Abstract
Cutaneous T-cell lymphomas (CTCL) are a heterogeneous group of non-Hodgkin lymphomas that are considered incurable. The role of allogeneic hematopoietic stem cell transplantation (HSCT) in the treatment of CTCL is not well defined but may provide potent graft-vs-lymphoma (GVL) activity independent of the conditioning therapy. We present outcomes of 12 extensively-pretreated patients with CTCL who underwent allogeneic HSCT using, most commonly, a reduced intensity conditioning (RIC) regimen. Median age at diagnosis of CTCL was 49 yrs, and median time to transplantation from diagnosis was 3.3 years. Transplant induced and maintained CR in 6 patients with active disease supporting the presence of a GVL effect. Transplant related mortality was low, and 42% of patients were alive and disease-free a median duration of 22 months after transplant. Two patients showed strong and direct evidence of a GVL effect with a direct response to withdrawal of immunosuppression or to donor leukocyte infusion (DLI). Our data show that HSCT can provide long-term disease control in patients with advanced CTCL otherwise refractory to immunotherapy and chemotherapy.
Keywords: CTCL, GVL, allogeneic, HSCT, DLI
Introduction
Cutaneous T-cell lymphomas (CTCL) are a heterogeneous group of non-Hodgkin lymphomas; the majority of cases are mycosis fungoides and/or Sezary syndrome; the remainder include a variety of subtypes which vary greatly in clinical behavior. All forms of CTCL are generally considered to be incurable. While mycosis fungoides may have a relatively indolent course (ref. 1), once the disease has progressed beyond early stages, it can behave in a highly aggressive manner. Sézary syndrome (circulating lymphoma cells greater than 1,000/mm3 and greater than 10% of peripheral blood leukocytes) has a median survival time of 31 months (ref. 2). Although there are numerous therapies available to treat CTCL, including newer biological therapies, CTCL is highly responsive to immune manipulation (ref. 3)(ref. 4–7). Responses to interferon α, interferon γ and interleukin-12 highlight that CTCL is influenced by host immune status (ref. 8)(ref. 9). Conventional treatments are at best temporizing with aggressive disease (ref. 10), and high dose chemotherapy with autologous HSCT for MF and SS has had disappointing results (ref. 11).
For many diseases, allogeneic HSCT is an ideal form of immunotherapy; however, the role for allogeneic HSCT in CTCL is not well defined. Allogeneic transplant is successful in part due to the GVL effect of the donor graft, independent of the conditioning regimen (ref. 12). Several small series describe durable remissions after allogeneic HSCT after both RIC and myeloablative conditioning (ref. 13, 14)(ref. 15). DLI has also been reported to be effective in producing a GVL effect but only when combined with several other modalities of therapy including chemotherapy (ref. 14). Nevertheless, long-term remissions, particularly in the setting of RIC allogeneic transplant, and the observation of responses with withdrawal of immunosuppression, support the presence of a potent GVL effect (ref. 14)(ref. 15).
We present outcomes of 12 consecutive patients who underwent allogeneic HSCT using, most commonly, a reduced intensity conditioning regimen for this rare lymphoid malignancy at our institution. The high response rate, durable remissions, and direct GVL induction with DLI all support a potent GVL effect in patients with MF and SS from allogeneic HSCT.
Methods
A retrospective review was performed of the University of Pennsylvania bone marrow transplant database to identify all patients who underwent allogeneic HSCT for cutaneous T cell lymphoma. Twelve patients were identified who were transplanted between 2004 to 2010. A chart review was performed to obtain data about diagnosis, staging treatment, transplantation and outcomes. This study was reviewed and approved by the institutional review board at the University of Pennsylvania.
Patient Selection
Patients were referred for transplant because of poor prognosis or progression after conventional therapy. They were generally heavily pretreated, having received a median of 8 non-chemotherapy, and 2 chemotherapy-based treatment modalities before being transplanted. Treatments are listed in Table 1. Only 3 patients were in CR at the time of transplant, while 1 had MRD detectable by flowcytometry in the bone marrow, 4 had chemo-responsive active disease, and 4 had progressive active disease.
Table 1.
Patient characteristics (sorted chronologically according to date of transplant)
| Pt | Sex/Age | Time Dx to SCT (yrs) | Diagnosis | Prior treatment (non-chemotherapy | Prior-treatment (chemotherapy) | Highest stage prior to HSCT | Disease status prior to HSCT | Conditioning regimen |
|---|---|---|---|---|---|---|---|---|
| 1 | F/57 | 2.8 | SS w/o large cell transformation (LCT) | B, C, EB, ECP, INF, Me, PUVA, S, Sar, Tacrolimus, TSEB | Alemtuzumab | IVA1 | Active disease, progressive | Flu/Cy |
| 2 | M/52 | 1.1 | MF w/o LCT | - | CHOP, HyperCVAD | IIB | CR | Flu/Bu |
| 3 | M/41 | 1.6 | Gamma-delta T-cell lymphoma (GDTL) | B, Hydroxychloroquine, S, TSEB | Denileukin Diftitox, HyperCVAD, Methotrexate | T3b | Active disease, chemo-responsive | Flu/Mel |
| 4 | F/43 | 0.8 | MF w/o LCT | S, INF, TSEB | - | IIB | MRD (marrow) | Cy/TBI |
| 5 | F/48 | 0.8 | GDTL | B, S | Alemtuzumab, Cytarabine, Cyclophosphamide, Methotrexate | T3b | Active disease, chemo-responsive | Cy/TBI |
| 6 | M/56 | 5.9 | MF with LCT | B, C, EB, Imiquimod, INF, M, S, TSEB, PUVA | Alemtuzumab, Denileukin Diftitox, ICE, Zanolimumab | IVA2 | Active disease, progressive | Flu/Bu |
| 7 | F/52 | 7.0 | MF with LCT | C, INF, Me, S, Tazarotene, TSEB, V | CHOP | IVB | CR | Flu/Bu |
| 8 | M/61 | 3.7 | MF w/o LCT | B, C, INF, PUVA, S, Sar, Tretinoin, TSEB, UVB | Alemtuzumab | IIIB | CR | Flu/Bu |
| 9 | F/44 | 3.9 | GDTL | B, EB, S, V | Cyclophosphamide, Denileukin Diftitox | T3b | Active disease, progressive | Flu/Bu |
| 10 | M/61 | 0.5 | SS with LCT | S, TSEB, UVB | CHOP, HyperCVAD, Alemtuzumab | IVA2 | Active disease, progressive | Flu/Bu |
| 11 | F/61 | 6.8 | SS w/o LCT | B, ECP, INF, M, PUVA, S, Sar, TSEB, V | Alemtuzumab | IVA2 | Active disease, chemo-responsive | Flu/Bu |
| 12 | F/54 | 6.8 | SS w/o LCT | B, ECP, Forodesine, INF, Pegfilgastrim, PUVA, Romidepsin, S, Tretinoin | Alemtuzumab | IVA2 | Active disease, chemo-responsive | Flu/Bu |
LCT = Large cell transformation
MF = Mycosis Fungoides
GDTL = Gamma-delta T-cell lymphoma
SS = Sézary Syndrome
TRM = Transplant-related mortality
B = Bexarotene
C = Carmustine (topical)
EB = Localized electron beam radiotherapy
ECP = Extracorporeal photopheresis
INF = Interferon-alpha/gamma
Me = Mechlorethamine (topical)
PUVA = Psoralen + Ultraviolet light A photochemotherapy
S = Steroids
Sar = Sargramostim
TSEB = Total skin electron beam radiation treatment
UVB = Ultraviolet light B phototherapy
V = Vorinostat
Transplantation
Seven of the patients (Patients #1 – #7) had HLA-identical sibling donors, while unrelated donors were identified for the rest. Of these, donors for 4 (Patients #8, Patients #10 – #12) were well matched by high resolution molecular typing at HLA A, B, C, DR and DQ, while Patient #9 had a single antigen mismatch.
RIC with fludarabine (120 mg/m2 over 4 days) and busulfan (6.4 mg/kg over 2 days) or fludarabine (same dose) and cyclophosphamide (3 gm/m2) was used in 10 cases. Conventional myeloablative conditioning with Cyclophosphamide (120 mg/kg over 2 days) and total body irradiation (TBI) 1200 cGy fractionated in 6 doses over 3 days was used in 2 young patients with excellent performance status and active disease prior to transplantation (see Table 1). GVHD prophylaxis consisted of a calcineurin inhibitor and methotrexate in all patients.
Statistics
Overall survival was measured as time from HSCT to death from any cause. Event-free survival was measured as time from HSCT to relapse, progression or death from any cause, whichever occurred first. Probabilities of OS and EFS, and survival curves, were generated using Kaplan-Meier estimates (ref. 16). Statistical analysis was performed using Statview.
Results
Patient characteristics
Patient characteristics, diagnosis and stage are shown in Table 1. Patients were staged using the ISCL/EORTC TNM staging system (ref. 17). There were 5 male and 7 female patients with a median age at diagnosis of CTCL of 49 yrs (range 40–61 yrs). Of the 12 patients, 5 had MF (stages IIB, IIB, IIIB, IVA2, IVB; 2 with nodal transformation), 4 had SS (one stage IVA1, three IVA2; 1 with nodal transformation), and 3 had Gamma-delta T-cell lymphoma (all T3b). Median time to transplantation from diagnosis was 3.3 yrs (range 0.5–7 yrs), with a median age at transplantation of 53 yrs (range 41–61 yrs). The median follow up is 15 months (range 1 to 45 months) for all patients and 24 months (range 13 to 45 months) for patients currently alive.
Engraftment
Neutrophil engraftment occurred with an ANC >500 at a median of 13 days (range 10–34 days) after HSCT. At day 100 after HSCT in 10 evaluable pts, median whole blood chimerism was 97%. Patient #1 was diagnosed with CNS PTLD within 100 days of HSCT and lost her graft after rapid tapering of immunosuppression. Patient #8 had decreasing donor chimerism 15 months after HSCT, and received DLI with subsequent achievement of 100% donor chimerism. Individual chimerism trends are described in Table 3.
Table 3.
Post-transplant donor chimerism (for patients surviving to 100 days)
| # | Day 100 | 6 months | 1 year | 2 years |
|---|---|---|---|---|
| 1 | 15 | NE (lost graft) | NE (lost graft) | NE (lost graft) |
| 2 | 90 (BM) | NE (Died month 7) | NE (Died month 7) | NE (Died month 7) |
| 3 | 100 | 100 | 100 | 100 |
| 4 | 93 (BM) | 85 | 88 (BM) | 100 |
| 6 | 100 (Day 60) | NE (Died month 5) | NE (Died month 5) | NE (Died month 5) |
| 7 | 85 | 99 | 100 | 100 |
| 8 | 97 (BM) | 98 | 94 | NE (F/U not reached) |
| 9 | 100 | NE (Died month 6) | NE (Died month 6) | NE (Died month 6) |
| 11 | 97 | 97 | 95 | NE (F/U not reached) |
| 12 | 97 (BM) | 97 | 100 | NE (F/U not reached) |
Listed chimerism is whole blood chimerism unless mentioned otherwise
BM = Bone marrow
NE = Not evaluable
Response and Survival Outcomes
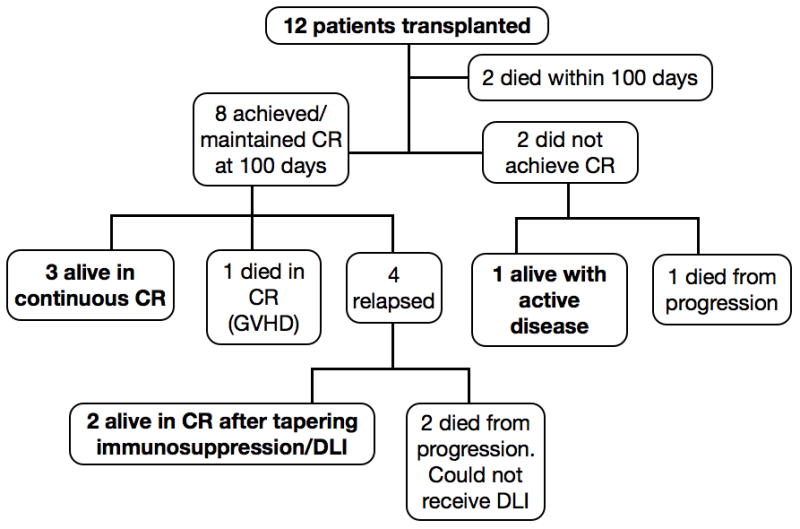
The outcomes of all patients are shown in Figure 1. Two patients (one with RIC and the other with myeloablative conditioning) died within the first 100 days from sepsis with active disease. At day 100, 8 of 10 evaluable patients were in complete remission (CR); transplant had induced and maintained CR in 6 pts with active disease. Three patients remain alive in continuous CR 16, 22 and 45 months after transplant. Four patients relapsed after achieving CR between 4 and 13 months after HSCT, but two of these re-achieved and maintained CR, one after tapering immunosuppression and the other after receiving DLI (without additional chemotherapy). Thus, of the 10 patients who survived to 100 days, 5 are alive in CR at last contact and 1 is alive with active disease.
Figure 1.
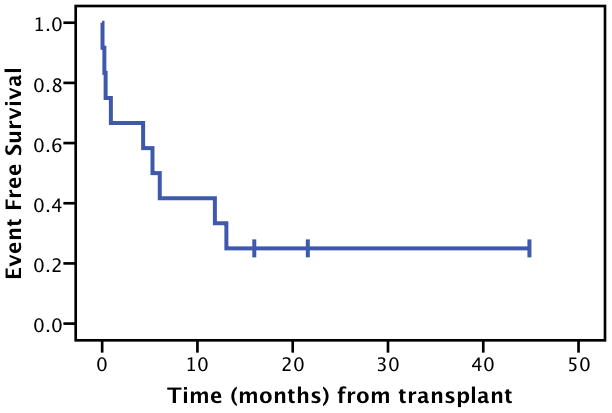
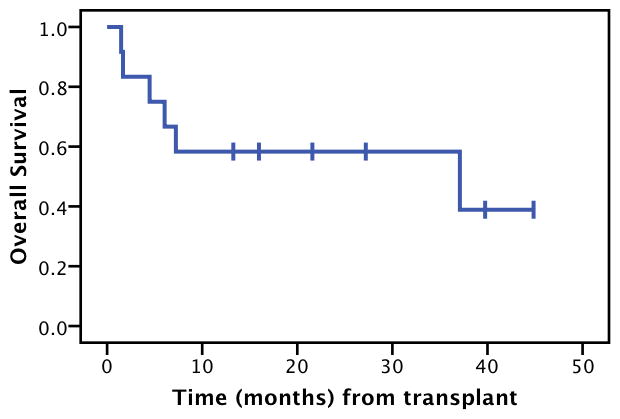
The median EFS for all pts was 5.3 months (Figure 2A). This underestimates achievement of CR, since two patients were able to re-achieve and maintain CR as mentioned above. Kaplan-Maier estimated 2-year OS was 58%, and estimated median overall survival was 37 months (Figure 2B).
Figure 2.
GVHD
9 of 12 patients developed acute GVHD, with 4 developing Grade III–IV GVHD. GVHD was the cause of death in 1 patient. Acute and chronic GVHD are detailed in Table 2.
Table 2.
Post-transplant outcomes
| Pt | Non-relapse/non- GVHD toxicity | Acute GVHD (Grade, Organ, and Stage) | Chronic GVHD | Disease Status at 100 days | Relapse (Y/N) | Treatment(s) since relapse | Current status | Time SCT to last follow up (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | CNS PTLD (responded to Rituximab), CMV antigenemia, Pneumonitis | - | - | CR | Y | Denileukin Diftitox, Alemtuzumab | Expired (progression) | 37 |
| 2 | Widely metastatic melanoma* | Grade II (Gut Stage 1) | - | CR | Y | - | Expired (progression and metastatic melanoma)* | 7 |
| 3 | Sinusitis, Osteoporosis, Herniated disk, Hearing loss, Steroid-induced glaucoma | Grade III (Gut Stage 3) | Severe chronic GVHD of Mouth, Musculoskeletal system and Lungs (Bronchiolitis obliterans) | CR | N | - | Alive, CR (continuous) | 45 |
| 4 | Steroid-induced DM | Grade II (Skin Stage 3, Gut Stage 1, Liver Stage 0) | - | CR | Y | DLI with re-achievement of CR. | Alive, CR | 39 |
| 5 | Bacterial sepsis | - | - | Expired | Y | - | Expired (Early TRM) | 2 |
| 6 | - | Grade III (Skin Stage 3, Liver Stage 2) | - | Prog-ression | Y | Tapering of immunosuppression (not effective), nodal XRT, Cyclophosphamide | Expired (progression) | 5 |
| 7 | - | Grade I (Skin Stage 1) | - | Relapse | Y | Tapering of immunosuppression, DLI (ineffective), Vorinostat, Topical carmustine, TSEB, Interferon gamma-1b, ICE | Alive, Active disease (chemo-responsive) | 27 |
| 8 | PICC-associated UE DVT, hypothyroidism, Herpes zoster | Grade III (Skin Stage 3, Liver Stage 0) | - | CR | N | - | Alive, CR (continuous) | 22 |
| 9 | Cholecystitis, bacterial sepsis | Grade IV (Gut Stage 4, Liver Stage 4-VOD) | - | CR | N | - | Expired in CR (GVHD) | 6 |
| 10 | Bacterial sepsis | Grade I (Skin Stage 2) | - | Expired | Y | - | Expired (Early TRM) | 1 |
| 11 | Hypothyroidism | - | - | CR | N | - | Alive, CR (continuous) | 16 |
| 12 | - | Grade I (Skin Stage 2) | - | CR | Y | Tapering of immunosuppression with re-achievement of CR | Alive, CR | 13 |
Patient #2 had a remote history of localized, resected melanoma, which resurfaced as widely metastatic disease after HSCT. Death was due to rapid progression of both CTCL and melanoma.
CMV: Cytomegalovirus
DVT: Deep Venous Thrombosis
ICE: Ifosfamide, Carboplatin, Etoposide
PTLD: Post-Transplant Lymphoproliferative Disease
TRM: Transplant Related Mortality
VOD: Veno-Occlusive Disease
GVL Activity
Patient #4 received DLI (2.2 × 10^8 nucleated cells/kg) at time of relapse (after tapering off immunosuppression) 13 months after HSCT, and achieved CR that is persistent 26 months since DLI. Patient #12 was on Tacrolimus at the time of relapse, and achieved CR with discontinuation of immune suppression. She remains in CR 8 months later. This provides clear evidence for a GVL effect, as neither of these patients received any accompanying chemo/systemic therapy that might explain achievement and maintenance of CR.
Of note, patient #7 received DLI (1.5 × 10^8 nucleated cells/kg) 6 months after HSCT, with minimal effect on disease progression. Patient #8 received DLI (10^7 nucleated cells/kg) 15 months after HSCT for decreasing donor chimerism (no evidence of relapse), with a subsequent achievement of 100% donor chimerism and continued CR.
Discussion
Although CTCL is often an indolent lymphoma, with numerous effective treatment options, it is generally incurable, and most patients eventually fail chemotherapy and biological therapies and will die of their disease (ref. 18). The prognosis is especially poor for patients with transformed CTCL and for patients who fail conventional therapy (ref. 19). Newer treatments like denileukin diftitox (ref. 20), vorinostat (ref. 3) and romidepsin (ref. 7), have recently shown promising results.
High dose therapy and autologous HSCT has been disappointing, and early relapses are common (ref. 11). The role for allogeneic HSCT in CTCL is unclear. A recent retrospective study from the EBMT included a heterogeneous group of patients who received both myeloablative and RIC regimens with estimated 3-year survival of 54% (ref. 21). Three other small reports further support the role of allogeneic HSCT in CTCL. Molina et al. described 8 patients with MF/SS who were treated with RIC:SCT and 6 patients remained alive without disease 33–108 months after transplant (ref. 13). Duvic et al. described outcomes in 19 recipients of an intensive though reduced intensity conditioning regimen that included total skin electron beam irradiation followed by allogeneic transplant, with 11 patients achieving sustained remissions, and several achieving stable disease after a combination of DLI, immunosuppression withdrawal, and/or chemotherapy (ref. 14). Delioukina et al. also showed similarly encouraging results for RIC:SCT in 11 patients with CTCL, and observed no further relapses/deaths after the first 18 months after HSCT (ref. 15).
We report our experience using, most commonly, a reduced intensity conditioning regimen followed by allogeneic stem cell transplant in 12 consecutive patients with extensively pretreated advanced CTCL. Response rates were high. Treatment related mortality was low. 2 patients who did not respond died from infectious complications within 100 days of transplant. GVHD accounted for death in one patient. The most common cause of death was disease progression in 5 patients.
42% of our patients were alive and disease-free a median duration of 22 months after transplant. While this percentage seems lower than other reports, it is difficult to compare patients across different retrospective studies. Furthermore, our outcomes provide strong and direct support the presence of a GVL effect in CTCL; 1 patient achieved CR after DLI alone for relapse and a second achieved sustained remission after withdrawal of immunosuppression.
Despite the limitations associated with this retrospective review of small numbers of patients, our outcomes support the presence of a meaningful GVL effect associated with RIC allogeneic stem cell transplant. With small numbers of patients it was not possible to associate the occurrence of graft versus host disease with a GVL effect.
Our experience shows that RIC HSCT can provide long-term disease control in patients with advanced CTCL otherwise refractory to immunotherapy and chemotherapy. Ideally, prospective studies will further define the role of allogeneic HSCT in this disease. However, given the limited transplant related mortality, consideration for earlier transplant should be given, with a focus on the use of newer agents for achieving minimal residual disease (MRD) prior to transplant. Interventions to decrease the risk of relapse should be considered, including the possibility of maintenance immunotherapy and/or planned DLI.
Figure 3.
Acknowledgments
This work was supported in part by grants from The Leukemia & Lymphoma Society (7000-02) and NIH (K24 CA11787901) (DLP).
Footnotes
Conflicts of Interest
Alain H. Rook - Therakos (Speaker’s bureau), HY Biopharma (Consultancy)
Ellen J Kim – TenX, Biocryst, Genmab, Glouchester, Celgene (Research funding), Eisai (Consultancy)
Other authors have no relevant conflicts of interest
References
- 1.van Doorn R, Van Haselen CW, van Voorst Vader PC, et al. Mycosis fungoides: disease evolution and prognosis of 309 Dutch patients. Arch Dermatol. 2000;136:504–510. doi: 10.1001/archderm.136.4.504. [DOI] [PubMed] [Google Scholar]
- 2.Bernengo MG, Quaglino P, Novelli M, et al. Prognostic factors in Sézary syndrome: a multivariate analysis of clinical, haematological and immunological features. Ann Oncol. 1998;9:857–863. doi: 10.1023/a:1008397323199. [DOI] [PubMed] [Google Scholar]
- 3.Duvic M, Talpur R, Ni X, et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL) Blood. 2007;109:31–39. doi: 10.1182/blood-2006-06-025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zain J, O’Connor OA. Targeting histone deacetyalses in the treatment of B- and T-cell malignancies. Invest New Drugs. 2010;28 (Suppl 1):S58–78. doi: 10.1007/s10637-010-9591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erter J, Alinari L, Darabi K, et al. New targets of therapy in T-cell lymphomas. Curr Drug Targets. 2010;11:482–493. doi: 10.2174/138945010790980376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant C, Rahman F, Piekarz R, et al. Romidepsin: a new therapy for cutaneous T-cell lymphoma and a potential therapy for solid tumors. Expert Rev Anticancer Ther. 2010;10:997–1008. doi: 10.1586/era.10.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whittaker SJ, Demierre MF, Kim EJ, et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:4485–4491. doi: 10.1200/JCO.2010.28.9066. [DOI] [PubMed] [Google Scholar]
- 8.Rook AH, Kuzel TM, Olsen EA. Cytokine therapy of cutaneous T-cell lymphoma: interferons, interleukin-12, and interleukin-2. Hematol Oncol Clin North Am. 2003;17:1435–48. ix. doi: 10.1016/s0889-8588(03)00109-6. [DOI] [PubMed] [Google Scholar]
- 9.Choi J, Foss F. Cutaneous T-cell lymphoma: Biologic targets for therapy. Curr Hematol Malig Rep. 2007;2:272–277. doi: 10.1007/s11899-007-0037-8. [DOI] [PubMed] [Google Scholar]
- 10.Horwitz SM. Novel therapies for cutaneous T-cell lymphomas. Clin Lymphoma Myeloma. 2008;8 (Suppl 5):S187–92. doi: 10.3816/CLM.2008.s.015. [DOI] [PubMed] [Google Scholar]
- 11.Bigler RD, Crilley P, Micaily B, et al. Autologous bone marrow transplantation for advanced stage mycosis fungoides. Bone Marrow Transplant. 1991;7:133–137. [PubMed] [Google Scholar]
- 12.Goldstein SC, Porter DL. Allogeneic immunotherapy to optimize the graft-versus-tumor effect: concepts and controversies. Expert Rev Hematol. 2010;3:301–314. doi: 10.1586/ehm.10.29. [DOI] [PubMed] [Google Scholar]
- 13.Molina A, Zain J, Arber DA, et al. Durable clinical, cytogenetic, and molecular remissions after allogeneic hematopoietic cell transplantation for refractory Sezary syndrome and mycosis fungoides. J Clin Oncol. 2005;23:6163–6171. doi: 10.1200/JCO.2005.02.774. [DOI] [PubMed] [Google Scholar]
- 14.Duvic M, Donato M, Dabaja B, et al. Total Skin Electron Beam and Non- Myeloablative Allogeneic Hematopoietic Stem-Cell Transplantation in Advanced Mycosis Fungoides and Sézary Syndrome. J Clin Oncol. 2010 doi: 10.1200/JCO.2009.25.8301. [DOI] [PubMed] [Google Scholar]
- 15.Delioukina M, Zain J, Palmer JM, Tsai N, Thomas S, Forman S. Reduced-intensity allogeneic hematopoietic cell transplantation using fludarabine-melphalan conditioning for treatment of mature T-cell lymphomas. Bone marrow transplantation. 2011 doi: 10.1038/bmt.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assn. 1958;53:457–481. [Google Scholar]
- 17.Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC) Blood. 2007;110:1713–1722. doi: 10.1182/blood-2007-03-055749. [DOI] [PubMed] [Google Scholar]
- 18.Lansigan F, Foss FM. Current and emerging treatment strategies for cutaneous T-cell lymphoma. Drugs. 2010;70:273–286. doi: 10.2165/11532190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Gardner JM, Evans KG, Musiek A, Rook AH, Kim EJ. Update on treatment of cutaneous T-cell lymphoma. Curr Opin Oncol. 2009;21:131–137. doi: 10.1097/CCO.0b013e3283253190. [DOI] [PubMed] [Google Scholar]
- 20.Prince HM, Duvic M, Martin A, et al. Phase III placebo-controlled trial of denileukin diftitox for patients with cutaneous T-cell lymphoma. J Clin Oncol. 2010;28:1870–1877. doi: 10.1200/JCO.2009.26.2386. [DOI] [PubMed] [Google Scholar]
- 21.Duarte RF, Canals C, Onida F, et al. Allogeneic hematopoietic cell transplantation for patients with mycosis fungoides and Sezary syndrome: a retrospective analysis of the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:4492–4499. doi: 10.1200/JCO.2010.29.3241. [DOI] [PubMed] [Google Scholar]


