Abstract
Some 400 million people worldwide are currently infected with the hepatitis B virus (HBV), and the infection is common in the Middle East. Another 170 million people around the globe presently live with chronic hepatitis C virus (HCV) infection. Both HBV and HCV represent a worldwide epidemic. Despite significant decline in the prevalence of HBV and HCV infection in Saudi Arabia, these viral diseases cause significant morbidity and mortality, and impose a great burden on the country's healthcare system. On the other hand, Saudi epidemiology studies have shown that the hepatitis A virus seroprevalence in the country has reduced considerably over the past two decades. The progress in mapping the epidemiological pattern of viral hepatitis in Saudi Arabia has not only aided our understanding of the disease, but has also exposed the small but relevant gaps in our identification of the intricate details concerning the disease's clinical expression. In this review, we aim to document the timeline of viral hepatitis epidemiology in Saudi Arabia, while summarizing the relevant published literature on the subject.
Keywords: Epidemiology, incidence, hepatitis B, hepatitis C, prevalence
An estimated 400 million people worldwide are currently infected with the hepatitis B virus (HBV), and approximately 500,000 to 700,000 die annually from this disease.[1,2] Infection early in life bestows a substantial risk of developing chronic disease later on. Hepatitis B virus infection is most commonly found in South-East Asia, the Middle and Far East, Southern Europe, and Africa. Another 130 to 170 million people around the globe presently live with chronic hepatitis C virus (HCV) infection.[1] Although HCV infection represents a worldwide epidemic, higher prevalence rates are seen in Africa, Latin America, and Central and South-East Asia.[3–6] An estimated 1.4 million new cases of hepatitis A virus (HAV) infection emerge globally each year according to the World Health Organization (WHO).[1] HAV infection is usually asymptomatic in young children, whereas symptomatic disease occurs more commonly among adults.
Despite significant decline in the prevalence of HBV and HCV infection in Saudi Arabia (discussed later), these viral diseases cause significant morbidity and mortality, and impose a great burden on the country's healthcare system. In 2007, the Saudi Ministry of Health (MOH) ranked viral hepatitis as the second most common viral disease after chickenpox, with almost 9000 new cases diagnosed in that year (52% HBV, 32% HCV, and 16% HAV).[7] Progress in mapping the epidemiological pattern of viral hepatitis in Saudi Arabia has accelerated dramatically over the last two decades. While this progress has aided our understanding of the disease, it has in the same vein exposed relevant gaps in our identification of the intricate details concerning disease clinical expression. For the past two decades our group, as well as others, have been involved in studying and documenting the epidemiology of viral hepatitis in Saudi Arabia. In this review, we attempt to document the timeline of viral hepatitis epidemiology in Saudi Arabia, while summarizing the relevant published literature on the subject, and estimating the probable disease burden based on available data.
HEPATITIS B
HBV was once considered hyper-endemic in the Kingdom of Saudi Arabia (KSA), where infection was acquired mainly through horizontal transmission early in life, and less commonly by vertical transmission similar to what is observed in other HBV-endemic countries.[8–16] From a historical perspective, the first large-scale community-based epidemiological study conducted on Saudi children showed a hepatitis B surface antigen (HBsAg) seroprevalence of approximately 7% and a > 70% prevalence of at least one HBV marker.[16]
The tale of vaccination
The outcome of the study above galvanized the Saudi health officials into action and triggered a successful collaboration between scientists and government agencies. To preclude transmission of HBV, prevent acute infection, and reduce the pool of a chronically infected population, a mass vaccination program against HBV was launched in 1989.[17] The Saudi government initiated a similar program in 1990 aimed at vaccinating all Saudi children at school entry. Mandatory vaccination of healthcare workers and hemodialysis patients were also introduced around this time. Government regulations stipulated that all children born after October 01, 1989 be vaccinated against HBV regardless of the mother's HBV status, thereby setting the stage for a preemptive strategy against HBV infection within the country. Part of this strategy involved integrating the HBV vaccine into the children's extended program of immunization (EPI). The vaccination schedule consisted of three pediatric doses of 10 μg Smith Kline and French (SKF) or 5 μg of Merck Sharp Dohme (MSD) recombinant HBV vaccine given at specified intervals (0-1-5 months) intramuscularly into the deltoid or thigh areas of infants. Vaccination details were recorded in the registries of the primary healthcare centers (PHCC) and on children's EPI cards. As a result of these programs, virtually all Saudi individuals aged 24 or younger as of October 2007 would potentially be vaccinated either at birth, or at school entry.
However, the main thrust of any strategy lies in its effective implementation, and its accomplishments must speak as the final arbiter. Toward this end, we have monitored the prevalence of HBV in Saudi children over the last two decades. The first post-vaccination follow-up study was performed on cohorts of children 2 years of age who had received the HBV vaccine at birth.[13] The second study was performed 8 years after starting the program on children aged 1-12 years who had been vaccinated either at birth or at school entry.[8] Both studies demonstrated a significant decline in the prevalence of HBsAg to negligible numbers, proving that the HBV vaccine was highly efficacious [Figure 1]. The third study published in 2008 verified long-term vaccine protection marked by complete absence of HBsAg or anti-hepatitis B core antigen (HBc) detection among a cohort of 1355 students of 10-12th grade randomly selected from high (Aseer), low (Al-Qaseem), and moderate (Madinah) HBV endemic regions [Figure 1].[18]
Figure 1.
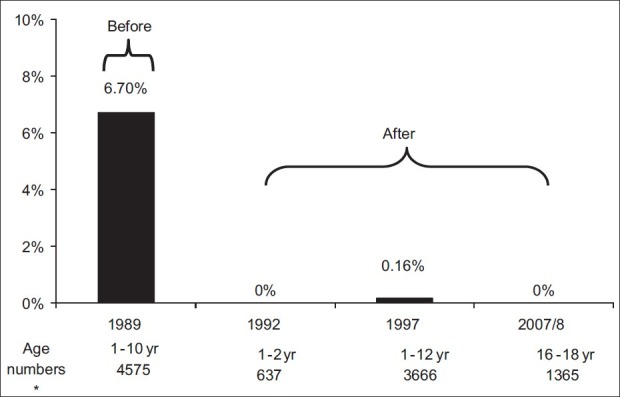
Prevalence of HBsAg among the Saudi population documented before and after introducing a nation-wide HBV vaccination program, over an 18-year period
Results from other non-affiliated independent studies support these findings. For example, among 74,662 individuals undergoing premarital screening between January and May of 2008, 1.31% subjects tested positive for HBV according to data gathered from the General Directorate for Communicable Diseases, MOH, in Riyadh.[19] Blood donor statistics suggest prevalence rates between 1.5% and 2.6% within the adult population.[20–22] Cross-community study data released in 2007 monitoring the influence of childhood HBV immunization revealed prevalence rates of 0.05% and 0.22% among children and adults, respectively. The average reported incidence was 0.15% with wide variations occurring between regions that ranged from 0.03% to 0.72%.[23,24]
Other epidemiological aspects
Epidemiological data in pregnant Saudi women indicate about 4% prevalence in this population overall. However, a recent study in five geographically distinct regions found virtually no HBsAg seroprevalence in pregnant women younger than 18 years of age.[15,18,25] Blood samples drawn from 755 pregnant Saudi women in a single-center study (June 2005 to June 2006) also indicated a low (1.6%) overall prevalence of seropositive HBsAg within this sub-population and a less than 5% prevalence of hepatitis B e-antigen (HBeAg) positivity.[26] Vertical transmission, therefore, seems to represent a low risk route of infection in neonates.
Gender represents an important risk factor for HBV infection within Saudi Arabia.[10] A retrospective analysis of patients in the Saudi Aramco Medical Services Organization (SAMSO) located in the Eastern Province of the KSA identified a 1.8 times greater likelihood of HBV infection in males vs. females. The same study did not report any significant gender biases in the case of HAV or HCV infections. In a report by Memish et al.,[27] incidence of HBV seropositivity stratified by gender in a separate report showed a higher prevalence of HBV infection in males vs. females of 123.0 vs. 85.5; (P < 0.001) per 100,000 served but not HCV (76.8 vs. 80.1 per 100,000; P = 0.110).[27]
In contrast to children, infection rates in adults have shown more regional variation due to non-uniformities in immunization and engagement in risky behaviors across different adult populations. Current prevalence of HBV markers among male Saudi blood donors was assessed in the Northwest region of Saudi Arabia in a study by El-Beltagy et al.[28] Of the 3192 subjects evaluated, 3.0% tested positive for HBsAg and 18.7% were positive for anti-HBc. The prevalence of HBV markers correlated inversely with socioeconomic status, in agreement with other assessments.[29] Significantly higher frequencies of HBV markers emerged for married subjects presumably due to advancing age, and among military subjects.[28] When comparing HBsAg seroprevalence rates in the northwest region to those in the other parts of the KSA, higher prevalence of HBsAg was found for both eastern (6.7%) and south-western (5.4%) regions, whereas the central city of Riyadh boasts the lowest prevalence rates (1.5%) of all.[29,30]
Despite the optimism surrounding the low HBV infection rates in the younger Saudi populations, the prevalence in older generations has not been well characterized, and remains a source of concern. A fair estimate of HBsAg prevalence among Saudis greater than 40 years of age falls in the 3-6% range based on the approximate 7% HBV prevalence rate initially documented from community-based studies in children in the late 80s. Much of the data generated on the prevalence of HBV has been in the context of blood donor studies, which despite their relative acceptability as reliable sources of epidemiological data, tend to underestimate seroprevalence since symptomatic individuals, or those with prior knowledge of infection, would selectively opt out from blood donation. Second, blood donors in the KSA are traditionally male, and hence the data derived from blood donor studies are likely to be skewed in favor of a male predominance, thus serving to under- or over-estimate disease prevalence. Actual prevalence can only be confirmed through a large community-based general population study centered on this age group, with an adequate sample size and geographical representation.
According to results from HBV genotyping studies, the majority of Saudi patients with chronic HBV infection carry genotype D and this subpopulation of patients demonstrates increased HBeAg negativity with advancing stages of liver disease. Most studies from Saudi Arabia indicate that the vast majority of Saudis are HBeAg negative by the age of 30.[31,32]
HEPATITIS C
Descriptions of HCV epidemiology in the KSA rely heavily upon HCV seroprevalence studies. These studies are typically cross-sectional in design and are done in select populations such as blood donors. Population-based studies are far more useful but are not feasible in most parts of the world, including KSA. Despite reported declines in HCV prevalence over the last 10 years, the disease constitutes a major public health problem in the country, especially among hemodialysis patients and intravenous drug users.[33,34] As yet, there is no large community-based study reporting on the actual prevalence of HCV in the KSA.
Prevalence in the general population
Prevalence assessed from Saudi blood donor screening centers indicates HCV infection rates of 0.4-1.1%.[21–23] Declines in HCV prevalence rates were also noted in the blood bank database of King Khalid University Hospital in Riyadh, from 0.58% in 1996 to 0.08% in 2006 (unpublished data; [Figure 2]). A summary report compiled by the WHO mentions 437,292 official reports of HCV infections among persons living in the KSA, giving an estimated prevalence of about 1.8%.[35]
Figure 2.
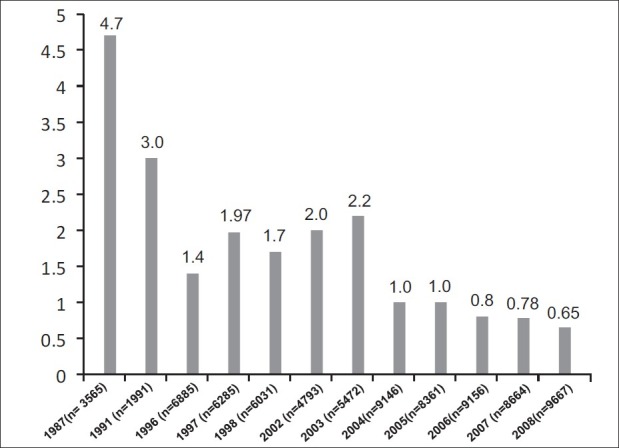
Changing patterns of hepatitis C prevalence in blood donors at the King Khalid University Hospital over 20 years
The prevalence of HCV in Saudi children formed the subject of a large cross-community study that involved a population-based survey of 4496 children from different regions of the country (aged 1-10). Of these children, 39 (0.90%) tested positive for HCV antibodies using a first-generation enzyme-linked immunosorbent assay (ELISA) kit, known to produce false positives.[36] During our HBV vaccination follow-up studies, we also tested children for HCV serology using a more reliable third-generation ELISA test coupled with a recombinant immunoblot assay (RIBA) for confirmation. The prevalence of HCV infection in Saudi children increased marginally from 0.04% in 1997 (aged 1-12 years) to 0.22% for adolescents aged 16-18 years in 2008 (unpublished results), reflecting worldwide increases in HCV prevalence accompanying aging populations [Table 1].[18] Similarly, Fakeeh et al.,[37] reported an increasing prevalence with age: 4.49% in children <15 years, 2.05% for those 15-21 years of age, 5.10% in 25-34-year olds, 8.64 in 35-44-year olds, 15.0% among those aged 45-54 years, and 11.9% in adults ≥55 years. However, the prevalence figures cited in this study are skewed in that the authors reported data from only one geographical region of the country and included Egyptian patients who are known to have a high rate of seropositivity. In addition, the study sample was formed from randomly selected hospital patients and, therefore, likely consists of ‘non-healthy subjects’. These factors would account for the alarmingly high rate of seropositivity observed among both children and adults. The number of cases of HCV infections reported to the Saudi MOH from 1994 to 2005 was proportionally much higher in adults (0.20%) compared with younger patients less than 15 years old (0.01%) despite similar mean population sizes.[23] Substantial differences between adult and pediatric populations were also identified by Memish et al.,[27] who reported an almost 45-fold higher annual incidence of HCV seropositivity in those ≥15 years vs. children <15 years of age.
Table 1.
Prevalence of HCV in children in three regions of Saudi Arabia at three points of time over a period of 18 years

Comparatively, the Saudi Arabian epidemiology literature contains more robust reporting of HCV incidence rather than overall prevalence rates. Nonetheless, it must be recognized that acute disease reporting systems can underestimate the incidence of HCV infection, even in those countries with well-established surveillance systems. A recent publication documenting numbers of adult HCV infections stratified by region in the KSA during 11 years of surveillance (1994-2005) showed variation in incidence across the regions and an overall average of 124 per 100,000 people.[23] The passive reporting nature of this investigation limits its generalization to the entire Saudi population. In addition, these data are likely to include duplicates and representation from many non-Saudi individuals since the patient identifiers used in the data collection are not very accurate. A more comprehensive study describing incidence trends of viral hepatitis seropositivity among the population served by the National Guard Health Affairs (NGHA) hospitals in the central, eastern, and western regions of the country was recently published.[27] Between 2000 and 2007, a total of 14,224 seropositive cases of viral hepatitis were reported to this surveillance system. The average annual incidence of seropositivity per 100,000 served population was highest for HBV (104.6), followed by HCV (78.4), and lowest for HAV (13.6). Saudis had higher HBV and HAV incidence, but lower HCV incidence compared with non-Saudis. Viral hepatitis incidence declined steadily by 20-30% for all three types during the entire 8 years of surveillance.
Government monitoring of HCV incidence among 13 Saudi Arabian administrative provinces from 1995 to 2006 showed considerable range in the number of cases reported to the MOH per region, with the highest prevalence expressed per capita occurring in Al-Baha and Jeddah (0.32%), and lowest in Jizan (0.016%).[23] Earlier studies in the KSA mirror these results. For instance, blood screening results from 528 blood donors in the Jeddah region reported a 1.7% prevalence of HCV infection, whereas another study of 557,815 Saudi residents in the Riyadh province found a 1.1% anti-HCV prevalence in adults.[33,38]
Reduction in transmissions acquired through transfusion-related means, as a consequence of more stringent stipulation for HCV testing implemented nationwide two decades ago, provides one explanation for the disparity in prevalence rates seen between adults and children. Additionally, general declines in both incidence and prevalence of HCV in the country may be attributed to safer blood transfusional practices; surgical, dental, and procedural practices; overall improvement in sanitation and a better standard of life; and screening of all expatriate populations entering the country.
Major studies conducted over a single decade from 1995 to 2004 identified genotype-4, followed by genotypes 1a and 1b, as the most prevalent genotypes in the country. Other genotypes, including 2a/2b, emerged from the Eastern Province and genotype-5 from the Western Province, whereas genotypes 3 and 6 remain extremely rare.[37,39–43]
Prevalence in special populations
In addition to the prevalence of hepatitis C in the general population, this infection represents a major health problem in a number of other special populations. For instance, hepatitis C represents the most common cause of liver disease in patients on dialysis. Those on maintenance hemodialysis (HD) are particularly at risk of contracting HCV due to impaired immunity prevailing in this population and a need for multiple blood transfusions. In the KSA, hemodialysis remains the most widely used form of renal replacement therapy and, furthermore, the number of patients in the country receiving HD treatment has been exponentially increasing from about 2085 patients in 1990 to more than 8000 patients in 2006 among 140 dialysis units.[34,44,45] Therefore, it is not surprising that the incidence and prevalence of HCV have shown an increase in the hemodialysis population over the past three decades.[34] During the 1990s, HCV prevalence in the country varied among HD centers, ranging from 15% to 90%.[34] In addition, the country witnessed a surge in HCV endemicity in the mid-1990s from 41% to 55% that paralleled the sudden expansion of HD services, including bringing in to use new HD machines and additional HD centers.[34] This change was in response to a significant increase in the number of patients with end-stage renal disease across the different regions of KSA.[45] The HCV prevalence rate has remained steady at 50% in recent years despite mounting demands for dialysis services, which may be attributed to better adherence to infection control policies and practices.[34] In fact, a recent single-center study that followed 36 seronegative hemodialysis patients from 2004 to 2008 treated under strict infection control guidelines reported a zero incidence of infection for the entire 5 years.[46] Another investigational study followed the epidemiology of HCV in the dialysis unit after initiating methods to reduce prevalence of the virus.[47] Adopted practices included strict adherence to universal infection control precautions, separation of HCV-positive patients from the negative ones, and using specially designated machines for the HD patients. The unit initiated further periodic testing for HCV RNA from July 2003. There have been no documented cases of sero-conversion from HCV-negative to HCV-positive since implementing these control measures in December 2005, and the overall prevalence of HCV RNA-positive patients has reduced to 6.5% at this particular unit.
A study in 1995 by Abu-Aisha et al.[48] investigating the role of dialysis machines in spreading disease concluded that HD machines were the most likely source of transmission of HCV infection, and therefore it was important to assign specific HD machines for anti-HCV-positive cases. Soyannwo et al.,[49] also determined that machine isolation policies rather than blood transfusions lead to wide-spread variation in HCV prevalence among different dialysis centers in Saudi Arabia.[49] Patient isolation represents a secondary important factor in controlling transmission of viral hepatitis in HD units.[50–52] For example, in one single-center study comparing HCV infection rates in the 4 years before and after establishing a specially designed center with complete isolation of HCV-positive from HCV-negative patients, the annual incidence of HCV infection reduced significantly from 2.4% to 0.2%.[52]
In addition to HD patients, intravenous drug users also represent a high HCV risk group in the KSA and the number of addicts is on the rise, as is the case worldwide.[23,41] The types of substances abused in the country include injectable drugs like heroin and cocaine, and non-injectable drugs such as cannabis and amphetamine-type stimulants. In 2000, an estimated 0.01% of the Saudi population >15 years of age used heroin and the annual admission to detoxification centers in Riyadh, Jeddah, Dammam, and Qassim ranged from 4740 to 6650 persons between 1996 and 2001.[23,41] Recent examination of viral infection among 344 Saudi injecting drug users reported a 38% HCV RNA detection rate.[53] The predominant HCV genotype was found to be genotype-1b. The incidence of HCV co-infections with HBV and/or TTV ranged from 2.6% to 24% in this study. An earlier study that evaluated socio-demographic characteristics of substance abuse in Saudi Arabia showed that 87% of addicts in a Jeddah detoxification center used alcohol or heroin and 91% of the heroin users injected the drug. The HCV infection prevalence among these patients was 69%.[54]
HEPATITIS A
Figures released from Saudi epidemiology studies consistently identify HAV as the most prevalent form of hepatitis among the three most common viral types. The anti-HAV prevalence rate in the KSA was recently estimated at 18.6%, a considerable reduction from the 90-100% rates reported only two decades ago in the adult population [Figure 3].[55–57] The rate of HAV infection among children aged 1-12 years has also reduced dramatically from 52% in 1989 to 25% in 1997 according to community-based studies.[58,59] A comparative study that monitored the prevalence of HAV infection in a single cohort of children from 1989 to 2008 and spread over three regions of Saudi Arabia found a significant linear decline from 53% in 1989 to 25% in 1997, and finally to 18.6% in 2008.[55] Likewise, data from a school-based seroprevalence study of 2399 Saudi children attending the National Guard schools in 2005 showed 28.9% seropositivity for anti-HAV-IgG among 4-18-year olds.[60]
Figure 3.
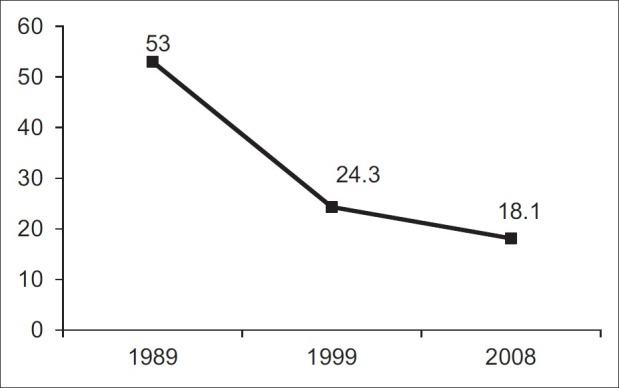
Changing patterns of hepatitis A prevalence within the Saudi population over 18 years
Data regarding the incidence of HAV in Saudi Arabia have also emerged. An evaluation of 14,224 cases of viral hepatitis documented between 2000 and 2007 at King Abdulaziz Medical City-NGHA in Riyadh demonstrated a significantly lower annual incidence of seropositivity for HAV (13.6) compared with HCV (78.4) or HBV (104.6) per 100,000 served population.[27] Moreover, the incidence of HAV seropositive cases among children followed a clear seasonal cyclical pattern, with peaks in March and September, likely as a result of more children being exposed during the mid-year and summer vacations.[27] Similar to HBV, gender is also an important risk factor for HAV infection in Saudi Arabia.[10] The study by Memish et al., found a higher prevalence of HAV infection in males vs. females HAV (14.7 vs. 12.4; P = 0.008) per 100,000 served compared with HCV. Other reports demonstrated either no gender biases in anti-HAV prevalence or higher positivity in Saudi males.[59,61] Culinary habits and preferences coupled with social practices that differ among Saudi males and females may explain the higher incidence of HAV infections seen in Saudi males.[55]
The prevalence of HAV differs according to the socioeconomic status of a population and may vary within a country depending on hygienic conditions.[62,63] Studies conducted since 2008 have revealed continued variations in anti-HAV-IgG prevalence among different regions of Saudi Arabia despite marked decreases in overall infection rates. Monitoring of patterns of HAV endemicity among children and adolescents in Saudi Arabia over 20 years has shown persistent and significant differences in HAV seroprevalences between three regions when classified according to socioeconomic status. In 2008, the prevalence of HAV stratified by class showed a 36.8% seropositivity among the lowest class compared with 9.6-16.6% and 5.9% for middle- and high-class adolescents aged 16-18 years, respectively.[55] A cross-sectional, sero-epidemiological investigation of HAV among children aged 1-6 years in the province of Northern borders between 2006 and 2007 revealed a 33.8% HAV-IgG prevalence overall.[64] The most significant independent predictors of infection included rural residence, non-Saudi nationality, and availability of safe municipal water. The nationality factor suggests a more rapid decline in HAV endemicity in the local population, which has a higher per capita income compared to other Middle Eastern countries.
Changes in the epidemiology of HAV infection are likely to be largely due to improvements in living conditions leading to fewer infected children.[10] During the period 1974-2003, the Saudi government's real-estate bank funded the building of 851,000 housing units bringing many Bedouins and rural citizens into urban areas with better sewage disposal and water sanitation systems.[55,65] The country also witnessed increases in GDP per capita from 1145 USD in 1970 to 10,853 USD in 2002. Mounting life expectancies from birth that climbed 17 years between 1970 and 2000 (53.9-70.9 years of age) further demonstrates improved socioeconomics within the country's borders.[55,65] The average age of HAV infection in the KSA has been increasing with time mainly due to these improvements in living conditions, consequently leading to more cases of symptomatic adults being diagnosed. According to recent data from a surveillance system monitoring trends in viral hepatitis incidence among NGHA-served regions from 2000 to 2007, HAV seropositivity rose by 61% during this period for those 15 years or older whereas rates in the <15 year-old cohort declined by 42%.[27]
The shift in the HAV epidemiologic status of KSA from a classic high to a moderate/low endemic country prompted the country's MOH to add the HAV vaccine to the list of infant/childhood immunizations in 2008.[60,66] Long-term follow-up of this strategy among different communities remains an important goal toward understanding the impact of HAV immunization in the KSA.
An estimation of the overall disease burden
According to data compiled in 2011 by the Central Department for Statistics and Information in Saudi Arabia ( www.cdsi.gov.sa), the number of Saudis is just below 20 million. Taking into consideration the information presented in this review, and classifying 60% of the Saudi population as being under 25 years of age, we can estimate that at least 60% of the Saudi population has been vaccinated for HBV either at birth or at school entry. As such, this segment of the population would have a negligible prevalence of infection (less than 0.1%). The remaining 40% (about 8 million) of the population may show varying trends in HBV prevalence depending on age and geographical area of birth and residence. Taking into account data from studies conducted on children in the late 80s (who now represent part of the adult population) and from blood donor screening studies, we can approximate HBV prevalence in the 3-6% range for older populations. This is commensurate with an overall HBV prevalence of about 2-4% in the general population (both younger vaccinated and older not vaccinated groups). Therefore, although the cycle of horizontal and childhood transmission of HBV has probably been broken and has significantly compromised the HBV epidemiological sequence, we estimate that we are still confronted with an estimated number of 240,000 Saudis who are chronically infected with the virus, many of whom may still be undiagnosed.
Although most studies indicate that the risk of developing significant liver disease in individuals infected with HBV lies somewhere between 20% and 40%, we must consider the possibility of a somewhat different natural history of HBV manifesting in the KSA. Disease progression unique to Saudi Arabia may arise due to a different average age for onset of spontaneous viral clearance among infected persons in this region. Several studies from Italy, where genotype-D also dominates, indicate that HBsAg-positive patients sero-convert mostly before the age of 20 years in contrast to Chinese patients, who convert mostly by the age of 40 years.[67–70] Furthermore, Chen et al.,[71] have demonstrated in a recent longitudinal study that patients with HBeAg sero-conversion before 30 years of age have a very low cumulative risk of developing cirrhosis and HCC. Our baseline study in 1991[13] and studies in young Saudi populations like pregnant women have shown that the majority of HBeAg-positive patients sero-convert before the age of 20 years. In addition, unpublished data from an HBV database, which has enrolled more than 1000 patients so far from three centers in Saudi Arabia, indicate that the majority (more than 70%) of patients have HBV DNA levels less than 10,000 IU/ml. Given that it is now well understood that HBV DNA level is strongly predictive of the risk of future cirrhosis and HCC,[72] it is likely that progression rates in Saudi Arabia will be less than what has been described in regions such as South-East Asia.
Unlike HBV, the prevalence of HCV in Saudi Arabia is much more difficult to quantify due to a veritable lack of recent literature. Given the available data discussed in this review, one can reasonably estimate an overall prevalence of HCV in the Saudi population to be 1-3%, but given that the majority of the population are in the younger age group (with a lower prevalence rate), an overall general prevalence of about 1% maybe more realistic, giving us an estimated number of patients of about 200,000, many of whom may still be undiagnosed.
Recognizing the limitations of the reported studies and the speculative nature of these assumptions, we can reasonably estimate that 20% of all chronic viral hepatitis patients will eventually develop cirrhosis in the next 20 years (240,000 hepatitis B patients and 200,000 hepatitis C patients), leading the Saudi healthcare system to confront an imposing burden of about 88,000 cirrhotic patients facing advanced and costly medical care. Given an estimated 10% annual risk of de-compensation (without treatment), we can further anticipate approximately 8800 decompensated cirrhotic patients per year becoming potential candidates for liver transplantation. Moreover, the likelihood of developing hepatocellular carcinoma (HCC) at an estimated 1-4% annual incidence in cirrhotic patients translates to around 1500 new cases of HCC per year. These patients need a multidisciplinary approach to care, intensive interventions, and costly treatments, which further inflates the burden on the kingdom's healthcare system.
It must be strenuously emphasized that this disease burden estimation does not take into account cirrhosis secondary to non-viral etiology that would likely impose a further strain related to liver disease alone on the healthcare system. Nonetheless, viral hepatitis remains the only transmissible but preventable cause of cirrhosis and HCC in Saudi Arabia. Collection and documentation of accurate epidemiological data therefore represent a critical step in the prevention and control of viral hepatitis in the KSA.
CONCLUSION
Saudi Arabia has witnessed steady prevalence declines for all three common hepatotropic viruses during the past 30 years. This may be attributed to better living conditions, childhood immunization against HBV, universal blood bank screening, and increased awareness of safe clinical and social practices. Clear distinctions in prevalence rates persist among different regions of the country particularly for HAV and HCV, and between genders in the case of HAV and HBV. The sub-population of injecting drug users is expected to grow and eventually become the highest risk group for either HBV or HCV infection. Despite the decline in prevalence, chronic liver disease will remain a huge burden to the Saudi healthcare system for years to come, with further challenges expected as a large number of undiagnosed cases begin to surface at an advanced stage of the disease. The proportion of patients with liver cirrhosis developing complications and requiring advanced care will rise in the next 20-40 years.[73] An initiative that includes early detection, proper timely assessment, and effective treatment represents an important goal. Realizing this goal will require a more coordinated national effort that includes increasing availability of liver transplantations to patients with end-stage liver disease.
Since direct measurement of HCV and HBV incidence is impractical, researchers in the KSA must rely upon mathematical models to infer trends in incidence. However, such undertakings can only occur in instances where population-based age-specific seroprevalence data are available, since these models rely on the assumption that current prevalence reflects the cumulative risk of acquiring infection. The remaining gaps in the viral hepatitis epidemiology story of the KSA rests principally on the performance of large epidemiological, community-based studies in HCV, and a similar study on HBV prevalence in the non-vaccinated older populations, coupled with an in-depth evaluation of the risk factors associated with both HBV and HCV acquisition in the current era.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sixty-Second World Health Assembly A62 / 22 Provisional Agenda Item. 2009. World Health Organization (WHO). Viral hepatitis: Report by the Secretariat. [Google Scholar]
- 2.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–38. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO). Initiative for Vaccine Research. 2010 [Google Scholar]
- 4.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–67. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 5.Xia GL, Liu CB, Cao HL, Bi SL, Zhan MY. Prevalence of hepatitis B and C virus infections in the general Chinese population.Results from a nationwide cross-sectional seroepidemiologic study of hepatitis A, B, C, D, and E virus infections in China, 1992. Int Hepatol Commun. 1996;5:62–73. [Google Scholar]
- 6.Frank C, Mohamed MK, Strickland GT, Lavanchy D, Arthur RR, Magder LS, et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355:887–91. doi: 10.1016/s0140-6736(99)06527-7. [DOI] [PubMed] [Google Scholar]
- 7.Saudi Arabia: Saudi Arabia Ministry of Health; Ministry of Health of Saudi Arabia (MOH). A review of health situation. The Annual Health Statistics Book. [Google Scholar]
- 8.Al-Faleh FZ, Al-Jeffri M, Ramia S, Al-Rashed R, Arif M, Rezeig M, et al. Seroepidemiology of hepatitis B virus infection in Saudi children 8 years after a mass hepatitis B vaccination programme. J Infect. 1999;38:167–70. doi: 10.1016/s0163-4453(99)90245-1. [DOI] [PubMed] [Google Scholar]
- 9.Sorrell MF, Belongia EA, Costa J, Gareen IF, Grem JL, Inadomi JM, et al. National institutes of health consensus development conference statement: Management of hepatitis B. Hepatology. 2009;49(5 Suppl):S4–12. doi: 10.1002/hep.22946. [DOI] [PubMed] [Google Scholar]
- 10.Al-Tawfiq JA, Anani A. Profile of viral hepatitis A, B, and C in a Saudi Arabian hospital. Med Sci Monit. 2008;14:CR52–6. [PubMed] [Google Scholar]
- 11.Arya SC, Ashraf SJ, Parande CM, el-Sayed M, Sahay R, Ageel AR, et al. Hepatitis B virus in Gizan, Saudi Arabia. J Med Virol. 1985;17:267–74. doi: 10.1002/jmv.1890170308. [DOI] [PubMed] [Google Scholar]
- 12.Parande CM, Arya SC, Ashraf SJ. Hepatitis B virus among Saudi children in Gizan, Saudi Arabia. Infection. 1986;14:223–5. doi: 10.1007/BF01644267. [DOI] [PubMed] [Google Scholar]
- 13.Al-Faleh FZ, Ayoola EA, Arif M, Ramia S, Al-Rashed R, Al-Jeffry M, et al. Seroepidemiology of hepatitis B virus infection in Saudi Arabian children: A baseline survey for mass vaccination against hepatitis B. J Infect. 1992;24:197–206. doi: 10.1016/0163-4453(92)93006-c. [DOI] [PubMed] [Google Scholar]
- 14.Shobokshi OA, Serebour FE. The aetiology of acute viral hepatitis in the western region of Saudi Arabia. Trans R Soc Trop Med Hyg. 1987;81:219–21. doi: 10.1016/0035-9203(87)90220-3. [DOI] [PubMed] [Google Scholar]
- 15.Al-Faleh FZ. Changing pattern of hepatitis viral infection in Saudi Arabia in the last two decades. Ann Saudi Med. 2003;23:367–71. doi: 10.5144/0256-4947.2003.367. [DOI] [PubMed] [Google Scholar]
- 16.Al-Faleh F. Hepatitis B infection in Saudi Arabia. Ann Saudi Med. 1988;8:474–80. [Google Scholar]
- 17.Al-Faleh FZ, Ayoola EA, Al-Jeffry M, Arif M, Al-Rashed RS, Ramia S. Integration of hepatitis B vaccine into the expanded program on immunization: The Saudi Arabian experience. Ann Saudi Med. 1993;13:231–6. doi: 10.5144/0256-4947.1993.231. [DOI] [PubMed] [Google Scholar]
- 18.Alfaleh F, Alshehri S, Alansari S, Aljeffri M, Almazrou Y, Shaffi A, et al. Long-term protection of hepatitis B vaccine 18 years after vaccination. J Infect. 2008;57:404–9. doi: 10.1016/j.jinf.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Alswaidi FM, O’brien SJ. Is there a need to include HIV, HBV and HCV viruses in the Saudi premarital screening program on the basis of their prevalence and transmission risk factors? J Epidemiol Community Health. 2010;64:989–97. doi: 10.1136/jech.2009.093302. [DOI] [PubMed] [Google Scholar]
- 20.Bashawri LA, Fawaz NA, Ahmad MS, Qadi AA, Almawi WY. Prevalence of seromarkers of HBV and HCV among blood donors in eastern Saudi Arabia, 1998-2001. Clin Lab Haematol. 2004;26:225–8. doi: 10.1111/j.1365-2257.2004.00601.x. [DOI] [PubMed] [Google Scholar]
- 21.Mehdi SR, Pophali A, Al-Abdul Rahim KA. Prevalence of hepatitis B and C and blood donors. Saudi Med J. 2000;21:942–4. [PubMed] [Google Scholar]
- 22.El-Hazmi MM. Prevalence of HBV, HCV, HIV-1, 2 and HTLV-I/II infections among blood donors in a teaching hospital in the Central region of Saudi Arabia. Saudi Med J. 2004;25:26–33. [PubMed] [Google Scholar]
- 23.Madani TA. Hepatitis C virus infections reported in Saudi Arabia over 11 years of surveillance. Ann Saudi Med. 2007;27:191–4. doi: 10.5144/0256-4947.2007.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madani TA. Trend in incidence of hepatitis B virus infection during a decade of universal childhood hepatitis B vaccination in Saudi Arabia. Trans R Soc Trop Med Hyg. 2007;101:278–83. doi: 10.1016/j.trstmh.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Al-Mazrou YY, Al-Jeffri M, Khalil MK, Al-Ghamdi YS, Mishkhas A, Bakhsh M, et al. Screening of pregnant Saudi women for hepatitis B surface antigen. Ann Saudi Med. 2004;24:265–9. doi: 10.5144/0256-4947.2004.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alrowaily MA, Abolfotouh MA, Ferwanah MS. Hepatitis B virus sero-prevalence among pregnant females in Saudi Arabia. Saudi J Gastroenterol. 2008;14:70–2. doi: 10.4103/1319-3767.39621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Memish ZA, Knawy BA, El-Saed A. Incidence trends of viral hepatitis A, B, and C seropositivity over eight years of surveillance in Saudi Arabia. Int J Infect Dis. 2010;14:115–20. doi: 10.1016/j.ijid.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 28.El Beltagy KE, Al Balawi IA, Almuneef M, Memish ZA. Prevalence of hepatitis B virus markers among blood donors in a tertiary hospital in Tabuk, northwestern Saudi Arabia. Int J Infect Dis. 2008;12:495–9. doi: 10.1016/j.ijid.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Ayoola AE, Tobaigy MS, Gadour MO, Ahmad BS, Hamza MK, Ageel AM. The decline of hepatitis B viral infection in South-Western Saudi Arabia. Saudi Med J. 2003;24:991–5. [PubMed] [Google Scholar]
- 30.Fathalla SE, Al-Jama AA, Al-Sheikh IH, Islam SI. Seroprevalence of hepatitis A virus markers in Eastern Saudi Arabia. Saudi Med J. 2000;21:945–9. [PubMed] [Google Scholar]
- 31.Hajeer AH, Al Knawy B, Alhaj-Hussein BT, Al-Rubiaan SD. Hepatitis B virus: A study of genotypes in an infected Saudi cohort. Br J Biomed Sci. 2007;64:93–4. doi: 10.1080/09674845.2007.11978099. [DOI] [PubMed] [Google Scholar]
- 32.Abdo AA, Al-Jarallah BM, Sanai FM, Hersi AS, Al-Swat K, Azzam NA, et al. Hepatitis B genotypes: Relation to clinical outcome in patients with chronic hepatitis B in Saudi Arabia. World J Gastroenterol. 2006;12:7019–24. doi: 10.3748/wjg.v12.i43.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shobokshi OA, Serebour FE, Al-Drees AZ, Mitwalli AH, Qahtani A, Skakni LI. Hepatitis C virus seroprevalence rate among Saudis. Saudi Med J. 2003;24:81–6. [PubMed] [Google Scholar]
- 34.Karkar A. Hepatitis C in dialysis units: The Saudi experience. Hemodial Int. 2007;11:354–67. doi: 10.1111/j.1542-4758.2007.00192.x. [DOI] [PubMed] [Google Scholar]
- 35.WHO. World Health Organization Regional Committee for the Eastern Mediterranean; The growing threats of hepatitis B and C in the Eastern Mediterranean Region: A call for action. 2009. [Last accessed on 2012 Oct 10]. Available from http://applications.emro.who.int/docs/EM_RC56_3_en.pdf .
- 36.Al-Faleh FZ, Ayoola EA, Al-Jeffry M, Al-Rashed R, Al-Mofarreh M, Arif M, et al. Prevalence of antibody to hepatitis C virus among Saudi Arabian children: A community-based study. Hepatology. 1991;14:215–8. [PubMed] [Google Scholar]
- 37.Fakeeh M, Zaki AM. Hepatitis C: Prevalence and common genotypes among ethnic groups in Jeddah, Saudi Arabia. Am J Trop Med Hyg. 1999;61:889–92. doi: 10.4269/ajtmh.1999.61.889. [DOI] [PubMed] [Google Scholar]
- 38.Abdelaal M, Rowbottom D, Zawawi T, Scott T, Gilpin C. Epidemiology of hepatitis C virus: A study of male blood donors in Saudi Arabia. Transfusion. 1994;34:135–7. doi: 10.1046/j.1537-2995.1994.34294143941.x. [DOI] [PubMed] [Google Scholar]
- 39.Al-Faleh FZ, Huraib S, Sbeih F, Al-Karawi M, Al-Rashed R, Al-Mofleh IA, et al. Hepatitis C virus genotypes in patients with chronic liver disease and haemodialysis patients from Saudi Arabia. J Viral Hepat. 1995;2:293–6. doi: 10.1111/j.1365-2893.1995.tb00044.x. [DOI] [PubMed] [Google Scholar]
- 40.Bosmans JL, Nouwen EJ, Behets G, Gorteman K, Huraib SO, Shaheen FA, et al. Prevalence and clinical expression of HCV-genotypes in haemodialysis patients of two geographically remote countries: Belgium and Saudi Arabia. Clin Nephrol. 1997;47:256–62. [PubMed] [Google Scholar]
- 41.Shobokshi OA, Serebour FE, Skakni L, Al-Saffy YH, Ahdal MN. Hepatitis C genotypes and subtypes in Saudi Arabia. J Med Virol. 1999;58:44–8. [PubMed] [Google Scholar]
- 42.Shobokshi OA, Serebour FE, Skakni LI. Hepatitis C genotypes/subtypes among chronic hepatitis patients in Saudi Arabia. Saudi Med J. 2003;24(Suppl 2):S87–91. [PubMed] [Google Scholar]
- 43.Al-Traif I, Handoo FA, Al-Jumah A, Al-Nasser M. Chronic hepatitis C. Genotypes and response to antiviral therapy among Saudi patients. Saudi Med J. 2004;25:1935–8. [PubMed] [Google Scholar]
- 44.Huraib S, Al-Rashed R, Aldrees A, Aljefry M, Arif M, Al-Faleh FA. High prevalence of and risk factors for hepatitis C in haemodialysis patients in Saudi Arabia: A need for new dialysis strategies. Nephrol Dial Transplant. 1995;10:470–4. doi: 10.1093/ndt/10.4.470. [DOI] [PubMed] [Google Scholar]
- 45.Saudi Center for Organ Transplantation. Annual Report. 2006 [Google Scholar]
- 46.Mohamed WZ. Prevention of hepatitis C virus in hemodialysis patients: Five years experience from a single center. Saudi J Kidney Dis Transpl. 2010;21:548–54. [PubMed] [Google Scholar]
- 47.Hussein MM, Mooij JM. Methods used to reduce the prevalence of hepatitis C in a dialysis unit. Saudi J Kidney Dis Transpl. 2010;21:909–13. [PubMed] [Google Scholar]
- 48.Abu-Aisha H, Mitwalli A, Huraib SO, Al-Wakeel J, Abid J, Yousif KI, et al. The effect of chemical and heat disinfection of the hemodialysis machines on the spread of hepatitis C virus infection: A prospective study. Saudi J Kidney Dis Transpl. 1995;6:174–8. [PubMed] [Google Scholar]
- 49.Soyannwo MA, Khan N, Kommajosyula S, Abdel Rahman AR, Khadaji M, Sing R, et al. Hepatitis C antibodies in haemodialysis and pattern of end-stage renal failure in Gassim, Saudi Arabia. Afr J Med Med Sci. 1996;25:13–22. [PubMed] [Google Scholar]
- 50.Saxena AK, Panhotra BR, Sundaram DS, Naguib M, Venkateshappa CK, Uzzaman W, et al. Impact of dedicated space, dialysis equipment, and nursing staff on the transmission of hepatitis C virus in a hemodialysis unit of the middle east. Am J Infect Control. 2003;31:26–33. doi: 10.1067/mic.2003.55. [DOI] [PubMed] [Google Scholar]
- 51.Al-Jiffri AM, Fadag RB, Ghabrah TM, Ibrahim A. Hepatitis C virus infection among patients on hemodialysis in Jeddah: A single center experience. Saudi J Kidney Dis Transpl. 2003;14:84–9. [PubMed] [Google Scholar]
- 52.Karkar A, Abdelrahman M, Ghacha R, Malik TQ. Prevention of viral transmission in HD units: The value of isolation. Saudi J Kidney Dis Transpl. 2006;17:183–8. [PubMed] [Google Scholar]
- 53.Alzahrani AJ, Dela Cruz DM, Obeid OE, Bukhari HA, Al-Qahtani A, Al-Ahdal MN. Molecular detection of hepatitis B, hepatitis C, and torque teno viruses in drug users in Saudi Arabia. J Med Virol. 2009;81:1343–7. doi: 10.1002/jmv.21487. [DOI] [PubMed] [Google Scholar]
- 54.Iqbal N. Substance dependence. A hospital based survey. Saudi Med J. 2000;21:51–7. [PubMed] [Google Scholar]
- 55.Al Faleh F, Al Shehri S, Al Ansari S, Al Jeffri M, Al Mazrou Y, Shaffi A, et al. Changing patterns of hepatitis A prevalence within the Saudi population over the last 18 years. World J Gastroenterol. 2008;14:7371–5. doi: 10.3748/wjg.14.7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ashraf SJ, Arya SC, Parande CM, Kristensen E. Hepatitis A virus among natives and expatriates in Saudi Arabia. J Med Virol. 1986;19:151–3. doi: 10.1002/jmv.1890190207. [DOI] [PubMed] [Google Scholar]
- 57.Talukder MA, Waller DK, Nixon P, Al-Admawy AM. Prevalence of antibody to hepatitis A virus in a Saudi Arabian hospital population. J Infect Dis. 1983;148:1167. doi: 10.1093/infdis/148.6.1167. [DOI] [PubMed] [Google Scholar]
- 58.Al-Faleh FZ, Al-Jeffry M, Ramia H, Al-Rashed RS, Arif MA, Mohammed OM, et al. Hepatitis A in Saudi Arabia: A comparative sero-epidemiological study. Saudi Med J. 1999;20:678–81. [PubMed] [Google Scholar]
- 59.Al Rashed RS. Prevalence of hepatitis A virus among Saudi Arabian children: A community-based study. Ann Saudi Med. 1997;17:200–3. doi: 10.5144/0256-4947.1997.200. [DOI] [PubMed] [Google Scholar]
- 60.Almuneef MA, Memish ZA, Balkhy HH, Qahtani M, Alotaibi B, Hajeer A, et al. Epidemiologic shift in the prevalence of hepatitis, A virus in Saudi Arabia: A case for routine hepatitis A vaccination. Vaccine. 2006;24:5599–603. doi: 10.1016/j.vaccine.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 61.Khalil M, Al-Mazrou Y, Al-Jeffri M, Al-Howasi M. Childhood epidemiology of hepatitis A virus in Riyadh, Saudi Arabia. Ann Saudi Med. 1998;18:18–21. doi: 10.5144/0256-4947.1998.18. [DOI] [PubMed] [Google Scholar]
- 62.Geneva: 2000. World Health Organization (WHO). Hepatitis A. [Google Scholar]
- 63.Feinstone SM. Hepatitis A virus. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. New York: Churchill Livingstone; 2000. pp. 1924–8. [Google Scholar]
- 64.El-Gilany A-H, Hammad S, Refaat K, Al-Enazi R. Seroprevalence of hepatitis A antibodies among children in a Saudi community. Asian Pacific J Trop Med. 2010;3:278–82. [Google Scholar]
- 65.Ministry of Economy and Planning Saudi Arabia. Human Development Report. 2003 [Google Scholar]
- 66.Tufenkeji H. Hepatitis A shifting epidemiology in the Middle East and Africa. Vaccine. 2000;18:S65–7. doi: 10.1016/s0264-410x(99)00468-5. [DOI] [PubMed] [Google Scholar]
- 67.Manno M, Camma C, Schepis F, Bassi F, Gelmini R, Giannini F, et al. Natural history of chronic HBV carriers in northern Italy: Morbidity and mortality after 30 years. Gastroenterology. 2004;127:756–63. doi: 10.1053/j.gastro.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 68.Bortolotti F, Guido M, Bartolacci S, Cadrobbi P, Crivellaro C, Noventa F, et al. Chronic hepatitis B in children after e antigen seroclearance: Final report of a 29-year longitudinal study. Hepatology. 2006;43:556–62. doi: 10.1002/hep.21077. [DOI] [PubMed] [Google Scholar]
- 69.Fattovich G, Brollo L, Giustina G, Noventa F, Pontisso P, Alberti A, et al. Natural history and prognostic factors for chronic hepatitis type B. Gut. 1991;32:294–8. doi: 10.1136/gut.32.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fattovich G. Natural history of hepatitis B. J Hepatol. 2003;39:50–8. doi: 10.1016/s0168-8278(03)00139-9. [DOI] [PubMed] [Google Scholar]
- 71.Chen YC, Chu CM, Liaw YF. Age-specific prognosis following spontaneous hepatitis B e antigen seroconversion in chronic hepatitis B. Hepatology. 2010;51:435–44. doi: 10.1002/hep.23348. [DOI] [PubMed] [Google Scholar]
- 72.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 73.Alghamdi AS, Sanai FM, Ismail M, Alghamdi H, Alswat K, Alqutub A, et al. SASLT practice guidelines: Management of hepatitis C virus infection. Saudi J Gastroenterol. 2012;18(suppl 1):1–32. doi: 10.4103/1319-3767.101155. [DOI] [PMC free article] [PubMed] [Google Scholar]


