Abstract
Background/Aim:
Helicobacter pylori is implicated in various gastroduodenal diseases and many tests are available for its detection. The present study attempted to document the morphological changes in the gastric mucosa induced by H. pylori colonization and correlate them with the severity of the infection. The study also compared various diagnostic tests and evaluated the different staining methods used for H. pylori detection, especially immunohistochemical identification.
Patients and Methods:
One hundred and two patients with dyspepsia were included. Enzyme-linked immunosorbent assay (ELISA) for H. pylori-specific immunoglobulin G (IgG), immunoglobulin A (IgA), and immunoglobulin M (IgM) was used. Rapid urease test was performed on endoscopic biopsy and it was stained with hematoxylin and eosin (H and E), modified Giemsa, and immunohistochemical stains.
Results:
A significant correlation was found between the density of H. pylori and severity of gastritis. A significant correlation was observed between serology (especially when used in combination, IgG and IgA) and status of H. pylori. Immunohistochemical staining enhanced the diagnostic yield of H. pylori detection.
Conclusions:
Immunohistochemistry (IHC) should be used judiciously, whereas simple and economical tests like modified Giemsa should be used routinely for the detection of H. pylori. Combined ELISA (IgG and IgA) should be preferred over single ELISA. Simultaneous morphological and serological detection of H. pylori is preferable as H. pylori may not be detected on morphology alone due to its patchy distribution in the stomach.
Keywords: Helicobacter pylori, immunohistochemistry, serology
Helicobacter pylori is a spiral Gram negative bacterium which was discovered by Marshall and Warren in 1982.[1] Studies have indicated that the presence of H. pylori is associated with a variety of gastrointestinal diseases including gastritis, duodenal and gastric ulcers, nonulcer dyspepsia, and gastric adenocarcinoma and lymphoma.[2–4] The removal of the organism by antimicrobial therapy is correlated with the resolution of symptoms and cure of diseases.[5]
The tests available for the diagnosis of H. pylori can be broadly divided into two types: invasive and noninvasive. Noninvasive tests include serological diagnosis, urea breath test (UBT), and stool antigen test. H. pylori-specific antibodies have been detected in the serum, saliva, and urine.[6,7] Invasive tests require an endoscopic gastric biopsy specimen and include rapid urease test, histological examination, and culture of the biopsy.
H. pylori can be seen in routine hematoxylin and eosin (H and E) staining, but many newer staining methods have been devised for better visualization of H. pylori, including immunohistochemical stains.[8,9]
The present study attempted to document the morphological changes in the gastric mucosa induced by the colonization of H. pylori and correlate them with the severity of the infection. The study also compared various diagnostic tests and evaluated the different staining methods used for the detection of H. pylori especially in relation to immunohistochemical identification.
PATIENTS AND METHODS
The present study was conducted in the department of pathology, Lady Hardinge Medical College and associated hospitals over a period of two years. All patients above 18 years of age, presenting with symptoms of dyspepsia and requiring an upper gastrointestinal endoscopy were included, comprising a total of 102 patients. Patients who had received antibiotics, proton pump inhibitors, H2 blockers within the past two months, or patients with a history of gastric resection/vagotomy, and those with complicated peptic ulcer disease were excluded. The study was approved by the institutional ethical board, and written informed consent was obtained from all patients.
A blood sample of all patients selected for endoscopy was taken and serum was stored at –20°C for H. pylori serology (ELISA for specific IgG, IgA, and IgM). Antibody index of each sample was calculated by dividing the optical density (OD) value of each sample by cutoff value. Antibody index < 0.9 indicates no detectable antibody, 1.1 implies borderline positive, and >1.1 indicates H. pylori infection. Endoscopic biopsies from antrum and corpus of stomach (2 biopsies) were performed in all patients. One biopsy was immediately subjected to a rapid urease test (Pronto Dry Kit). The rest were preserved in 10% buffered formalin to be used for histopathological examination. Routine H and E staining, modified Giemsa staining, and immunohistochemistry were performed on tissue sections in each case.
Histologic features such as gastric mucosal changes for any evidence of gastritis, and presence or absence of H. pylori and so on were studied on H and E-stained sections for all cases. These were also graded according to the updated Sydney system (1994) using the visual analog scale.[10] Tissue sections were stained with modified Giemsa, the method suggested by Gray et al.[11]
The tissue sections were also assessed for the presence of H. pylori infection by immunohistochemical staining using polyclonal anti-H. pylori antibody and polymer-HRP based (detection system). The slides were examined for the presence of H. pylori in the mucus and in the gastric pits and were also graded according to the following criteria: Grade 0 (0 bacteria/oil immersion field), Grade 1 (19 bacteria/oil immersion field), Grade 2 (20-29 bacteria/oil immersion field), Grade 3 (30-99 bacteria/oil immersion field) and Grade 4 ≥ 100 bacteria/oil immersion field).[12]
RESULTS
The study group comprised 102 patients with a mean age of 37.4 years (19-80 years) and male to female ratio of 1:1 approximately (52 males vs. 50 females). The most common symptom encountered was epigastric pain which was seen in 96% cases, followed by nausea, vomiting, or both.
Upper gastrointestinal biopsies were endoscopically normal in most of the cases (83%); 8% cases had mild hyperaemia of mucosa, 8% had mild antral gastritis, and 1% had severe antral gastritis.
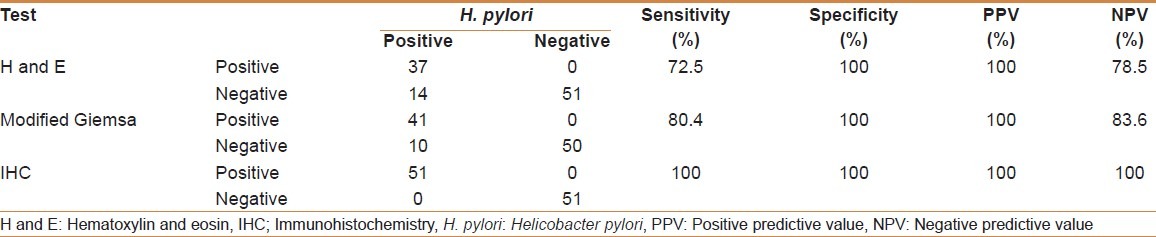
For the purpose of analysis, a case was defined as positive for H. pylori if bacteria were seen on any of the following: H and E, modified Giemsa, and immunohistochemistry (IHC), of which IHC was taken as the gold standard. Of 102 cases, a total of 51 cases were positive for H. pylori on any one or more of the three tests. Of these, 37 had visible H. pylori on H and E, 41 had visible H. pylori on modified Giemsa, and all 51 were positive on IHC [Table 1].
Table 1.
Comparison of modified Giemsa, H and E, and IHC
Rapid urease test was positive in 70% cases. It showed a sensitivity of 74.5% and a positive predictive value of 54.3%.
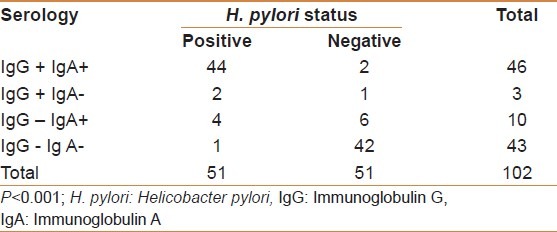
Serum ELISA for H. pylori was positive in 68 patients. Out of these, IgG type was positive in 49 (72%), IgA in 56 (82.3%), and IgM in 25 (36.8%) cases. Serum ELISA for IgG antibodies against H. pylori correlated significantly (P < 0.001) with the presence of bacteria on histology (H and E, modified Giemsa, and IHC). Of 53 cases which were negative for IgG ELISA, only four showed H. pylori on H and E (false negatives)[Table 2]. The biopsy findings also correlate well with IgG status, as 43 (89.5%) of 48 cases with both gastritis and positive H. pylori had IgG antibodies to H. pylori in their serum. All the cases that did not have gastritis and which were negative for H. pylori had a negative ELISA for serum IgG antibodies to H. pylori (P < 0.001). IgG ELISA was found to have a sensitivity and specificity of 90.2% and 94.1%, respectively.
Table 2.
Comparison of H. pylori status with combined IgG and IgA serology
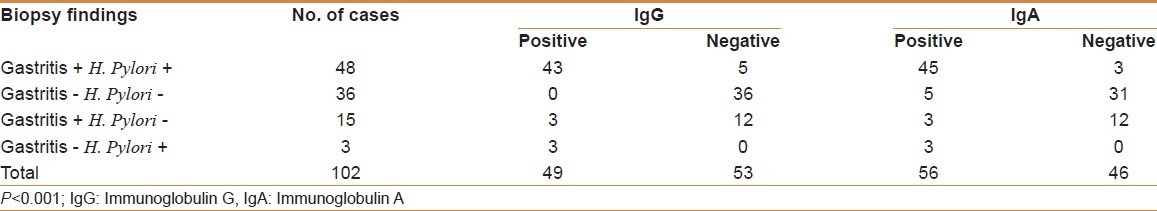
Serum ELISA for IgA antibodies also correlated significantly (P < 0.001) with H and E, modified Giemsa, and IHC. Of 44 cases which were negative for IgA ELISA, only two showed H.pylori on H and E (false negatives). Forty-five (93.75%) of 48 cases with both gastritis and positive H. pylori had IgA antibodies to H.pylori in their serum. Only five cases who did not have gastritis and who were negative for H. pylori had a positive ELISA for serum IgA antibodies to H. pylori (P < 0.001) [Table 3]. IgA ELISA was found to have a sensitivity and specificity of 94.1 and 84.3%, respectively. The present study found that the positive and negative predictive values of the combined IgG and IgA were higher than those of IgG or IgA alone, being 95.7 and 97.7%, respectively.
Table 3.
Correlation between biopsy findings and IgG and IgA status
Serum ELISA for IgM antibodies against H. pylori did not correlate significantly with the presence of bacteria on H and E, modified Giemsa, and IHC. Of 25 patients positive by IgM serology, only 12 (48%) showed visible H. pylori on H and E.
Chronic superficial gastritis was seen in 54 (53%) cases, of which 22 (40.7%) showed activity. Chronic atrophic gastritis was seen in nine (8.8%) cases. On scoring inflammation, acute inflammation was seen in 22 cases (19 mild and 3 moderate grade). However, this finding did not have a significant correlation with the presence of H. pylori, as only 12 cases (54.5%) of these 22 were positive for H. pylori (P = 0.113). Sixty-three cases showed chronic inflammation, of which 33 had mild, 24 had moderate, and six had marked chronic inflammation. Chronic inflammation score correlated significantly with H. pylori status (P < 0.001).
A total of 16 cases (15.7%) showed the presence of lymphoid follicles in addition to chronic inflammation. However, the presence of lymphoid follicles was not significantly correlated with the presence of H. pylori as only nine of these 16 patients were positive for H. pylori (P = 0.393). Intestinal metaplasia was seen in two cases (1.9%), both being mild. One case showed the presence of H. pylori with a positive serology. However, H. pylori were not seen overlying the metaplastic epithelium. The other case was serologically positive but did not show H. pylori on morphology. Glandular atrophy was seen in nine cases, of which eight were mild and one was moderate. Out of these nine cases, seven were positive on serology, whereas only five (55.5%) showed H. pylori on morphology.
H. pylori were seen in 37 cases on H and E [Figure 1], of which 29 showed mild (Grade 1), four showed moderate (Grade 2), and four showed marked (Grade 3) presence of H. pylori. H and E had a sensitivity and specificity of 72.5 and 100%, respectively. Modified Giemsa [Figure 2] was positive in 41 cases with a sensitivity and specificity of 80.4 and 100%, respectively. Modified Giemsa showed more concordance than H and E and rapid urease test with IHC.
Figure 1.
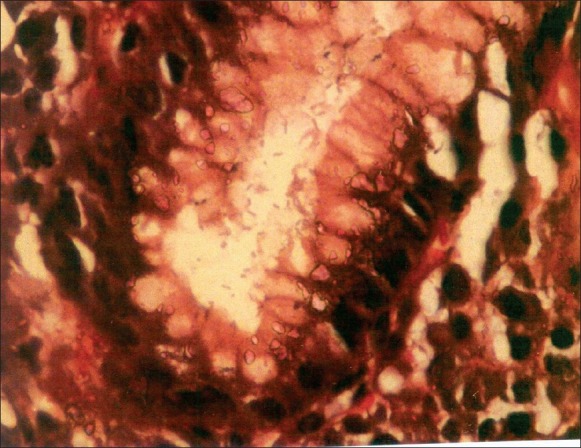
Photomicrograph showing numerous Helicobacter pylori within the gastric pit (H and E, 1000×)
Figure 2.
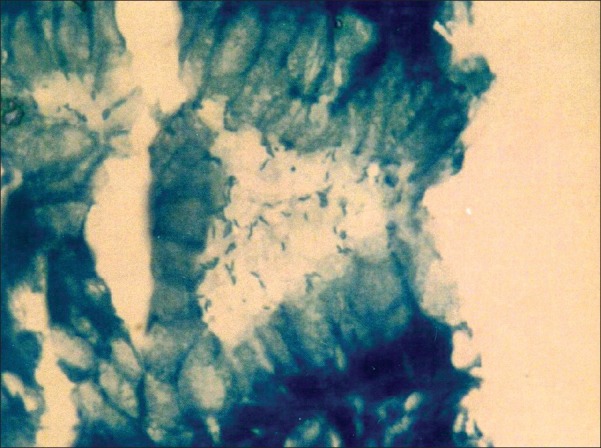
Photomicrograph showing numerous Helicobacter pylori within the gastric pit (modified Giemsa, 1000×)
Immunohistochemistry was positive in 51 cases, of which 42 cases had a grade of 1+. The remaining nine cases had grades between 2+ and 4+ [Figure 3]. Thus, IHC increased the diagnostic yield of modified Giemsa by a further 19.6% and of H and E by 27.5%, respectively. Comparison of modified Giemsa, H and E, and IHC are shown in Table 1.
Figure 3.
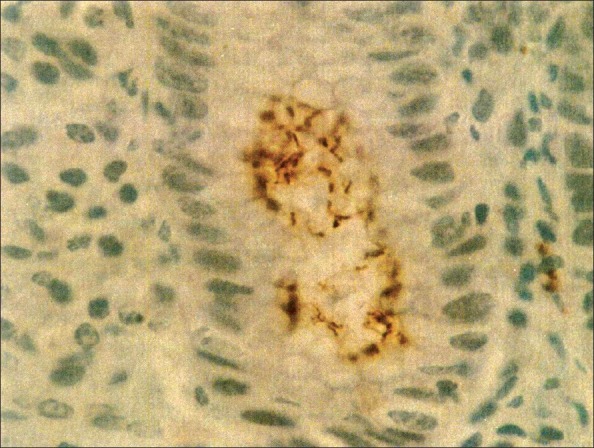
Photomicrograph showing Helicobacter pylori grade 3+ (IHC, 1000×)
DISCUSSION
H. pylori enjoys a worldwide distribution, though the prevalence strongly varies between developing and developed countries; it is more than 80 and 30%, respectively.[13] A recent report from India indicates that almost 80% of the population is infected with H. pylori.[14] Since its discovery, H. pylori has been implicated as a potential cause of nonulcer dyspepsia in a subset of patients.[15] Epidemiologic studies have clearly demonstrated a major etiologic role of H. pylori for several gastroduodenal diseases including gastric ulcer, duodenal ulcer, gastric MALT lymphoma (MALT: Mucosa-associated lymphotic tissue), and distal gastric cancer.[16]
Recently, we came across a few studies comparing the various diagnostic tests for H. pylori.
Peng et al.[17] reported that accuracy of capsule UBT was higher than conventional UBT and serology (98 vs. 93 and 88%, respectively). Similar accuracy for serological test was reported by Rahman et al.[18] Tzeng et al.[19] did not find any significant difference in sensitivity, specificity, and positive predictive value for the four diagnostic tests for H. pylori, namely, H and, Giemsa, rapid urease test, and imprint cytology (P > 0.05).
Very few studies[20,21] have correlated the serology of H. pylori with morphological changes and density of H. pylori on IHC with serology. In the present study, cases of nonulcer dyspepsia were taken and the morphological changes in the gastric mucosa induced by the colonization of H. pylori were documented and their correlation was done with anti-H. pylori serology and the severity of infection. The study also compared various diagnostic tests and evaluated the different staining methods used for H. pylori detection.
The rapid urease test was found to be of less value in diagnosing H. pylori infection in our study with a sensitivity of 74.5% which is comparable to the findings of Tokunaga et al.,[22] Malik et al.,[23] and Ceken et al.[24] On the contrary, Calvet et al.[25] and Redeen et al.[26] reported a much higher sensitivity of 94 and 90%, respectively. Moreover, Redeen et al.[26] recommended rapid urease test as the first choice as the result could be obtained within hours.
Siddique et al.[27] showed that increasing the number of gastric antral biopsies from one to four significantly improves the sensitivity of the CLO test (rapid urease test), eliminates sampling error, and hastens the time needed by the test to become positive for the diagnosis of H. pylori infection. About half of the patients (52%) had a positive CLO test in group 1 (1 biopsy), compared to 68% in group 2 (2 biopsies), 76% in group 3 (3 biopsies), and 96% in group 4 (4 biopsies) (Group 1 vs. 4 P < 0.01).
We found the seroprevalence of H. pylori infection to be 66.7%, which was comparable to two studies by Kate et al.[28,29] Serum ELISA for IgG antibodies against H. pylori correlated significantly with the presence of bacteria on histology (H and E, modified Giemsa, and IHC) which is in accordance with Booth et al.[30] and Perez-Perez et al.[20] Hashemi et al.[31] studied the diagnostic accuracy of four different staining methods on touch cytology, and stated that rapid urease test should still be acknowledged as the primary test for diagnosing H. pylori following upper gastrointestinal endoscopy.
In the present study, the sensitivity, specificity, positive predictive values and negative predictive values of IgG ELISA and IgA ELISA were similar to Urita et al.[32] and Martin-de-Argila et al.[33] On the contrary, She et al.[34] found much lower values for sensitivity, specificity, and positive predictive values as they used stool antigen test as the gold standard. Lindsetmo et al.[35] also found a much lower specificity of anti-H. pylori IgG and IgA (32-50% among the peptic ulcer patients and 58-71% among the controls). The specificity of combined IgG and IgA ELISA (82.4%) in our study fell somewhere between the specificity found by Martin-de-Argila et al.[33] (85.3%) and De Wouw et al.[21] (79%). We found that 72.1% cases positive for H. pylori on serology had gastritis on morphology which is comparable to Perez-Perez et al.[20] and Booth et al.[30]
Although in adults, ELISA has proven to be highly accurate in diagnosing H. pylori infection, it has demonstrated variable accuracy in children. Leal et al.[36] conducted a systematic review and meta-analysis to assess the accuracy of antibody-based detection tests for the diagnosis of H. pylori infection in children. In-house ELISA with whole-cell antigen tests showed the highest overall performance: Sensitivity, 94% (95% confidence interval (CI): 90.2–96.7) and specificity, 96.4% (95% CI: 94.2–97.9), whereas ELISA commercial tests varied widely in performance (test for heterogeneity P < 0.0001). Me Graud[37] compared four diagnostic tests, that is, UBT, stool antigen test, and antibody detection in serum and urine in comparison with biopsy based tests in children and adolescents. The positive and negative predictive values for the serological tests were 76.4 and 98.3%, respectively, comparable to results in adults.
In the present study, there was a significant correlation between the severity of gastritis and the grade of H. pylori infection on IHC (P < 0.001) which is in accordance with several other authors.[12,20,22]
Acute inflammation was seen in 22 cases in our study. However, this finding did not have a significant correlation with the presence of H. pylori as only 12 cases (54.5%) were positive for H. pylori (P = 0.113). On the contrary, Perez-Perez et al.[20] and Shafii et al.[38] found a significantly higher activity in H. pylori-positive cases as compared to negative cases.
Although H. pylori can be visualized in H and Estained sections in most of the infected gastric biopsies, when the bacterial load is low, they can be missed. In such cases, other stains like modified Giemsa, Wright's, Warthin-Starry, and so on can be useful. Immunohistochemical detection of H. pylori in cases with very low density of infection has proved to be a very effective diagnostic modality in recent years. In our study, modified Giemsa fared better than H and E and was positive in 41 (80.4%) of 51 cases which were positive for H. pylori. Loffeld et al.[39] found Giemsa to be positive in 78% patients and IHC in 89% and recommended Giemsa staining as a routine detection method. Similar results were obtained by Tokunaga et al.[22]
We recommend that IHC should be judiciously used, and simple and economical tests like modified Giemsa should be used routinely for the detection of H. pylori. IHC should be used if there are no constraints of resources and it should be used only in cases where other staining modalities have failed to detect H. pylori. Combined ELISA (IgG and IgA) should be preferred over single ELISA for the detection of H. pylori infection. IgM ELISA was found to be of little diagnostic utility and it is recommended that the use of IgM serology can be avoided. Multiple biopsies should preferably be taken because of the highly patchy distribution of H. pylori due to which its presence can be underestimated. Simultaneous morphologic and serological detection of H. pylori is preferable as it may not be detected on morphology alone due to its patchy distribution in the stomach. Secondly, ELISA if done alone, may overestimate the presence of active H. pylori infection as antibody titers can remain elevated even after the eradication of H. pylori. Moreover, precancerous morphological changes associated with H. pylori infection may be missed if serology alone is performed.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–5. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 2.Blaser MJ. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990;161:626–33. doi: 10.1093/infdis/161.4.626. [DOI] [PubMed] [Google Scholar]
- 3.Wotherspoon A, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori associated gastritis and primary B cell Lymphoma. Lancet. 1991;338:1175–6. doi: 10.1016/0140-6736(91)92035-z. [DOI] [PubMed] [Google Scholar]
- 4.Graham DY, Klein PD, Evans DG, Fiedorek SC, Evans DJ, Jr, Adam E, et al. Helicobacter pylori: Epidemiology, relationship to gastric cancer and the role of infants in transmission. Eur J gastroenterol Hepatol. 1992;4(suppl):S1–6. [Google Scholar]
- 5.Kumar M, Yachha SK, Aggarwal R, Shukla S, Pandey R, Prasad KN, et al. Healing of chronic antral gastritis: Effect of sucralfate and colloidal bismuth subcitrate. Ind J Gastroenterol. 1996;15:90–3. [PubMed] [Google Scholar]
- 6.IC Wu, DC Wu, CY Lu, Kuo CH, Su YC, Yu FJ, et al. Comparison of serum and urine ELISA methods for the diagnosis of Helicobacter pylori- a prospective pilot study. Hepatogastroenterology. 2004;51:1736–41. [PubMed] [Google Scholar]
- 7.Luzza F, Maletta M, Imeneo M, Marcheggiano A, Iannoni C, Biancone L, et al. Salivary specific immunoglobulins G in the diagnosis of Helicobacter pylori infection in dyspeptic patients. Am J Gastroenterol. 1995;90:1820–3. [PubMed] [Google Scholar]
- 8.Laine L, Lewin DN, Naritoku W, Cohen H. Prospective comparison of H and E, Giemsa and genta stains for the diagnosis of Helicobacter pylori. Gastrointest Endosc. 1997;45:463–7. doi: 10.1016/s0016-5107(97)70174-3. [DOI] [PubMed] [Google Scholar]
- 9.Genta RM, Robason GO, Graham DY. Simultaneous visualization of Helicobacter pylori and gastric morphology: A new stain. Hum Pathol. 1994;25:221–6. doi: 10.1016/0046-8177(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 10.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis.The updated Sydney system. Am J Surg Pathol. 1996;20:1161–81. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Gray SF, Wyatt JI, Rathbone BJ. Simplified techniques for identifying Campylobacter pyloridis. J Clin Pathol. 1986;39:1279. doi: 10.1136/jcp.39.11.1279-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tokunaga Y, Shirahase H, Yamamoto E, Gouda Y, Kanaji K, Ohsumi K. Semiquantitative evaluation for diagnosis of Helicobacter pylori infection in relation to histological changes. Am J Gastroenterol. 1998;93:26–9. doi: 10.1111/j.1572-0241.1998.026_c.x. [DOI] [PubMed] [Google Scholar]
- 13.Atherton JC, Blaster MJ. Helicobacter pylori infections. In: Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, editors. Harrison's principles of internal medicine. 15th ed. New York: Mc Graw-Hill Company; 2001. pp. 960–2. [Google Scholar]
- 14.Poddar U, Yaccha SK. Helicobacter pylori in children: An Indian perspective. Indian Pediatr. 2007;44:761–70. [PubMed] [Google Scholar]
- 15.Sahay P, Axon AT. Non-ulcer dyspepsia: Does Helicobacter pylori matter? Postgrad Med J. 1995;71:262–4. doi: 10.1136/pgmj.71.835.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malary HM, Nyren O. Epidemiology of Helicobacter pylori infection. Helicobacter. 2003;8:8–12. doi: 10.1046/j.1523-5378.2003.00163.x. [DOI] [PubMed] [Google Scholar]
- 17.Peng NJ, Lai KH, Lo GH, Hsu PI. Comparison of noninvasive diagnostic tests for Helicobacter pylori Infection. Med Princ Pract. 2009;18:57–61. doi: 10.1159/000163048. [DOI] [PubMed] [Google Scholar]
- 18.Rahman SH, Azam MG, Radman MA, Arfin MS, Alam MM, Bhuiyan TM, et al. Non-invasive diagnosis of H. pylori infection: Evaluation of serological tests with and without current infection marker CIM. World J Gastroenterol. 2008;28:1231–6. doi: 10.3748/wjg.14.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tzeng JE, Lin YL, Chung SM, Chu YT. Comparison of four diagnostic methods for Helicobacter pylori. Tzu Chi Med J. 2005;17:339–43. [Google Scholar]
- 20.Perez-Perez GI, Brown WR, Cover TL, Dunn BE, Cao P, Martin JB. Correlation between serological and mucosal inflammatory responses to Helicobacter pylori. Clin Diagn Lab Immunol. 1994;1:325–9. doi: 10.1128/cdli.1.3.325-329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Wouw BA, De Boer WA, Jansz AR, Roymans RT, Staals AP. Comparison of three commercially available enzyme linked immunosorbent assays and biopsy dependent diagnosis for detecting Helicobacter pylori infection. J Clin Microbiol. 1996;34:94–7. doi: 10.1128/jcm.34.1.94-97.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tokunaga Y, Shirahase H, Yamamoto E, Inao R, Hamaguchi S, Kanaji K, et al. Modified rapid urease test for H. pylori detection in relation to an immunohistochemical stain. J Gastroenterol Hepatol. 2000;15:617–21. doi: 10.1046/j.1440-1746.2000.02213.x. [DOI] [PubMed] [Google Scholar]
- 23.Malik GM, Mubarik M, Kadla SA. Helicobacter pylori infection in endoscopy biopsy specimens of gastric antrum: Laboratory diagnosis and comparative efficacy of three diagnostic tests. Diagn Ther Endosc. 1999;6:25–9. doi: 10.1155/DTE.6.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ceken N, Yurtsever SG, Baran N, Alper E, Buyrac Z, Unsal B. Comparison of Helicobacter pylori antibody detection in stool with other diagnostic tests for infection. Asian Pac J Cancer Prev. 2011;12:1077–81. [PubMed] [Google Scholar]
- 25.Calvet X, Sanchez-Delgado J, Montserrat A, Lario S, Ramirez-Lazaro MJ, Quesada M, et al. Accuracy of diagnostic tests for Helicobacter pylori: A reappraisal. Clin Infect Dis. 2009;48:1385–91. doi: 10.1086/598198. [DOI] [PubMed] [Google Scholar]
- 26.Redeen S, Petersson F, Kechagias S, Mardh E, Borch K. Natural history of chronic gastritis in a population-based cohort. Scand J Gastroenterol. 2010;45:540–9. doi: 10.3109/00365521003624151. [DOI] [PubMed] [Google Scholar]
- 27.Siddique I, Al-Mekhaizeem K, Alateeqi N, Memon A, Hasan F. Diagnosis of Helicobacter pylori: Improving the sensitivity of CLOtest by increasing the number of gastric antral biopsies. J Clin Gastroenterol. 2008;42:356–60. doi: 10.1097/MCG.0b013e31802b650d. [DOI] [PubMed] [Google Scholar]
- 28.Kate V, Ananthakrishnan N. Helicobacter pylori and gastric carcinoma: Evidence for the link. Natl Med J India. 2000;13:329. [PubMed] [Google Scholar]
- 29.Kate V, Ananthakrishnan N, Badrinath S, Ratnakar C. Prevalence of Helicobacter pylori infection in disorders of the upper gastrointestinal tract in south India. Natl Med J India. 1998;11:5–8. [PubMed] [Google Scholar]
- 30.Booth L, Holdstock G, MacBride H, Hawtin P, Gibson JR, Ireland A, et al. Clinical importance of Campylobacter pylorides and associated serum IgG and IgA antibody responses in patients undergoing upper gastrointestinal endoscopy. J Clin Pathol. 1986;39:215–9. doi: 10.1136/jcp.39.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashemi MR, Rahnavardi M, Bikdeli B, Zahedani MD, Iranmanesh F. Touch cytology in diagnosing Helicobacter pylori: Comparison of four staining methods. Cytopathology. 2008;19:179–84. doi: 10.1111/j.1365-2303.2007.00417.x. [DOI] [PubMed] [Google Scholar]
- 32.Urita Y, Hike K, Torii N, Kikuchi Y, Kurakata H, Kanda E, et al. Comparison of serum IgA and IgG antibodies for detecting Helicobacter pylori infection. Intern Med. 2004;43:548–52. doi: 10.2169/internalmedicine.43.548. [DOI] [PubMed] [Google Scholar]
- 33.Martin-de-Argila C, Boixeda D, Canton R, Valdezate S, Mir N, De Rafeal L, et al. Usefulness of the combined IgG and IgA antibody determinations for serodiagnosis of Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 1997;9:1191–6. [PubMed] [Google Scholar]
- 34.She RC, Wilson AR, Litwin CM. Evaluation of Helicobacter pylori IgG, IgA and IgM serologic testing compared stool antigen testing. Clin Vaccine Immunol. 2009;16:1253–5. doi: 10.1128/CVI.00149-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindsetmo RO, Johnsen R, Eide TJ, Gutteberg T, Husum HH, Revhaug A. Accuracy of Helicobacter pylori serology in two peptic ulcer populations and in healthy controls. World J Gastroenterol. 2008:145039–45. doi: 10.3748/wjg.14.5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leal YA, Flores LL, Garcı×a-Corte×s BL, Cedillo-Rivera R, Torres J. Antibody-based detection tests for the diagnosis of Helicobacter pylori infection in children: A meta-analysis. PLos One. 2008;3:e3751. doi: 10.1371/journal.pone.0003751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MéGraud F. Comparison of non-invasive tests to detect Helicobacter Pylori infection in children and adolescents: Results of a multicenter European study. J Pediatr. 2005;146:198–203. doi: 10.1016/j.jpeds.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 38.Shafii M, Nikzad SE, Kasiri H, Naghipour M. Histopathological evaluation of chronic gastritis with and without Helicobacter pylori colonization: A study from Iran. Malays J Pathol. 2008;30:27–30. [PubMed] [Google Scholar]
- 39.Loffeld R, Stobberingh E, Flendrig JA, Arends JW. Helicobacter pylori in gastric biopsy specimens.comparison of culture, modified giemsa stain and immunohistochemistry. A retrospective study. J Pathol. 1991;165:69–73. doi: 10.1002/path.1711650111. [DOI] [PubMed] [Google Scholar]


