Abstract
Recently, a second copy of a gene encoding proclavaminate amidinohydrolase (pah1), an enzyme involved in the early stages of clavulanic acid and clavam metabolite biosynthesis in Streptomyces clavuligerus, was identified and isolated. Using Southern analysis, we have now isolated second copies of the genes encoding the carboxyethylarginine synthase (ceaS) and β-lactam synthetase (bls) enzymes. These new paralogues are given the gene designations ceaS1 and bls1 and are located immediately upstream of pah1 on the chromosome. Furthermore, sequence analysis of the region downstream of pah1 revealed a second copy of a gene encoding ornithine acetyltransferase (oat1), thus indicating the presence of a cluster of paralogue genes. ceaS1, bls1, and oat1 display 73, 60, and 63% identities, respectively, at the nucleotide level to the original ceaS2, bls2, and oat2 genes from the clavulanic acid gene cluster. Single mutants defective in ceaS1, bls1, or oat1 were prepared and characterized and were found to be affected to variable degrees in their ability to produce clavulanic acid and clavam metabolites. Double mutants defective in both copies of the genes were also prepared and tested. The ceaS1/ceaS2 and the bls1/bls2 mutant strains were completely blocked in clavulanic acid and clavam metabolite biosynthesis. On the other hand, oat1/oat2 double mutants still produced some clavulanic acid and clavam metabolites. This may be attributed to the presence of the argJ gene in S. clavuligerus, which encodes yet another ornithine acetyltransferase enzyme that may be able to compensate for the lack of OAT1 and -2 in the double mutants.
The genus Streptomyces comprises gram-positive filamentous soil-dwelling organisms, which are renowned for their ability to produce a wide variety of chemically distinct antibiotics and secondary metabolites (25). When grown in soy medium, Streptomyces clavuligerus produces a variety of β-lactam compounds, including cephamycin C, penicillin N, clavulanic acid, and at least four other clavams (9, 17). S. clavuligerus is used commercially for the production of the clinically important β-lactamase inhibitor clavulanic acid on an industrial scale (21). Although clavulanic acid and the other clavams are structurally related to each other, only clavulanic acid is inhibitory to β-lactamases (3). The β-lactamase inhibitory activity of clavulanic acid has been attributed to its 3R, 5R stereochemistry, which differs from the 5S stereochemistry of all of the other known clavams (hereafter referred to as 5S clavams) produced by S. clavuligerus (21).
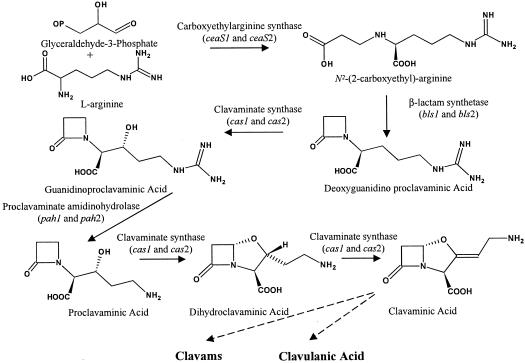
Although separate pathways produce cephamycin C and clavulanic acid, the two gene clusters involved in their biosynthesis are found grouped on the S. clavuligerus chromosome, forming a supercluster (1, 19, 45). Clavulanic acid and the 5S clavams arise by the condensation of l-arginine and glyceraldehyde-3-phosphate (24). This reaction is catalyzed by the enzyme carboxyethylarginine synthase and leads to the formation of N2-(2-carboxyethyl)arginine, the first dedicated intermediate in the biosynthesis of clavulanic acid and the 5S clavams (24) (Fig. 1.). Carboxyethylarginine then undergoes intramolecular ring closure to form the β-lactam ring-containing intermediate, deoxyguanidinoproclavaminate. This ring formation is mediated by the action of the enzyme β-lactam synthetase (β-LS) (2, 28) encoded by bls, the second gene in the clavulanic acid gene cluster (18, 19, 20). Subsequently, deoxyguanidinoproclavaminate is hydroxylated in the first of three reactions catalyzed by the enzyme clavaminate synthase (CAS) to form guanidinoproclavaminate (6). Guanidinoproclavaminate is then converted to proclavaminate by the removal of the guanidino group in a reaction catalyzed by the enzyme proclavaminate amidinohydrolase (PAH) (15). Next, CAS mediates the formation of the bicyclic nucleus of clavulanic acid and the 5S clavams in a two-step reaction involving oxidative cyclization, followed by desaturation to form clavaminate (5, 40). Clavaminate is thought to be the branch point between the biosynthetic pathways leading to clavulanic acid and the 5S clavams (14). The pathway beyond clavaminate is not well characterized, and the only other known intermediate between clavaminate and clavulanic acid is clavaldehyde (33). Clavaldehyde has the same stereochemistry as clavulanic acid, shows β-lactamase inhibitory activity, and is reduced to clavulanic acid by the action of the enzyme clavulanic acid dehydrogenase (CAD) (33). However, the mechanism by which clavaminate undergoes stereochemical inversion and side chain modification to form clavaldehyde is unknown. Similarly, the reactions leading from clavaminate to the 5S clavams are not known (3).
FIG. 1.
Early steps of clavulanic acid and 5S clavam metabolite biosynthesis.
In addition to genes encoding enzymes with clearcut roles in clavulanic acid biosynthesis, a gene (orf6) apparently encoding an ornithine acetyltransferase (OAT) is also present within the clavulanic acid gene cluster. Although its function in clavulanic acid and clavam metabolite biosynthesis is unclear, mutation of orf6 has been shown to decrease metabolite production (20). Recently, orf6 was shown to encode a protein with OAT activity (23). OATs are normally involved in arginine biosynthesis (12), and since arginine is a precursor of clavulanic acid and the 5S clavams, perhaps orf6, hereafter called oat, functions to increase the flux of arginine into the pathway.
S. clavuligerus possesses two CAS isozymes, encoded by two separate paralogous genes, cas1 and cas2 (4, 27). cas2 is located within the clavulanic acid gene cluster (1, 19, 45), whereas cas1 is located elsewhere on the chromosome, surrounded by genes that are involved in 5S clavam but not clavulanic acid biosynthesis (27, 32). The two cas paralogues are regulated differently, and transcriptional studies have shown that cas2 is transcribed in both complex soy and defined starch-asparagine (SA) medium, whereas cas1 is transcribed exclusively in soy medium (34). The continued production of some clavulanic acid and 5S clavams on soy medium even when cas2 was disrupted by insertional inactivation indicated that cas1 could partially complement the cas2 mutation (34). Very similar phenotypes were observed when individual mutants defective in each of ceaS, bls, pah, and oat from the clavulanic acid gene cluster were prepared and analyzed, suggesting that paralogues may also exist for these genes (20). This was recently shown to be true for pah, since a paralogue was isolated and characterized (22a). The pah paralogues were designated as pah1 and pah2, with pah2 located adjacent to cas2 in the clavulanic acid gene cluster. Preliminary studies indicated that pah1, on the other hand, is not located in the vicinity of cas1 (22a).
In the present study we report the isolation and characterization of paralogues for the ceaS, bls, and oat genes, all of which are located in the region of the S. clavuligerus chromosome flanking pah1. Mutants defective in each of the paralogous genes, and double mutants defective in both genes, were prepared by targeted gene disruption and tested for their abilities to produce clavulanic acid and 5S clavam metabolites.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and culture conditions
All bacterial strains and plasmids used in this study are described in Table 1. Cultures of Escherichia coli were grown in liquid culture in Luria broth (LB) and maintained on LB agar medium at 37°C (42). Plasmid-containing cultures were supplemented with ampicillin (100 μg/ml), apramycin (50 μg/ml), chloramphenicol (25 μg/ml), or kanamycin (50 μg/ml). S. clavuligerus was maintained either on MYM (43) or ISP4 medium agar plates (Difco, Detroit, Mich.) at 28°C. Plasmid-bearing Streptomyces cultures were supplemented with apramycin (25 μg/ml), kanamycin (50 μg/ml), or thiostrepton (5 μg/ml for S. clavuligerus and 50 μg/ml for S. lividans). Cultures for the isolation of chromosomal DNA were grown in Trypticase soy broth supplemented with 1% starch. Cultures for analysis of clavulanic acid and 5S clavam metabolite production were grown on both SA medium and on soy medium as described previously (34). All Streptomyces liquid cultures were grown at 28°C on a rotary shaker at 250 rpm.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain, plasmid, or cosmid | Descriptiona | Source reference |
|---|---|---|
| Strains | ||
| Bacillus sp. strain ATCC 27860 | Indicator strain for alanyl clavam bioassay | 36 |
| E. coli ESS | Indicator strain for cephamycin C bioassay | A. L. Demain, Drew University, Madison, N.J. |
| E. coli BW25113/pIJ790 | Recombination host for Redirect PCR targeting system | 16 |
| K. pneumoniae ATCC 15380 | Indicator strain for clavulanic acid bioassay | 37 |
| S. clavuligerus NRRL3585 | Wild type | Northern Regional Research Laboratory, Peoria, Ill. |
| S. clavuligerus 4B and 4B-C | ceaS2 disruption mutant (ceaS2::apr) | 20 |
| S. clavuligerus O2FS | ceaS2 frameshift mutant | This study |
| S. clavuligerus orf3::apr | bls disruption mutant | 20 |
| S. lividans TK24 | Plasmid-less cloning host (SLP2− SLP3−), str-6 | D. A. Hopwood, John Innes Institute, Norwich, United Kingdom |
| Plasmids and cosmids | ||
| 6G9 | pWE15-derived cosmid carrying DNA fragment encoding ceaS1, bls1, pah1, and oat1 and flanking sequences | 22a |
| 14E10 | pWE15-derived cosmid carrying DNA fragment encoding ceaS1, bls1, pah1, and oat1 and flanking sequences | 22a |
| p2.8-18 | pUC18 containing 2.8-kb EcoRI fragment carrying part of ceaS1 | This study |
| p5K-6 | pUC118 containing 4.3-kb NcoI fragment carrying pah1, oat1, and upstream sequences but with an internal KpnI fragment deleted to remove pah1 | This study |
| p5.7 | pUC118 containing 5.7-kb EcoRI fragment carrying the 3′ end of ceaS1, all of bls1, pah1, and oat1, and flanking sequences | This study |
| p5.7-T | p5.7 containing tsr from pTSR#8 inserted at FseI site within bls1 | This study |
| p5.7-TH | E. coli-Streptomyces shuttle vector formed by fusing p5.7T to pJOE829 | This study |
| pApOrf6 | Disruption construct carrying oat2 disrupted with apr | 20 |
| pCAD2-3 | pUC120 carrying ceaS2 and bls2 | 20 |
| pCAD2-3(L1-5) | pCAD2-3 but with a frameshift mutation in ceaS2 | This study |
| pCAD2-3(L1-5)486 | E. coli-Streptomyces shuttle vector: pCAD2-3(L1-5) fused to pIJ486 | This study |
| pFDNeo-S | pUC18 carrying neo from Tn5 | 13 |
| pIJ486 | Streptomyces plasmid vector, Tsrr | D. A. Hopwood |
| pIJ773 | Template plasmid for PCR targeting; acc(3)IV plus oriT | 16 |
| pJOE829 | Streptomyces plasmid vector; Hygr | J. Altenbucher, University of Stuttgart, Stuttgart, Germany |
| pNEO5K-6A | p5K-6 containing neo from pFDNeo-S inserted at RsrII site in oat1 | This study |
| pTSR#8 | pUC118 containing tsr gene from pIJ702 | 1 |
| pUC18 | E. coli cloning vector; Ampr | Stratagene |
| pUC118 | E. coli phagemid; Ampr | 44 |
| pUC120Apr | pUC120 containing the apr gene flanked by NcoI sites | 34 |
Hygr, hygromycin resistance; Ampr, ampicillin resistance.
DNA isolation, manipulation, and Southern analysis
Plasmid DNA isolation from Escherichia coli cultures, restriction endonuclease digestion, ligation, generation of blunt-ended fragments, and E. coli transformation were carried out by standard procedures (42). In all subsequent procedures, when DNA fragments with incompatible ends were to be ligated, they were first made blunt by treatment with the Klenow fragment of DNA polymerase I. The QIAquick gel extraction kit (Qiagen, Inc.) was used for the isolation of DNA fragments separated by agarose gel electrophoresis. Plasmid and genomic DNA isolation from Streptomyces spp. and preparation and transformation of Streptomyces lividans protoplasts were conducted as described earlier (25). Preparation and transformation of S. clavuligerus protoplasts was as described by Paradkar and Jensen (34). Studies using the PCR were performed with the Expand High-Fidelity PCR system according to the manufacturer's instructions (Roche). Conjugative transfer of DNA from E. coli into S. clavuligerus was carried out as described for S. coelicolor (25), except that AS-1 medium supplemented with 10 mM MgCl2 was used for the isolation of exconjugants (7). Southern analysis of S. clavuligerus DNA fragments and the labeling of double-stranded DNA probes with [α-32P]dCTP by nick translation was conducted as described by Sambrook et al. (42).
Isolation and DNA sequence of ceaS1, bls1, and oat1
The ceaS1 and the bls1 genes were located on EcoRI fragments subcloned from the cosmids 14E10 and 6G9, respectively. The 2.85-kb EcoRI insert from plasmid p2.8-18 carries the 5′ end of ceaS1; the rest of ceaS1 was found on the 5.7-kb EcoRI insert of p5.7, which also encodes bls1. DNA sequence of the ceaS1 and bls1 regions of plasmids p2.8-18 and p5.7 was obtained by a combination of subcloning and analysis with both universal and sequence-specific primers. Similarly, the DNA sequence of oat1 was obtained by analysis of appropriate subclones isolated from the 4.3-kb NcoI fragment previously found to encode pah1 (22a).
All DNA sequence information was confirmed on both strands, and sequence information was obtained to cross all junctions of subclones in order to ensure that no small fragments were lost during subcloning. Sequencing reactions were carried out by using the DYEnamic ET terminator cycle sequencing kit (Amersham Pharmacia, Baie d'Urfe, Quebec, Canada) by the Molecular Biology Service Unit, University of Alberta.
DNA sequence analysis
The nucleotide sequence data obtained were compiled and analyzed by using GeneTools 1.0 (BioTools, Inc.). Prediction of open reading frames (ORFs) based on codon preference was done with the online program FramePlot 2.3.2 (http://watson.nih.go.jp/∼jun/cgi-bin/frameplot.p1). Similarity and homology searches were performed by using the online basic local alignment search tool (BLAST) program at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/BLAST/). The PROSITE online program at the ExPASy home page was used to search for specific peptide motifs (http://ca.expasy.org/prosite/).
Creation of targeted gene replacement mutants
The plasmid pCAD2-3 was used to prepare ceaS2 frameshift mutants (ceaS2-Fs). pCAD2-3 was linearized by digestion at the unique NotI site located 674 bp from the proposed start codon of ceaS2. The linearized plasmid was made blunt by treatment with the Klenow fragment of DNA polymerase I and then recircularized to give pCAD2-3(L1-5) with a 4-bp insertion resulting in a +1 frameshift mutation in ceaS2. The Streptomyces vector pIJ486 was fused to pCAD2-3(L1-5) at the HindIII site to give the E. coli-Streptomyces shuttle vector pCAD2-3(L1-5)486, which was passed through S. lividans TK24 and then transformed into the S. clavuligerus ceaS2::apr mutant, 4B. ceaS2::apr-4B has a disruption in the ceaS2 gene resulting from insertion of an apramycin resistance gene cassette (apr) (20). Apramycin- and thiostrepton-resistant (Aprr and Tsrr, respectively) transformants were allowed to sporulate twice on nonselective medium to isolate Aprs and Tsrs mutants. Southern analysis was then used to confirm the replacement of the original ceaS2::apr by ceaS2-Fs. An 855-bp EcoRI-NotI fragment which contained the 5′ region of ceaS2 and some upstream sequence was used as the ceaS2-specific probe.
ceaS1 mutants were prepared by using the Redirect PCR targeting system described by Gust et al. (16). The Redirect PCR targeting materials were supplied by Plant Bioscience, Ltd., Norwich, United Kingdom. The primers KTA14 (5′-CCATCCCGCGCCCGTCCGTGCGAAGGAGATCTCCATGATTCCGGGGATCCGTCGACC) and KTA15 (5′-CGGGGCCGGGCATGGTGAACTCGTCCTCCACGGTGGTCATGTAGGCTGGAGCTGCTT) were used to amplify the disruption cassette from the template plasmid pIJ773. The disruption cassette comprised the acc3(IV) gene, conferring Aprr, and an RK2 origin of transfer (oriT) flanked by DNA sequence homologous to regions immediately upstream and downstream of ceaS1. The cosmid 14E10 was used to prepare the ceaS1 disruption construct in E. coli BW25113/pIJ780 (16), and the entire gene was deleted from the cosmid and replaced by the disruption cassette (ΔceaS1::apr) to produce the mutant cosmid 14E10-AP. 14E10-AP was then introduced into wild-type S. clavuligerus by conjugation, and exconjugants were selected based on Aprr and kanamycin sensitivity (Kans) on AS-1 medium supplemented with 10 mM MgCl2 (7). These isolates were allowed to sporulate under nonselective conditions to isolate unigenomic Aprr Kans spores.
ceaS1/ceaS2 double mutants were prepared as described for the ceaS1 mutants, except that the mutant cosmid 14E10-AP was conjugated into the ceaS2-Fs mutant strain, O2FS, instead of the wild type. Disruption of the wild-type copy of ceaS1, in the ceaS1 mutants and in the ceaS1/ceaS2 double mutants, was confirmed by Southern hybridization. A 777-bp EcoRI-NruI fragment internal to ceaS1 was used as the ceaS1-specific probe.
bls1 mutants were prepared by isolating the thiostrepton resistance gene cassette (tsr) from pTSR#8 as an EcoRI/HindIII fragment and inserting it into the FseI site of p5.7, located within bls1, 507 bp from the proposed start codon, to give p5.7-T (tsr in the opposite orientation to bls1). The construct was converted to an E. coli-Streptomyces shuttle vector (p5.7-TH) by fusing p5.7-T to pJOE829 at their HindIII sites. p5.7-TH was passed through S. lividans and into wild-type S. clavuligerus to generate gene replacement mutants, as described previously (34). Replacement of the wild-type copy of bls1 by the tsr-disrupted copy was confirmed by Southern hybridization. A 1,862-bp NcoI fragment, including bls1 and 407 bp of upstream sequence, was used as the bls1-specific probe. In addition to the isolation of bls1 mutant strains, cured wild-type strains were also isolated as controls. These control strains were derived from primary transformants that subsequently lost the targeting vector without undergoing gene replacement versus the same primary transformants that underwent gene replacement to produce the mutants.
The bls1/bls2 double mutants were prepared by transformation of the bls2 mutant strain (originally called bls::apr [20]) with the bls1 disruption construct, p5.7-TH. Aprr Tsrr transformants were selected, and the bls1/bls2 double mutation was confirmed by Southern analysis.
oat1 was disrupted by linearization of p5K-6 at the RsrII site located in the middle of oat1 and ligation to a PstI/EcoRI fragment from pFDNeo-S carrying the neomycin resistance cassette (neo). The resulting plasmid, pNEO5K-6A (neo in the same orientation as oat1), was digested with BamHI and fused to pIJ486 digested with BglII to yield an E. coli-Streptomyces shuttle disruption construct. The disruption construct was then passed through S. lividans and into wild-type S. clavuligerus to generate gene replacement mutants, essentially as described by Paradkar and Jensen (34). Southern analysis was used to confirm the oat1 disruption. A 319-bp SalI fragment internal to oat1 was used as the oat1-specific probe. In addition to the oat1 mutants, cured wild-type strains were also isolated as controls as was described for the bls1 mutants.
oat1/oat2 double mutants were generated by transforming protoplasts of the oat1::neo mutant strain prepared in the present study with the oat2::apr disruption construct pApOrf6, prepared earlier (20). Southern analysis was then used to verify the replacement of the genomic wild-type copies of oat1 and oat2 with the plasmid encoded neo and apr disrupted copies, respectively. A 531-bp NruI fragment internal to oat2 was used as the oat2-specific probe.
HPLC analyses of culture filtrates
High-performance liquid chromatographic (HPLC) analysis of culture supernatants after imidazole derivatization was performed as described earlier (34).
Bioassays and growth assays
The production of clavulanic acid was also detected by bioassays with Klebsiella pneumoniae ATCC 15380 as the indicator organism as described previously (Jensen et al., unpublished). Cephamycin C was detected in culture supernatants by bioassay against the indicator organism E. coli ESS (22). The production of alanylclavam was also assayed with Bacillus sp. ATCC 27860 as the indicator organism (34).
The extent of growth of S. clavuligerus in fermentation medium was determined by using an indirect assay for deoxyribose (10).
Nucleotide sequence accession number
The nucleotide sequence encompassing ceaS1, bls1, and oat1 from S. clavuligerus has been deposited in GenBank under the accession number AY426768.
RESULTS
Location of paralogues for ceaS, bls, and oat
Recently, it was shown that there is a second copy of the gene encoding PAH in S. clavuligerus (22a). Together with the previously discovered cas1 and cas2 paralogues (41), the pah genes provide the second example of a pair of paralogous genes encoding enzymes involved in the early stages of the shared clavulanic acid-5S clavam biosynthetic pathway (Fig. 1). Furthermore, when ceaS, bls, pah2, cas2, and oat mutant strains were examined for clavulanic acid production (20, 32), their phenotypes could best be explained if all of these genes, not just pah2 and cas2, also have paralogues.
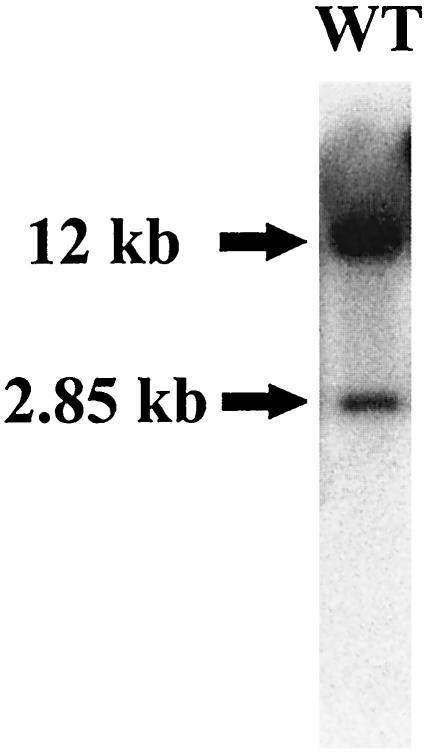
To investigate the possibility of a second ceaS gene in S. clavuligerus, chromosomal DNA from wild-type S. clavuligerus was digested with EcoRI and analyzed by Southern hybridization with a ceaS-specific probe. The 855-bp EcoRI-NotI fragment from pCAD2-3, which contained the 5′ region of ceaS and some upstream coding sequence, was used as the ceaS specific probe. A DNA fragment of 12 kb hybridized strongly with the probe, while a 2.85-kb fragment gave a weaker hybridization signal (Fig. 2). From previous studies it was known that the ceaS gene in the clavulanic acid gene cluster is carried on a 12-kb EcoRI fragment (20). The 2.85-kb EcoRI fragment was therefore postulated to encode a second copy of ceaS, the putative ceaS paralogue.
FIG. 2.
Southern analysis of genomic DNA from wild-type S. clavuligerus after EcoRI digestion. The fractionated and blotted genomic DNA was probed with a ceaS2-specific probe.
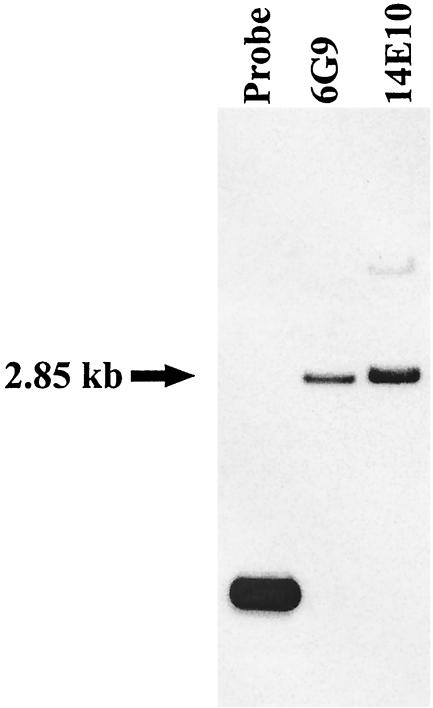
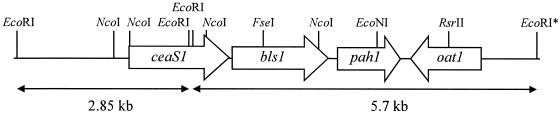
Previous DNA sequence analyses had not located any putative paralogues in the regions flanking cas1 (32), and pah1 is apparently not linked to cas1. However, it seemed possible that other paralogues, if they exist, might be clustered with pah1. Cosmids 14E10 and 6G9, previously shown to carry pah1 and flanking regions of the chromosome, were digested with EcoRI and subjected to Southern analysis with the ceaS-specific probe. A 2.85-kb hybridizing fragment was observed in both of the cosmids (Fig. 3), and the fragment was subcloned for further study. Sequence analysis indicated the presence of an incomplete ORF showing similarity to the 5′ end of ceaS, located at one end of this fragment. The same EcoRI digests of the cosmids 14E10 and 6G9 were also screened with a probe specific for the gene encoding β-lactam synthetase (bls), to search for a bls paralogue in the same region (data not shown). The 582-bp BglII-NcoI fragment from pCAD2-3 was used as the bls-specific probe. A 5.7-kb fragment seen in digests of the cosmid 6G9 but not in 14E10, hybridized to the probe, and was therefore subcloned and partially sequenced. At one end of this fragment the 3′ end of the ceaS-like ORF was found. The complete ORF was 1,668 bp in size, and the predicted amino acid sequence of this ORF showed 66% identity (77% similarity) to CeaS. Downstream of the ceaS-like ORF, separated by an intergenic region of 23 bp, a 1,584-bp ORF was found that displayed 49% identity (59% similarity) to β-LS at the amino acid level (Table 2). The previously described pah1 gene was found to be located immediately downstream and in the same orientation as the bls-like ORF, separated by a gap of 314 bp (Fig. 4). These newly found ORFs were designated ceaS1 and bls1, respectively, due to their linkage to pah1.
FIG. 3.
Southern analysis of cosmids 6G9 and 14E10 with a ceaS2-specific probe. Lane 1, ceaS2 probe (control); lanes 2 and 3, EcoRI-digested 6G9 and 14E10, respectively.
TABLE 2.
Three newly sequenced ORFs found adjacent to pah1, extending the S. clavuligerus paralogue gene cluster
| ORF | Size (bp/aa)a | % G+C content | Molecular mass of encoded protein (Da) | Similarity to known proteins from S. clavuligerusb | % Identityb |
|---|---|---|---|---|---|
| ceaS1 | 1,668/555 | 70.1 | 59,078 | Carboxyethylarginine synthase | 66 |
| bls1 | 1,584/527 | 75.6 | 55,042 | β-LS | 49 |
| oat1 | 1,176/391 | 76.8 | 39,747 | OAT | 47 |
aa, amino acids.
Similarities at the amino acid level were determined by searching the database by using the BLASTp online program.
FIG. 4.
Diagram of the genes encoded by the paralogue gene cluster thought to be involved in the early stages of clavulanic acid and 5S clavam metabolite biosynthesis. Only restriction sites referred to in the text are shown. EcoRI* denotes an EcoRI site arising from the multiple cloning site of the cosmid vector, pWE15. (The diagram is not to scale.)
The pah1 gene was initially found on a 4.3-kb NcoI fragment that also contained flanking sequences from the S. clavuligerus chromosome (22a). When the region downstream of pah1 was sequenced, an 1,176-bp ORF was found separated by an intergenic gap of 124 bp. The predicted amino acid sequence of this ORF showed 47% identity (58% similarity) to OAT from the clavulanic acid gene cluster. This ORF was therefore called oat1 to distinguish it from oat2, which lies adjacent to cas2 in the clavulanic acid gene cluster. Like OAT2, the predicted OAT1 protein also contains an ArgJ family domain, and oat1 was predicted to be transcribed in the orientation opposite to pah1 transcription (Fig. 4). S. clavuligerus also possesses another known OAT encoded by argJ, which is part of the arginine biosynthetic gene cluster (38) and the predicted OAT1 showed 29% identity to ArgJ.
The gene cluster comprising ceaS1, bls1, pah1, and oat1 is hereafter referred to as the paralogue gene cluster (Fig. 4) to distinguish it from the clavulanic acid gene cluster which comprises ceaS2, bls2, pah2, cas2, and oat2 in addition to other genes involved in clavulanic acid and 5S clavam metabolite synthesis.
Generation of a ceaS2 frameshift mutant
In order to study the involvement of ceaS1 in clavulanic acid and 5S clavam metabolite biosynthesis, ceaS1 single and ceaS1/ceaS2 double mutants were prepared. However, as a first step, a new ceaS2 single-mutant strain was constructed in which the apr resistance cassette of the original ceaS2::apr mutant (20) was replaced by a simple frameshift mutation. This enabled the Aprr gene cassette to be used in the preparation of the ceaS1 disruption mutant. The new ceaS2 mutant was generated by the introduction of a plasmid construct, pCAD2-3(L1-5)486 carrying a frameshifted mutant copy of ceaS2 (ceaS2-Fs), into the previously prepared ceaS2::apr mutant strain (20), and by screening for double-crossover events with a loss of Aprr.
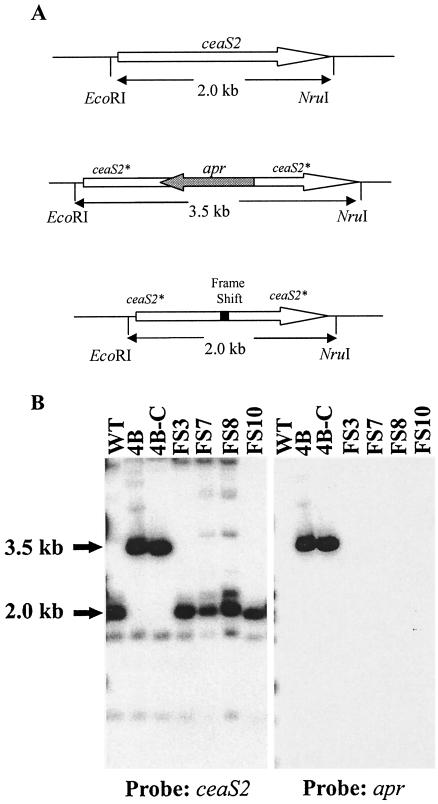
To confirm the replacement of the apr disrupted copy of ceaS2 by ceaS2-Fs, genomic DNA from the new ceaS2 mutant and wild-type strains was analyzed by Southern hybridization after digestion with EcoRI and NruI. When the EcoRI-NotI fragment from pCAD2-3 (includes both the ceaS2 and bls2 genes) was used as a ceaS2-specific probe, a 2.0-kb fragment hybridized to the probe in the wild-type and the new ceaS2-Fs mutant samples (Fig. 5). In contrast, the same probe hybridized to a 3.5-kb fragment in samples from the parental ceaS2::apr mutants (Fig. 5). When the same blot was stripped and reprobed with apr from pUC120Apr as a probe, no hybridizing bands were seen in lanes containing DNA from the wild type and the ceaS2-Fs mutants, whereas a 3.5-kb fragment hybridized to the probe in lanes containing DNA from the ceaS2::apr mutant (Fig. 5). These results were consistent with the replacement of ceaS2::apr by ceaS2-Fs on the S. clavuligerus chromosome.
FIG. 5.
Southern analysis of the ceaS2-Fs mutant. (A) Diagram of the ceaS2 region of the S. clavuligerus chromosome in the wild type, the ceaS2::apr mutant, and the ceaS2-Fs mutant. The gray arrow represents the apr disruption cassette, and the open arrow represents ceaS2 with the direction of transcription represented by the direction of the arrowheads. The solid bar represents the frame shift mutation, and the fine lines represent the rest of the S. clavuligerus chromosome. (B) Southern analysis of EcoRI- and NruI-digested genomic DNA from S. clavuligerus wild-type and ceaS2 mutant strains. DNA from the wild type, from ceaS2::apr mutants 4B and 4B-C, and from ceaS2-Fs mutants FS3, FS7, FS8, and FS10 was probed with a ceaS2-specific probe and an apr-specific probe.
Three ceaS2-Fs mutants were fermented in SA and soy media in single shake flask cultures, along with wild-type and parental ceaS2::apr strains. Supernatants from 72- and 96-h cultures were analyzed by HPLC and bioassays for clavulanic acid and 5S clavam metabolite production. HPLC analysis showed that the ceaS2-Fs mutants produced clavulanic acid at levels of up to 13% compared to the wild-type strain in soy medium. Various amounts of clavam metabolites were also detected in these culture supernatants, but production of all of the detectable clavam metabolites was depressed. As predicted earlier, both the ceaS2::apr and ceaS2-Fs mutants were completely blocked in clavulanic acid and clavam metabolite biosynthesis in SA medium (20).
Generation of a ceaS1 mutant
The ceaS1 mutant was created by using the recently described Redirect PCR targeting system (16). The PCR primers used were designed as such that ceaS1 was completely deleted and replaced by the acc(3)IV + oriT cassette (ΔceaS1::apr).
Five mutants were isolated and confirmed by Southern analysis (Table 3) and then characterized by fermentation in SA and soy media. On HPLC analysis of SA and soy culture supernatants, a reduction in clavulanic acid production compared to the wild-type strain was observed (Table 4). 5S clavam metabolite biosynthesis also varied in soy medium, but no specific pattern was identified. The ceaS1 disruption did not have any effect on alanylclavam or cephamycin production, as indicated by bioassays.
TABLE 3.
Hybridization profiles from Southern analyses of wild-type and mutant strains
| Mutant | Restriction endonuclease(s) | Probea | Hybridizing fragment (kb)
|
|
|---|---|---|---|---|
| Parentb | Mutant | |||
| ceaS2-Fs | EcoRI/NruI | ceaS2 | 3.5 | 2.0 |
| apr | 3.5 | None | ||
| ΔceaS1::apr | NcoI | ceaS1 | 1.2c | Nonec |
| apr | None | 3.4 | ||
| ΔceaS1::apr/ceaS2-Fs | NcoI | ceaS1 | 1.2c | Nonec |
| apr | None | 3.4 | ||
| bls1::tsr | NcoI | bls1 | 1.9c | 3.0c |
| tsr | None | 3.0 | ||
| bls1::tsr/bls2::apr | NcoI | bls1 | 1.9c | 3.0c |
| tsr | None | 3.0 | ||
| oat1::neo | BglII | oat1 | 4.3 | 2.4 and 2.9 |
| neo | None | 2.4 and 2.9 | ||
| oat1::neo/oat2::apr | BglII | oat2 | 6.5 | 7.95 |
| neo | None | 7.95 | ||
Gene-specific probes are described in Materials and Methods. An approximately 1.5-kb fragment from pUC120Apr was used as the apramycin-specific probe. An approximately 1-kb EcoRI-HindIII fragment from pTSR#8 was used as the thiostrepton-specific probe. An approximately 1-kb BamHI-HindIII fragment from pFDNeo-S was used as the neomycin-specific probe.
ceaS2::apr was the parental strain for the ceaS2-Fs mutant, ceaS2-Fs was the parental strain for the ΔceaS1::apr/ceaS2-Fs double mutant, bls2::apr was the parental strain for the bls1::tsr/bls2::apr double mutant, and oat1::apr was the parental strain for the oat1::neo/oat2::apr double mutant. For all other mutants, the parental strain was the wild type.
Faint cross-hybridizing bands were observed which can be attributed to the presence of the respective paralogues.
TABLE 4.
Clavulanic acid production by single and double mutants of ORFs located in the paralogue gene cluster
| Wild type or mutant | Clavulanic acid produceda (% relative to wild type)
|
|
|---|---|---|
| SA medium | Soy medium | |
| Wild type | 100 | 100 |
| ceaS2-Fs | 0 | 5-13 |
| ΔceaS1::apr | 17-63 | 2-63 |
| ceaS2-Fs/ΔceaS1::apr | 0 | 0 |
| bls1::tsr | 103-162 | 107-173 |
| bls1::tsr/bls2::apr | 0 | 0 |
| oat1::neo | 30-154 | 57-145 |
| oat1::neo/oat2::apr | 12-62 | 31-67 |
The amount of clavulanic acid produced after 96 h of growth was measured by HPLC analysis.
Generation of an S. clavuligerus ceaS1 and ceaS2 double mutant
Since both the ceaS1 and the ceaS2 single mutants produced some clavulanic acid and 5S clavams, a ceaS1/ceaS2 double-mutant strain was prepared to verify that the two genes are indeed true paralogues. The ceaS1/ceaS2 double mutant was prepared by conjugating the mutant cosmid 14E10-AP containing ΔceaS1::apr into the ceaS2-Fs mutant strain. Three parental ceaS2-Fs mutants were used to isolate six ceaS1/ceaS2 mutants, and Southern analysis confirmed that gene disruption had taken place in each case (Table 3).
The six isolated ceaS1/ceaS2 double mutants were analyzed for clavulanic acid, 5S clavam, and cephamycin production after 72 and 96 h of growth in SA and soy medium. No clavulanic acid or 5S clavam production was detected in either SA or soy culture supernatants by HPLC or bioassays. Bioassays also indicated that the ceaS1/ceaS2 mutants were unaffected in cephamycin biosynthesis.
Generation of a bls1 mutant
The bls1 mutant was prepared by insertion of a Tsrr gene cassette into the bls1 gene in the opposite orientation to bls1. Nine mutants were isolated, verified by Southern analysis (Table 3), and analyzed for their ability to produce clavulanic acid and clavam metabolites. On HPLC analysis of SA and soy culture supernatants, the mutants appeared to be little affected in clavulanic acid or 5S clavam metabolite biosynthesis. In SA medium the bls1 mutants produced between 102 to 312% and 103 to 162% of the wild-type levels of clavulanic acid after 72 and 96 h of growth, respectively. Similar results were obtained when soy culture supernatants were analyzed. After 72 and 96 h of growth in soy medium, the mutants produced between 50 to 247% and 107 to 173% of the wild-type levels clavulanic acid. Once again, the level of 5S clavams varied from mutant to mutant, with some bls1 mutants accumulating elevated levels of 5S clavam metabolites in soy culture, whereas others produced much reduced levels. At 96 h clavam-2-carboxylate and 2-hydroxymethyl clavam production varied from 10 to 301% and 5 to 355%, respectively, compared to cured wild-type controls. Cephamycin production was again unaffected in the bls1 mutant strains.
Generation of an S. clavuligerus bls1 and bls2 double mutant
When bls1 and bls2 were knocked out individually, the mutants still retained the ability to produce clavulanic acid (20). To establish unequivocally the involvement of both bls1 and bls2 in clavulanic acid biosynthesis, a bls1/bls2 double-mutant strain was prepared and tested for its ability to produce clavulanic acid and clavam metabolites. On analysis of culture supernatants from bls1/bls2 double mutants grown in soy and SA media, no clavulanic acid or 5S clavam production was detected by HPLC or bioassays. Although clavulanic acid and clavam metabolite biosynthesis was completely abolished in these mutants, they still produced wild-type levels of cephamycin.
Generation of an oat1 mutant
The involvement of oat1, which lies immediately downstream of pah1 (Fig. 4), in clavulanic acid and clavam metabolite biosynthesis was investigated by preparing a mutant with neo disrupting the oat1 gene. The oat1 mutation was verified by Southern analysis (Table 3), and its effect was surveyed by fermenting different oat1 mutants in soy and SA medium along with wild-type cured strains. After 72 h of growth, clavulanic acid production was between 28 to 86% and 14 to 87% in SA and soy medium, respectively, compared to cured wild-type strains. After 96 h of growth, even more variation in clavulanic acid production was observed in both media compared to the cured wild-type controls. In SA medium clavulanic acid production varied from 30 to 154% and in soy medium from 57 to 145% compared to the wild-type strain. No specific trend in 5S clavam production was observed, and cephamycin production was unaffected in the oat1 mutants.
Generation of an oat1 and oat2 double mutant
Previously, when supernatants from oat2 mutant cultures were analyzed, the mutants still produced clavulanic acid in soy medium, although at levels only 40% of that produced by the wild-type strain (20). To determine whether the ability of the oat2 mutant to produce clavulanic acid could be attributed to the presence of oat1, we prepared oat1/oat2 double-mutant strains of S. clavuligerus and studied their effect on clavulanic acid and 5S clavam biosynthesis. This was done by introducing the previously prepared oat2::apr disruption (20) into the S. clavuligerus oat1::neo mutant strain prepared in the present study. Seven mutants were isolated, verified by Southern analysis (Table 3), and characterized by fermentation. After 72 h of growth, HPLC analyses revealed that clavulanic acid production by the double mutant was between 3 to 9% and 24 to 56% of wild-type levels, in SA and soy medium, respectively. A similar decrease in clavulanic acid production was also observed in the culture supernatants analyzed after 96 h of growth (Table 4). Again, there was a high degree of variation in the levels of 5S clavam metabolites produced, and no specific trend was observed. Cephamycin production was unaffected in the oat1/oat2 mutants.
DISCUSSION
This study extends the work of Jensen et al. (20), who found that S. clavuligerus ceaS, bls, pah2, cas2, and oat mutants, when prepared individually, still retained some ability to produce clavulanic acid and 5S clavam metabolites in complex soy medium but not in defined SA medium. In the case of CAS and PAH, it is known that there are two copies of the genes encoding each of these enzymes present in S. clavuligerus (22a, 27). Based on similarities in the observed phenotypes of the ceaS, bls, pah2, oat, and cas2 mutants, it was proposed that paralogues may also exist for ceaS, bls, and oat (20).
In the present study we report the isolation and characterization of three additional paralogues of genes encoding proteins involved in the early stages of clavulanic acid and 5S clavam biosynthesis. The genes ceaS1, bls1, and oat1 were found grouped together, along with the previously reported pah1 (22a), in a cluster designated the paralogue gene cluster. The paralogue cluster is distinct from the clavulanic acid gene cluster that encodes ceaS2, bls2, and oat2 and other genes involved in clavulanic acid and clavam metabolite biosynthesis (20, 26, 29). ceaS1, bls1, and oat1 are 73, 60, and 63% identical to ceaS2, bls2, and oat2, respectively, while the predicted proteins encoded by ceaS1, bls1, and oat1 are 66, 49, and 47% identical to those encoded by ceaS2, bls2, and oat2, respectively. From the paralogue gene cluster, pah1 shows the highest level of identity to its paralogous counterpart pah2, which was 71% at the amino acid level (22a).
OAT catalyzes the formation of ornithine by transferring an acetyl group from N-acetylornithine to glutamate, a key step in the biosynthesis of arginine (12). Recently, orf6 from the clavulanic acid gene cluster, referred to as oat2 in the present study, was shown to display OAT activity (23). In addition, S. clavuligerus contains another OAT, ArgJ, that is encoded by the arginine biosynthetic gene cluster (39), making the predicted OAT1 the third OAT so far known to be present in S. clavuligerus. Although argJ is presumably required for arginine biosynthesis, oat2 and oat1 are associated with genes involved in the biosynthesis of clavulanic acid and the 5S clavams, which utilize arginine as a precursor. To test whether oat1 was involved in the biosynthesis of clavulanic acid or the clavams, an oat1 mutant was prepared, but no marked decrease in clavulanic acid or 5S clavam production by the oat1 mutant strain was observed. Since the oat1 mutant still has wild-type copies of oat2 and argJ, it is possible that OAT2 and ArgJ can compensate for the oat1 mutation. Alternatively, OAT1 activity may not be essential for clavulanic acid or 5S clavam metabolite biosynthesis. To investigate the former hypothesis, we prepared an oat1/oat2 double-mutant strain and saw that the levels of clavulanic acid and 5S clavams produced by the double mutant dropped in both SA and soy media compared to the wild-type strain, although some production still remained (Table 4). If oat1 and oat2 indeed encode OATs involved in providing arginine for clavulanic acid and clavam biosynthesis, then the residual production of these metabolites by the oat1/oat2 double mutant may be attributed to the presence of wild-type ArgJ in this mutant. Although a role for oat1 and oat2 in providing increased precursor availability for metabolite synthesis seems most plausible, their role in some unidentified step in clavulanic acid or 5S clavam biosynthesis cannot be ruled out.
All of the known or putative OATs sequenced to date contain the autoproteolytic cleavage motif KGXGMXXPX-(M/L)AT(M/L)L, with cleavage taking place between the alanine and threonine residues (11). OAT2 is expressed as a 42-kDa peptide that undergoes posttranslational autoproteolytic cleavage to form a small 19-kDa subunit and a large 25-kDa subunit, which oligomerize to form an 84-kDa heterotetramer (23). The cleavage occurs between alanine 180 and threonine 181 residues of the motif 169KGVGMLEPDMATLL183 (23). A similar motif, 168KGAGMLAPGLATTLL181, is also found in S. clavuligerus ArgJ, but posttranslational cleavage has yet to be demonstrated. The predicted amino acid sequence of OAT1 in this region is 167KGPGTGPAEQDDRSTL182, which deviates from the consensus sequence, and it is also missing the adjacent alanine and threonine residues where cleavage is thought to take place. Therefore, more work is required to confirm whether oat1 actually encodes an active OAT or if it could have some other, yet-unknown function in clavulanic acid or clavam biosynthesis.
The ceaS1 and the bls1 genes described here were isolated by screening S. clavuligerus chromosomal DNA, using sequences from ceaS2 and bls2 as probes, respectively. When ceaS2 was first sequenced, it showed striking similarity to genes encoding acetohydroxyacid synthases (AHAS) based on observed homologies and conservation of five of the eight amino acids forming the active center of AHAS (35). Subsequently, CeaS2 was shown to catalyze the thiamine pyrophosphate (TPP)-dependent condensation of glyceraldehyde-3-phosphate and l-arginine to form carboxyethylarginine, the first reaction in the clavulanic acid-clavam biosynthetic pathways (24). These five amino acids, associated with the active centers of AHAS enzymes, which are found in CeaS2 (69E,132Q,472G,499N, and 503G), are also conserved in CeaS1 (45E,112Q,446G,472N, and 476G). Since CeaS2 utilizes TPP, it also contains the TPP binding motif (446IGAQMARPDQPTFLIAGDGG465), and a similar TPP binding motif (428MAAQIARPGEPVFLIAGDGG447) is also present in the predicted CeaS1.
β-Lactam synthetase, as the name suggests, is responsible for the formation of the β-lactam ring of clavulanic acid and the clavams (2, 28). It catalyzes the second reaction in the clavulanic acid and 5S clavam biosynthetic pathway and requires ATP and Mg2+ (2, 28). Crystallographic studies on β-LS2 have identified certain amino acids that are involved in substrate binding and catalysis (30, 31), and these amino acids were also found to be conserved in β-LS1 (Fig. 6). Both β-LS2 and β-LS1 show similarities to asparagine synthases (AS-B) from different organisms. Asparagine synthases belong to a family of enzymes called Ntn amidotransferases that have a conserved cysteine residue at their N terminus (8). This conserved cysteine residue is missing at the N terminus of both β-LS2 and β-LS1 (Fig. 6) and, in the case of β-LS2, the absence of this residue is consistent with its function in clavulanic acid and 5S clavam metabolite biosynthesis rather than asparagine biosynthesis (2, 28). Therefore, based on homologies and the presence of conserved residues, it seems probable that β-LS1 may perform a function similar to β-LS2 in S. clavuligerus.
FIG. 6.
Similarities between regions of β-LS1 and β-LS2. Heavy shading indicates conserved residues, and light shading indicates similar residues. Amino acids that have been shown to be involved in substrate binding, either directly or indirectly, are indicated by dots. The boxed residues represent a loop thought to form a part of the active site of β-LS2.
Due to the high levels of similarities between the predicted CeaS1 and β-LS1 proteins and the characterized CeaS2 and β-LS2, respectively, similar functions were envisioned for these pairs of proteins. To test whether ceaS1 and bls1 were involved in clavulanic acid or clavam biosynthesis, ceaS1 and bls1 mutants were prepared and tested individually. Since the ceaS1 and the bls1 mutants still contain functional wild-type copies of ceaS2 and bls1, respectively, any clavulanic acid or clavams produced by these mutants can be attributed to the presence of these genes. Large variations in the levels of 5S clavam production were observed in the bls1 mutant. It is possible that the production of these metabolites may be extremely sensitive to minor variations in culture conditions. Despite our best efforts to produce replicate cultures, these variations persist and are observed in all of our fermentation studies. To confirm the hypothesis that ceaS1 and ceaS2 and bls1 and bls2 were indeed true paralogues, ceaS1/ceaS2 and bls1/bls2 double mutants were prepared and characterized. Both the ceaS1/ceaS2 and the bls1/bls2 double mutants were found to be completely blocked in clavulanic acid and clavam biosynthesis, lending further evidence to the paralogue hypothesis.
Results from the present study and from other work (22a) show that the genes encoding enzymes involved in the early stages of clavulanic acid and clavam metabolite biosynthesis, at least up to the level of clavaminic acid, are duplicated in S. clavuligerus. At present, the paralogue gene cluster contains paralogues of four genes from the clavulanic acid gene cluster in a similar but not an identical arrangement to their counterparts in the clavulanic acid gene cluster (20). Surprisingly, cas1, the paralogous counterpart of cas2, is found elsewhere on the S. clavuligerus chromosome and is surrounded by genes that are involved exclusively in 5S clavam metabolite biosynthesis and not in the biosynthesis of clavulanic acid (32). In addition to the striking absence of the cas1 paralogue, the relative orientation of oat1 is opposite to that of oat2 with respect to its neighboring genes. Therefore, the paralogue gene cluster does not appear to have arisen from a direct duplication of a portion of the clavulanic acid gene cluster; rather, a somewhat more complex evolution is indicated.
The reasons why S. clavuligerus has two sets of genes encoding enzymes involved in the early part pathway that is shared between clavulanic acid and the 5S clavams is not clear. One explanation could be to provide a gene dosage effect and thereby increase the level of clavaminate production, which is a precursor of both clavulanic acid and the 5S clavams. A second possibility is that the two sets of paralogues have arisen to serve separate, parallel biosynthetic pathways, which happen to share intermediates up to the level of clavaminate. To date there is no evidence for paralogues of other genes in the clavulanic acid gene cluster, based on mutational phenotypes and genetic studies (20), suggesting that these other genes are dedicated to the biosynthesis of either clavulanic acid or the 5S clavams with no need for duplication and increased gene dosage (20). Therefore, in conclusion, the genes involved in clavulanic acid and clavam biosynthesis are now shown to be grouped into three distinct gene clusters in S. clavuligerus, the clavulanic acid gene cluster, the cas1-associated clavam gene cluster and the paralogue gene cluster, with no evidence of linkage between the clusters. Duplication of all of the genes encoding enzymes involved in the early shared steps of the pathway is evident, with paralogues to the original clavulanic acid gene cluster genes located in both the cas1 clavam gene cluster and in the newly described paralogue gene cluster.
Acknowledgments
This study was supported by grants from the Canadian Institutes of Health Research and the Natural Sciences and Engineering Research Council of Canada. K.T. was supported by a studentship from the Alberta Heritage Foundation for Medical Research.
REFERENCES
- 1.Aidoo, K. A., A. Wong, D. C. Alexander, R. A. Rittammer, and S. E. Jensen. 1994. Cloning, sequencing, and disruption of a gene from Streptomyces clavuligerus involved in clavulanic acid biosynthesis. Gene 147:41-46. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann, B. O., R. Li, and C. A. Townsend. 1998. β-Lactam synthetase: a new biosynthetic enzyme. Proc. Natl. Acad. Sci. USA 95:9082-9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baggaley, K. H., A. G. Brown, and C. J. Schofield. 1997. Chemistry and biosynthesis of clavulanic acid and other clavams. Nat. Prod. Rep. 14:309-333. [DOI] [PubMed] [Google Scholar]
- 4.Baggaley, K. H., S. W. Elson, N. H. Nicholson, and J. T. Sime. 1990. Studies on the biosynthesis of clavaminic acid. Part 4. Synthetic routes to the monocyclic β-lactam precursor, proclavaminic acid. J. Chem. Soc. Perkin Trans. I. 1990:1513-1520. [Google Scholar]
- 5.Baldwin, J. E., R. M. Adlington, J. S. Bryans, A. O. Bringhen, J. B. Coates, N. P. Crouch, M. D. Lloyd, C. J. Schofield, S. W. Elson, K. H. Baggaley, R. Cassels, and N. H. Nicholson. 1991. Isolation of dihydroclavaminic acid, an intermediate in the biosynthesis of clavulanic acid. Tetrahedron 47:4089-4100. [Google Scholar]
- 6.Baldwin, J. E., M. D. Lloyd, B. Wha-Son, C. J. Schofield, S. W. Elson, K. H. Baggaley, and N. H. Nicholson. 1993. A substrate analog study on clavaminic acid synthase: possible clues to the biosynthetic origin of proclavaminic acid. J. Chem. Soc. Chem. Commun. 500-502.
- 7.Baltz, R. H. 1980. Genetic recombination by protoplast fusion in Streptomyces. Dev. Indust. Microbiol. 21:43-54. [Google Scholar]
- 8.Brannigan, J. A., G. Dodson, H. Duggleby, P. C. E. Moody, J. L. Smith, D. R. Tomchick, and A. G. Murzin. 1995. A protein catalytic framework with an N-terminal nucleophile is capable of self-activation. Nature 378:416-419. [DOI] [PubMed] [Google Scholar]
- 9.Brown, A. G., D. Butterworth, M. Cole, G. Hanscombe, J. D. Hood, C. Reading, and G. N. Robinson. 1976. Naturally occurring beta-lactamase inhibitors with antibacterial activity. J. Antibiot. 29:668-669. [DOI] [PubMed] [Google Scholar]
- 10.Burton, K. 1957. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem. J. 62:315-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crabeel, M., D. Charlier, R. Cunin, and N. Glansdorff. 1979. Cloning and endonuclease restriction analysis of argF and of the control region of the argECBH bipolar operon in Escherichia coli. Gene 5:207-231. [DOI] [PubMed] [Google Scholar]
- 12.Cunin, R., N. Glansdorff, A. Pierard, and V. Stalon. 1986. Biosynthesis and metabolism of arginine in bacteria. Microbiol. Rev. 50:314-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denis, F., and R. Brzezinski. 1991. An improved aminoglycoside resistance gene cassette for use in gram-negative bacteria and Streptomyces. Federation Eur. Microbiol. Soc. Microbiol. Lett. 81:261-264. [DOI] [PubMed] [Google Scholar]
- 14.Egan, L. A., R. W. Busby, D. IwataReuyl, and C. A. Townsend. 1997. Probable role of clavaminic acid as the terminal intermediate in the common pathway to clavulanic acid and the antipodal clavam metabolites. J. Am. Chem. Soc. 119:2348-2355. [Google Scholar]
- 15.Elson, S. W., K. H. Baggaley, M. Davison, M. Fulston, N. H. Nicholson, G. D. Risbridger, and J. W. Tyler. 1993. The identification of three new biosynthetic intermediates and one further biosynthetic enzyme in the clavulanic acid pathway. J. Chem. Soc. Chem. Commun. 1993:1212-1214. [Google Scholar]
- 16.Gust, B., G. L. Challis, K. Fowler, T. Kieser, and K. F. Chater. 2003. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc. Natl. Acad. Sci. USA 100:1541-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgens, C. E., and R. E. Kastner. 1971. Streptomyces clavuligerus sp. nov., a β-lactam antibiotic producer. Int. J. Syst. Bacteriol. 21:326-331. [Google Scholar]
- 18.Hodgson, J. E., A. P. Fosberry, N. S. Rawlinson, H. N. M. Ross, R. J. Neal, J. C. Arnell, A. J. Earl, and E. J. Lawlor. 1995. Clavulanic acid biosynthesis in Streptomyces clavuligerus: gene cloning and characterization. Gene 166:49-55. [DOI] [PubMed] [Google Scholar]
- 19.Jensen, S. E., D. C. Alexander, A. S. Paradkar, and K. A. Aidoo. 1993. Extending the β-lactam biosynthetic gene cluster in Streptomyces clavuligerus, p. 169-176. In R. H. Baltz, G. D. Hegeman, and P. L. Skatrud (ed.), Industrial microorganisms: basic and applied molecular genetics. American Society for Microbiology, Washington, D.C.
- 20.Jensen, S. E., K. J. Elder, K. A. Aidoo, and A. S. Paradkar. 2000. Enzymes catalyzing the early steps of clavulanic acid biosynthesis are encoded by two sets of paralogous genes in Streptomyces clavuligerus. Antimicrob. Agents Chemother. 44:720-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen, S. E., and A. S. Paradkar. 1999. Biosynthesis and molecular genetics of clavulanic acid. Antonie Leeuwenhoek 75:125-133. [DOI] [PubMed] [Google Scholar]
- 22.Jensen, S. E., D. W. Westlake, and S. Wolfe. 1982. Cyclization of δ-(l-α-aminoadipyl)-l-cysteinyl-d-valine to penicillins by cell-free extracts of Streptomyces clavuligerus. J. Antibiot. 35:483-490. [DOI] [PubMed] [Google Scholar]
- 22a.Jensen, S. E., A. Wong, A. Griffin, and B. Barton. 2004. Streptomyces clavuligerus has a second copy of the gene encoding proclavaminate amidinohydrolase. Antimicrob. Agents Chemother. 48:192-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kershaw, N. J., H. J. McNaughton, K. S. Hewitson, H. Hernandez, J. Griffin, C. Hughes, P. Greaves, B. Barton, C. V. Robinson, and C. J. Schofield. 2002. ORF6 from the clavulanic acid gene cluster of Streptomyces clavuligerus has ornithine acetyltransferase activity. Eur. J. Biochem. 269:2052-2059. [DOI] [PubMed] [Google Scholar]
- 24.Khaleeli, N., R. F. Li, and C. A. Townsend. 1999. Origin of the β-lactam carbons in clavulanic acid from an unusual thiamine pyrophosphate-mediated reaction. J. Am. Chem. Soc. 121:9223-9224. [Google Scholar]
- 25.Kieser, T., M. J. Bibb, M. J. Buttner, K. F. Chater, and D. A. Hopwood. 2000. Practical Streptomyces genetics. John Innes Foundation, Norwich, England.
- 26.Li, R., N. Khaleeli, and C. A. Townsend. 2000. Expansion of the clavulanic acid gene cluster: identification and in vivo functional analysis of three new genes required for biosynthesis of clavulanic acid by Streptomyces clavuligerus. J. Bacteriol. 182:4087-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marsh, E. N., M. D. Chang, and C. A. Townsend. 1992. Two isozymes of clavaminate synthase central to clavulanic acid formation: cloning and sequencing of both genes from Streptomyces clavuligerus. Biochemistry 31:12648-12657. [DOI] [PubMed] [Google Scholar]
- 28.McNaughton, H. J., J. E. Thirkettle, Z. H. Zhang, C. J. Schofield, S. E. Jensen, B. Barton, and P. Greaves. 1998. β-Lactam synthetase: implications for β-lactamase evolution. Chem. Commun. 1998:2325-2326. [Google Scholar]
- 29.Mellado, E., L. M. Lorenzana, M. Rodriguez-Saiz, B. Diez, P. Liras, and J. L. Barredo. 2002. The clavulanic acid biosynthetic cluster of Streptomyces clavuligerus: genetic organization of the region upstream of the car gene. Microbiology 149:1427-1438. [DOI] [PubMed] [Google Scholar]
- 30.Miller, M. T., B. O. Bachmann, C. A. Townsend, and A. C. Rosenzweig. 2002. The catalytic cycle of β-lactam synthetase observed by x-ray crystallographic snapshots. Proc. Natl. Acad. Sci. USA 99:14752-14757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller, M. T., B. O. Bachmann, C. A. Townsend, and A. C. Rosenzweig. 2001. Structure of β-lactam synthetase reveals how to synthesize antibiotics instead of asparagine. Nat. Struct. Biol. 8:684-689. [DOI] [PubMed] [Google Scholar]
- 32.Mosher, R. H., A. S. Paradkar, C. Anders, B. Barton, and S. E. Jensen. 1999. Genes specific for the biosynthesis of clavam metabolites antipodal to clavulanic acid are clustered with the gene for clavaminate synthase 1 in Streptomyces clavuligerus. Antimicrob. Agents Chemother. 43:1215-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicholson, N. H., K. H. Baggaley, R. Cassels, M. Davison, S. W. Elson, M. Fulston, J. W. Tyler, and S. R. Woroniecki. 1994. Evidence that the immediate biosynthetic precursor of clavulanic acid is its N-aldehyde analog. J. Chem. Soc. Chem. Commun. 1994:1281-1282. [Google Scholar]
- 34.Paradkar, A. S., and S. E. Jensen. 1995. Functional analysis of the gene encoding the clavaminate synthase 2 isoenzyme involved in clavulanic acid biosynthesis in Streptomyces clavuligerus. J. Bacteriol. 177:1307-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez-Redondo, R., A. Rodriguez-Garcia, J. F. Martin, and P. Liras. 1999. Deletion of the pyc gene blocks clavulanic acid biosynthesis except in glycerol-containing medium: evidence for two different genes in formation of the C3 unit. J. Bacteriol. 181:6922-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pruess, D. L., and M. Kellett. 1983. Ro-22-5417, a new clavam antibiotic from Streptomyces clavuligerus. I. Discovery and biological activity. J. Antibiot. 36:208-212. [DOI] [PubMed] [Google Scholar]
- 37.Reading, C., and M. Cole. 1977. Clavulanic acid: a beta-lactamase-inhibiting beta-lactam from Streptomyces clavuligerus. Antimicrob. Agents Chemother. 11:852-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez-Garcia, A., A. de La Fuente, R. Perez-Redondo, J. F. Martin, and P. Liras. 2000. Characterization and expression of the arginine biosynthesis gene cluster of Streptomyces clavuligerus. J. Mol. Microbiol. Biotechnol. 2:543-550. [PubMed] [Google Scholar]
- 39.Rodriguez-Garcia, A., M. Ludovice, J. F. Martin, and P. Liras. 1997. Arginine boxes and the argR gene in Streptomyces clavuligerus: evidence for a clear regulation of the arginine pathway. Mol. Microbiol. 25:219-228. [DOI] [PubMed] [Google Scholar]
- 40.Salowe, S. P., W. J. Krol, D. Iwata-Reuyl, and C. A. Townsend. 1991. Elucidation of the order of oxidations and identification of an intermediate in the multistep clavaminate synthase reaction. Biochemistry 30:2281-2292. [DOI] [PubMed] [Google Scholar]
- 41.Salowe, S. P., E. N. Marsh, and C. A. Townsend. 1990. Purification and characterization of clavaminate synthase from Streptomyces clavuligerus: an unusual oxidative enzyme in natural product biosynthesis. Biochemistry 29:6499-6508. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 43.Stuttard, C. 1982. Temperate phages of Streptomyces venezuelae:lysogeny and host specificity shown by phages SV1 and SV2. J. Gen. Microbiol. 128:115-121. [Google Scholar]
- 44.Vieira, J., and J. Messing. 1987. Production of single stranded plasmid DNA. Methods Enzymol. 153:3-11. [DOI] [PubMed] [Google Scholar]
- 45.Ward, J. M., and J. E. Hodgson. 1993. The biosynthetic genes for clavulanic acid and cephamycin production occur as a ‘super-cluster’ in three Streptomyces. Federation Eur. Microbiol. Soc. Microbiol. Lett. 110:239-242. [DOI] [PubMed] [Google Scholar]