Abstract
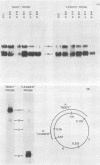
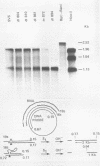
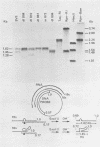
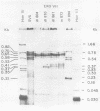
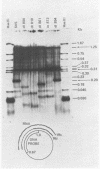
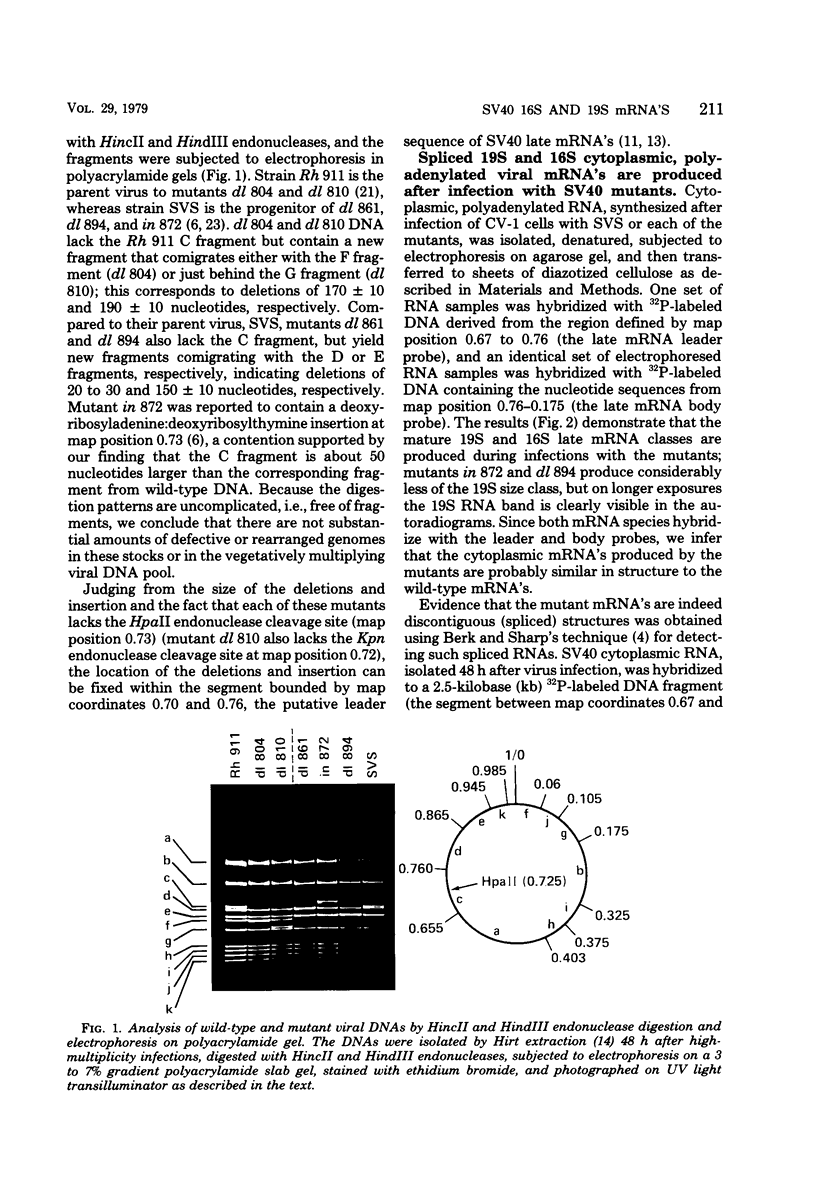
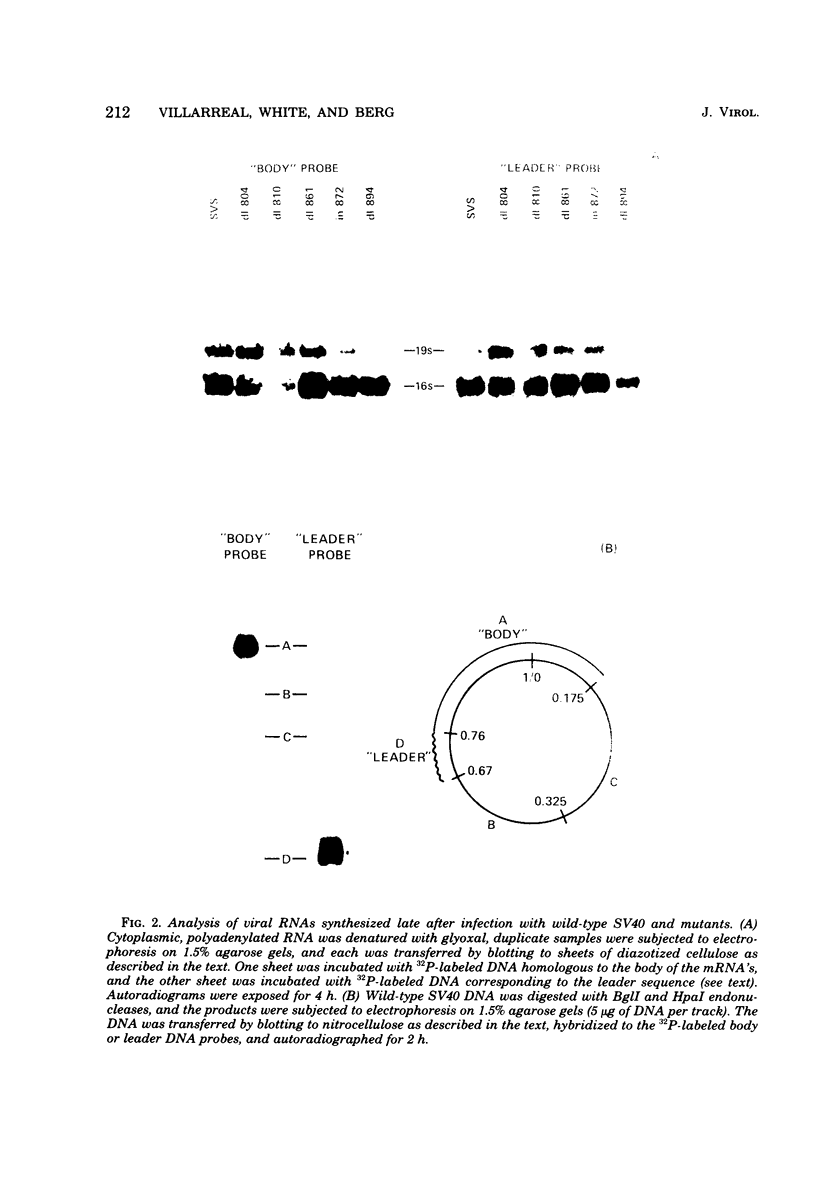
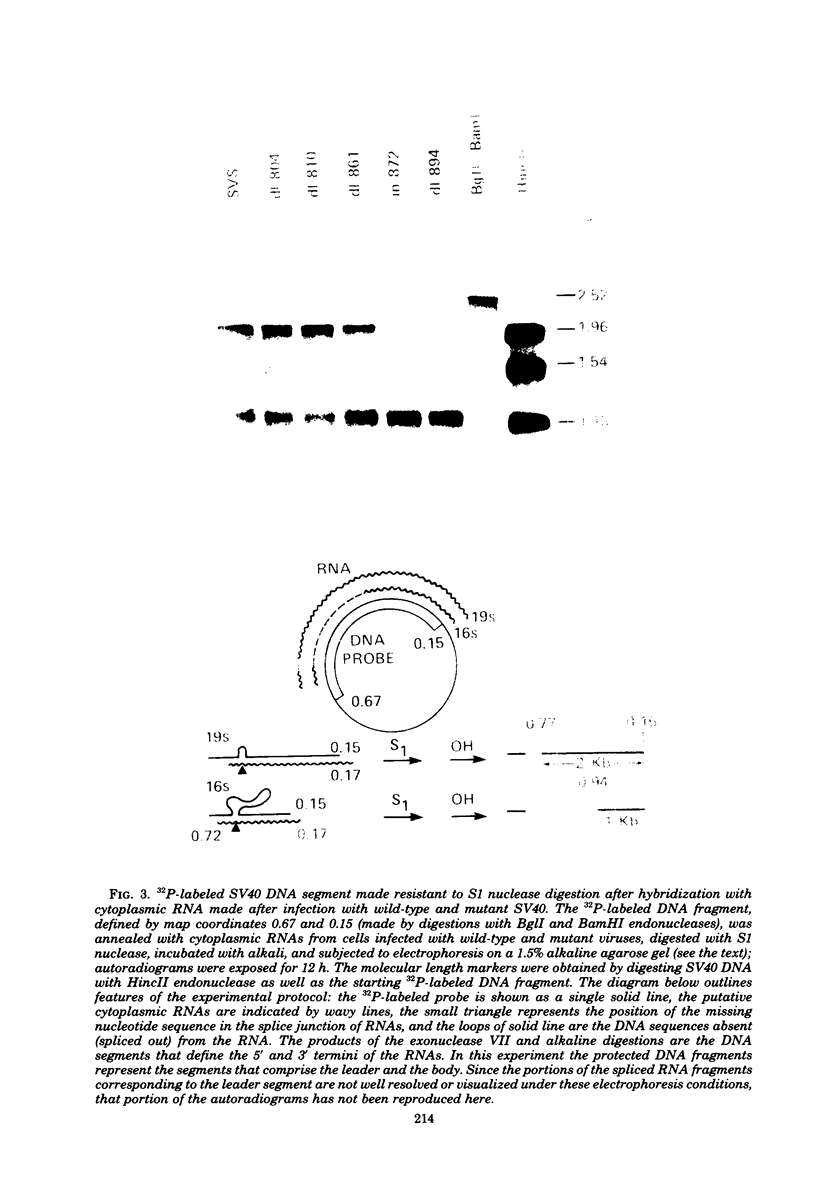
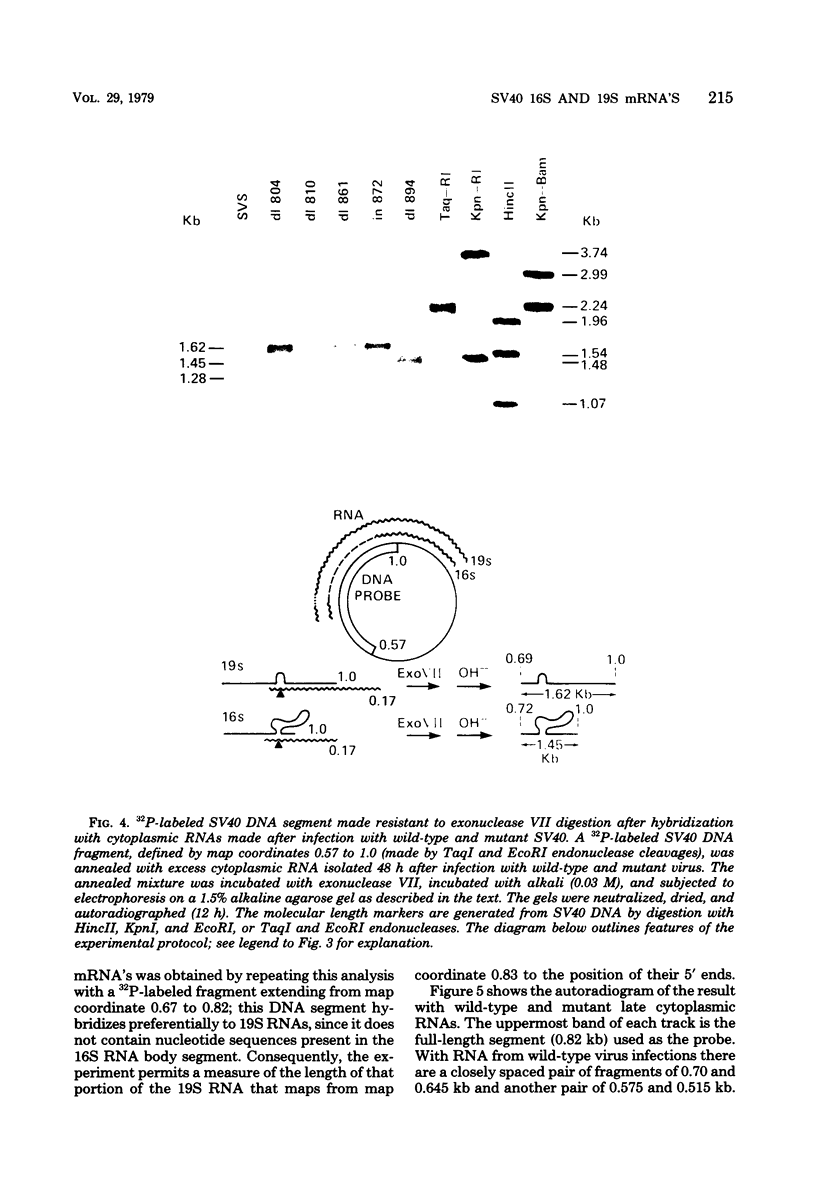
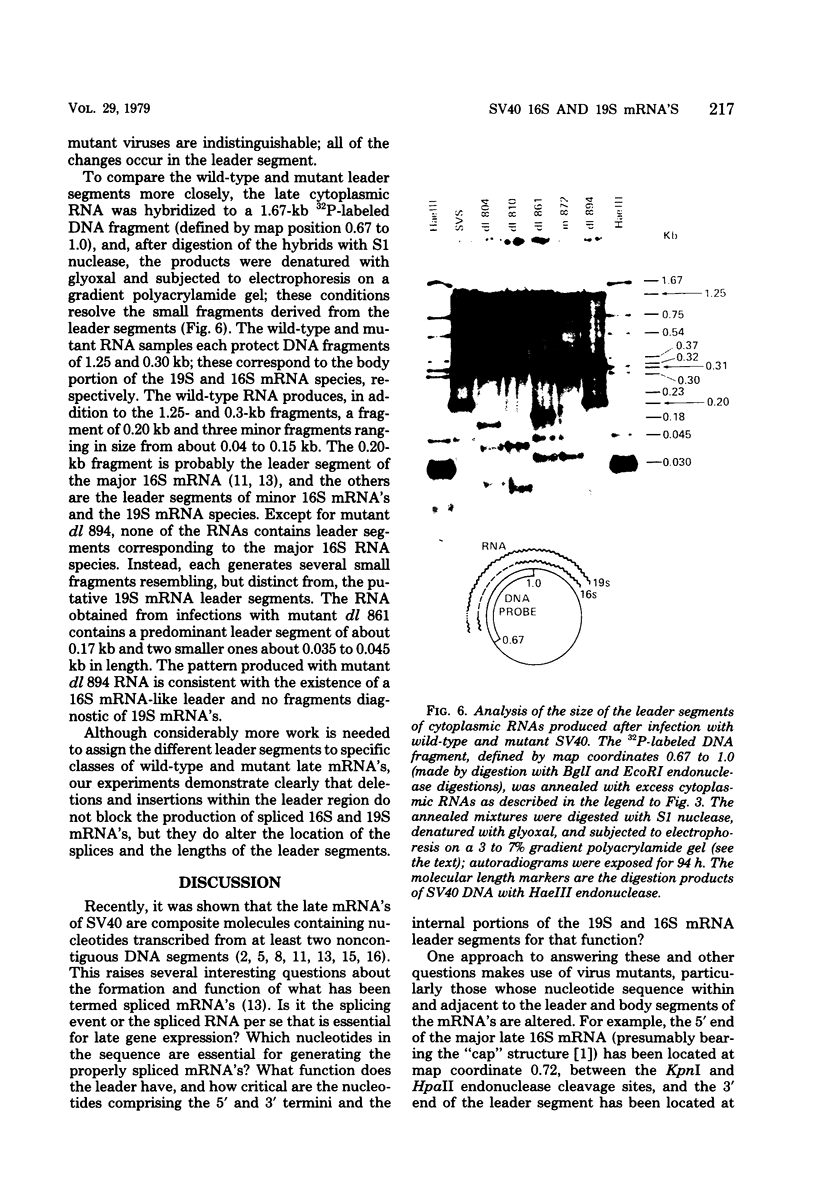
We have analyzed the structure of the late cytoplasmic RNAs made after infection with wild-type simian virus 40 and a set of viable mutants, four of which have deletions and one an insertion within the nucleotide sequence specifying the leader segment of the 16S and 19S mRNA's. The principal findings are: (i) simian virus 40 16S and 19S mRNA's made during infections with wild-type virnds and possibly in the nucleotide sequence comprising the "leader" segments. (II) "Spliced" 16S and 19S mRNA's are made during infections with each of the mutants although, in some cases, the ratio of 19S to 16S mRNA species is reduced. (iii) The deletion or insertion of nucleotides within the DNA segment defined by map position 0.70 to 0.75 causes striking alterations in the types of leader structures in the late mRNAs. (iv) Many of the late RNA leader segments produced after infection with the mutants appear to be multiply spliced, i.e., instead of the major 200- to 205-nucleotide-long leader segment present in wild-type 16S mRNA, the RNAs produced by several of the deletion mutants have leaders with whort discontiguous segments.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y., Dhar R., Laub O., Horowitz M., Khoury G. Novel mechanism for RNA maturation: the leader sequences of simian virus 40 mRNA are not transcribed adjacent to the coding sequences. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3686–3690. doi: 10.1073/pnas.74.9.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni Y., Dhar R., Laub O., Horowitz M., Khoury G. Novel mechanism for RNA maturation: the leader sequences of simian virus 40 mRNA are not transcribed adjacent to the coding sequences. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3686–3690. doi: 10.1073/pnas.74.9.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni Y. Methylated SV40 mRNAs. FEBS Lett. 1975 Jul 1;54(3):363–367. doi: 10.1016/0014-5793(75)80940-9. [DOI] [PubMed] [Google Scholar]
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratosin S., Horowitz M., Laub O., Aloni Y. Electron microscopic evidence for splicing of SV40 late mRNAs. Cell. 1978 Apr;13(4):783–790. doi: 10.1016/0092-8674(78)90228-3. [DOI] [PubMed] [Google Scholar]
- Carbon J., Shenk T. E., Berg P. Construction in vitro of mutants of simian virus 40: insertion of a poly(dA-dT) segment at the Hemophilus parainfluenza II restriction endonuclease cleavage site. J Mol Biol. 1975 Oct 15;98(1):1–15. doi: 10.1016/s0022-2836(75)80097-0. [DOI] [PubMed] [Google Scholar]
- Casey J., Davidson N. Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucleic Acids Res. 1977;4(5):1539–1552. doi: 10.1093/nar/4.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celma M. L., Dhar R., Pan J., Weissman S. M. Comparison of the nucleotide sequence of the messenger RNA for the major structural protein of SV40 with the DNA sequence encoding the amino acids of the protein. Nucleic Acids Res. 1977 Aug;4(8):2549–2550. [PMC free article] [PubMed] [Google Scholar]
- Chase J. W., Richardson C. C. Exonuclease VII of Escherichia coli. Mechanism of action. J Biol Chem. 1974 Jul 25;249(14):4553–4561. [PubMed] [Google Scholar]
- Cole C. N., Landers T., Goff S. P., Manteuil-Brutlag S., Berg P. Physical and genetic characterization of deletion mutants of simian virus 40 constructed in vitro. J Virol. 1977 Oct;24(1):277–294. doi: 10.1128/jvi.24.1.277-294.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P. K., Reddy V. B., Swinscoe J., Choudary P. V., Lebowitz P., Weissman S. M. The 5'-terminal leader sequence of late 16 S mRNA from cells infected with simian virus 40. J Biol Chem. 1978 May 25;253(10):3643–3647. [PubMed] [Google Scholar]
- Goff S. P., Berg P. Excision of DNA segments introduced into cloning vectors by the poly(dA-dT) joining method. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1763–1767. doi: 10.1073/pnas.75.4.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegeman G., Fiers W. Evidence for 'splicing' of SV40 16S mRNA. Nature. 1978 May 4;273(5657):70–73. doi: 10.1038/273070a0. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Lavi S., Groner Y. 5'-Terminal sequences and coding region of late simian virus 40 mRNAs are derived from noncontiguous segments of the viral genome. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5323–5327. doi: 10.1073/pnas.74.12.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg U., Persson T. Messenger RNA isolation with poly(U) agarose. Methods Enzymol. 1974;34:496–499. doi: 10.1016/s0076-6879(74)34061-x. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz J. E., Berg P. Defective simian virus 40 genomes: isolation and growth of individual clones. Virology. 1974 Nov;62(1):112–124. doi: 10.1016/0042-6822(74)90307-9. [DOI] [PubMed] [Google Scholar]
- Mertz J. E., Berg P. Viable deletion mutants of simian virus 40: selective isolation by means of a restriction endonuclease from Hemophilus parainfluenzae. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4879–4883. doi: 10.1073/pnas.71.12.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T. E., Rhodes C., Rigby P. W., Berg P. Biochemical method for mapping mutational alterations in DNA with S1 nuclease: the location of deletions and temperature-sensitive mutations in simian virus 40. Proc Natl Acad Sci U S A. 1975 Mar;72(3):989–993. doi: 10.1073/pnas.72.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B., Sandeen D. The ribonuclease activity of crystallized pancreatic deoxyribonuclease. Anal Biochem. 1966 Feb;14(2):269–277. doi: 10.1016/0003-2697(66)90137-0. [DOI] [PubMed] [Google Scholar]