Abstract
GW433908 is the water-soluble, phosphate ester prodrug of the human immunodeficiency virus type 1 protease inhibitor amprenavir (APV). A high-yield synthesis of GW433908 is achieved by phosphorylation of the penultimate precursor of APV with phosphorous oxychloride (POCl3) in pyridine. A single-dose pharmacokinetic study of GW433908 sodium salt in dogs showed that APV exposure was similar to that achieved with an equivalent molar dose of the APV clinical formulation (Agenerase) and that systemic exposure to the prodrug was minimal (0.3% of the APV exposure). However, the sodium salt of GW433908 was a hygroscopic, amorphous solid and thus not suitable for pharmaceutical development. The calcium salt was a developable crystalline solid, but oral dosing afforded only 24% of the APV exposure in dogs compared with Agenerase. Acidification of the dog stomach by coadministration of HCl increased the bioavailability of the calcium salt to levels near those of the sodium salt. Single-dose administration of GW433908 calcium salt in dogs and rats produced portal vein GW433908 concentrations that were maximally 1.72 and 0.79% of those of APV concentrations, respectively. Furthermore, GW433908 had poor transepithelial flux and APV showed significant flux across human-derived Caco-2 cell monolayers (a model of intestinal permeability). Taken together, these results suggest that GW433908 is primarily metabolized to APV at or in the epithelial cells of the intestine and that the prodrug is not substantially absorbed. Based in part on these findings, GW433908 was advanced to clinical development.
The widespread use of human immunodeficiency virus (HIV) protease inhibitors in combination antiretroviral regimens has been temporally associated with marked declines in HIV-related morbidity and mortality (3, 4, 6, 11, 12, 16, 19). Protease inhibitor-containing antiretroviral regimens can effect significant reductions from baseline in viral load and improve CD4+ T-cell counts and immune function (7, 17, 18, 22, 26). However, as with all chronic conditions (5), medication regimen adherence in HIV-AIDS is challenging for patients, and imperfect adherence can lead to more rapid virologic rebound and emergence of drug resistance (1, 9, 14, 15, 20, 21, 24).
Amprenavir (APV) is one of seven commercially available HIV protease inhibitors (23). APV-based therapy possesses several favorable clinical attributes (e.g., twice-daily administration without regard to food, a unique resistance pathway that may preserve future protease inhibitor treatment options, and potentially fewer metabolic effects than other currently marketed protease inhibitors). However, because of the inherent low aqueous solubility of APV, a high ratio of excipients to drug is required in the capsule formulation to aid in maintaining gastrointestinal tract solubility and ultimately absorption. Therefore, the marketed formulation of APV (Agenerase) has a substantial pill burden.
Several studies have indicated that a high pill burden reduces antiretroviral adherence and, consequently, virologic control (2, 25). Therefore, we initiated a research program to identify a water-soluble prodrug of APV that can be formulated with a lower excipient-to-drug ratio and thus a lower pill burden. From this program, GW433908 was discovered and showed systemic APV levels similar to those achieved with Agenerase when administered as an aqueous solution to rats (C. T. Baker, P. R. Chaturvedi, M. R. Hale, G. Bridson, A. Heiser, E. S. Furfine, A. Spaltenstein, and R. D. Tung. Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 916, 1999). Herein we describe, in part, the preclinical development of GW433908. The objectives of these studies were to identify a developable salt form, a suitable nonrodent species for toxicological evaluation, and a scalable synthetic route and to provide insight into the mechanism of prodrug activation.
MATERIALS AND METHODS
Chemistry
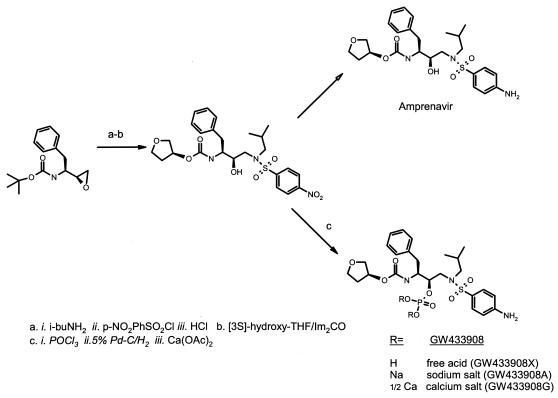
GW433908 was synthesized as outlined in Fig. 1. The overall yield of GW433908 calcium salt from the commercially available starting material, (1S)-tert-butyl-N-[1-((2S)-2-oxiranyl)-2-phenylethyl]carbamate, was about 41% of the theoretical yield.
FIG. 1.
Synthesis of GW433908.
Preparation of N-(3S-amino-2R-hydroxy-4-phenylbutyl)-N-isobutyl-4-nitrobenzenesulfonamide hydrochloride
A mixture of material (1S)-tert-butyl-N-[1-((2S)-2-oxiranyl)-2-phenylethyl]carbamate (about 450 kg, 1,709 mol) and an excess of isobutylamine were heated at reflux in toluene for a minimum of 2.5 h. The bulk of the excess isobutylamine was removed by distillation. Residual levels of isobutylamine were then reduced either by codistillation with toluene or by washing with an aqueous solution of ammonium chloride. Triethylamine (173 to 199 kg, 1,709 to 1,965 mol) was added, and then the mixture was heated to at least 90°C. To the resulting solution a solution of 4-nitrobenzenesulfonylchloride (378 to 435 kg, 1,709 to 1,965 mol) in toluene was added. The resultant solution was cooled to not less than 80°C and washed with water. The concentrated hydrochloric acid (about 304 kg) was charged while the temperature was maintained at ∼80°C and then subjected to reflux for a minimum of 1.5 h. The toluene solution was azeotropically dried, and then ethanol was added. The mixture was heated to ∼80°C, to achieve solution, and then cooled and stirred for a minimum of 3 h. The product was isolated by filtration, washed with ethanol, and then dried under vacuum at a jacket temperature of up to 100°C. The yield was about 548 kg (70% of theoretical value).
Preparation of (3S)-tetrahydrofuran-3-yl-N-(1S,2R)-1-benzyl-2-hydroxy-3-{isobutyl[(4-nitrophenyl)sulfonyl]amino}propylcarbamate
A solution of (S)-tetrahydrofuran-3-ol (∼50 kg, 567 mol) in ethyl acetate was added to N,N′-carbonyldiimidazole (∼88.4 kg, 545 mol) in ethyl acetate while the temperature was maintained below 50°C, and the mixture was stirred for not less than 1 h. In a separate reactor, a solution of the product from the previous step above (∼208 kg, 454 mol) in ethyl acetate was dried by azeotropic distillation. The solution of the activated carbonate was added. The mixture was heated to boiling and concentrated to not less than 1,000 liters by distillation. The boiling was continued for 10 to 16 h. Ethanol (not less than 312 liters) was added, and then the mixture was cooled to below 5°C and stirred for not less than 2 h. The desired product was isolated by filtration, the cake was washed with ethanol, and then the product was dried under vacuum at a jacket temperature of up to 80°C. The yield was ∼194 kg (80% of theoretical value, corrected for solvent content).
Preparation and purification of GW433908
Phosphorus oxychloride (85 to 103 liters, 2.5 to 3.0 eq) was added to the product of the previous step (∼200 kg, corrected for solvent content), pyridine (230 to 355 liters, 8 to 12 eq), and methylisobutylketone (MIBK). The mixture was stirred for at least 2 h and then added to diluted hydrochloric acid. This mixture was heated at above 50°C for at least 2 h and then cooled. The phases were separated, and the aqueous phase was back-extracted with MIBK. The combined MIBK solutions were washed with water and concentrated by distillation; the intermediate was extracted into aqueous sodium hydroxide. The aqueous phase was separated off and then washed with ethyl acetate.
A catalytic amount of 5 or 10% Pd-C catalyst (approximately 50% wet paste) was mixed with the aqueous solution of the sodium salt and methanol. The mixture was stirred under a hydrogen atmosphere at below 35°C until no further hydrogen uptake was observed. The catalyst was removed using a suitable filter that could be precoated with filter aid and then washed with methanol.
The combined filtrate and washings were heated to ∼50°C, and a solution of calcium acetate (50 to 65 kg, 0.8 to 1.1 eq) in water was added over at least 1 h at ∼50°C. The suspension was stirred at ∼50°C for approximately 30 min and then cooled to below 25°C. Further methanol could be added at this point. The solid was filtered and washed with a mixture of methanol and water and then water. Water was added, and the slurry was stirred at above 70°C. The slurry was then cooled below 70°C and filtered. The slurry procedure and filtration could be repeated, and then the solid product was washed with acetone. The product was dried under vacuum with a jacket temperature of below 60°C. The yield was about 170 to 230 kg (60 to 88% of theoretical value). The product could be milled and sieved through a suitable sieve.
Spectroscopic and analytical data
Elemental analysis for GW433908, calcium salt, and pentahydrate (C, H, N, P, S) showed the following for 750-MHz, 1H nuclear magnetic resonance (5% DCl-D2O): 0.80 (d, 3H), 0.82 (d, 3H), 1.91 (m, 1H), 1.99 (m, 1H), 2.15 (m, 1H), 2.70 (m, 1H), 2.98 (dd, 1H), 3.09 (dd, 1H), 3.13 (dd, 1H), 3.38 (dd, 1H), 3.46 (d, 1H), 3.6 to 3.7 (m, 2H), 3.82 (m, 1H), 3.89 (m, 1H), 4.22 (m, 1H), 4.58 (m, 1H), 5.00 (m, 1H), 7.30 (m, 1H), 7.33 (m, 2H), 7.37 (m, 2H), 7.69 (m, 1H), 8.04 (m, 1H). GW433908 exists as an approximately 2:1 mixture of rotamers (restricted rotation about CN amide partial double bond). Peaks quoted above are of the major rotamer. The 188.7-MHz 13C nuclear magnetic resonance data were as follows: 22.19, 22.27, 29.20, 34.77, 37.17, 52.50, 56.79, 60.18, 69.82, 75.56, 78.60, 80.42, 127.60, 129.68, 131.53, 132.21, 132.30, 137.25, 140.75, 141.61, and 160.10. Electrospray mass spectrometry showed 586.3 (M+ + 1). For the qualitative assay of susceptibility to alkaline phosphatase, a solution of ca. 10 mg of GW433908 in ca. 5 ml of pH 8 phosphate buffer was treated with ca. 100 U of bovine alkaline phosphatase (Sigma Biochemical, St. Louis, Mo.). Within 10 min, a white precipitate was observed. The material was collected by filtration and identified as APV by spectroscopic and chromatographic comparison with a commercial sample.
Dependence of GW433908 solubility on pH
The aqueous solubility of GW433908 was determined for the free acid (GW433908X), bis-sodium salt (GW433908A), and calcium salt (GW433908G) early in the development process. Only single points were assayed per pH-solvent, and quantification was performed with the UV spectrometric assay, described below. For GW433908A (bis-sodium) solubility was estimated by visual inspection. The aqueous solubility of the calcium salt (GW433908G) was determined in duplicate experiments. Samples were assayed three or four times over 10 to 18 days to demonstrate equilibrium, with the average of the last two pHs and solubility values reported. Quantification was performed with a high-pressure liquid chromatography (HPLC) assay as follows: solubilities were determined by equilibrating excess solid in the solvent of interest at 25°C. Solid was separated from solution by filtration with Gelman 0.45-μm-pore-size nylon filters (UV assay) or Millipore 0.45-μm-pore-size polyvinylidene fluoride filters (HPLC assay). For the UV assay, samples were diluted in pH 9 borate buffer to approximately 0.05 mg/ml and absorbances were measured using a Perkin-Elmer Lambda 18 UV-visible light spectrophotometer. Sample concentrations were calculated using a standard curve of the drug in pH 9 borate buffer. For the HPLC assay, samples were diluted in methanol to approximately 0.2 mg/ml and injected onto a Phenomenex Luna C18 (2) chromatography column (50 mm by 2 mm; 3 μm) at 40°C. Samples were eluted from the column using a gradient mobile phase (A, 0.05% trifluoroacetic acid in water; B, 0.05% trifluoroacetic acid in acetonitrile; gradient 0% B to 95% B over 8 min with a 2-min reequilibration). Sample concentrations were analyzed using a Hewlett-Packard HP1100 HPLC chromatograph with UV detection (220 nm), with peak areas compared to that of an external standard.
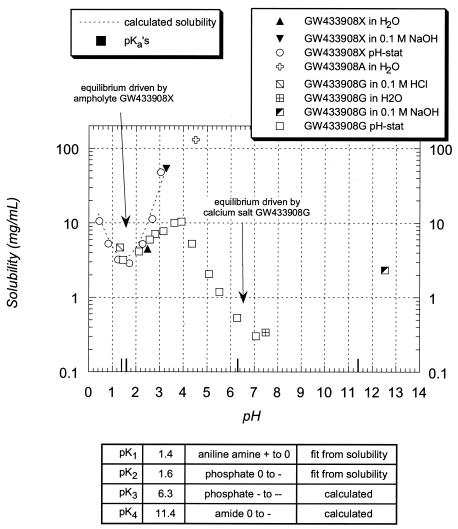
The negative logarithms of the equilibrium constants (pKs) of GW433908 were determined as follows: pK1 and pK2 were calculated by using a least-squares model in the program Micromath Scientist for Windows (version 2.01) to fit the solubility data for the pH range 0.4 to 3.3; pK3 and pK4 were calculated with the ACD/Labs pKa DB (version 3.0) software.
Animals
Male beagle dogs, weighing 10 to 14 kg, were purchased from Marshall Research Animals (North Rose, N.Y.). One dog had a portal vein cannula surgically implanted. Male Han Wistar rats, weighing 0.22 to 0.26 kg, were purchased from Taconic Farms (Germantown, N.Y.). Rats were acclimated for 1 week prior to surgical placement of a portal vein cannula and allowed to recover from surgery for at least 36 h prior to dosing. Dosing and blood collection were conducted at GlaxoSmithKline (International Development BioMetabolism, Research Triangle Park, N.C.).
The protocol and any amendment(s) or procedures involving the care and use of animals in these studies were reviewed and approved by GlaxoSmithKline's Institutional Animal Care and Use Committee prior to the experiment. During the study, the care and use of animals were performed in accordance with the guidelines of the U.S. National Research Council.
Pharmacokinetic studies. (i) APV exposure and bioavailability from clinical formulation and prodrug formulations
A crossover study design, with at least a 7-day washout period between doses, was used to administer single doses of APV and four oral preparations of GW433908 to three male beagle dogs. Animals were weighed and fasted for 16 h before and 8 h after each dose.
Four GW433908 formulations were tested: (i) hard-gelatin capsules containing the free acid of GW433908, (ii) hard-gelatin capsules containing the bis-sodium salt of GW433908, (iii) tablets containing the calcium salt of GW433908 without citrate (administered on two separate occasions), and (iv) the calcium salt with citrate. The free acid of GW433908 (60%) was combined with lactose (35.7%), Ac-Di-Sol (croscarmellose sodium; 3.3%), and sodium lauryl sulfate (1.0%). The bis-sodium salt capsules did not contain excipients. Two compressed tablet preparations containing the calcium salt of GW433908 were synthesized. In preparation A, the calcium salt (56.7%) was combined with Avicel (microcystalline cellulose; 39.4%) and crospovidone (3.9%). In preparation B, the calcium salt (56.4%) was combined with Avicel (29.7%), crospovidone (4.0%), and citric acid (10%). The dogs received two hard-gelatin capsules containing the free acid (total GW433908 free acid dose, 360 mg), one hard-gelatin capsule containing the sodium salt (total GW433908 sodium salt dose, 250 mg), and two tablets (without and with citric acid) containing the calcium salt (total GW433908 calcium salt dose, 418 and 434 mg, respectively). Except for the sodium salt form (at ca. 220 mg of APV equivalent dose), the GW433908 dose of each formulation was equivalent to approximately 300 mg of APV. Immediately prior to the repeat dosing with tablets containing the calcium salt of GW433908 formulated without citric acid, the dogs were administered 100 ml of 0.05 N HCl (pH 1.7) by gavage.
A total of 11 serial whole-blood samples were collected over 24 h (t = 0 [predose], 0.25, 0.50, 1.0, 2.0, 3.0, 4.0, 6.0, 8.0, 12.0, and 24.0 h) for the determination of plasma APV concentrations. Each 2.5-ml whole-blood sample was obtained from the cephalic catheter and collected into a sodium citrate-containing glass Vacutainer tube. Plasma was separated by refrigerated centrifugation and stored frozen at −20°C until analyzed.
Historical APV pharmacokinetic data for the same dogs were used to determine relative bioavailability. Doses of APV (300 mg in vitamin E-TPGS [d-alpha tocopherol polyethylene glycol 1000 succinate), polyethylene glycol 400, and propylene glycol) were administered orally in two soft-gelatin capsules. Samples were collected and handled as described above.
(ii) GW433908 portal vein sampling study
A single dose of an oral suspension of the calcium salt of GW433908 (28.0 mg/ml; 22.8 mg of free acid/ml) in 0.5% hydroxypropylmethylcellulose (prepared in 0.1% Tween 80) was administered by gavage to seven male Han Wistar rats and one male beagle dog for portal vein sampling. The rats were divided into three groups with each group having different blood collection times as described below. Prior to dosing, the dog was administered 100 ml of 0.05 N HCl solution to produce a favorable gastric environment for GW433908 calcium salt dissolution. Rats received a single dose of 112 mg of GW433908 calcium salt/kg of body weight (91.3 mg of GW433908 free acid/kg, 4 ml/kg), and the dog received a single dose of 35 mg of GW433908 calcium salt/kg (28.5 mg of GW433908 free acid/kg, 1.25 ml/kg).
For determination of GW433908 free acid and APV concentrations, serial whole-blood samples (0.5 ml) were collected from rats in tubes containing sodium citrate according to the following composite schedule: t = 0 (predose), 1.0, 4.0, and 12.0 h (group 1); t = 0.25, 2.0, 6.0, and 24.0 h (group 2); and t = 0.50, 3.0, and 8.0 h (group 3). Eleven serial whole-blood samples (1.8 ml) were collected from the dog over 24 h (t = 0 [predose], 0.25, 0.50, 1.0, 2.0, 3.0, 4.0, 6.0, 8.0, 12.0, and 24.0 h). Each whole-blood sample was obtained from the portal vein cannulae and collected into sodium citrate-containing glass Vacutainer tubes. Plasma was separated by refrigerated centrifugation and stored frozen at −20°C until analyzed.
Assays for concentrations of APV and GW433908 free acid in plasma
Plasma APV concentrations after administration of APV and the free acid and calcium salt formulations of GW433908 (study 1) were analyzed with a validated method with fluorescence detection (excitation at 245 nm, emission at 340 nm). APV was extracted from thawed plasma samples (0.5 ml of plasma combined with 0.5 ml of internal standard solution) by solid-phase extraction with a Waters MilliLab Workstation and C18 Sep-Pak cartridges. After extraction, 50-μl samples were injected onto a Waters Symmetry C18 chromatography column (150 by 3.9 mm) at 40°C. Samples were eluted from the column with a mobile phase of 43% acetonitrile in water (43:57, vol/vol) at a constant flow rate of 0.8 ml/min. Samples were spiked with an internal standard solution (VB 11599, 5 μg/ml; Vertex Pharmaceuticals). Peak areas for APV and internal standards, as well as linear regression of the standards, were determined with HP 1090 ChemStation software version A.03.01. APV concentrations in control and unknown standards were determined from the regression curve (1/C2 weighting). The lower limit of quantitation (LLOQ) for the assay was 0.05 μg/ml. Recovery of APV with this method was 86 to 94%, and recovery of the internal standard was 91 to 98%. Estimates of intra-assay precision (percent coefficient of variation [CV]) and accuracy (percent bias) determined with spiked validation rat control samples for APV were 1.1 to 4.4% and −4.2 to −1.9%, respectively. Estimates of intra-assay precision (percent CV) and accuracy (percent bias) determined with spiked validation dog control samples for APV were 1.4 to 5.6% and −3.5 to 8.2%, respectively.
APV and GW433908 concentrations in plasma following oral administration of the sodium salt formulation of GW433908 (study 1) were determined by reversed-phase HPLC analysis with tandem mass spectrometric detection (HPLC-MS-MS) with an HP1100 HPLC system interfaced to a Sciex API-365 triple quadrupole mass spectrometer (MDS Sciex, Concord, Ontario, Canada). Plasma samples (100 μl) were deproteinated by addition of 500 μl of ethanol-acetonitrile (1:1, vol/vol) followed by centrifugation (15,800 × g, 4°C, 5 min). The resulting supernatants were evaporated to dryness, and the dried extracts were reconstituted to the original sample volume with 20% acetonitrile in 0.1% aqueous acetic acid adjusted to pH 5.4 with ammonium hydroxide (initial mobile phase). The plasma extracts (10 μl) were injected onto a Luna reversed-phase C18 column (100 by 2.0 mm, 5 μm; Phenomenex) at room temperature. Samples were eluted with a 5-min linear gradient from 20 to 95% acetonitrile in 0.1% acetic acid, pH 5.4, followed by a 4-min isocratic elution with 95% acetonitrile-0.1% acetic acid, pH 5.4, at a constant flow rate of 0.2 ml/min. GW433908 and APV eluted at approximately 6.5 and 7.0 min, respectively, and were detected by positive-ion multiple reaction monitoring, monitoring the transitions m/z 586.3 to m/z 418.2 and m/z 506.3 to m/z 245.2, respectively, at a collision energy of 30 eV. Comparison of the nominal standard concentrations to the peak areas was performed with 1/C2-weighted calibration curves generated with the MacQuan program. Peak areas were used to back-calculate the concentrations for each plasma sample. The LLOQ for the assay was 0.3 ng/ml.
For APV and GW433908 free acid concentrations in rat plasma after administration of the calcium salt formulation of GW433908 (study 2), the analysis was accomplished by using a validated method with tandem mass spectrometric detection (HPLC-MS-MS) on a Shimadzu 10VP series HPLC system interfaced to a Sciex API IIIPLUS triple quadrupole mass spectrometer. MS-MS analyses were done by multiple reaction monitoring in the positive-ion mode. Samples (250 μl) were combined with 250 μl of the internal standard working solution [(13C6)APV and (13C6)GW433908G]. After extraction, 3- to 5-μl samples (APV analyses) and 20-μl samples (GW433908X analyses) were injected onto a Keystone Aquasil reversed-phase C18 chromatography column (100 by 2.0 mm) at room temperature. Samples were eluted from the column with a mobile phase of acetonitrile-water (55:45 for APV and 15:85 for GW433908 free acid) containing 0.02 g of ammonium acetate/liter at a constant flow rate of 0.30 or 0.25 ml/min, respectively. Comparison of the nominal standards to the peak areas was performed with 1/C2 weighted calibration curves generated with the MacQuan program (version 1.6a6). Peak areas were used to back-calculate the concentrations for each plasma sample. The LLOQ for the assay was 10 ng/ml for APV and 5 ng/ml for GW433908 free acid. Estimates of intra-assay precision (percent CV) and accuracy (percent bias) determined with spiked validation rat control samples for APV were 1.4 to 8.1% and −0.2 to 11.2%, respectively. Estimates of intra-assay precision (percent CV) and accuracy (percent bias) determined with spiked validation rat control samples for GW433908 free acid were 0.8 to 12.5% and −8.8 to 8.1%, respectively.
For APV and GW433908 free acid concentrations in dog plasma after administration of the calcium salt formulation of GW433908 (study 2), the analysis was accomplished by using a validated method with tandem mass spectrometric detection (HPLC-MS-MS) on a Waters HPLC system interfaced to a Sciex API 300 triple quadrupole mass spectrometer. MS-MS analyses were done by multiple reaction monitoring in the positive-ion mode. Samples (250 μl) were combined with 250 μl of the internal standard working solution [(13C6)APV and (13C6)GW433908G]. After extraction, 5 to 50 μl was injected onto a Keystone Aquasil reversed-phase C18 chromatography column (100 by 2.1 mm) at room temperature. Samples were eluted from the column with a mobile phase of acetonitrile-water (55:45 for APV and 15:85 for GW433908 free acid) containing 0.02 g of ammonium acetate/liter at a constant flow rate of 0.35 or 0.25 ml/min, respectively. Comparison of the nominal standards to the peak areas was performed with 1/C2 weighted calibration curves generated with the MacQuan program. Peak areas were used to back-calculate the concentrations for each plasma sample. The LLOQ for the assay was 10 ng/ml for APV and 5 ng/ml for GW433908 free acid. Estimates of intra-assay and interassay precision (percent CV) and accuracy (percent bias) determined with spiked validation dog control samples for APV were 2.4 to 4.5 and 0.9 to 2.3% and −0.3 to 3.0%, respectively. Estimates of intra-assay and interassay precision (percent CV) and accuracy (percent bias) determined with spiked validation dog control samples for GW433908 free acid were 2.6 to 11.3 and 1.6 to 2.9% and −1.2 to 5.4%, respectively.
Pharmacokinetic analysis
For both study 1 and study 2, a noncompartmental model (WinNonlin Professional Network Edition software, version 1.5; Scientific Consulting Inc., Apex, N.C.) was used to calculate single-dose pharmacokinetic parameters for APV after oral dosing with GW433908 formulations. The maximum concentration in plasma (Cmax) and the time to reach Cmax (Tmax) were identified by inspection of each plasma concentration-time profile. The area under the plasma concentration time-curve (AUC0-24) was calculated by using the linear trapezoidal rule, and the AUC extrapolated to infinity (AUC0-∞) was calculated by dividing the concentration at the last measurable time point (Clast) by the apparent terminal elimination rate constant (λz) and adding this value to the AUC24. In study 1, the relative bioavailability (F) was calculated as the ratio of the values determined in the test formulations to the baseline values determined in the same dogs in a previous study. In study 2, the t1/2 was calculated as 0.693/λz and in the determination of the ratio of GW433908 free acid exposure (AUC0-∞) to APV exposure (AUC0-∞), the APV value was multiplied by a factor of 1.158 to correct for the differences in molecular weight.
Statistical analysis
Only descriptive statistical analysis was performed for the pharmacokinetic study in dogs.
GW433908 in vitro permeability-metabolism study
Caco-2 cells were purchased from the American Type Culture Collection (Manassas, Va.) and used between passage number 16 and 26. Cells were maintained in modified Eagle's medium (Mediatech, Herndon, Va.) containing 10% (vol/vol) fetal bovine serum (GIBCO, Grand Island, N.Y.) and 1% (vol/vol) nonessential amino acids (Sigma) at 37°C in an atmosphere of 5% CO2 and 95% relative humidity. Cells were passaged at 80 to 90% confluence (approximately every 7 days) with Trypsin-EDTA solution (0.05% trypsin plus 0.53 mM EDTA; Sigma). For the transport assay, cells were seeded on Biocoat wells (1.0-μm-pore-size, fibrillar collagen-coated polyethylene terephthalate filters; Becton Dickinson Labware, Bedford, Mass.) at 100,000 cells per well. After seeding, cells were maintained with Biocoat medium according to procedures described by the manufacturer.
Three days after seeding, the cell medium was removed and replaced with transport buffer (Hanks' balanced salt solution containing 25 mM glucose and 25 mM HEPES; Sigma). Monolayers were allowed to equilibrate for approximately 1 h at 37°C. After the equilibration period, the transport buffer was removed and dosing solutions containing GW433908 were added to either the apical side of the monolayer, for absorptive direction studies, or the basolateral side of the monolayer, for secretory direction studies. Blank transport buffer was added to the compartment on the opposing side of the monolayer. Dosing solutions contained a final dimethyl sulfoxide concentration of 0.5% (vol/vol). Each treatment was done in triplicate (n = 3 wells).
After 1 h, donor and receiver compartments were sampled. Aliquots (100 μl) from the donor compartment were diluted 40-fold with the aqueous mobile phase (95% of 0.1% acetic acid in water adjusted to pH 4.6 with ammonium hydroxide, 5% acetonitrile). Donor and receiver samples (5 μl) were analyzed on a Luna C18 column (Phenomenex; 50 by 2 mm, 5 μm) with a mobile phase of 95% of 0.1% acetic acid in water adjusted to pH 4.6 with ammonium hydroxide-5% acetonitrile (A) and 0.1% acetic acid in acetonitrile (B) at a flow rate of 240 μl/min. A linear gradient was started from the initial conditions of 20% B to 95% B at 2.5 min. Tandem mass spectrometric detection of GW433908 was achieved in the positive-ion mode with a Sciex 365 mass spectrometer, monitoring the ion transition m/z 586.3 to m/z 418.2 using a collision energy of 30 eV. APV was detected by monitoring the ion transition m/z 506.3 to m/z 245.1. GW433908 and APV concentrations in each donor and receiver sample were determined from a peak area versus concentration standard curve. The LLOQ for GW433908 was 0.005 μM; the LLOQ for APV was 0.001 μM.
RESULTS
Identifying a developable form of GW433908
A crystalline salt form with high aqueous solubility was desired for further development of GW433908. The solubility of the sodium salt and the free acid was high (≥3 mg/ml) from pH 1 to 8 (Fig. 2). The aqueous solubility of the calcium salt of GW433908 was strongly pH dependent. The solubility was very low at pH 7 (0.3 mg/ml) compared with the peak solubility between pH 3 and 4 (maximum of 54 mg/ml at pH 3.3). Furthermore, the solubility remained relatively high at pH values below 3.
FIG. 2.
Dependence of solubility of Ca salt of GW433908 on pH.
Attempts to crystallize the sodium salt or the free acid failed, and all isolated forms of these salts were amorphous. In addition, the sodium salt was extremely hygroscopic, drawing at least 50% of its starting weight in moisture. In contrast, the calcium salt formed a stable crystalline solid that was a hydrate with five water molecules per GW433908 molecule.
Comparison of the pharmacokinetic parameters of various salt forms of GW433908
Development of GW433908 required that it be delivered as a solid dosage form, preferably a tablet. Therefore, we selected the sodium salt, the free acid, and the calcium salt of GW433908 to determine the relative APV exposure after oral administration of a solid dose (in gelatin capsules or a tablet) and of the APV clinical formulation (Agenerase) to beagle dogs (Table 1). The estimated APV exposure (AUC) and Cmax after administration of the sodium salt in a gelatin capsule were similar to APV AUC and Cmax values following Agenerase administration. Surprisingly, APV exposure and Cmax after administration of the calcium salt formulations of GW433908 were very low compared with those of Agenerase (24 and 19%, respectively). The variability among these AUC and Cmax values was also extremely high compared with that of any of the other salt forms or APV.
TABLE 1.
Estimated APV pharmacokinetic parameters after single-oral-dose administration of different GW433908 formulations in dogs
| Formulationa | APV pharmacokinetic parameterb
|
||||
|---|---|---|---|---|---|
| AUC0-∞c (μg · h/ml) | Cmaxc (μg/ml) | Tmax (h) | t1/2 (h) | Relative bioavailability (%) | |
| APVd,e | 26.2 ± 5.61 | 7.23 ± 1.14 | 1-2 | 3.4 ± 1.5 | 100 |
| GW433908X | 16.8 ± 4.22 | 3.28 ± 1.30 | 1-6 | 3.0 ± 1.0 | 64.2 ± 9.50 |
| GW433908A | 20.3 ± 3.52 | 7.68 ± 2.13 | 2-3 | 1.9 ± 0.8 | 82.2 ± 34.9 |
| GW433908G | 6.94 ± 7.35 | 1.43 ± 1.54 | 1-4 | 3.6 ± 1.4 | 24.1 ± 24.2 |
| GW433908G (with HCl gavage) | 15.8 ± 5.06 | 4.48 ± 1.14 | 2-4 | 1.4 ± 0.3 | 59.4 ± 7.82 |
| GW433908G (with citric acid) | 7.94 ± 6.21 | 2.37 ± 1.81 | 0.5-2 | 2.8 ± 1.5 | 28.2 ± 19.3 |
Formulations: GW433908X, free acid; GW433908A, bis-sodium salt; GW433908G, calcium salt. Three dogs were used for each compound.
Values are means ± standard deviations.
Data were corrected for equivalence to 25 mg of APV/kg.
Agenerase clinical formulation (23.0% vitamin E-TPGS, 60.2% polyethylene glycol 400, 4.6% propylene glycol).
Historical APV data in the same dogs.
The pH in stomachs of fasting dogs is variable and can reach as high as pH 7 (13). Given the lower solubility of the calcium salt at higher pH, it was conceivable that the low and variable APV exposure from the calcium salt resulted from decreased solubility at the higher pH levels in the dog stomach. Therefore, coadministration of HCl or citric acid was used in an attempt to increase the solubility of the calcium salt in the stomachs of the fasting dogs and ultimately increase APV exposure (Table 1). The mean estimate of relative bioavailability of the calcium salt in fasting beagle dogs more than doubled with predosing acidification of the stomach (59 versus 24% of value for clinical formulation of APV). The addition of citric acid to a tablet formulation of the calcium salt was not able to achieve the same improvement.
GW433908 metabolic conversion to APV
Systemic exposure to GW433908 was low; however, whether the liver was significantly exposed was not conclusively established because the observed low systemic exposure could be the result of first-pass metabolism. Portal vein sampling for GW433908 free acid and APV concentrations was conducted following oral administration of GW433908 calcium salt to rats (n = 7) and one dog. The portal vein GW433908 (free acid) exposure and Cmax in rats (0.3 and 0.79% of APV values, respectively) and the dog (0.85 and 1.72% of APV values, respectively) were very low (Table 2). Therefore, the liver is not highly exposed to GW433908 and most of the prodrug is converted to APV prior to portal vein exposure, most likely in the gastrointestinal tract.
TABLE 2.
Estimated APV and GW433908 free acid pharmacokinetic parameters (in the portal vein) after administration of single oral doses of GW433908 calcium salt to portal vein-cannulated Han Wistar rats and a beagle dog
| Parameter | Speciesa
|
|||
|---|---|---|---|---|
| Ratb
|
Dog
|
|||
| APV | GW433908 | APV | GW433908 | |
| AUC0-∞ (h · μg/ml) | 44.6 | 0.156c | 8.60 | 0.0848 |
| Cmax (μg/ml) | 7.00 | 0.0637 | 6.00 | 0.120 |
| Tmax (h) | 2.0 | 0.5 | 0.5 | 0.25 |
| t1/2 (h) | 1.6 | NDe | 0.5 | 0.4 |
| AUC ratiod (GW433908/APV) | 0.003 | 0.0085 | ||
| Cmax ratiod (GW433908/APV) | 0.0079 | 0.0172 | ||
GW433908 dose: 112 mg/kg in rat and 35 mg/kg in dog.
Values for rats are composite means.
AUC24.
Molar basis.
ND, not determined.
GW433908 in vitro permeability-metabolism study
The permeability of GW433908 was assessed by its ability to cross a Caco-2 cell monolayer. GW433908 did not substantially cross the Caco-2 cell monolayer after application of 10 or 100 μM to the apical side (Table 3). APV, however, did cross the monolayer >50-fold faster than GW433908 after application of 100 μM GW433908 to the apical side. Similar results were found after application of GW433908 to the basolateral side. Furthermore, apical application of GW433908 generated a significant amount of APV in the compartment where it was applied. By contrast, GW433908 was not substantially hydrolyzed to APV after application to the basolateral side.
TABLE 3.
GW433908 and APV amounts in apical or basolateral compartments following application of GW433908 to Caco-2 monolayers
| Applied dose (μM) for side | Amt (nmol) in compartment analyzeda
|
|||
|---|---|---|---|---|
| Apical
|
Basolateral
|
|||
| GW433908 | APV | GW433908 | APV | |
| Apical | ||||
| 10 | 2.4 ± 0.2 | 0.44 ± 0.04 | <0.008 | 0.01 ± 0.01 |
| 100 | 40 ± 1.7 | 9.4 ± 0.2 | <0.008 | 0.4 ± 0.1 |
| Basolateral | ||||
| 10 | <0.002 | 0.11 ± 0.01 | 11.4 ± 0.8 | 0.03 ± 0.01 |
| 100 | 0.01 ± 0.01 | 0.3 ± 0.03 | 204 ± 8 | 1.0 ± 0.1 |
Values are the means ± standard deviations of three individual cell monolayers.
DISCUSSION
In an ongoing effort to improve HIV medicines, we initiated an effort to identify a more compact APV dosing form to increase medication adherence and ultimately optimize the drug's efficacy and durability of response. Because the pill burden of APV is the result of its relatively low aqueous solubility (high excipient-to-drug ratio), a program was designed to generate prodrugs with increased solubility; GW433908, the phosphate ester prodrug of APV, was ultimately identified (Baker et al., 39th ICAAC). Subsequent to the discovery that GW433908 was an effective oral prodrug of APV in rats (data not shown) and had markedly improved aqueous solubility, there were numerous other attributes of the molecule required for further development, including bioavailability in a nonrodent species, a stable crystal form, limited systemic exposure to the intact prodrug, a scalable synthetic route, and a similar or better toxicology profile than that of APV.
The dog was identified as a nonrodent species that achieved high APV concentrations following GW433908 administration. This provided not only additional confidence that the prodrug would work similarly in humans but also a second, required, species for toxicological evaluation of the drug. Furthermore, the very low systemic exposure to intact prodrug, demonstrated in two species (rat and dog), suggested that the safety of GW433908 would be similar to that of APV.
To minimize the excipient-to-drug ratio, GW433908 needed to be delivered as a solid dosage form, preferably a tablet. Crystalline drug substance is preferred for the development of tablets, but the aqueous solubility of crystalline forms must remain high. Whereas the free acid and sodium salt were the most soluble over the relevant physiological pH range (1 to 8), neither could be isolated as a crystalline solid. In addition, the sodium salt was extremely hygroscopic. The calcium salt had reduced solubility compared to the other forms, particularly at high pH (i.e., pH 7), but produced a crystalline form (as a stable pentahydrate). The reduced solubility of the calcium salt at high pH was initially not of distinct concern because the pH of the stomach is typically low where dissolution should occur and the solubility of the salt was thought to be adequate at stomach pH values.
Surprisingly, when the calcium salt was administered to fasted dogs, the bioavailability was very low compared with that of the other salt forms or Agenerase. One explanation was that the pH in stomachs of fasted dogs can reach 7 (13), which may have resulted in variable and incomplete dissolution of the calcium salt. Lower dissolution of the calcium salt would result in lower APV exposure; therefore, in a second experiment, a volume of dilute hydrochloric acid was administered to the dogs prior to administration of the calcium salt. Such predosing acidification of the stomach markedly increased APV exposure in the dogs; a weaker acid (such as citric acid) apparently cannot achieve the same results. The pH in fasted human stomachs is approximately 2 (13), providing confidence that the calcium salt would yield adequate APV exposures in humans. In fact, the first study with humans indicated that the oral administration of the calcium salt resulted in APV exposure similar to that achieved with Agenerase (C. Falcoz, J. M. Jenkins, C. Bye, K. B. Kenney, S. Studenberg, H. Fuder, and W. T. Prince, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 918, 1999). Systemic exposure to GW433908 was generally less than 3% of that of APV in rats and dogs (data not shown). However, despite low systemic exposure to the intact prodrug, it was not clear if the liver was highly exposed because first-pass metabolism could result in decreased systemic exposure. Therefore, the portal vein blood of rats and dogs was analyzed for APV and GW433908 free acid after oral administration of GW433908 calcium salt. GW433908 portal vein exposure was minimal compared with APV (0.3 and 0.85% of that of APV exposure in rats and one dog, respectively). These results suggested that the liver was not exposed to high concentrations of GW433908 and that the primary site of prodrug hydrolysis to APV was prior to the portal vein, most likely the gastrointestinal tract.
Further evidence that GW433908 is converted to APV in the gastrointestinal tract was provided by showing that Caco-2 cell monolayers hydrolyzed GW433908 to APV. The Caco-2 cell monolayer system, which contains alkaline phosphatase primarily in the apical membrane, is a model for intestinal absorption (10). After application of GW433908 to the apical side of Caco-2 cells, GW433908 did not detectably cross the monolayer, whereas APV did. Furthermore, GW433908 was hydrolyzed on the apical side of the monolayer. We speculate that this hydrolysis is catalyzed by alkaline phosphatase. Moreover, we subsequently determined that GW433908 was a substrate for purified alkaline phosphatase and alkaline phosphatase present in rat, dog, and human intestinal brush border membrane (S. D. Studenberg, E. S. Furfine, C. C. Boehlert, C. R. Delozier, C. D. Smith, J. L. Woolley, Abstr. Front. Drug Dev. Antiretrovir. Ther., abstr. 069, 2000). Combined with the low portal vein and low systemic GW433908 exposure after oral administration, these results suggest that the intestinal tract is the primary site of prodrug activation (and that alkaline phosphatase is the catalytic component), resulting in minimal absorption of intact GW433908. Because GW433908 free acid has minimal systemic exposure (including the liver), the safety profile should be quite similar to that of the parent drug. Preliminary results of the toxicology of GW433908 calcium salt indeed indicate that GW433908 has a toxicity and safety profile similar to that of APV (data not shown).
For additional confidence to develop GW433908, a scalable synthetic route for drug manufacturing was required. Such a route was identified for GW433908 by inserting only one additional stage into the present manufacturing process for APV. In this step, the phosphate is added to the immediate APV precursor on the hydroxyl moiety by using POCl3 in pyridine.
The aqueous solubility properties of the calcium salt of GW433908 at physiologic pH of the gastrointestinal tract allow for a compact tablet formulation of the APV prodrug, requiring few excipients. Because the plasma APV exposure obtained with the GW433908 calcium salt tablet was found to be comparable to that obtained with Agenerase, it is expected that GW433908 calcium salt in combination antiretroviral regimens will provide virologic control and improved immune function comparable to those observed with Agenerase-containing regimens (8, 17). Equally important, the compact formulation of GW433908 calcium salt will likely facilitate antiretroviral adherence, a key factor in durable virologic suppression. Together with the well-established antiviral potency and the resistance as well as safety profiles of amprenavir, the preclinical studies presented here provided excellent support for the progression of GW433908 into clinical development.
Acknowledgments
We gratefully acknowledge extensive technical assistance and/or helpful discussions with Misty Burnette, Cindy Wilson, Arup Roy, Graham Whitesell, Lian Huang, Ryan Klein, Peter Varlashkin, Mark Patrick, Steve Kutz, Pat Wheelan, Ian Armitage, Tim Tippin, Brigid Kane, and Chari Smith.
REFERENCES
- 1.Bangsberg, D. R., F. M. Hecht, E. D. Charlebois, A. R. Zolopa, M. Holodniy, L. Sheiner, J. D. Bamberger, M. A. Chesney, and A. Moss. 2000. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS 14:357-366. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett, J. A., R. DeMasi, J. Quinn, C. Moxham, and F. Rousseau. 2001. Overview of the effectiveness of triple combination therapy in antiretroviral-naïve HIV-1 infected adults. AIDS 15:1369-1377. [DOI] [PubMed] [Google Scholar]
- 3.Brodt, H. R., B. S. Kamps, P. Gute, B. Knupp, S. Staszewski, and E. B. Helm. 1997. Changing incidence of AIDS-defining illnesses in the era of antiretroviral combination therapy. AIDS 11:1731-1738. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1997. Update: trends in AIDS incidence—United States, 1996. Morb. Mortal. Wkly. Rep. 46:861-864. [PubMed] [Google Scholar]
- 5.Claxton, A. J., J. Cramer, and C. Pierce. 2001. A systematic review of the associations between dose regimens and medication compliance. Clin. Ther. 23:1296-1310. [DOI] [PubMed] [Google Scholar]
- 6.Detels, R., A. Munoz, G. McFarlane, L. A. Kingsley, J. B. Margolick, J. Giorgi, L. K. Schrager, and J. P. Phair for the Multicenter AIDS Cohort Study Investigators. 1998. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. JAMA 280:1497-1503. [DOI] [PubMed] [Google Scholar]
- 7.Gulick, R. M., J. W. Mellors, D. Havlir, J. J. Eron, A. Meibohm, J. H. Condra, F. T. Valentine, D. McMahon, C. Gonzalez, L. Jonas, E. A. Emini, J. A. Chodakewitz, R. Isaacs, and D. D. Richman. 2000. 3-year suppression of HIV viremia with indinavir, zidovudine, and lamivudine. Ann. Intern. Med. 133:35-39. [DOI] [PubMed] [Google Scholar]
- 8.Haubrich, R., M. Thompson, R. Schooley, W. Lang, A. Stein, D. Sereni, M. E. van der Ende, F. Antunes, D. Richman, G. Pagano, L. Kahl, A. Fetter, D. J. Brown, N. Clumeck, and the Amprenavir PROAB 2002 Study Team. 1999. Phase II safety and efficacy study of amprenavir in combination with zidovudine and lamivudine in HIV-infected patients with limited antiretroviral experience. AIDS 13:2411-2420. [DOI] [PubMed] [Google Scholar]
- 9.Haubrich, R. H., S. J. Little, J. S. Currier, D. N. Forthal, C. A. Kemper, G. N. Beall, D. Johnson, M. P. Dube, J. Y. Hwang, J. A. McCutchan, and the California Collaborative Treatment Group. 1999. The value of patient-reported adherence to antiretroviral therapy in predicting virologic and immunologic response. AIDS 13:1099-1107. [DOI] [PubMed] [Google Scholar]
- 10.Hidalgo, I. J., T. J. Raub, and R. T. Borchardt. 1989. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 96:736-749. [PubMed] [Google Scholar]
- 11.Hogg, R. S., K. V. Health, B. Yip, K. J. P. Cralb, M. V. O'Shaugnessy, M. T. Schechter, and J. S. G. Montaner. 1998. Improved survival among HIV-infected individuals following initiation of antiretroviral therapy. JAMA 279:450-454. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan, J. E., D. Hanson, M. S. Dworkin, T. Frederick, J. Bertolli, M. L. Lindegren, S. Holmberg, and J. L. Jones. 2000. Epidemiology of human immunodeficiency-virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin. Infect. Dis. 30:S5-S14. [DOI] [PubMed] [Google Scholar]
- 13.Kararli, T. T. 1995. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm. Drug Dispos. 16:351-380. [DOI] [PubMed] [Google Scholar]
- 14.Le Moing, V., G. Chene, M. P. Carrieri, A. Alioum, F. Brun-Vezinet, L. Piroth, J. P. Cassuto, J.-P. Moatti, F. Raffi, C. Leport, and the Aproco Study Group. 2002. Predictors of virological rebound in HIV-1-infected patients initiating a protease inhibitor-containing regimen. AIDS 16:21-29. [DOI] [PubMed] [Google Scholar]
- 15.Low-Beer, S., B. Yip, M. V. O'Shaughnessy, R. S. Hogg, and J. S. Montaner. 2000. Adherence to triple therapy and viral load response. J. Acquir. Immune Defic. Syndr. 23:360-361. [DOI] [PubMed] [Google Scholar]
- 16.Murphy, E. L., A. C. Collier, L. A. Kalish, S. F. Assmann, M. F. Para, T. P. Flanigan, P. N. Kuman, L. Mintz, F. R. Wallach, and G. J. Nemo for the Viral Activation Transfusion Study Investigators. 2001. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann. Intern. Med. 135:17-26. [DOI] [PubMed] [Google Scholar]
- 17.Murphy, R. L., R. M. Gulick, V. DeGruttola, R. T. D'Aquila, J. J. Eron, J.-P. Sommadossi, J. S. Currier, L. Smeaton, I. Frank, A. M. Caliendo, J. G. Gerber, R. Tung, and D. R. Kuritzkes for the AIDS Clinical Trials Group 347 Study Team. 1999. Treatment with amprenavir alone or amprenavir with zidovudine and lamivudine in adults with human immunodeficiency virus infection. J. Infect. Dis. 179:808-816. [DOI] [PubMed] [Google Scholar]
- 18.Opravil, M., R. W. Cone, M. Fischer, P. L. Vernazza, S. Bassetti, P. Lorenzi, L. R. Bisset, P. Ott, W. Huber, M. C. Knuchel, M. Roos, R. Luthy, R. Weber, and the Swiss HIV Cohort Study. 2000. Effects of early antiretroviral treatment on HIV-1 RNA in blood and lymphoid tissue: a randomized trial of double versus triple therapy. J. Acquir. Immune Defic. Syndr. 23:17-25. [DOI] [PubMed] [Google Scholar]
- 19.Pallela, F. J., Jr., K. M. Delaney, A. C. Moorman, M. O. Loveless, J. Fuhrer, G. A. Satten, D. J. Aschman, S. D. Holmberg, and the HIV Outpatient Study Investigators. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 338:853-860. [DOI] [PubMed] [Google Scholar]
- 20.Paterson, D. L., S. Swindells, J. Mohr, M. Brester, E. N. Vergis, C. Squler, M. M. Wagener, and N. Singh. 2000. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann. Intern. Med. 133:21-30. [DOI] [PubMed] [Google Scholar]
- 21.Raboud, J. M., M. Harris, S. Rae, J. S. Montaner, et al. 2002. Impact of adherence on duration of virological suppression among patients receiving combination antiretroviral therapy. HIV Med. 3:118-124. [DOI] [PubMed] [Google Scholar]
- 22.Saag, M. S., P. Tebas, M. Sension, M. Conant, R. Myers, S. K. Chapman, R. Anderson, and N. Clendeninn for the Viracept Collaborative Study Group. 2001. Randomized, double-blind comparison of two nelfinavir doses plus nucleosides in HIV-infected patients (Agouron study 511). AIDS 15:1971-1978. [DOI] [PubMed] [Google Scholar]
- 23.Sadler, B. M., and D. S. Stein. 2002. Clinical pharmacology and pharmacokinetics of amprenavir. Ann. Pharmacother. 36:102-118. [DOI] [PubMed] [Google Scholar]
- 24.Singh, N., S. M. Berman, S. Swindells, J. C. Justis, J. A. Mohr, C. Squier, and M. M. Wagener. 1999. Adherence of human immunodeficiency virus-infected patients to antiretroviral therapy. Clin. Infect. Dis. 29:824-830. [DOI] [PubMed] [Google Scholar]
- 25.Stone, V. E. 2001. Strategies for optimizing adherence to highly active antiretroviral therapy: lessons from research and clinical practice. Clin. Infect. Dis. 33:865-872. [DOI] [PubMed] [Google Scholar]
- 26.Walmsley, S., B. Bernstein, M. King, J. Arribas, G. Beall, P. Ruane, M. Johnson, D. Johnson, R. Lalonde, A. Japour, S. Brun, and E. Sun for the M98-863 Study Team. 2002. Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection. N. Engl. J. Med. 346:2039-2046. [DOI] [PubMed] [Google Scholar]