images available online at www.liebertpub.com/tea
Discussion
The use of human microvascular ECs is limited by the low availability of the source, the reduced proliferation rates, and the small number of the isolated cells that are often contaminated by fibroblasts and other stromal cells.43 In addition, the benefits of mature ECs, as well as of other endothelial progenitors, as part of tissue-engineered constructs, over the anostomosis, perfusion, and survival of those constructs are still not clear. The present study emphasizes the possibility of obtaining endothelial-like and osteogenic cells from a single-cell source by selecting a subpopulation residing within the adipose tissue that expresses a marker associated with pluripotency, the SSEA-4. This approach, in addition to providing new insights regarding the differentiation potential of the SSEA-4+ subpopulation present in the SVF of adipose tissue, might be of high value for defining innovative bone TE strategies. The coexistence of different cell subpopulations expressing CD105, CD90, CD73, CD31, CD34, and SSEA-4 within SVF confirmed previous works.26,29 Interestingly, a subpopulation comprising almost of 3% of the SVF was found to express the SSEA-4 marker, associated to the pluripotent character of other stem cells.36 This marker was used to successfully select the subpopulation of interest at the time of the SVF isolation. In fact, the percentage of hASCs, expressing SSEA-4, cultured both in α-MEM and EGM-2 MV medium, was reduced along the passages. Likewise, the selected subpopulation also lost the SSEA-4 expression along the passages and under the same culture conditions. While in the culture with the EGM-2 MV medium, this feature could be associated to the differentiation of the SSEA-4+hASCs into the endothelial lineage, in the culture in α-MEM was connected with the acquirement of a mesenchymal phenotype characterized by fibroblast-like morphology of the cells and the expression of mesenchymal cell surface markers. This phenotypic pattern was kept stable along passages and allowed the osteogenic differentiation when cells were cultured in osteo-inductive conditions.
Our data suggest that the composition of the culture medium exerted major effects on the differentiation of SSEA-4+hASCs. The EGM-2 MV medium, in particular, has been often presented as a suitable cell culture environment to trigger the endothelial differentiation of embryonic stem cells21 and MSCs44 or the maturation of EPCs.45 We were also able to confirm that the selected SSEA-4+hASCs were differentiating into endothelial-like cells as demonstrated by their morphology, markers profile, and in vitro capacity to form tubular-like structures, when cultured in EGM-2 MV. In opposition, this subpopulation cultured in α-MEM did not demonstrate this capacity of differentiation into the endothelial lineage. Therefore, these results confirm that factors present in EGM-2 MV, but not in α-MEM, such as angiogenic factors, fibroblast growth factors, and VEGFs, might be involved in the endothelial differentiation of the SSEA-4+hASCs subpopulation. Others have used VEGF in concentrations that range from 1046 to 50 ng/mL23,45,47 to induce and lead endothelial differentiation. However, concentrations of 50 ng/mL of VEGF might significantly impair the therapeutic application of those cells. EGM-2 MV, with a concentration of VEGF lower than 5 ng/mL,48 allowed deriving, from the SSEA-4+hASCs subpopulation, a population of cells in which around 80% expressed the CD31and CD34 markers at passage 1 and more than 95% at passage 4. Moreover, cells were concomitantly expressing CD105, while lacking the expression of CD45, altogether a characteristic markers profile of ECs.49–51
Recent investigations have clearly confined the expression of CD34 in association with the expression of CD105, CD73, and CD31 to microvascular-like ECs.52–54 The fact that 95% of the differentiated SSEA-4+hASCs were CD34+ and within the control macrovascular HUVECs population (95% CD31+) only 13% expressed CD34, reinforces that the cells generated by the differentiation of SSEA-4+hASCs possess a microvascular endothelial-like phenotype. Interestingly, during the endothelial differentiation of SSEA-4+hASCs, the levels of expression of CD90 rapidly decreased. Being a counter receptor for the leukocyte integrin Mac-1 (CD11b/CD18) present in polymorphonuclear neutrophils and in monocytes, the expression of CD90 by ECs is only expected upon activation,55–57 which might indicate a resting state of the obtained cells. Future studies should be performed to analyze the molecular mechanism that governs the endothelial differentiation and the influence of other growth factors or other stimuli to trigger this process. An evaluation of the endothelial-specific markers and adhesion molecules upon stimulation with inflammatory cytokines would better assess the behavior of the endothelial-like SSEA-4+hASCs.
SSEA-4+hASCs can also give rise to fibroblastic-like cells, phenotypically resembling MSCs when cultured in α-MEM. Besides the expression (>90%) of CD90, CD73, and CD105 and the absence of CD34- and CD45-positive cells, these cells were able to differentiate, in addition to the endothelial lineage, into osteoblasts and chondrocytes (data not shown). The osteogenic differentiation of hASCs and SSEA-4+hASCs was followed at the molecular and protein levels by the expression of the osteogenic-related markers (OCN and OPN)58 and by subsequent deposition and mineralization of the extracellular matrix. For each cell population, the inverse expression profile of OPN and OCN, respectively, with higher levels at earlier and later time points, confirmed the expected progress of the osteogenic differentiation. Moreover, altogether these results confirm the advanced stage of osteogenesis59 in the later culturing time points.
Overall, the discussed results underline the high potential of SSEA-4+hASCs subpopulation. EGM-2 MV was sufficient to trigger the differentiation of SSEA-4+hASCs into the endothelial lineage giving rise to microvascular-like ECs, while the same culture conditions were not sufficient to trigger the endothelial differentiation of hASCs. Moreover, the capacity of the SSEA-4+hASCs to differentiate toward the osteogenic lineage is similar to the hASCs. Numerous advantages derive from the use of SSEA-4+hASCs among which is the relatively easy retrieval of the SSEA-4+cells, while preserving their differentiation potential. Furthermore, the acceptance of adipose tissue as an abundant source of cells allows overcoming the potential issue of the low percentage (<2%) of cells that SSEA-4+cells subpopulation represents within the SVF. Under this context, we consider that obtaining relevant number of SSEA-4+cells to be applied in the future in a bone TE strategy to improve tissue vascularization constitute a realistic scenario.
Conclusions
This study reports that human adipose tissue contains a subpopulation defined by the expression of the pluripotent marker, SSEA-4, that can be obtained using an immunomagnetic selection. Culturing the SSEA-4+hASCs in EGM-2 MV can generate cells with a mature microvascular-like endothelial profile. In addition, the same subpopulation can undergo osteogenic differentiation, as observed by an intense extracellular matrix deposition and mineralization. Therefore, this subpopulation contains cells that under specific culture condition can give rise to both osteoblastic and microvascular endothelial-like cells. We demonstrated that from a single-cell source and by selecting the appropriate subpopulation, it is possible to obtain the relevant types of cells envisioning engineering vascularized bone tissue.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Portuguese Foundation for Science and Technology (FCT) for the grants SFRH/BD/42968/2008 through the MIT-Portugal Program (S.M.Mihaila), SFRH/BPD/45206/2008 (A.M.Frias) and SFRH/BD/44893/2008 (R.P.Pirraco) and Find&Bind research project NMP4-SL-2009-229292.
Disclosure Statement
Authors have nothing to disclose.
References
- 1.Vallee M. Cote J.F. Fradette J. Adipose-tissue engineering: taking advantage of the properties of human adipose-derived stem/stromal cells. Pathol Biol (Paris) 2009;57:309. doi: 10.1016/j.patbio.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 2.De Ugarte D.A. Morizono K. Elbarbary A. Alfonso Z. Zuk P.A. Zhu M., et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 3.Liu T.M. Martina M. Hutmacher D.W. Hui J.H.P. Lee E.H. Lim B. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells. 2007;25:750. doi: 10.1634/stemcells.2006-0394. [DOI] [PubMed] [Google Scholar]
- 4.Gimble J.M. Katz A.J. Bunnell B.A. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helder M.N. Knippenberg M. Klein- Nulend J. Wuisman P.I. Stem cells from adipose tissue allow challenging new concepts for regenerative medicine. Tissue Eng. 2007;13:1799. doi: 10.1089/ten.2006.0165. [DOI] [PubMed] [Google Scholar]
- 6.Wagner W. Wein F. Seckinger A. Frankhauser M. Wirkner U. Krause U., et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Strem B.M. Hedrick M.H. The growing importance of fat in regenerative medicine. Trends Biotechnol. 2005;23:64. doi: 10.1016/j.tibtech.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Zuk P.A. Zhu M. Mizuno H. Huang J. Futrell J.W. Katz A.J., et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 9.Anghileri E. Marconi S. Pignatelli A. Cifelli P. Galie M. Sbarbati A., et al. Neuronal differentiation potential of human adipose-derived mesenchymal stem cells. Stem Cells Dev. 2008;17:909. doi: 10.1089/scd.2007.0197. [DOI] [PubMed] [Google Scholar]
- 10.Prunet- Marcassus B. Cousin B. Caton D. Andre M. Penicaud L. Casteilla L. From heterogeneity to plasticity in adipose tissues: site-specific differences. Exp Cell Res. 2006;312:727. doi: 10.1016/j.yexcr.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 11.Planat- Benard V. Menard C. Andre M. Puceat M. Perez A. Garcia-Verdugo J.M., et al. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res. 2004;94:223. doi: 10.1161/01.RES.0000109792.43271.47. [DOI] [PubMed] [Google Scholar]
- 12.Moon J.J. West J.L. Vascularization of engineered tissues: approaches to promote angio-genesis in biomaterials. Curr Top Med Chem. 2008;8:300. doi: 10.2174/156802608783790983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain R.K. Au P. Tam J. Duda D.G. Fukumura D. Engineering vascularized tissue. Nat Biotechnol. 2005;23:821. doi: 10.1038/nbt0705-821. [DOI] [PubMed] [Google Scholar]
- 14.Kaigler D. Krebsbach P.H. Wang Z. West E.R. Horger K. Mooney D.J. Transplanted endothelial cells enhance orthotopic bone regeneration. J Dental Res. 2006;85:633. doi: 10.1177/154405910608500710. [DOI] [PubMed] [Google Scholar]
- 15.Levenberg S. Rouwkema J. Macdonald M. Garfein E.S. Kohane D.S. Darland D.C., et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 16.Hegen A. Blois A. Tiron C.E. Hellesoy M. Micklem D.R. Nor J.E., et al. Efficient in vivo vascularization of tissue-engineering scaffolds. J Tissue Eng Regen Med. 2011;5:e52. doi: 10.1002/term.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S. von Recum H. Endothelial stem cells and precursors for tissue engineering: cell source, differentiation, selection, and application. Tissue Eng Part B Rev. 2008;14:133. doi: 10.1089/teb.2007.0304. [DOI] [PubMed] [Google Scholar]
- 18.Asahara T. Murohara T. Sullivan A. Silver M. vander Zee R. Li T., et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 19.Mead L.E. Prater D. Yoder M.C. Ingram D.A. Isolation and characterization of endothelial progenitor cells from human blood. Curr Protoc Stem Cell Biol. 2008 doi: 10.1002/9780470151808.sc02c01s6. Chapter 2: Unit 2C.1. [DOI] [PubMed] [Google Scholar]
- 20.Hristov M. Erl W. Weber P.C. Endothelial progenitor cells - Isolation and characterization. Trends Cardiovasc Med. 2003;13:201. doi: 10.1016/s1050-1738(03)00077-x. [DOI] [PubMed] [Google Scholar]
- 21.Blancas A.A. Lauer N.E. McCloskey K.E. Endothelial differentiation of embryonic stem cells. Curr Protoc Stem Cell Biol. 2008 doi: 10.1002/9780470151808.sc01f05s6. Chapter 1:Unit 1F.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rufaihah A.J. Haider H.K. Heng B.C. Ye L. Toh W.S. Tian X.F., et al. Directing endothelial differentiation of human embryonic stem cells via transduction with an adenoviral vector expressing the VEGF(165) gene. J Gene Med. 2007;9:452. doi: 10.1002/jgm.1034. [DOI] [PubMed] [Google Scholar]
- 23.Oswald J. Boxberger S. Jorgensen B. Feldmann S. Ehninger G. Bornhauser M., et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22:377. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 24.Chen M.Y. Lie P.C. Li Z.L. Wei X. Endothelial differentiation of Wharton's jelly-derived mesenchymal stem cells in comparison with bone marrow-derived mesenchymal stem cells. Exp Hematol. 2009;37:629. doi: 10.1016/j.exphem.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Zhang P. Baxter J. Vinod K. Tulenko T.N. Di Muzio P.J. Endothelial differentiation of amniotic fluid-derived stem cells: synergism of biochemical and shear force stimuli. Stem Cells Dev. 2009;18:1299. doi: 10.1089/scd.2008.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rada T. Reis R.L. Gomes M.E. Distinct stem cells subpopulations isolated from human adipose tissue exhibit different chondrogenic and osteogenic differentiation potential. Stem Cell Rev. 2011;7:64. doi: 10.1007/s12015-010-9147-0. [DOI] [PubMed] [Google Scholar]
- 27.Rada T. Reis R.L. Gomes M.E. Novel method for the isolation of adipose stem cells (ASCs) J Tissue Eng Regen Med. 2009;3:158. doi: 10.1002/term.141. [DOI] [PubMed] [Google Scholar]
- 28.Zimmerlin L. Donnenberg V.S. Pfeifer M.E. Meyer E.M. Peault B. Rubin J.P., et al. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010;77:22. doi: 10.1002/cyto.a.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Astori G. Vignati F. Bardelli S. Tubio M. Gola M. Albertini V., et al. “In vitro” and multicolor phenotypic characterization of cell subpopulations identified in fresh human adipose tissue stromal vascular fraction and in the derived mesenchymal stem cells. J Translational Med. 2007;5:55. doi: 10.1186/1479-5876-5-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sengenes C. Miranville A. Maumus M. de Barros S. Busse R. Bouloumie A. Chemotaxis and differentiation of human adipose tissue CD34+/CD31- progenitor cells: role of stromal derived factor-1 released by adipose tissue capillary endothelial cells. Stem Cells. 2007;25:2269. doi: 10.1634/stemcells.2007-0180. [DOI] [PubMed] [Google Scholar]
- 31.Yamashita J. Itoh H. Hirashima M. Ogawa M. Nishikawa S. Yurugi T., et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 32.Miranville A. Heeschen C. Sengenes C. Curat C.A. Busse R. Bouloumie A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 33.Sengenes C. Lolmede K. Zakaroff- Girard A. Busse R. Bouloumie A. Preadipocytes in the human subcutaneous adipose tissue display distinct features from the adult mesenchymal and hematopoietic stem cells. J Cell Physiol. 2005;205:114. doi: 10.1002/jcp.20381. [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Estrada O.M. Munoz- Santos Y. Julve J. Reina M. Vilaro S. Human adipose tissue as a source of Flk-1+ cells: new method of differentiation and expansion. Cardiovasc Res. 2005;65:328. doi: 10.1016/j.cardiores.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 35.Fischer L.J. Mc Ilhenny S. Tulenko T. Golesorkhi N. Zhang P. Larson R., et al. Endothelial differentiation of adipose-derived stem cells: effects of endothelial cell growth supplement and shear force. J Surg Res. 2009;152:157. doi: 10.1016/j.jss.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riekstina U. Cakstina I. Parfejevs V. Hoogduijn M. Jankovskis G. Muiznieks I., et al. Embryonic stem cell marker expression pattern in human mesenchymal stem cells derived from bone marrow, adipose tissue, heart and dermis. Stem Cell Rev. 2009;5:378. doi: 10.1007/s12015-009-9094-9. [DOI] [PubMed] [Google Scholar]
- 37.Henderson J.K. Draper J.S. Baillie H.S. Fishel S. Thomson J.A. Moore H., et al. Preimplantation human embryos and embryonic stem cells show comparable expression of stage-specific embryonic antigens. Stem Cells. 2002;20:329. doi: 10.1634/stemcells.20-4-329. [DOI] [PubMed] [Google Scholar]
- 38.Shamblott M.J. Axelman J. Wang S. Bugg E.M. Littlefield J.W. Donovan P.J., et al. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci U S A. 1998;95:13726. doi: 10.1073/pnas.95.23.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heins N. Englund M.C. Sjoblom C. Dahl U. Tonning A. Bergh C., et al. Derivation, characterization, and differentiation of human embryonic stem cells. Stem Cells. 2004;22:367. doi: 10.1634/stemcells.22-3-367. [DOI] [PubMed] [Google Scholar]
- 40.Zuk P.A. Zhu M. Ashjian P. De Ugarte D.A. Huang J.I. Mizuno H., et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaffe E.A. Nachman R.L. Becker C.G. Minick C.R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 292001:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arts C.H. Blankensteijn J.D. Heijnen-Snyder G.J. Verhagen H.J. Hedeman Joosten P.P. Sixma J.J., et al. Reduction of non-endothelial cell contamination of microvascular endothelial cell seeded grafts decreases thrombogenicity and intimal hyperplasia. Eur J Vasc Endovasc Surg. 2002;23:404. doi: 10.1053/ejvs.2002.1604. [DOI] [PubMed] [Google Scholar]
- 44.Zhou J. Lin H. Fang T. Li X. Dai W. Uemura T., et al. The repair of large segmental bone defects in the rabbit with vascularized tissue engineered bone. Biomaterials. 2010;31:1171. doi: 10.1016/j.biomaterials.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 45.Eggermann J. Kliche S. Jarmy G. Hoffmann K. Mayr- Beyrle U. Debatin K.M., et al. Endothelial progenitor cell culture and differentiation in vitro: a methodological comparison using human umbilical cord blood. Cardiovasc Res. 2003;58:478. doi: 10.1016/s0008-6363(03)00252-9. [DOI] [PubMed] [Google Scholar]
- 46.Ye C. Bai L. Yan Z.Q. Wang Y.H. Jiang Z.L. Shear stress and vascular smooth muscle cells promote endothelial differentiation of endothelial progenitor cells via activation of Akt. Clin Biomech (Bristol, Avon) 2008;23(Suppl 1):S118. doi: 10.1016/j.clinbiomech.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 47.Quirici N. Soligo D. Caneva L. Servida F. Bossolasco P. Deliliers G.L. Differentiation and expansion of endothelial cells from human bone marrow CD133(+) cells. Br J Haematol. 2001;115:186. doi: 10.1046/j.1365-2141.2001.03077.x. [DOI] [PubMed] [Google Scholar]
- 48.Ferreira L.S. Gerecht S. Shieh H.F. Watson N. Rupnick M.A. Dallabrida S.M., et al. Vascular progenitor cells isolated from human embryonic stem cells give rise to endothelial and smooth muscle-like cells and form vascular networks in vivo. Circ Res. 2007;101:286. doi: 10.1161/CIRCRESAHA.107.150201. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka F. Otake Y. Yanagihara K. Kawano Y. Miyahara R. Li M., et al. Correlation between apoptotic index and angiogenesis in non-small cell lung cancer: comparison between CD105 and CD34 as a marker of angiogenesis. Lung Cancer. 2003;39:289. doi: 10.1016/s0169-5002(02)00534-2. [DOI] [PubMed] [Google Scholar]
- 50.Theuerkauf I. Zhou H. Fischer H.P. Immunohistochemical patterns of human liver sinusoids under different conditions of pathologic perfusion. Virchows Archiv. 2001;438:498. doi: 10.1007/s004280000364. [DOI] [PubMed] [Google Scholar]
- 51.Nagatsuka H. Hibi K. Gunduz M. Tsujigiwa H. Tamamura R. Sugahara T., et al. Various immunostaining patterns of CD31, CD34 and endoglin and their relationship with lymph node metastasis in oral squamous cell carcinomas. J Oral Pathol Med. 2005;34:70. doi: 10.1111/j.1600-0714.2004.00227.x. [DOI] [PubMed] [Google Scholar]
- 52.Zakrzewicz A. Grafe M. Terbeek D. Bongrazio M. Auch- Schwelk W. Walzog B., et al. L-selectin-dependent leukocyte adhesion to microvascular but not to macrovascular endothelial cells of the human coronary system. Blood. 1997;89:3228. [PubMed] [Google Scholar]
- 53.Muller A.M. Hermanns M.I. Skrzynski C. Nesslinger M. Muller K.M. Kirkpatrick C.J. Expression of the endothelial markers PECAM-1, vWf, and CD34 in vivo and in vitro. Exp Mol Pathol. 2002;72:221. doi: 10.1006/exmp.2002.2424. [DOI] [PubMed] [Google Scholar]
- 54.Fina L. Molgaard H.V. Robertson D. Bradley N.J. Monaghan P. Delia D., et al. Expression of the CD34 gene in vascular endothelial cells. Blood. 1990;75:2417. [PubMed] [Google Scholar]
- 55.Wetzel A. Chavakis T. Preissner K.T. Sticherling M. Haustein U.F. Anderegg U., et al. Human Thy-1 (CD90) on activated endothelial cells is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18) J Immunol. 2004;172:3850. doi: 10.4049/jimmunol.172.6.3850. [DOI] [PubMed] [Google Scholar]
- 56.Saalbach A. Haustein U.F. Anderegg U. A ligand of human thy-1 is localized on polymorphonuclear leukocytes and monocytes and mediates the binding to activated thy-1-positive microvascular endothelial cells and fibroblasts. J Invest Dermatol. 2000;115:882. doi: 10.1046/j.1523-1747.2000.00104.x. [DOI] [PubMed] [Google Scholar]
- 57.Lee W.S. Jain M.K. Arkonac B.M. Zhang D. Shaw S.Y. Kashiki S., et al. Thy-1, a novel marker for angiogenesis upregulated by inflammatory cytokines. Circ Res. 1998;82:845. doi: 10.1161/01.res.82.8.845. [DOI] [PubMed] [Google Scholar]
- 58.Marom R. Shur I. Solomon R. Benayahu D. Characterization of adhesion and differentiation markers of osteogenic marrow stromal cells. J Cell Physiol. 2005;202:41. doi: 10.1002/jcp.20109. [DOI] [PubMed] [Google Scholar]
- 59.Stains J.P. Civitelli R. Cell-to-cell interactions in bone. Biochem Biophys Res Commun. 2005;328:721. doi: 10.1016/j.bbrc.2004.11.078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.