Abstract
Background/Aim:
Endosonography is a distinct method for evaluating the structural lesions of the gastrointestinal (GI) tract, particularly the pancreatobilliary region. This procedure has made a fundamental change in the diagnosis of pancreatic mass lesion through providing fine needle aspiration. This study aims at evaluating the results and efficacy of endoscopic ultrasound fine needle aspiration (EUS-FNA) in patients with pancreatic solid mass.
Patients and Methods:
The present study is an observational, prospective case series nature, evaluated patients with pancreatic solid mass referred to Imam Khomeini educational hospital in Tehran for a duration of one year since November 2010. In order to determine the false negative cases, the patients were followed-up from 6 to 12 months.
Results:
EUS-FNA was conducted on all 53 patients without any complication. The majority of patients included in the study were males (68%) and 81% of patients had a mass in the head of pancreas. The result of cytopathology revealed 36 adenocarcinomas (68%), 7 other malignancies (13%), benign lesions (6%) and 7 non-diagnostic cases (13%). The frequency of non-diagnostic results was significantly high in masses smaller than 3 cm (6 vs. 1, P < 0.002). Patients with non-diagnostic result were younger than those with malignant cytopathology (52 ± 7.5 vs. 66 ± 7.5 years, P < 0.001).. Sensitivity, specificity, positive predictive value, negative predictive value and accuracy of this procedure concerning Adenocarcinoma were 88%, 100%, 100%, 70% and 90%, respectively.
Conclusion:
EUS – FNA is an effective and safe procedure in histopathologic diagnosis of pancreatic tumors. This procedure is useful in all pancreatic mass cases including resectable and non-resectable ones.
Keywords: Endosonography, fine needle aspiration, pancreatic neoplasm
Majority of pancreatic solid masses are adenocarcinoma.[1] However, few less invasive malignancies such as neuroendocrine tumors and benign lesions such as autoimmune pancreatitis can manifest as a solid mass in pancreas.[2] The pancreas is a retroperitoneal organ and tissue sampling from pancreatic masses can be associated with limitations and complications such as tumoral cell seeding, pancreatitis due to parenchyma damage, and trauma to adjacent vessels and organs.[3,4]
The best diagnostic modality for assessment of pancreatic solid efficacy of endoscopic ultrasound fine needle aspiration (EUS-FNA).[5] This method can determine the tumor location, size, and invasion to adjacent organs and provides safe and accurate fine needle aspiration (FNA). Concerning malignancy in pancreatic solid masses, the sensitivity, specificity and accuracy of this method are 85%, 100% and 60 to 94%, respectively.[6–13] Histopathologic result of EUS-FNA in this setting has revealed 87% malignancy, 13-14% benign lesion and 11-28% non-diagnostic.[12,13] EUS-FNA accuracy is dependant on the endosonographist and pathologist's experience. Pancreatitis that sometimes occurs in association with adenocarcinoma, can lead to sampling error and mistakes in histopathologic assessment.[14,15]
This study aims at evaluating the results and efficacy of EUS-FNA in patients with pancreatic solid mass.
PATIENTS AND METHODS
The present study which is of a descriptive, prospective and case series nature, evaluated patients with pancreatic solid mass, referred to Imam Khomeini educational hospital in Tehran for a duration of one year since November 2010. In order to determine the false negative cases, the patients have been followed-up from 6 to 12 months. Cystic or solid-cystic masses are not studied.
All procedures were carried out by a single gastroenterologist. All patients were placed under conscious sedation using oropharyngeal topical anesthetic and I.V. midazolam and fentanyl with or without propofol. The echo endoscope used was curved linear array (Olympus GF-UC 24OP-AL5 Tokyo, Japan.) with Aloka Prosound SSD-5000 (Aloka, Tokyo, Japan) processor. EUS-guided FNA was carried out using a single use aspiration 22-G 13-mm Wilson-Cook Quick needle (Wilson-Cook GI Endoscopy, Winston-Salem, NC, USA). Approximately 7 ± 2 to 10 back-and-forth passages were performed while maintaining aspiration in the needle.
Aspirated samples were evaluated either by means of cytological smears or cell blocks. All cytological samples were interpreted by one experienced cytopathologists. The cytology samples were reported as “positive”, “suspicious for malignancy”, “atypical”, “negative”, or “non-diagnostic” on the official pathology report. Some non-adenocarcinoma tumors were diagnosed based on morphology and immunocytochemical staining. Aspirate specimens containing inadequate cellular material or cellular atypia were defined as “non-diagnostic”.
In 5 patients, the final diagnosis was established by histological assessment of the second EUS-FNA or a surgical specimen. Patients followed-up for 12 months without any evidence of malignancy, were defined as non-malignant (true negative).
Statistical analysis
The significance level was 5% for all statistical procedures. Numerical variables were expressed as mean ± SD and comparative analysis between them was performed by Student's t-test. All categorical data were analyzed by Chi-square test with Yates correction and Fischer's exact test. Concerning the diagnosis obtained by EUS-FNA, sensitivity, specificity, positive and negative predictive values, and accuracy were calculated with a 2 × 2 table.
RESULTS
53 patients with pancreatic solid mass were enrolled in the present study. Patient's demographic information, clinical and imaging finding, and pathologic results are depicted in Tables 1 and 2. The mean age of the patients was 61 years (range 24–90 years). The lesion was situated in the head of the pancreas in 43 cases (81%).
Table 1.
Demographic and clinical finding in 53 studied patients
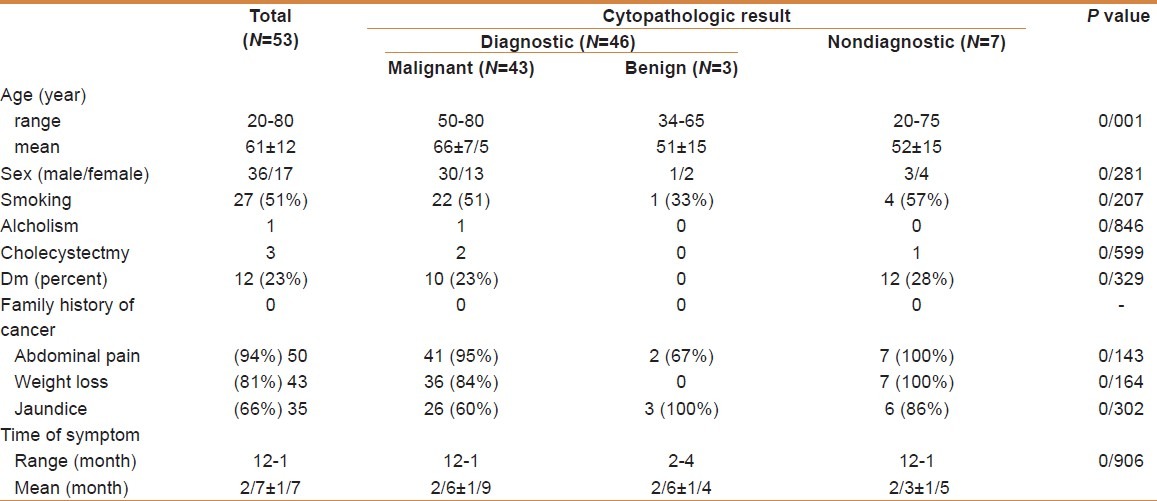
Table 2.
Endoscopic ultrasonography findings and cytopathological results
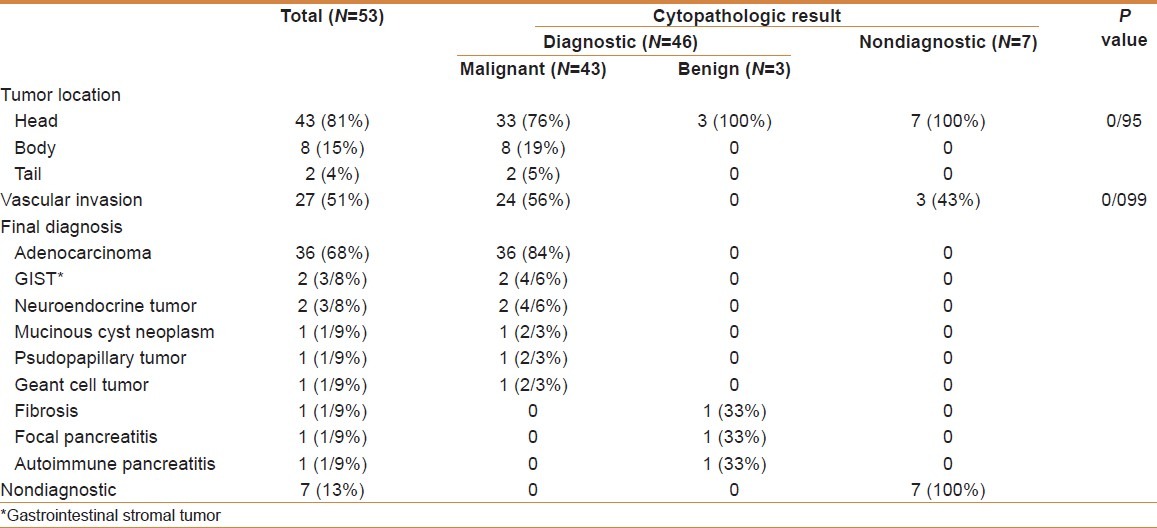
The mean lesion size was 41 mm (range 20-105 mm). Cytological examination was performed in all 53 cases without any complication [Figure 1]. The final diagnosis was obtained in 50 patients: by first EUS-FNA in 43 cases, by surgical specimen in 2 cases, by a second EUS-FNA in 3 cases, by a CT-scan guided biopsy in one case, by an ascites cytology in one case and by clinical and radiological follow-up for at least 12 months. Finally EUS-FNA was diagnostic in 46 cases (87%).
Figure 1.
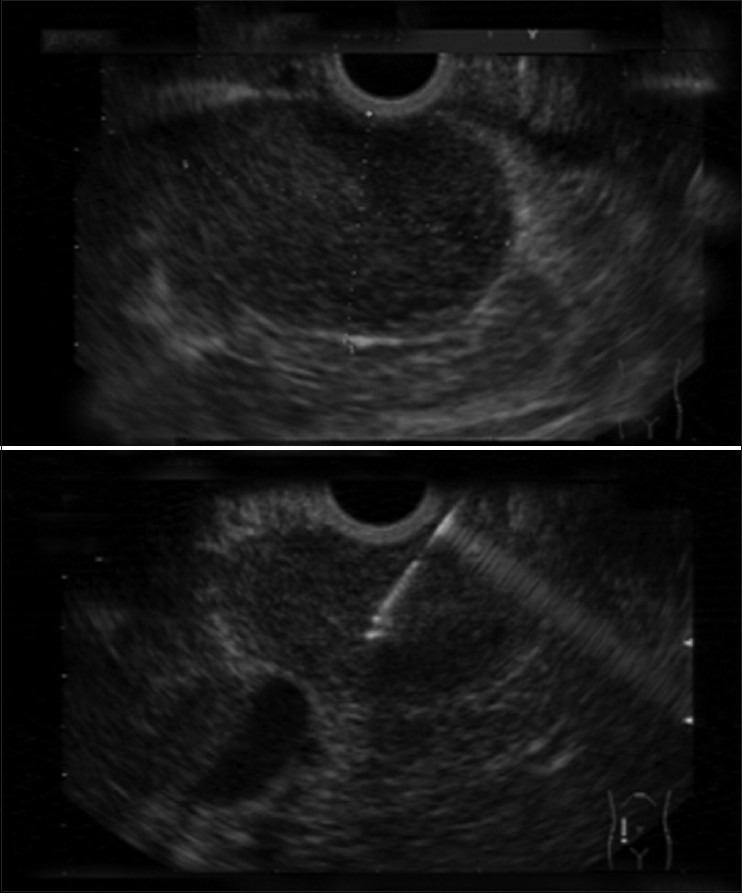
Hypoechoic mass in the head of the pancreas diagnosed as adenocarcinoma in EUS_FNA (aspiration needle is seen inside the mass in bottom image)
Among these 50 patients, 38 patients (72%) died with evidence of malignant progression of their disease in 12 month follow-up. In 3 patients with an initial diagnosis of benign disease (i.e., autoimmune pancreatitis), the status remained stable or improved.
Second FNA was performed in five patients with non-diagnostic primary specimen; results of these second FNAs were adenocarcinoma in 3 cases, focal pancreatitis in one patient and non-diagnostic in one case. A pancreatic CT scan in one patient was normal but EUS-FNA revealed a 2.6 cm adenocarcinoma in the head of the pancreas. In another patient that was diagnosed as chronic pancreatitis by CT guided biopsy, the EUS-FNA revealed adenocarcinoma.
The most common (68%) cytopathologic result of EUS-FNA was adenocarcinoma. But few uncommon tumors and benign lesions were diagnosed by this method too [Table 2]. The pathologic diagnosis of two gastrointestinal stromal tumors (GIST), two neuroendocrine tumors and one pseudo papillary tumor were confirmed by immunohistochemistry (IHC).
There was no cystic component in two mucinous cyst neoplasms and this diagnosis was made histopathologically.Autoimmune pancreatitis was diagnosed by numerous plasma cells in FNA specimen and clinical setting. Focal pancreatitis was diagnosed by evidence of chronic inflammation and lack of malignancy in FNA specimen and clinical setting.
All patients were followed-up for 12 months, especially those with benign and/or non-diagnostic cytopathologic results were observed closely. Symptoms and CT scan findings of the patient with autoimmune pancreatitis resolved by oral prednisolone in 3 months. Two patients that were diagnosed as focal pancreatitis and fibrosis by EUS-FNA fared well and did not have clinical or imaging evidence of malignancy during 12 months of follow up.
Cytopathologic result of EUS-FNA was non-diagnostic in seven cases; tumor was in the head of the pancreas in all of these patients. Four cases from these ones had adenocarcinoma, lymphoma, metastasis from colon cancer and peritoneal carcinomatosis that were diagnosed by whipple surgery, supra-clavicle lymph node excision, CT guided biopsy, and ascites fluid cytology, respectively. Three of the remaining patients died without histopathologic diagnosis.
Concerning adenocarcinoma, the sensitivity, specificity, PPV, NPV, and accuracy of EUS-FNA was 88%, 100%, 100%, 70% and 90%, respectively (sensitivity before re-FNA was 80%).
Several variables such as age, sex, smoking, alcohol use, diabetes mellitus, tumor characteristic and serum CA19-9 were evaluated. Only age and tumor size had significant correlation with cytopathologic result of EUS-FNA; the frequency of non-diagnostic results was significantly high in masses smaller than 3 cm (6 vs. 1, P < 0.002) and patients with non-diagnostic result were younger than those with malignant cytopathology (52 ± 7.5 vs. 66 ± 7.5 years, P < 0.001)
DISCUSSION
Over the last decades, the incidence of pancreatic cancer has increased.[16] Prognosis of this tumor remains poor despite rapid improvements in imaging technologies and therapeutic modalities. Curative treatment is dependent on early diagnosis. EUS-FNA is a reference method for diagnosis of the pancreatic neoplasm such as adenocarcinoma or non-adenocarcinoma tumors.[17,18]
Sensitivity and specificity of this method is dependent on the pathologist's experience and endosonographist's skill. Pancreatitis that sometimes is associated with tumor can decrease the diagnostic accuracy of tissue sampling including EUS-FNA.[19]
Indications of EUS-FNA in pancreatic malignancy include: detection of small tumors that cannot be seen on CT scan or MRI,[20] biopsy from lesions that are surrounded by blood vessels,[20] detection of lymph nodes involvement,[21] biopsy from small lesions in the left lobe of the liver that are suspected to be metastasis,[22] diagnosis of peritoneal carcinomatosis with ascites fluid aspiration[23] and celiac nerve block in patients with severe pain.[24]
Considering low negative predictive value of EUS-FNA in pancreatic malignancy, some clinicians offer surgery for resectable tumors without attempting FNA. These physicians recommend FNA only for advanced non-resectable tumors because pathologic diagnosis is essential for starting chemotherapy.[25,26] As noted in this present study, there are various benign lesions and non-adenocarcinoma tumors that manifest as pancreatic mass. Surgery in benign lesions such as autoimmune pancreatitis and few malignancies such as lymphoma is unnecessary. And in masses where surgery is necessary, extent of surgical resection is different according to tumor pathology. So FNA can be recommended for all of the pancreatic masses.
In this present study, frequency of advanced disease was higher than similar studies.[27–30] This can be due to delay in referring the patients for diagnostic EUS-FNA.
In this study and other similar studies, most of the pancreatic masses were adenocarcinoma in the head of the pancreas.[16,21] Frequency of non-diagnostic results was greater in masses of the head rather than body and/or tail of the pancreas. This remarkable difference that may be due to angulations of the tip of the endoscope in the duodenum and decrease in efficacy of FNA suction was not statistically significant. (7 vs. 1, P = 0.95)
The patients with malignant FNA result were older than patients with non-diagnostic result (66 ± 7.5 years vs. 52 ± 15 years, P < 0.001). Similar results have been shown by Fisher et al, and may be related to the higher incidence of pancreatic cancer in old age.[31]
Frequency of non-diagnostic results was greater in masses smaller than 3 cm rather than the larger tumors (6 vs. 1, P < 0.002). This finding was also shown by Williams et al, but not by Fisher et al, and may be related to endosonographist's experience and skills.[21,31]
Tissue sampling and diagnosis of neuroendocrine tumors by FNA is difficult because these tumors are small in size, and are hypervascular.[32] But in this present study two neuroendocrine tumors were diagnosed by EUS-FNA; one of them was 5 cm and another was 10 cm in diameter. The reason for the diagnosis is perhaps the larger size of the tumor.
Pancreas lymphoma and metastasis to the pancreas manifest as pancreatic mass and can be diagnosed by EUS-FNA.[12,13] But one pancreatic lymphoma case and one case with metastasis from the colon cancer were not diagnosed in the present study; in both cases pancreatic mass diameter was less than 3 centimeters and is perhaps the cause of failure of the diagnosis.
Sensitivity, specificity, PPV, NPV and accuracy of EUS-FNA concerning adenocarcinoma were 88%, 100%, 100%, 70% and 90%, respectively. These values are consistent with previous studies.[12,13,26,33–38]
Cellular atypia results were considered as adenocarcinoma in some of the previous studies, but in our study they are considered as non-diagnostic. As regards to malignancy, the high PPV (100%) and relatively low NPV (70%) show that the diagnosis of malignancy by EUS-FNA is valid, but that non-diagnostic results of this method does not exclude malignancy. Moreover, since surgery was carried out for only three cases of adenocarcinoma, we were unable to match the results of EUS staging with surgical findings.
CONCLUSION
EUS-FNA is an effective and safe procedure for histopathologic assessment of the pancreatic solid tumors including malignant or non-malignant, and resectable or non-resectable ones. Young patients, small tumor size, and inadequate skill and experience of endosonographist may lead to non-diagnosis by EUS-FNA. If the first FNA is non-diagnostic, a second FNA can help in diagnosing the tumor pathologically. However, surgical resection may be a good option in resectable tumors.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Klimstra DS. Nonductal neoplasms of the pancreas. Mod Pathol. 2007;20(Suppl 1):S94–112. doi: 10.1038/modpathol.3800686. [DOI] [PubMed] [Google Scholar]
- 3.Pinto MM, Avila NA, Criscuolo EM. Fine needle aspiration of the pancreas.A five-year experience. Acta Cytol. 1988;32:39–42. [PubMed] [Google Scholar]
- 4.Welch TJ, Sheedy PF, Johnson CD, Johnson CM, Stephens DH. CT-guided biopsy: Prospective analysis of 1,000 procedures. Radiology. 1989;171:493–6. doi: 10.1148/radiology.171.2.2704815. [DOI] [PubMed] [Google Scholar]
- 5.LeBlanc JK, Espada R, Ergun G. Non-small cell lung cancer staging techniques and endoscopic ultrasound: Tissue is still the issue. Chest. 2003;123:1718–25. doi: 10.1378/chest.123.5.1718. [DOI] [PubMed] [Google Scholar]
- 6.Legmann P, Vignaux O, Dousset B, Baraza AJ, Palazzo L, Dumontier I, et al. Pancreatic tumors: Comparison of dual-phase helical CT and endoscopic sonography. Am J Roentgenol. 1998;170:1315–22. doi: 10.2214/ajr.170.5.9574609. [DOI] [PubMed] [Google Scholar]
- 7.Akahoshi K, Chijiiwa Y, Nakano I, Nawata H, Ogawa Y, Tanaka M, et al. Diagnosis and staging of pancreatic cancer by endoscopic ultrasound. Brit J Radiol. 1998;71:492–6. doi: 10.1259/bjr.71.845.9691893. [DOI] [PubMed] [Google Scholar]
- 8.Cannon ME, Carpenter SL, Elta GH, Nostrant TT, Kochman ML, Ginsberg GG, et al. EUS compared with CT, MRI, and angiography and the influence of biliary stenting on staging accuracy of ampullary neoplasms. Gastrointest Endosc. 1999;50:27–33. doi: 10.1016/s0016-5107(99)70340-8. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad NA, Lewis JD, Ginsberg GG, Rosato EF, Morris JB, Kochman ML. EUS in preoperative staging of pancreatic cancer. Gastrointest Endosc. 2000;52:463–8. doi: 10.1067/mge.2000.107725. [DOI] [PubMed] [Google Scholar]
- 10.Meining A, Dittler HJ, Wolf A, Lorenz R, Schusdziarra V, Siewert JR, et al. You get what you expect? A critical appraisal of imaging methodology in endosonographic cancer staging. Gut. 2002;50:599–603. doi: 10.1136/gut.50.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soriano A, Castells A, Ayuso C, Ayuso JR, de Caralt MT, Gines MA, et al. Preoperative staging and tumor resectability assessment of pancreatic cancer: Prospective study comparing EUS, helical CT, MRI, and angiography. Am J Gastroenterol. 2004;99:492–501. doi: 10.1111/j.1572-0241.2004.04087.x. [DOI] [PubMed] [Google Scholar]
- 12.Ardengh JC, Lopes CV, de Lima LF, de Oliveira JR, Venco F, Santo GC, et al. Diagnosis of pancreatic tumors by endoscopic ultrasound-guided fine-needle aspiration. World J Gastroenterol. 2007;13:3112–6. doi: 10.3748/wjg.v13.i22.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson JL, Kalade A, Prasad S, Cade R, Thomson B, Banting S, et al. Diagnosis of solid pancreatic masses by endoscopic ultrasound-guided fine-needle aspiration. Intern Med J. 2009;39:32–7. doi: 10.1111/j.1445-5994.2008.01633.x. [DOI] [PubMed] [Google Scholar]
- 14.Alsibai KD, Denis B, Bottlaender J, Kleinclaus I, Straub P, Fabre M. Impact of cytopathologist expert on diagnosis and treatment of pancreatic lesions in current clinical practice.A series of 106 endoscopic ultrasound-guided fine needle aspirations. Cytopathology. 2006;17:18–26. doi: 10.1111/j.1365-2303.2006.00312.x. [DOI] [PubMed] [Google Scholar]
- 15.Varadarajulu S, Tamhane A, Eloubeidi MA. Yield of EUS-FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc. 2005;62:728–36. doi: 10.1016/j.gie.2005.06.051. [DOI] [PubMed] [Google Scholar]
- 16.Helmstaedter L, Riemann JF. Pancreatic cancer—EUS and early diagnosis. Arch Surg. 2008;393:923–7. doi: 10.1007/s00423-007-0275-1. [DOI] [PubMed] [Google Scholar]
- 17.Warshaw AL, Gu ZY, Wittenberg J, Walkman AC. Preoperative staging and assessment of resectability of pancreatic cancer. Arch Surg. 1990;125:230–3. doi: 10.1001/archsurg.1990.01410140108018. [DOI] [PubMed] [Google Scholar]
- 18.Gan SI, Rajan E, Adler DG, Baron TH, Anderson MA, Cash BD, et al. ASGE Standards of Practice Committee; Role of EUS. Gastrointest Endosc. 2007;66:425–34. doi: 10.1016/j.gie.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 19.DelMaschio A, Vanzulli A, Sironi S, Castrucci M, Mellone R, Staudacher C, et al. Pancreatic cancer versus chronic pancreatitis: Diagnosis with CA 19-9 assessment, US, CT, and CT-guided fine-needle biopsy. Radiology. 1991;178:95–9. doi: 10.1148/radiology.178.1.1984331. [DOI] [PubMed] [Google Scholar]
- 20.Gress F, Gottlieb K, Sherman S, Lehman G. Endoscopic ultrasonography-guided fine-needle aspiration biopsy of suspected pancreatic cancer. Ann Intern Med. 2001;34:459–64. doi: 10.7326/0003-4819-134-6-200103200-00010. [DOI] [PubMed] [Google Scholar]
- 21.Williams DB, Sahai AV, Aabakken L, Penman ID, van Velse A, Webb J, et al. Endoscopic ultrasound guided fine needle aspiration biopsy: A large single centre experience. Gut. 1999;44:720–6. doi: 10.1136/gut.44.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.TenBerge J, Hoffman BJ, Hawes RH, Van Enckevort C, Giovannini M, Erickson RA, et al. EUS-guided fine needle aspiration of the liver: Indications, yield, and safety based on an international survey of 167 cases. Gastrointest Endosc. 2002;55:859–62. doi: 10.1067/mge.2002.124557. [DOI] [PubMed] [Google Scholar]
- 23.Chang KJ, Albers CG, Nguyen P. Endoscopic ultrasound-guided fine needle aspiration of pleural and ascitic fluid. Am J Gastroenterol. 1995;90:148–50. [PubMed] [Google Scholar]
- 24.Gunaratnam NT, Sarma AV, Norton ID, Wiersema MJ. A prospective study of EUS-guided celiac plexus neurolysis for pancreatic cancer pain. Gastrointest Endosc. 2001;54:316–24. doi: 10.1067/mge.2001.117515. [DOI] [PubMed] [Google Scholar]
- 25.Saftoiu A, Vilmann P. Role of endoscopic ultrasound in the diagnosis and staging of pancreatic cancer. J Clin Ultrasound. 2009;37:1–17. doi: 10.1002/jcu.20534. [DOI] [PubMed] [Google Scholar]
- 26.Hartwig W, Schneider L, Diener MK, Bergmann F, Büchler MW, Werner J. Preoperative tissue diagnosis for tumors of the pancreas. Br J Surg. 2009;96:5–20. doi: 10.1002/bjs.6407. [DOI] [PubMed] [Google Scholar]
- 27.O’Malley ME, Boland GW, Wood BJ, Fernandez-del Castillo C, Warshaw AL, Mueller PR. Adenocarcinoma of the head of the pancreas: Determination of surgical unresectability with thin-section pancreatic-phase helical CT. AJR Am J Roentgenol. 1999;173:1513–8. doi: 10.2214/ajr.173.6.10584794. [DOI] [PubMed] [Google Scholar]
- 28.Lu DS, Reber HA, Krasny RM, Kadell BM, Sayre J. Local staging of pancreatic cancer: Criteria for unresectability of major vessels as revealed by pancreatic-phase, thin-section helical CT. AJR Am J Roentgenol. 1997;168:1439–43. doi: 10.2214/ajr.168.6.9168704. [DOI] [PubMed] [Google Scholar]
- 29.Kalser MH, Barkin J, MacIntyre JM. Pancreatic cancer; Assessment of prognosis by clinical presentation. Cancer. 1985;56:397–402. doi: 10.1002/1097-0142(19850715)56:2<397::aid-cncr2820560232>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 30.Bakkevold KE, Arnesjo B, Kambestad B. Carcinoma of the pancreas and papilla of vater: Presenting symptoms, signs and diagnosis related to stage and tumor site. Scand J Gastroenterol. 1992;27:317–25. doi: 10.3109/00365529209000081. [DOI] [PubMed] [Google Scholar]
- 31.Fisher L, Segarajasingam DS, Stewart C, Deboer WB, Yusoff IF. Endoscopic ultrasound guided fine needle aspiration of solid pancreatic lesions: Performance and outcomes. J Gastroenterol Hepatol. 2009;24:90–6. doi: 10.1111/j.1440-1746.2008.05569.x. [DOI] [PubMed] [Google Scholar]
- 32.Voss M, Hammel P, Molas G, Palazzo L, Dancour A, O’Toole D, et al. Value of endoscopic ultrasound guided fine needle aspiration biopsy in the diagnosis of solid pancreatic masses. Gut. 2000;46:244–9. doi: 10.1136/gut.46.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rocca R, De Angelis C, Daperno M, Carucci P, Ravarino N, Bruno M, et al. Endoscopic ultrasound-fine needle aspiration (EUS-FNA) for pancreatic lesions: Effectiveness in clinical practice. Dig Liver Dis. 2007;39:768–74. doi: 10.1016/j.dld.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Touchefeu Y, Lerhun M, Coron E, Alamdari A, Heymann MF, Mosnier JF, et al. EUS-FNA for the diagnosis of solid pancreatic masses: The impact on patient-management strategy. Aliment Pharmacol Ther. 2009;30:1070–7. doi: 10.1111/j.1365-2036.2009.04138.x. [DOI] [PubMed] [Google Scholar]
- 35.Imaoka H, Yamao K, Bhatia V, Shimizu Y, Yatabe Y, Koshikawa T, et al. Rare pancreatic neoplasms: The utility of endoscopic ultra sound guided fine-needle aspiration—a large single center study. J Gastroenterol. 2009;4:146–53. doi: 10.1007/s00535-008-2282-6. [DOI] [PubMed] [Google Scholar]
- 36.Volmar KE, Vollmer RT, Jowell PS, Nelson RC, Xie HB. Pancreatic FNA in 1000 cases: A comparison of imaging modalities. Gastrointest Endosc. 2005;61:854–61. doi: 10.1016/s0016-5107(05)00364-0. [DOI] [PubMed] [Google Scholar]
- 37.Mirbagheri A, Mansuri S, Abuzari M, Radmehr M, Jazaieri H, Hormozdi M, et al. Results and complications of endoscopic ultrasound guided-fna in patients with pancreatic and gastrointestinal sub mucosal lesions. Govaresh. 2005;10:194–8. [Google Scholar]
- 38.DiMagno EP, Reber HA, Tempero MA. AGA technical review on the epidemiology, diagnosis, and treatment of pancreatic ductal adenocarcinoma.American Gastroenterological Association. Gastroenterology. 1999;117:1464–84. doi: 10.1016/s0016-5085(99)70298-2. cited in the text. [DOI] [PubMed] [Google Scholar]


