Abstract
Providing anaesthesia for the separation surgery of conjoined twins presents unique challenges to the managing anaesthesiologists. The low incidence of such surgeries and anatomical variations in each type of conjoined twins makes each separation surgery a unique experience. This report features the anaesthetic plan and challenges faced in performing the separation surgery of a set of thoraco-omphalopagus twins in a rural secondary hospital in a remote location in India.
Keywords: Anaesthesia, anaesthetic management, conjoined twins, thoraco-omphalopagus twins, twin separation surgery
INTRODUCTION
A set of female thoraco-omphalopagus conjoined twins were born at a rural secondary hospital in a remote location in India. The twins were delivered by emergency caesarean section and they cried well after birth. Their combined weight at birth was 3.7 kg. Though their neonatal period was uneventful, at the age of 6 and 8 months, Twin B developed pneumonia on two separate occasions, which was successfully treated. There was no other significant history. Though individually both girls were underweight for their age, they were feeding well and healthy, and when scheduled for surgery, at age 11 months, the twins combined weight was 12.5 kg.
The hospital had become home to the twins as the parents had abandoned them and they were being looked after by the hospital staff ever since. The idea of surgery at a better resourced centre was considered and then deferred because the twins had become an integral part of the community and it was felt that if we could provide expert care with all the facilities required, we ought to keep them in the midst of the loving and caring community in which they had grown up. The hospital is a multispecialty 250-bedded unit, routinely performing obstetric, general surgery, orthopaedic, plastic and oncosurgical procedures. Though paediatric patients are routinely operated there, the hospital was not resourced for this complex and high-risk surgery, nor for the post-operative care.
A core planning team consisting of doctors, nurses, paramedics, administrators and public relations officers was formed from within the hospital. They put together a team of specialists from within the hospital, from other Indian centre's and abroad. The team included paediatric surgeons, a cardiothoracic surgeon, a plastic surgeon, anaesthesiologists, paediatric intensivists and neonatologists. The ancillary services including personnel, laboratory backup, equipment and disposables were taken into consideration. The public relations officers garnered financial support from the media and equipment support from the medical companies. All of this, along with expert medical care provided by various disciplines, helped convert the rural secondary hospital into a temporary tertiary paediatric care unit.
CASE REPORT
The 11-month-old twin girls were joined from manubrium to umbilicus by a 15 cm vertical and 8-10 cm horizontal elliptical skin bridge, which was slightly off centre. Hence when the twins lay down, Twin A was to the right and was deficient more on the left precordium whereas Twin B was to the left and was deficient more on the right precordium [Figure 1]. The twins lay facing each other with one set of upper limbs and one set of lower limbs at the back. Twin A was the smaller twin and had mild micrognathia. Twin B had right-sided hemi-facial bony hypoplasia. Both twins had adequate mouth opening and neither had snoring or mouth breathing. The Chest X-ray [Figure 2] and computed tomography (CT) scan of the chest revealed separate rib cages for both, which were deficient anteriorly. A CT scan of the abdomen confirmed sharing of a wide bridge of liver, as seen on ultrasound abdomen. Each had a separate gall bladder and porta hepatis. Colour doppler over the liver bridge revealed multiple venous channels with preferential flow from Twin A to Twin B. A 12 lead electrocardiogram showed electrical activity from both hearts, with two separate QRS complexes [Figure 3]. With this it was inferred that both hearts had separate normal electrical conduction pathways. Blood investigations revealed haemoglobin of 9.6 gm% for Twin A and 10.9 gm% for Twin B, with normal biochemistry values for both. Blood and blood products including fresh frozen plasma, platelets and cryoprecipitate were reserved for surgery.
Figure 1.
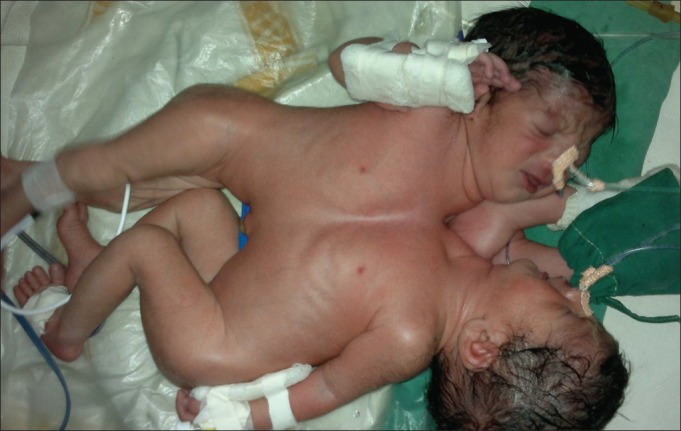
Twins at birth
Figure 2.
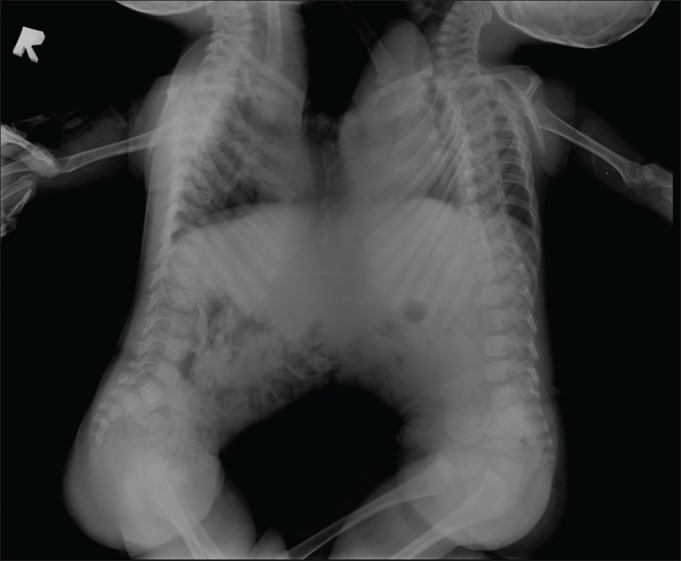
Chest X-ray
Figure 3.
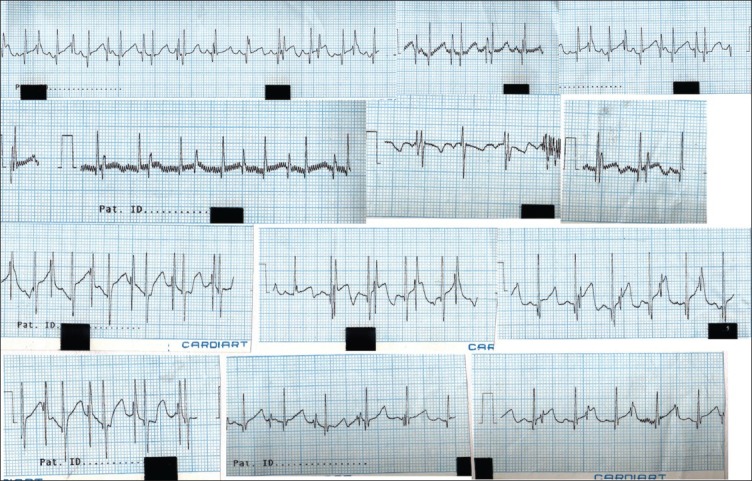
ECG showing 2 QRS complexes
Planning and preparation
Meticulous planning went into the preparation for the surgery and post-op care [Figure 4]. A mindmap was created to ensure that nothing was forgotten. One specialist for each of the specialties visited the hospital and looked at the feasibility of doing the surgery. Each was then asked to provide a list of equipment they thought might be needed for the surgery. The lists were categorised as “must have”, “would like” and “ideal”. The media and equipment companies were then contacted and all requirements were fulfilled. Since access to the village was limited, as it was 4 hours from the nearest airport, every piece of equipment and disposable had to be available on site.
Figure 4.
Mind map
Two days before surgery, the surgical and anaesthetic teams discussed the steps of the surgery and the problems anticipated at each step. The individual groups then discussed problems specific to their specialty and drew up a detailed plan of action.
Simulation
On the day before the planned surgery, a simulation of the significant steps was done [Figure 5]. Two dolls were joined together using tape. Monitoring lines, old endotracheal tubes, anaesthetic circuits, i.v. sets and central venous catheters were used to simulate the numerous lines and tubes that would be used. Monitoring cables and the lines to each twin were isolated using colour-coded wraps allowing the twins, once separated to be lifted in one smooth motion without entangling of the lines. The dolls were then separated and the transfer of one “twin” to another operating table was done using a sterile, draped transfer trolley. Finally the transfer to the paediatric ICU (PICU) was also simulated with equipment including transfer monitors.
Figure 5.
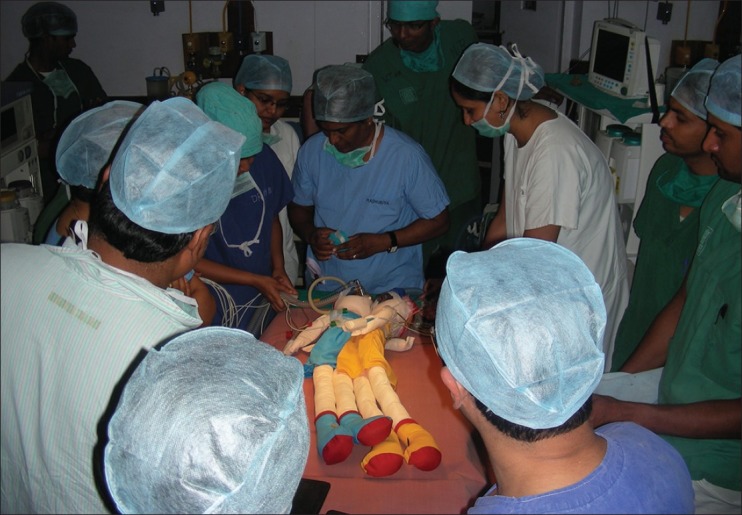
Simulation
Separation procedure
On the day of surgery, the twins were premedicated with oral ketamine 3 mg/kg and midazolam 0.5 mg/kg each, mixed with honey. After 20 minutes, they were wheeled into the operating room. Anaesthesia teams consisting of two anaesthesiologists and one anaesthesia technician were separately designated for each twin. Anaesthesia was delivered to Twin A using a Fabius® Plus (Drager, Lubeck, Germany) workstation and to Twin B using an Aespire 7900 (GE Healthcare, Waukesha, WI, USA) workstation. Monitors were IntelliVue MP 40 (Phillips, Best, The Netherlands) monitors. Non-invasive monitoring with electrocardiography (ECG), oxygen saturation (SpO2), end-tidal carbon dioxide (EtCO2) and non-invasive blood pressure (NIBP) was started and baseline parameters were noted. Both twins were simultaneously induced with sevoflurane in oxygen-nitrous oxide mixture, using an Ayre's T-piece. Peripheral intravenous lines were secured in the upper limbs with 22 G cannulae. All drugs and intravenous fluids were calculated on the total weight basis and half given to each twin. Fentanyl 2 μg/kg was given and ventilation checked with assistance. Muscle relaxants were avoided at induction as the close proximity of the infants’ faces potentially made airway management and intubation difficult. There was also a possibility of crossover of drugs from one twin to the other through the patent liver venous channels. The twins were both intubated orally first and then nasally, one after the other. Once the nasal cuffed (Kimberly Clarke Microcuff™) endotracheal tubes size 4.0 were confirmed for position and fixed, atracurium 0.5 mg/kg was administered. Arterial lines were secured in the right radial and left radial for Twin A and B, respectively. The internal jugular vein (IJV) cannulation was done with triple lumen catheters, 5.5 Fr, under ultrasound guidance with careful positioning and support. It was found that the right IJV of Twin A overlay the right carotid artery and the left IJV of Twin B was medial to the left carotid artery. A second peripheral venous cannula was placed on the foot of each twin with long extensions for access. Nasogastric tubes, nasopharyngeal temperature probes and urinary catheters were inserted. All the tubing, urinary catheters, wires, machines and equipment were colour-coded with coloured electric tape (Twin A red and Twin B green) and then securely wrapped in covers to help easy segregation during separation and transport. From mid thigh downwards, their legs were wrapped in cotton padding and covered in Opsite™ (Smith and Nephew, London, UK) for temperature control as well as easy separation. They were then placed on a warming blanket.
Anaesthesia was maintained on air, oxygen and isoflurane. The lungs were ventilated by pressure control ventilation set at 12-15 cm of H2O, able to deliver tidal volume of 7-10 ml/kg body weight. Intraoperative monitoring included 3-lead ECG, pulse oximetry, non-invasive BP, invasive BP (IBP), continuous central venous pressure (CVP), core temperature, EtCO2, agent analyser, airway pressures (Paw) and urine output. The initial ECG reflected the QRS complexes of both the twins in each ECG, which reverted to normal after they were physically separated. Hence, alarm limits were set based on pulse rate as determined by pulse oximetry rather than the ECG and the ECG trace was closely monitored for arrhythmias. Intravenous maintenance fluid used was Ringer's lactate with 1% dextrose, administered via syringe pump at 2-4 ml/kg/h. Remaining fluid requirement per hour including losses was replaced with Ringer's lactate solution via burette set. Blood loss was replaced with fresh whole blood in 100 ml aliquots. Arterial blood gases including haematocrit, electrolytes, blood sugar and lactate were monitored using i-STAT® System cartridges (Abbot Laboratories, Illinois, USA) in the operating room. Values were checked after induction, after pericardial closure, after liver division and after separation during abdominal closure. Blood sugars appeared to be low and this was corrected with 5% dextrose. Blood gases and lactate were remarkably stable.
The surgical preparation of the twins included positioning for adequate exposure while protecting the pressure points. After anaesthetic preparation was complete, the twins were lifted and surgically prepped on the posterior side first, placed on sterile sheets and then prepped anteriorly. Initially, one surgical team comprising the cardiothoracic and paediatric surgeon started the chest separation. The twins shared a single pericardial sac, which was divided. Twin A required a bovine pericardial patch (St Jude Medical®) to cover the pericardial defect, while closure of the pericardium was possible in Twin B. The surgery then went to the second phase of separation. The diaphragm was first divided and the liver exposed. A Pringle's manoeuvre was performed in Twin B where a temporary occlusion of the hepatic artery and portal vein within the hepatoduodenal ligament causing a temporary ischemia of Twin B's liver. The resulting line of demarcation then defined the line of resection. The liver was meticulously divided, with careful attention to the vascular channels. At this time, it was noticed that the blood pressure and CVP of Twin A increased and that of twin B decreased, as compared to baseline. This was due to the reduced blood flow from Twin A to Twin B from the venous channels in the liver and was corrected by infusing more fluid including colloid and blood to Twin B to compensate for the reduced venous return. The intraoperative fluid and blood requirement for Twin A was significantly lower than for Twin B. After 6 h of surgery, the twins were finally separated; the abdominal wall defect was temporarily closed by medical grade plastic bags, which were made by cutting sterile blood collection bags and suturing them to the wound edges [Figure 6]. Twin B safely transferred to the other operating table [Figure 7].
Figure 6.
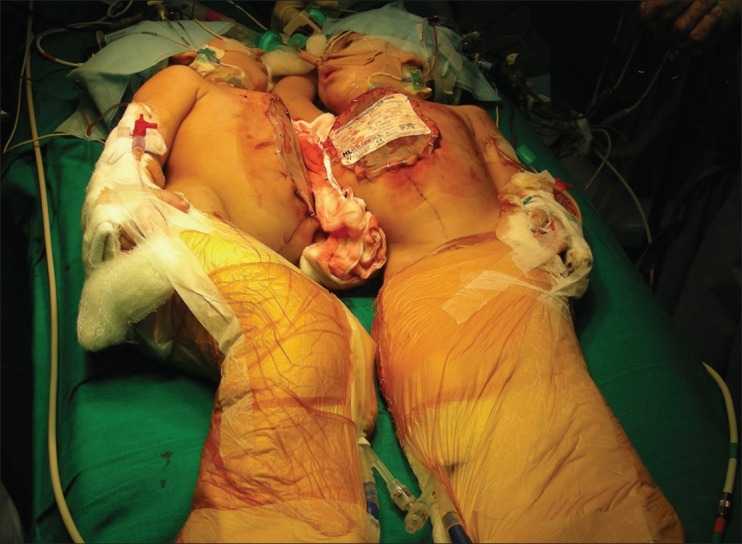
Separated twins
Figure 7.
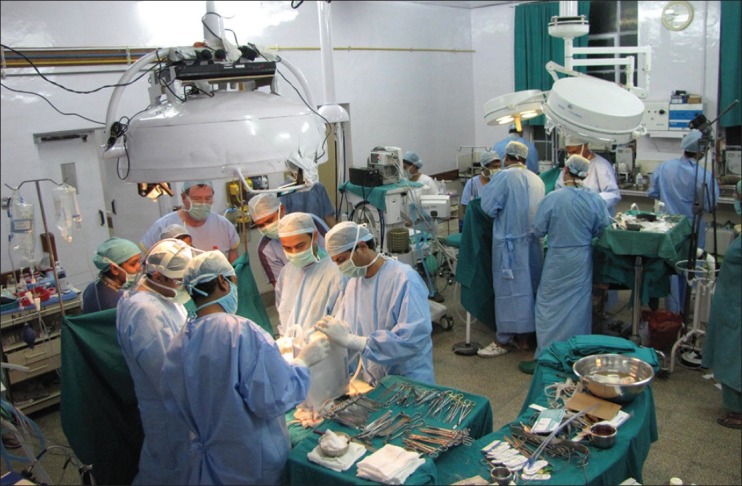
Twins on separate operating tables
After separation, both babies were re-prepped and re-draped. Abdominal wall closure was possible for Twin A after mobilising the lax abdominal skin and using bovine pericardial patch for the peritoneal defect. For Twin B, the chest closure followed by abdominal closure using bovine pericardial patch for the peritoneal defect was attempted. Upon closure, the CVP increased 5 mmHg above the baseline, Paw increased by 5 mmHg and systolic IBP fell by 20 mmHg. Intra-abdominal pressure (IAP) was then measured by the urinary catheter three-way stopcock connected to a transducer, and was found to have increased to 15 mmHg. IAP up to 12 mmHg was accepted. On reopening the chest, the vitals signs were restored. These haemodynamic changes were thought to be a result of reduced space within the thoracic cavity, causing a tamponade effect on heart. Inotropic support with dopamine was added at 5 μg/kg/min. The thymus was removed to debulk the chest anteriorly, and the chest and abdominal wall defect was left open and covered with sterile vacuum-assisted closure device (V.A.C® therapy system, KCI Licensing Inc., Sparks, Maryland, USA) to enhance growth of granulation tissue. For both the twins, the anterior chest wall was supported by porcine dermal collagen implant, Permacol™ (Covidien Surgicals, Dublin, Ireland) in place of missing sternum. The entire procedure took 12 h.
Twin A was transferred first to the PICU with complete monitoring using a Jackson Rees T-piece circuit. Twin B was similarly transferred to the PICU 45 min after Twin A. Both were electively ventilated postoperatively, with post-operative analgesia and sedation being provided by opiate (morphine) and midazolam infusions.
DISCUSSION
The birth of conjoined twins has always fascinated man. The incidence is small, i.e. 1:50,000 pregnancies, but the true incidence is 1:250,000 as 60% of the twins succumb in utero.[1] Hence, there is paucity of literature on the anaesthetic management of conjoined twins.
Conjoined twins are classified according to the location of the tissue that links the twins. Twenty-eight percent of conjoined twins are classified as thoraco-omphalopagus.[2] Antenatal ultrasound can detect the presence of conjoined twins in almost all cases as early as 12 weeks of gestation.[2]
Preoperative assessment of the twins to evaluate the extent of organ sharing is the first and most important step.
To prepare for the surgery, extensive imaging was performed. CT scans showed that the girls had separate hearts; their livers were fused and their intestines were adjacent to each other, but their digestive systems functioned separately. Their sternums were joined but their ribs were separate. ECGs showed discordant rate and rhythm with two separate QRS complexes that ruled out any electrophysiological connection.[3]
Kingston et al.[4] suggest dynamic biliary scintigraphy with Tc99m hepatobiliary iminodiacetic acid (HIDA) scan to demonstrate independent biliary drainage system. It was not required in our twins as separate gall bladders and porta hepatis were visualised on imaging. Liver sharing is found in 80% of omphalopagus babies.[5] Kingston et al. suggest initial liver assessment with abdominal ultrasound followed by magnetic resonance imaging (MRI) or contrast-enhanced computerised tomography (CECT). A CECT was performed in the twins and the intravenous injection of contrast in one twin delineated the liver parenchyma belonging to that twin. CECT also helped delineate the vascular anatomy, venous drainage and biliary system, which was separate in each of the twins.
Presence or absence of cross-circulation is an important determining factor in planning the induction as well as haemodynamics after separation. For the preoperative evaluation of cross-circulation, Toyoshima et al.[6] injected a bolus of indigo carmine and the pigment appeared in the urine of the other twin. They also undertook radioimmune (RI) angiography, which showed that radionuclides in one twin were similar to those in the other after 5-10 min. In our set of twins, it was found that there were large venous channels with blood flow from Twin A to Twin B, but Doppler studies as well as the CECT showed that the livers were individually perfused and that one twin was not dependent on the other for its blood supply. The venous contribution from Twin A to Twin B explained why Twin A was the smaller of the two. Intraoperatively, ligation of these venous channels caused signs of hypovolemia, with lowering of the CVP and BP in Twin B, while Twin A became normovolemic/hypervolemic. Twin B also required more fluids, colloid and blood to maintain the higher cardiac output that she was used to. Therefore, we recommend determination of presence or absence of and amount of cross-circulation occurring between twins which will give a good idea of what to expect intraoperatively, when vascular channels are divided.
Muscle relaxants were not used until the airway was secured. Awake intubation has also been reported with some haemodynamic compromise in the other twin due to coughing of the first child.[6] In our case, nasotracheal intubation was performed in consideration of subsequent repositioning during surgery as well as the requirement for postoperative ventilation.
Central venous access was obtained using triple lumen catheters in the IJV of both twins, to provide reliable venous access and the ability to measure central venous pressures. We anticipated difficulties due partly to the positioning of the children, and used ultrasound guidance for locating the IJV. The IJV in Twin A overlay the internal carotid artery, as might have been anticipated by the positioning of the child, and significant rotation of the neck, however the medial positioning of the IJV in Twin B was unexpected and would have been difficult, if not impossible to access without ultrasound guidance.
Greenberg et al. have described the use of caudal epidural along with general anaesthesia in omphalopagus conjoined twins separation.[7] However, they also mention that early extubation as a potential advantage of postoperative regional analgesia may not always be possible as these babies have a high chance of postoperative respiratory failure. We avoided regional anaesthesia due to practical difficulties in positioning for the epidural and managed postoperative analgesia with opioid infusions.
Closure of the chest can give rise to a number of problems. Deficient ribs and sternum can change the dynamics of the chest wall, giving rise to a simulated flail chest. The chest wall requires support and the child may need prolonged post-op ventilation. Permacol was used to provide “structure” to the chest wall in our twins, both of whom were electively ventilated postoperatively. Another problem we noted was the severe haemodynamic compromise seen in Twin B after pericardial and chest wall closure. It is essential to monitor intra-arterial, central venous, peak airway and intra-abdominal pressures to diagnose the problem early and take corrective action immediately.
CONCLUSION
Surgery for the separation of conjoined twins presents many challenges. This procedure was the culmination of several months of complex planning involving specialists from nearly every part of the hospital. But the overall success depends on detailed preparation, the involvement of caring and expert personnel, and appropriate technology.
Note: Sadly Twin B succumbed to respiratory complications 14 days post-operatively. Twin A continues to be well and thriving 2 months later.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Coran A. Paediatric surgery. In: Spitz L, Kiely EM, Pierro A, editors. Chapter 131. Conjoined Twins. 7th ed. Philadelphia, USA: Elsevier Saunders; 2012. pp. 1725–38. [Google Scholar]
- 2.Fagan CJ. Antepartum diagnosis of conjoined twins by ultrasonography. AJR Am J Roentgenol. 1977;129:921–2. doi: 10.2214/ajr.129.5.921. [DOI] [PubMed] [Google Scholar]
- 3.Wu MH, Lai YC, Lo HM, Hsu YH, Lue HC. Assessment of electromyocardial continuity in conjoined (thoracopagus) twins. Am J Cardiol. 1992;69:830–2. doi: 10.1016/0002-9149(92)90521-y. [DOI] [PubMed] [Google Scholar]
- 4.Kingston CA, McHugh K, Kumaradevan J, Kiely EM, Spitz L. Imaging in the preoperative assessment of conjoined twins. Radiographics. 2001;21:1187–208. doi: 10.1148/radiographics.21.5.g01se011187. [DOI] [PubMed] [Google Scholar]
- 5.Spitz L. Conjoined twins. Br J Surg. 1996;83:1028–30. doi: 10.1002/bjs.1800830803. [DOI] [PubMed] [Google Scholar]
- 6.Toyoshima M, Fujihara T, Hiroki K, Namatame R, Ka K, Ooe K. Evaluation of cross circulation in conjoined twins. Masui. 1993;42:1347–50. [Article in Japanese] [PubMed] [Google Scholar]
- 7.Greenberg M, Frankville DD, Hilfiker M. Separation of omphalopagus conjoined twins using combined caudal epidural-general anesthesia. Can J Anaesth. 2001;48:478–82. doi: 10.1007/BF03028313. Erratum in: Can J Anaesth 2001;48:931. [DOI] [PubMed] [Google Scholar]


