Abstract
A heritable phenotype resulting from the self-administration of cocaine in rats was delineated. We observed delayed acquisition and reduced maintenance of cocaine self-administration in male, but not female, offspring of sires that self-administered cocaine. Brain-derived neurotrophic factor (BDNF) mRNA and protein were increased in the medial prefrontal cortex (mPFC) and there was an increased association of acetylated histone H3 with BDNF promoters only in the male offspring of cocaine-experienced sires. Administration of a BDNF receptor antagonist (the TrkB receptor antagonist ANA-12) reversed the diminished cocaine self-administration in male cocaine-sired rats. In addition, the association of acetylated histone H3 with BDNF promoters was increased in the sperm of sires that self-administered cocaine. Collectively, these findings indicate that voluntary paternal ingestion of cocaine results in epigenetic reprograming of the germline resulting in profound effects on mPFC gene expression and resistance to cocaine reinforcement in male offspring.
A growing body of evidence indicates that ancestral environmental perturbations can influence the physiology and behavior of descendants. Although the majority of studies of this sort focus on maternal effects1–3, there are examples of paternal phenotype transmission between generations. For example, progeny of male mice fed a low-protein diet exhibit elevated hepatic expression of genes involved in lipid and cholesterol biosynthesis4, whereas exposing male rats to a high fat diet results in pancreatic beta cell dysfunction in female offspring5. Moreover, early prenatal stress reprograms the male germline resulting in dysmasculinization in second-generation offspring6. Notably, transgenerational influences of ancestral environment are evident in humans since epidemiological data link exposure to a famine in grandfathers to obesity and cardiovascular disease two generations later7,8.
In terms of drugs of abuse, the adult offspring of female rats exposed to morphine during adolescence display increases in anxiety (female offspring), enhancement of morphine-induced analgesia (male offspring), and augmented behavioral sensitization to morphine (male and female offspring)9,10. Maternal exposure to cocaine decreases global DNA methylation in the hippocampus of male offspring11, whereas paternal cocaine administration results in impaired working memory in female offspring12 and hyperactivity and increases in perseveration in a t-maze among male progeny13. The implications of these findings for the descendants of drug dependent individuals are profound. Therefore, we established a rat model to examine the influence of paternal cocaine self-administration on gene expression, chromatin remodeling and cocaine reinforcement in the progeny. We examined paternal transmission in order to avoid the influence of in utero cocaine exposure and the potential influence of prior cocaine experience by dams on maternal behavior.
RESULTS
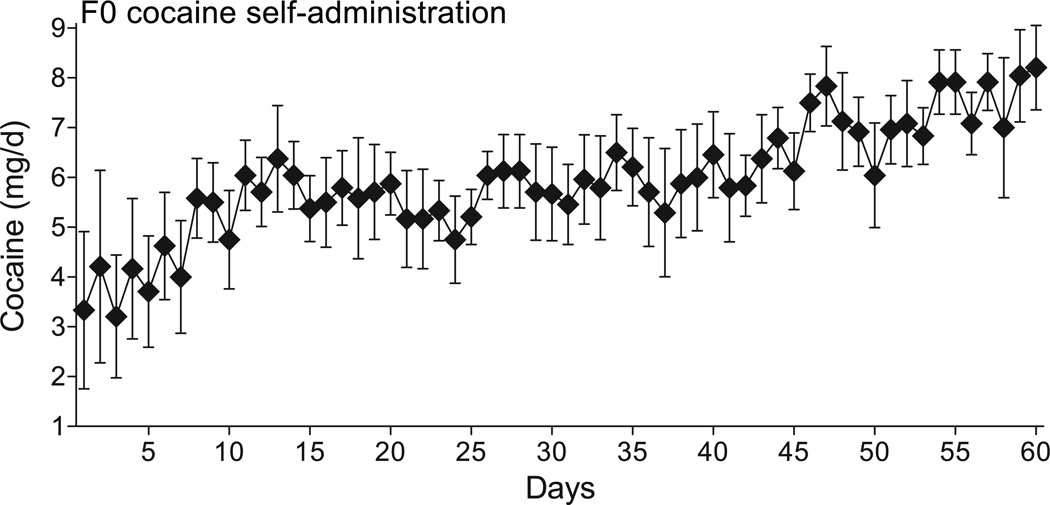
In order to develop a model for the inter-generational influence of cocaine self-administration on gene expression, chromatin remodeling and behavior we allowed male Sprague-Dawley rats to self-administer i.v. cocaine (0.25 mg/infusion, not adjusted for animal weight) for 60 days, the duration of rat spermatogenesis; control rats received yoked i.v. saline injections. The mean (±s.e.m.) number of infusions per day was 23.79±0.5. Rats initially self-administered between 3–4 mg (approximately 0.5 mg/kg/infusion) of cocaine per day with the daily intake escalating to 7–8 mg/day after 60 days of self-administration (see Fig. 1). In this experiment, the average cocaine dose was approximately 0.7 mg/kg/infusion. The day after the last self-administration session, the F0 males were mated with naïve females resulting in 13 litters from cocaine-experienced sires and 13 litters from saline control sires.
Figure 1.
Cocaine self-administration by the F0 sires. The data are expressed as mean±s.e.m. mg cocaine consumed on each of the 60 days of self-administration. Note that the behavior stabilizes in the second week and subsequently escalates after approximately 45 days of self-administration
Reduced cocaine intake in male cocaine-sired rats
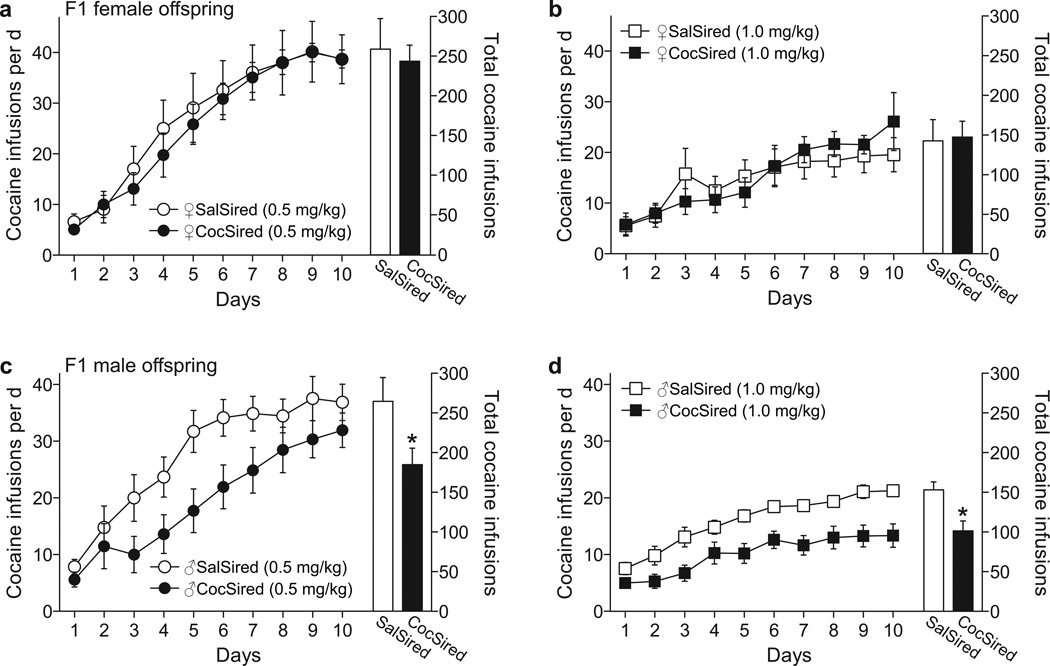
When they reached approximately 60 days of age, we implanted jugular catheters into 1–3 male and female offspring from each litter. After 7 days of recovery we assessed the acquisition of cocaine self-administration under a fixed ratio 1 (FR1) schedule of reinforcement. Under an FR1 schedule all lever presses resulted in cocaine administration. The results indicated no difference in the rate of acquisition or the level of cocaine intake among female offspring of cocaine-experienced males (♀CocSired) relative to controls (♀SalSired) (Fig. 2a,b). However, we observed significantly delayed acquisition of 0.5 and 1.0 mg/kg cocaine self-administration by ♂CocSired rats relative to ♂SalSired rats (Fig. 2c,d). Moreover, when cocaine self-administration reached asymptote, we saw significantly decreased intake of 1.0 mg/kg cocaine under an FR1 schedule in ♂CocSired relative to ♂SalSired rats (Fig. 2d). These data were analyzed with separate two-way analyses of variance (ANOVAs) with a between subjects factor of treatment (saline- vs. cocaine-sired) and repeated measures over time (days). For the male offspring, the results of the low dose analyses revealed significant main effects of time [F(9,252)=39.37, p<0.0001] and siring [F(1,28)=4.278, p<0.048]. The results of the high dose analyses showed significant main effects of time [F(9,180)=22.28, p<0.0001] and siring [F(1,20)=10.69, p<0.0038]. We analyzed the total number of infusions for each cocaine dose with separate unpaired t-tests. The results of these analyses revealed significant differences between treatments for both the low [t(28)=2.068; p<0.048] and high doses of cocaine [t(20)=3.269; p<0.0038] among male offspring (Fig. 2c,d).
Figure 2.
There were no differences in the acquisition and maintenance of 0.5 mg/kg (a) or 1.0 mg/kg (b) cocaine self-administration between the female saline-sired (♀SalSired) and cocaine-sired (♀CocSired) groups. With female progeny there also was no difference between groups when cocaine was self-administered under a progressive ratio schedule (c). In contrast, there was delayed acquisition of 0.5 (d) and 1.0 (e) mg/kg cocaine self-administration and reduced asymptotic intake of 1.0 mg/kg cocaine (e) in the male offspring of cocaine-experienced sires. Data are expressed as the mean (±s.e.m.) cocaine infusions per daily two-hour session. The asterisks represent significant differences between the SalSired and CocSired groups (p<0.05). 15 male and 11 female rats per treatment were tested at the low cocaine dose and 11 male and 9 female subjects per treatment were exposed to the higher dose of cocaine.
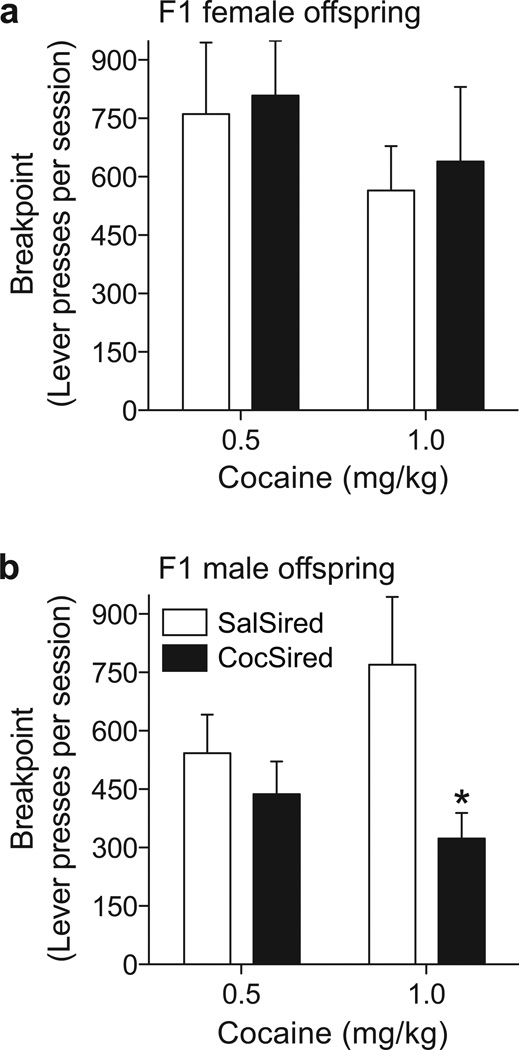
Following the acquisition of cocaine self-administration, all subjects switched to a progressive ratio (PR) schedule. Under a PR schedule the response requirement for each subsequent drug delivery increases until the subject fails to meet a requirement. In the current experiments, the breakpoint was operationally defined as the total number of responses prior to the termination of the session or the final completed ratio. In our experiments, sire cocaine exposure did not influence cocaine self-administration under a PR schedule among female offspring (Fig. 3a). However, results showed reduced breakpoint (responses) in ♂CocSired rats compared to ♂SalSired rats at the higher [t(18)=2.399, p<0.0275] but not the lower dose of cocaine (Fig. 3b). The final completed ratio data revealed similar results [♂SalSired 0.5 mg/kg cocaine: 105.47±14.43 (mean±SEM); ♂CocSired 0.5 mg/kg cocaine: 81.73±14.01; ♂SalSired 1.0 mg/kg cocaine: 134.90±25.90; ♂CocSired 1.0 mg/kg cocaine: 60.50±11.53]. We tested 15 male rats and 11 female rats per treatment at the low cocaine dose; we exposed 11 male and 9 female subjects per treatment to the higher dose of cocaine. The lack of group differences at the lower cocaine dose was likely related to the fact that ♂CocSired and ♂SalSired rats displayed similar levels of self-administration at the end of the acquisition period. In contrast, ♂CocSired rats self-administered significantly less cocaine than ♂SalSired animals when responding for the high cocaine dose reached asymptote.
Figure 3.
When cocaine was self-administered under a progressive ratio schedule, there were no differences between the breakpoints of female CocSired and SalSired rats at either cocaine dose (a). The breakpoint for 1.0 mg/kg cocaine was significantly reduced in male cocaine-sired relative to saline-sired (♂SalSired) rats (b). Data are expressed as the mean (±s.e.m.) lever presses per session. The asterisks represent significant differences between the SalSired and CocSired groups (p<0.05).
Normal intake of sucrose in male cocaine-sired rats
The delayed acquisition of cocaine self-administration among cocaine-sired rats could be due to a general learning deficit. In order to test for potential operant learning impairment, we trained naïve F1 littermates to self-administer sucrose pellets under an FR1 schedule. This operant task represents an ideal control because the behavior the animals must learn is identical to the cocaine self-administration paradigm, i.e. lever pressing. There were no differences in the acquisition of food self-administration between the SalSired and CocSired male offspring (see Supplementary Fig. 1a). These data were analyzed with a mixed factors ANOVA (repeated measures over day), which revealed a significant main effect of day [F(4,100)=11.82, p<0.001] but no significant main effect of sire and no significant interaction. Time course data were not available for all animals, but representative rats from each group on Day 1 and Day 5 are shown in Supplementary Figures 1b and 1c, respectively (n=5). Analysis of the Day 1 data revealed no significant main effects or interaction. For Day 5, a mixed factors ANOVA (repeated measure over time) showed a significant main effect of time [F(11,88)=16.44, p<0.001] but no significant main effect of sire and no significant interaction.
In order to assess the specificity of the decreased breakpoint for cocaine self-administration in CocSired relative to SalSired rats (see Fig. 2) we assessed self-administration of sucrose under a PR schedule immediately following acquisition of sucrose self-administration in one of the two cohorts tested (see above). Results indicated no significant difference between CocSired and SalSired groups when breakpoint was defined as lever presses per session (see Supplementary Fig. 2) or last ratio completed (SalSired=66.8±8.62; CocSired=74.25±19.05; mean±SEM).
Sires have no influence on maternal behavior
In evolutionary biology the differential allocation hypothesis posits that the phenotypic quality of a male that a female mates with can influence the subsequent maternal investment in their offspring14. Although the vast majority of the work in this area is in birds15, there is evidence that male quality may influence maternal investment in rodents. For example, in experiments using feral mice, the offspring of a dam bred with a sire she did not prefer are less viable and exhibit impaired performance on tests of predator avoidance, aggression and nest construction relative to offspring resulting from breeding with preferred males16,17. Notably, the behavioral and epigenetic mechanisms accounting for these effects were not examined in these studies. In experiments performed in mice, there is a significant positive correlation between paternal open field behavior and behavior in the open field of female, but not male, offspring18. Importantly, paternal effects on offspring behavior are not related to differences in maternal care18. Clearly, very little is known about paternal influence on maternal behavior and the subsequent effects on offspring in rodents. Nonetheless, we examined this factor in the current experiments since potential changes in maternal behavior could substantially impact the subsequent self-administration of cocaine by offspring19.
There was no difference in maternal behaviors between cocaine- and saline-sired litters (see Supplementary Fig. 2). Thus, the percent time dams spent licking and grooming their pups (P3–P7) was nearly identical between F1 CocSired (16.2±0.9%) and SalSired (13.7±3.2%) litters. The time spent off pups was 40.4±9.8% for CocSired and 39.1±5.8% for SalSired. The total time spent nursing the pups was 59.6±9.8% and 60.8±5.8% for CocSired and SalSired, respectively. Finally, the amount of time spent in the arched back nursing position was 42.5±4.7% for the CocSired and 50.1±7.1% for the SalSired.
Increased mPFC BDNF protein and mRNA in male CocSired rats
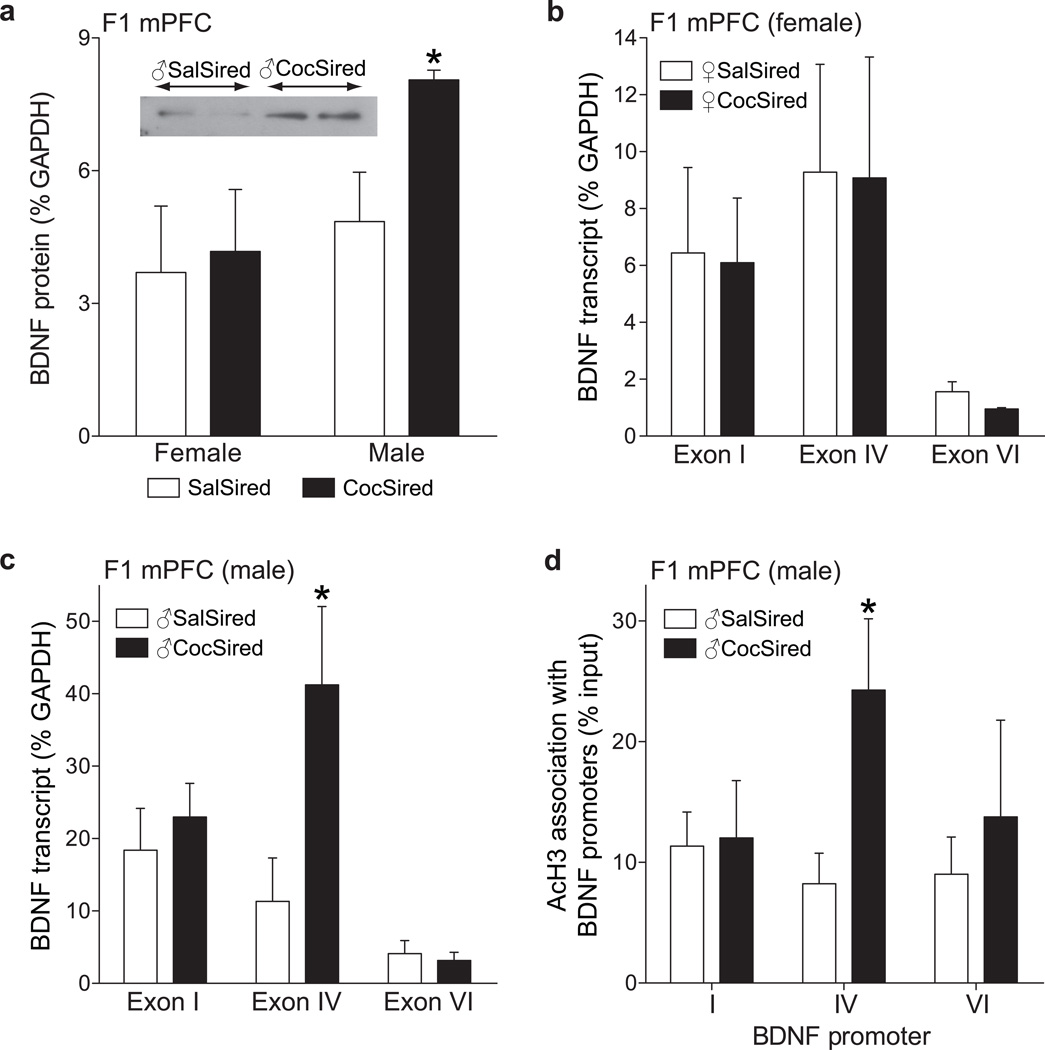
Previous work shows that increased BDNF in the medial prefrontal cortex (mPFC) blunts the behavioral effects of cocaine20,21. Therefore, we examined BDNF in the mPFC (including the anterior cingulate, prelimbic and infralimbic cortices) of offspring that were not exposed to cocaine. BDNF protein levels in the mPFC were measured using western blot analysis. BDNF protein levels in the mPFC of ♂CocSired rats were significantly increased relative to ♂SalSired rats (F1 generation) (Fig. 4a). These data were analyzed with an unpaired t-test, which revealed a significant difference between groups [t(10)=2.811, p<0.0184]. There were 6 subjects per group. There were no differences in protein expression levels in the ♀CocSired rats relative to controls (♀SalSired) (Fig. 4a).
Figure 4.
a, BDNF protein was increased in the mPFC of male, but not female, CocSired relative to SalSired rats. The inset includes representative Western bots from the two male offspring groups. b, No change in BDNF mRNA expression in female CocSired relative to SalSired rats. c, Increased expression of BDNF exon IV-containing transcript in the mPFC of male CocSired rats. d, Increased association of AcH3 with BDNF promoter IV in the mPFC of male CocSired rats. All data are expressed as mean (±s.e.m.). The asterisks indicate significant differences (p<0.05) between the SalSired and CocSired groups.
BDNF mRNA expression levels in the mPFC were measured using qPCR. There were no differences in the expression of BDNF exons in ♀CocSired vs. ♀SalSired rats (Fig. 4b). In contrast, there was a significant increase in BDNF exon IV in ♂CocSired rats compared to controls (Fig. 4c). The BDNF mRNA data from males were analyzed with a mixed factors two-way ANOVA, which revealed a significant main effect of exon [F(2,12)=14.75; p<0.0006] as well as a significant exon × siring interaction [F(2,12)=7.181; p<0.0089]. Fisher’s post-hoc analyses revealed a significant difference in BDNF exon IV expression between ♂CocSired and ♂SalSired animals (p<0.05). ChIP-qPCR was used to assess the association of BDNF exons with acetylated histone H3 (H3K9K14ac2, AcH3) in male offspring (Fig. 4d). These data were analyzed with a two-way mixed factors ANOVA that showed a marginal effect of exon [F(2,12)=3.675; p<0.0569] and a significant siring × exon interaction [F(2,12)=7.873; p<0.0065]. Fisher’s post-hoc tests showed increased association of AcH3 with BDNF exon IV (p<0.05). These results indicated that there was increased association of AcH3 with BDNF promoter IV in the mPFC of ♂CocSired rats.
ANA-12 reversed the cocaine resistance phenotype
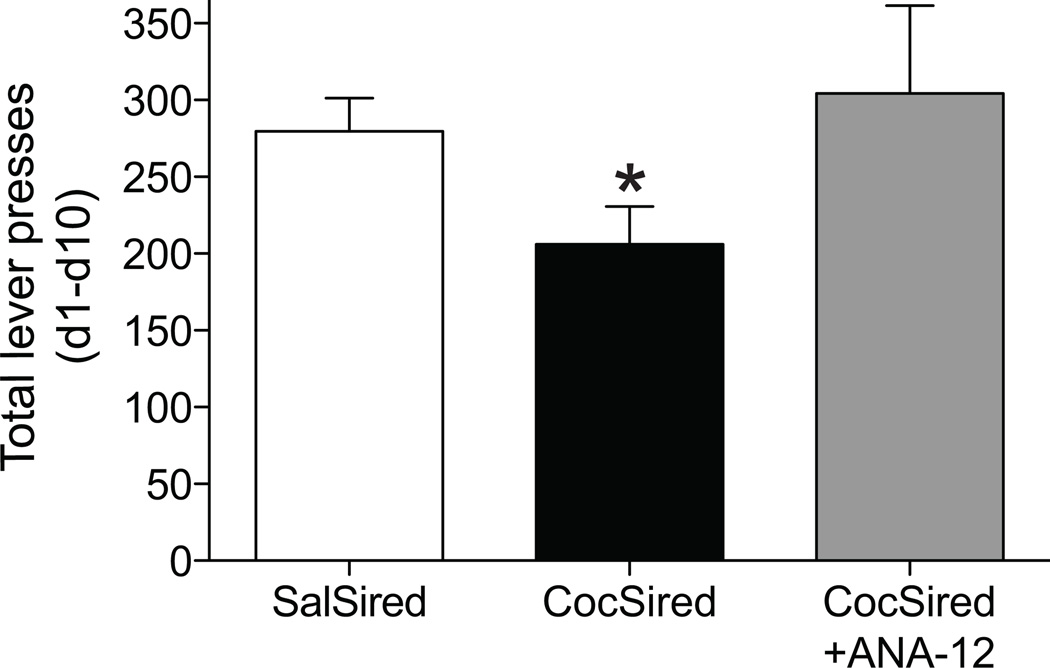
Increased BDNF levels in the mPFC of ♂CocSired (see Fig. 4) may contribute to the decreased acquisition of cocaine self-administration in these animals. BDNF signals primarily through TrkB receptors22. In order to test the hypothesis that increased BDNF activation of TrkB receptors suppressed cocaine self-administration in CocSired rats, the selective TrkB receptor antagonist (ANA-12; 0.5 mg/kg, i.p.) or its vehicle (100% DMSO) was administered systemically 4 hours prior to each of 10 daily cocaine (0.5 mg/kg/infusion) self-administration sessions. The ANA-12 dose and administration parameters were based on the original paper describing the development of this drug23. Results indicated that ANA-12 reversed the diminished self-administration of cocaine in ♂CocSired rats (Fig. 5), which suggests that enhanced BDNF expression in the mPFC reduced cocaine reinforcement in the male offspring of cocaine-experienced sires.
Figure 5.
Pretreatment with the TrkB antagonist ANA-12 normalized the decreased acquisition of cocaine self-administration in CocSired rats. The data are expressed as the mean (±s.e.m.) lever presses summed across the 10 days of cocaine self-administration. The asterisk represents a significant difference from both other groups (Fisher’s LSD, p<0.05). The number of rats per treatment was as follows: SaSired=16, CocSired=21, CocSired+ANA-12=8.
In this experiment, some SalSired rats in the control groups received DMSO and others did not; there were no significant behavioral differences produced by DMSO so these data sets were combined to produce one control group. Some animals do not acquire cocaine self-administration, usually due to faulty catheters. In order to control for this possibility, a criterion for acquisition is imposed. Thus, if an animal fails to average at least 3 lever presses per session (i.e. a total of 30 lever presses over 10 days) those data are excluded from the analyses. In the data included in Fig. 1, no rats met this criterion. However, in the experiment summarized in Fig. 5, 5 subjects (2 CocSired, 2 SalSired, 1 CocSired+ANA-12) were excluded from the one-way ANOVA. Analysis of these data indicated a marginally significant main effect of treatment [F(2,40)=3.0579, p<0.0581]. Planned comparisons (Fisher’s LSD) showed that the responding in the CocSired group was significantly different from both the SalSired and CocSired plus ANA-12 treatments (p<0.05).
Increased sperm BDNF promoter acetylation in cocaine sires
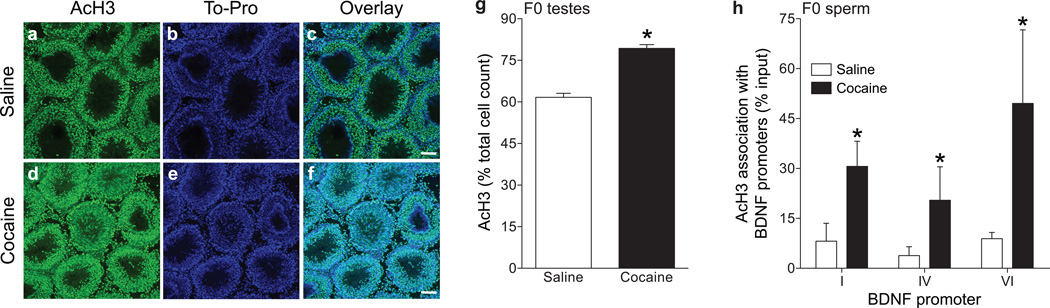
In our experiments, the sires played no role in rearing their offspring and mating with cocaine-experienced sires did not influence maternal behaviors (see Supplementary Fig. 3). It is possible that information may have been passed through the paternal germline. Initial qualitative analysis of AcH3 expression in the testes of saline- and cocaine-exposed sires indicated substantially increased levels in rats that self-administered cocaine; AcH3 expression was localized in the nuclei as expected (Fig. 6a–f). Quantitative analysis of the AcH3 staining intensity per cell was performed on the testes of three saline (1057 cells total) and three cocaine (938 cells total) rats. Results indicated that there was a significant increase in AcH3 staining (intensity/cell) in the testes of cocaine- relative to saline-experienced sires [t(4)=8.959, p<0.0009] (Fig. 6g).
Figure 6.
Representative sections of testes from saline- (a) and cocaine- (b) exposed rats showing clear qualitative increases in AcH3 (a,b) in the tubules as measured by immunohistochemistry. Staining of To-Pro DNA dye (c,d) and the overlay of To-Pro and AcH3 (e,f) in saline- and cocaine-experienced rats, respectively, shows co-expression as expected (scale bars=100 µM). g, Quantitative assessment of the percent of total cell counts (±s.e.m.) showed a significant increase in AcH3 staining (intensity/cell) in the testicular tubules of cocaine relative to saline rats (p<0.05). h, Increased association of AcH3 with BDNF promoters I, IV and VI among cocaine exposed sires, expressed as % input ±s.e.m. The asterisks denote a significant main effect of treatment (p<0.05).
In order to determine if paternal cocaine exposure produced relevant changes in sperm specifically, we examined the association of AcH3 with BDNF promoters I, IV and VI. There were no differences in total H3 levels in the sperm of cocaine-experienced sires relative to saline controls (data not shown). ChIP-qPCR was used to examine the association of AcH3 with BDNF promoters I, IV and VI in cocaine exposed sires. Results indicated that there was an increased association of AcH3 with BDNF promoters in the sperm of sires that self-administered cocaine relative to saline controls (Fig. 6h). These data were analyzed with a two-way repeated measures ANOVA, which revealed a significant main effect of treatment [F(1,4)=11.11, p<0.029].
DISCUSSION
The current experiments examined cocaine self-administration in the adult offspring of sires exposed to cocaine or saline. The results indicated that there was no difference in the rate of acquisition or the level of cocaine intake among female offspring of cocaine-experienced males. In sharp contrast, we observed delayed acquisition and reduced maintenance of cocaine self-administration in ♂CocSired rats. Following the acquisition of self-administration, subjects switched to a PR schedule. Under this schedule, ♂CocSired (but not ♀CocSired) rats did not work as hard as controls to receive infusions of cocaine, which suggested decreased reinforcing effectiveness of cocaine among the male offspring of sires that self-administered cocaine. These differences in cocaine self-administration among male offspring were not due to operant learning deficits since the acquisition of sucrose self-administration and the reinforcing effectiveness of sucrose did not differ between naïve ♂CocSired and ♂SalSired rats.
Several physiological differences could underlie the differential responses to cocaine between male and female CocSired rats. Notably, males secrete testosterone during late gestation and early neonatal periods resulting in sexually dimorphic characteristics in reproductive endocrinology, brain nuclei and adult behavioral traits24. Of particular interest, the bed nucleus of the stria terminalis, a brain region implicated in stress and addiction25,26, is larger in males than females27. Cycling gonadal hormones in female rats may also play a role in that estrogen enhances the effects of cocaine28,29 whereas progesterone has the opposite effect30. Thus, interactions between intergenerational effects of cocaine on gene transcription and subsequent hormonal influences likely contribute to the differential behavioral phenotypes of male and female CocSired rats.
The present behavioral results are apparently at odds with human epidemiological data indicating that cocaine addiction is heritable31–33. However, drug availability and myriad other environmental factors are fundamental aspects of addiction. Moreover, human genetics studies are by definition correlational. Therefore, the genetic basis for the influence of family history as a risk factor for the development of addiction should be interpreted cautiously34. That said, there is little doubt that addiction is influenced by genetic and environmental (and potentially epigenetic) factors; however, the exact contribution of each of these factors is difficult to discern based on human data. In contrast, our animal experiments focused exclusively on the influence of cocaine experience in sires (in the absence of any other drug of abuse) and controlled for environmental influences to an extent that is impossible with human studies.
Cocaine alters the expression of BDNF in various limbic nuclei, which influences cocaine-mediated behaviors including cocaine self-administration and cocaine seeking20,35. For example, cocaine self-administration followed by seven days of forced abstinence increases BDNF mRNA and protein levels in the rat mPFC20. Altered BDNF levels in the mPFC have functional consequences. Thus, a single, exogenous BDNF infusion into the mPFC suppresses the reinstatement of cocaine seeking21,36. Moreover, decreasing mPFC BDNF levels using RNA interference dramatically enhances the cocaine breakpoint under a PR schedule, suggesting that increases in mPFC BDNF levels counteract the reinforcing effectiveness of cocaine20. Since the present data indicated that the acquisition of cocaine self-administration was delayed/reduced in the male progeny of cocaine-experienced sires, we speculated that increased BDNF levels in the mPFC might underlie this drug resistance phenotype.
The present results revealed that BDNF protein was increased in the mPFC of male, but not female, CocSired relative to SalSired rats. The increased mPFC BDNF protein in ♂CocSired rats was associated with a selective increase in BDNF exon IV-containing transcript as well as increased association of AcH3 with BDNF promoter IV in the mPFC of ♂CocSired rats. Histone acetylation results in an open chromatin configuration, which enhances transcription20,37. Thus, increased acetylation of histone H3 associated with a specific promoter is one mechanism underlying increased BDNF expression in the mPFC of ♂CocSired rats. Moreover, administration of a TrkB receptor antagonist during cocaine self-administration reversed the delayed acquisition of cocaine self-administration in ♂CocSired rats, which suggests that increased BDNF signaling in the mPFC (and possibly other nuclei) blunts the reinforcing effect of cocaine in the male offspring of cocaine exposed sires.
In our experiments, the dams interacted with the sires only during a very limited breeding period. The sires played no role in rearing their offspring and mating with cocaine-experienced sires did not influence maternal behaviors. This raises the question of how paternal cocaine exposure influenced the behavior of the male offspring. One possibility is that cocaine might produce functional changes in sperm. Following subcutaneous injection, the concentration of cocaine in the testes is second only to the brain38 due to specific and saturable binding of cocaine to uncharacterized proteins in the testes39. These findings led us to hypothesize that repeated cocaine exposure might influence regulators of DNA structure associated with the BDNF gene in the sperm. Consistent with this general notion, exposure of dams (F0) to vinclozolin results in promoter-specific changes in the sperm epigenome in F3 generation rats. Alterations in the sperm epigenome represent a means for the epigenetic inheritance of adult onset diseases associated with ancestral vinclozolin exposure40. In the current experiments, we observed no difference in total sperm histone H3 levels between cocaine and saline exposed sires. During spermatogenesis most histones are replaced by protamines, which allows for denser packaging of DNA. Our data indicated that histone H3 replacement by protamines in sperm was not influenced by cocaine. Significantly, we measured increased association of AcH3 with BDNF promoters I, IV and VI in the sperm of F0 rats that self-administered cocaine.
It is becoming increasingly clear that spermatozoa are not merely passive carriers of the paternal genome with little epigenetic influence on offspring41. In fact, retained spermatozoal histones (i.e. the 10–15% of histones that are not replaced by protamines) are highly acetylated and these epigenetic marks are transmitted to the oocyte where they influence the onset of zygotic gene expression and regulate gene expression during early embryogenesis41. Thus, cocaine-induced changes in sperm histone acetylation represent a plausible mechanism for the intergenerational inheritance of the observed cocaine resistance phenotype.
In conclusion, the present findings indicated that paternal cocaine self-administration resulted in decreased acquisition and maintenance of cocaine self-administration in male offspring. Enhancement of acetylation-associated increases in BDNF transcription in the mPFC appeared to underlie, at least in part, the expression of this cocaine resistance phenotype. Importantly, increased mPFC BDNF mRNA and protein were observed in male offspring and not female cocaine-sired progeny, which did not display the cocaine resistance phenotype. We observed increased acetylation of histones associated with BDNF promoters in the sperm of sires that self-administered cocaine, which represents an epigenetic mechanism that may underlie the transmission of cocaine-associated information from male progenitors to their male descendants. That is, cocaine reprograms the male germline through an epigenetic mechanism (i.e. acetylation) resulting in the inheritance of a neuronal and associated behavioral phenotype, which is characterized by reduced cocaine reinforcement in male progeny.
Online Methods
Animals and housing
For the F0 generation, we obtained male and female Sprague-Dawley rats (Rattus norvegicus) weighing 250–300 g from Taconic Laboratories (Germantown, NY). Animals remained individually housed except for 1 week of pair housing during the mating period. Food and water were available ad libitum. Researchers employed a 12/12 hr light/dark cycle with the lights on at 7:00 a.m. and all experimental procedures occurred during the light cycle.
Materials
All self-administration experiments used Med-Associates (East Fairfield, VT) instrumentation enclosed within ventilated, sound attenuating chambers. All apparatus contained response levers, stimulus lights, food pellet dispensers and injection pumps.
Surgery
Researchers administered 80 mg/kg ketamine and 12 mg/kg xylazine to anesthetize the rats. After threading the catheter subcutaneously over the shoulder blade, routing to a mesh backmount platform (CamCaths, Cambridge, UK) and suturing below the skin between the shoulder blades, we positioned an indwelling silastic catheter into the right jugular vein and sutured it in place. Investigators flushed catheters daily with an antibiotic (Timentin, 0.93 mg/ml) dissolved in heparinized saline and sealed with plastic obturators when not in use.
F0 Cocaine Self-Administration
Cocaine was gifted from National Institute on Drug Abuse (Rockville, MD) and dissolved in bacteriostatic saline. Following a 7-day recovery from surgery, we placed the F0 males in operant chambers and allowed them to lever press for cocaine infusions (0.25 mg cocaine/56 µl saline/infusion over 5 sec; the cocaine dose was not adjusted for animal weight). We utilized an FR1 schedule of reinforcement to train rats to lever press for cocaine. A 20-sec timeout period followed each cocaine infusion. We limited the rats to 75 infusions per daily 2-hour session. Self-administration continued for a total of 60 days.
Breeding
Twenty-four hours after the last day of self-administration, we placed male rats into a cage with naïve females. The animals remained co-housed for 7 days. Three out of 18 F0 males continued receiving daily self-administration sessions during the breeding period. There were no differences in the behavior of the offspring so the groups were combined.
Sperm Extraction/Double Swim Up Assay
Researchers sacrificed F0 males and sperm was extracted as described previously42. Briefly, the testes, epididymis, and reproductive fat pad were removed. We dissected the cauda epididymis and made longitudinal slices to release sperm. Sperm-saturated solution settled for 30 minutes at 37°C. We transferred the supernatant containing sperm and allowed it to settle for another 10 minutes at 37°C. We removed the supernatant and centrifuged it for 5 minutes at 4,000 RPM at 4°C.
Licking and grooming
Investigators examined maternal behavior in the mothers of the F1 generation similar to previous studies43, 44. We recorded observations twice a day from P3 to P14. During each 60-minute observation period, we quantified the time dams spend in each of five different measures (mother licking or grooming any pup, mother nursing in an arched position, blanket posture, or passive posture, mother off pups).
F1 Self-Administration
We weaned and group housed offspring at P26. When the offspring reached P60, we singly housed and prepared some rats for behavioral experiments. Following a 7-day recovery from surgery, we placed cocaine- and saline-sired rats in operant chambers and allowed them to lever press for cocaine (0.5 or 1.0 mg/kg/56 µl saline/infusion over 5 sec). Animals received daily 2-hour self-administration sessions for a total of 10 days under an FR1 schedule. Following 10 days of FR1 self-administration, animals switched to an FR5 schedule for 2 days and then to a progressive ratio (PR) schedule. Under the PR schedule, the response requirement for each subsequent drug delivery increased until the subject failed to meet a requirement. The response requirement for the ith reinforcement was R(i)=[5e0.2i–5] and the session expired when an animal took >30 minutes to satisfy the response requirement. We operationally defined the breakpoint as the total number of responses or the final completed ratio.
Tissue Extraction
We sacrificed the offspring not used for self-administration studies, removed their brains and dissected the mPFC. The mPFC samples encompassed the anterior cingulate, prelimbic and infralimbic cortices as in our previous work6. All samples were frozen at −80°C for subsequent analysis.
Sucrose Self-Administration
Researchers maintained animals at 85° of free feeding body weight for 3 days prior to self-administration. Animals lever pressed for sucrose pellets on an FR1 schedule for 5 days with no limit to the number of pellets earned. On the 6th day, some animals switched to an FR5 schedule for two days and then shifted to a PR schedule of reinforcement (for details, see above).
Western Blots
We homogenized samples in 200 µl of lysis buffer (1% Nonidet P–40, 20 mM Tris, pH 8.0, 137 mM NaCl, 10% glycerol, 1 mM PMSF, sodium butyrate 1mM, and protease inhibitors) at 4°C. After centrifugation, we collected the supernatant and measured protein levels by Bradford assay. We boiled 15–20 µ g of each sample for 5 min before separation on 10–20% SDS-polyacrylamide gel, and transfer to nitrocellulose membranes. We blocked immunoblots with 5% nonfat dry milk dissolved in TBST for 60 min, before incubating overnight (4°C) with specific antibodies: anti-BDNF (Cat# ARP 41970_P050, Aviva Systems Biology, San Diego; 1:700); anti-di acetyl lysine 9 and lysine 14 histone H3 (Cat# 06-599, Millipore, Billerica, MA; 1:2000); anti-GAPDH (Cat # MAB374, Millipore, Billerica, MA; 1:700). We washed membranes in TBST before incubation with the secondary antibody (HRP-conjugated goat anti-rabbit IgG, Jackson ImmunoResearch Laboratories, West Grove, PA), additional washes, and visualization using the ECL detection system (NEN, Boston, MA).
Immunohistochemistry
We permeabilized 25 µm sections in 0.5% Triton X–100 for 20 min and incubated with 10% normal goat serum for 1 hr prior to overnight incubation (4°C) with anti-AcH3 (Cat# 06-599, Millipore; 1:500) prepared in GDB buffer (1% gelatin solution, 5% Triton X-100). After washing in 0.5% Triton X-100 for 1 h, we incubated sections in Alexa488 goat-anti rabbit IgG (1:1000) in GDB for 1 h (RT) followed by incubation in To-Pro DNA dye (1:1000) for 30 min. We washed sections with 0.1 M PBS for 1 hr and mounted with Vectashield. We examined AcH3 immunoreactivity on a confocal microscope, acquired images and measured intensity of staining using ImageJ software.
RNA extraction and RT-PCR
Investigators extracted RNA using RNeasy kit (Qiagen, Valencia, CA) according to manufacturer’s instructions. We performed reverse transcription reactions with the Superscript First Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA) and used specific primers to quantitate gene expression compared to a standard curve. The exon specific BDNF primers were designed based on previously published results45, 46. BDNF exon I: forward 5’-AAGCCGAACTTCTCACATGATGA-3’, reverse 5’-TGCAACCGAAGTATGAAATAACCATAG-3’; BDNF exon IV: forward, 5’-CTGCCTAGATCAAATGGAGCTTCT-3’, reverse 5’-GGAAATTGCATGGCGGAGGTAA-3’; BDNF exon VI: forward 5’-TTTGGGGCAGACGAGAAAGC-3’, reverse 5’-GGCAGTGGAGTCACATTGTTGTC-3’; GAPDH: forward 5’-AACAGCAACTCCCATTCTTC-3’, reverse 5’-TGGTCCAGGGTTTCTTACTC-3’. We performed quantitative real time-PCR in an iCycler (Bio-Rad) with the use of SYBR-green PCR Master Mix (Applied Biosystems, Foster City, CA) through 50 PCR cycles (95°C for 30 sec, 57°C for 60 sec, 72°C for 90 sec). We selected the threshold cycle for each sample from the linear range and converted to a starting quantity by interpolation from a standard curve run on the same plate for each set of primers. We normalized the mRNA levels for each well to GAPDH and verified single PCR products by assessing that the melting temperature of the product had a single value.
Chromatin Immunoprecipitation (ChIP)
We recently published detailed methodology for performing ChIP experiments47, 48. Briefly, we cut into pieces, weighed (< 30 mg) and deposited into tubes mPFC from both hemispheres of an individual cocaine- or saline-sired rat. We added formaldehyde (10 µl of 1% formaldehyde to 1 mg tissue) before incubating brain pieces for 10 min at 37°C. We washed brain tissue twice with ice-cold PBS containing protease inhibitors (PI, Complete Mini protease inhibitor cocktail tablets, Roche, Indianapolis, IN), and then suspended in SDS lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris-HCl, pH 8.1) containing PI at a ratio of 10 µl buffer for each mg of brain. Following 10 min incubation, we sonicated brain lysates to shear lengths of 200–1000 base pair DNA fragments49. We pooled the resulting homogenates from one brain, centrifuged for 10 min at 13,000 g at 4°C and collected 200 µl aliquots of the suspension. We diluted each sample 10-fold with dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris-HCl, pH 8.1, 167 mM NaCl), and designated 20 µl (1%) of the diluted sample as ‘Input’ DNA. Samples were either processed immediately or stored at −80°C.
For immunoprecipitation, we pre-cleared 2000 µl of each sample with 80 µl of salmon sperm DNA/Protein A-agarose 50% slurry (Upstate Biotechnology, Lake Placid, NY) by incubating at 4°C for 30 min with gentle agitation before overnight incubation (4°C) with 5 µg of AcH3 antibody (Cat# 06-599, Millipore, Billerica, MA). Negative controls included no antibody and IgG (Jackson, West Grove, PA). We included mock immunoprecipitation conditions (mouse IgG) as a control. After immunoprecipitation, we added 80 µl of salmon sperm DNA/protein A-agarose 50% slurry to samples, and collected immunocomplexes for 1 h at 4°C on a rocking platform. After pelleting agarose (1000 rpm, 4°C, 2 min), we washed sequentially (4 min each) the chromatin-antibody/protein A-agarose complexes with 1 ml each of: low salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.1, 150 mM NaCl), high salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.1, 500 mM NaCl), LiCl buffer (0.25 M LiCl, 1% NP-40, 1% deoxycholate, 1 mM EDTA, 10 mM Tris-HCl, pH 8.1), and TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0). We eluted by incubating samples in 250 µl buffer (1% SDS, 0.1 M NaHCO3) for 15 min at room temperature; elution was repeated and eluates combined. We reversed cross-links by adding 20 µl 5 M NaCl to the pooled eluates and heating to 65°C for 4 h. We diluted the Input DNA to a volume of 500 µl and reversed cross-links. After cross-link reversal, we digested all samples (ChIP and Input) with 20 µg proteinase K (1 h, 45°C), and recovered DNA with phenol/chloroform extraction and ethanol precipitation. We resuspended DNA pellets in 25 µl of sterile water.
We used 1 µl of ChIP-derived DNA as template in 20 µl reactions containing 10 µl 2X SYBR Green Master Mix (Applied Biosystems, Foster City, CA) and 0.5 µM of each primer. RtPCR was performed using an iCycler (Bio-Rad, Hercules, CA), with continuous SYBR Green monitoring according to the manufacturer’s recommendations, using iCycler software. Cycling parameters for all amplifications were as follows: 60 cycles of 95°C for 30 sec, 57°C for 30 sec, and 72°C for 45 sec, followed by melt-curve analysis (55°C for 10 sec × 80 cycles). We performed all PCR reactions in triplicate and included negative controls (no DNA) as well as positive controls (serial dilutions of known amounts of genomic DNA).
We estimated DNA sequence quantities from threshold amplification cycle numbers (Tc) using iCycler software. For every gene, we calculated a ΔTc value for each sample by subtracting the Tc value for the immunoprecipitated sample from the Tc value for the corresponding Input DNA to normalize for differences in ChIP sample aliquots. We expressed DNA quantities as percentages of corresponding Input using the equation: (Antibody ChIP as a percentage of Input) = 2(ΔTc) × 100. Finally, we compared DNA quantities (normalized to Input) for immunoprecipitated vs. mock-immunoprecipitated samples; we considered only immunoprecipitated samples containing >1.5 times as much DNA sufficient DNA for analysis.
We interrogated Input and IP samples with gene promoter-specific primers in triplicate reactions in real-time PCR analysis as previously described47–50. The following exon specific BDNF primers were designed based on previously published sequences45–47 and used for real-time PCR analysis. BDNF exon I: forward 5’-GCAGTTGGACAGTCATTGGTAACC-3’, reverse 5’-ACGCAAACGCCCTCATTCTG-3’; BDNF exon IV: forward 5’-AACAAGAGGCTGTGACACTATGCTC-3’, reverse 5’-CAGTAAGTAAAGGCTAGGGCAGGC-3’, BDNF exon VI: forward 5’-TTTGGGGCAGACGAGAAAGC-3’, reverse 5’-GGCAGTGGAGTCACATTGTTGTC-3’.
Statistics
We analyzed all data with analyses of variance (ANOVAs) or t-tests. For the ANOVAs, we made pairwise comparisons with Fisher’s LSD (p<0.05).
Supplementary Material
Acknowledgements
We thank Rachel Schassburger, Thomas Hopkins, Blake Kimmey, Shayna Friedman, Alycia Lee, Shayna Darnell and Gavin Sangrey for technical assistance, and Lisa Briand for advice on experimental design. This work was supported by grants from the National Institutes of Health (R01s DA15214, DA22339, DA33641, K02 DA18678, K01 DA30445, F31 DA31535, T32s DA28874 and MH86599).
Footnotes
Author Contributions: F.M.V., S.L.W., H.D.S. and G.S.-V. performed experiments. F.M.V. and R.C.P. analyzed the data, prepared the figures and wrote the first draft of the manuscript. All authors designed experiments and edited the manuscript.
Author Information: The authors declare no competing financial interests.
REFERENCES
- 1.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunn GA, Bale TL. Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice. Endocrinology. 2009;150:4999–5009. doi: 10.1210/en.2009-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Champagne FA. Epigenetic influence of social experiences across the lifespan. Dev Psychobiol. 2010;52:299–311. doi: 10.1002/dev.20436. [DOI] [PubMed] [Google Scholar]
- 4.Carone BR, et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell. 2010;143:1084–1096. doi: 10.1016/j.cell.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng SF, et al. Chronic high-fat diet in fathers programs beta-cell dysfunction in female rat offspring. Nature. 2010;467:963–966. doi: 10.1038/nature09491. [DOI] [PubMed] [Google Scholar]
- 6.Morgan CP, Bale TL. Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage. J Neurosci. 2011;31:11748–11755. doi: 10.1523/JNEUROSCI.1887-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaati G, Bygren LO, Edvinsson S. Cardiovascular and diabetes mortality determined by nutrition during parents' and grandparents' slow growth period. Eur J Hum Genet. 2002;10:682–688. doi: 10.1038/sj.ejhg.5200859. [DOI] [PubMed] [Google Scholar]
- 8.Pembrey ME, et al. Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet. 2006;14:159–166. doi: 10.1038/sj.ejhg.5201538. [DOI] [PubMed] [Google Scholar]
- 9.Byrnes EM. Transgenerational consequences of adolescent morphine exposure in female rats: effects on anxiety-like behaviors and morphine sensitization in adult offspring. Psychopharmacology (Berl) 2005;182:537–544. doi: 10.1007/s00213-005-0122-4. [DOI] [PubMed] [Google Scholar]
- 10.Byrnes JJ, Babb JA, Scanlan VF, Byrnes EM. Adolescent opioid exposure in female rats: transgenerational effects on morphine analgesia and anxiety-like behavior in adult offspring. Behav Brain Res. 2011;218:200–205. doi: 10.1016/j.bbr.2010.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novikova SI, et al. Maternal cocaine administration in mice alters DNA methylation and gene expression in hippocampal neurons of neonatal and prepubertal offspring. PLoS One. 2008;3:e1919. doi: 10.1371/journal.pone.0001919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He F, Lidow IA, Lidow MS. Consequences of paternal cocaine exposure in mice. Neurotoxicol Teratol. 2006;28:198–209. doi: 10.1016/j.ntt.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Abel EL, Moore C, Waselewsky D, Zajac C, Russell LD. Effects of cocaine hydrochloride on reproductive function and sexual behavior of male rats and on the behavior of their offspring. J Androl. 1989;10:17–27. doi: 10.1002/j.1939-4640.1989.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 14.Burley N. The differential-allocation hypothesis: an experimental test. Am Nat. 1988;132:611–628. [Google Scholar]
- 15.Sheldon TA, Smith PC. Equity in the allocation of health care resources. Health Econ. 2000;9:571–574. doi: 10.1002/1099-1050(200010)9:7<571::aid-hec555>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 16.Drickamer LC, Gowaty PA, Holmes CM. Free female mate choice in house mice affects reproductive success and offspring viability and performance. Anim Behav. 2000;59:371–378. doi: 10.1006/anbe.1999.1316. [DOI] [PubMed] [Google Scholar]
- 17.Gowaty PA, et al. The hypothesis of reproductive compensation and its assumptions about mate preferences and offspring viability. Proc Natl Acad Sci U S A. 2007;104:15023–15027. doi: 10.1073/pnas.0706622104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alter MD, et al. Paternal transmission of complex phenotypes in inbred mice. Biol Psychiatry. 2009;66:1061–1066. doi: 10.1016/j.biopsych.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martini M, Valverde O. A single episode of maternal deprivation impairs the motivation for cocaine in adolescent mice. Psychopharmacology (Berl) 2011;219:149–158. doi: 10.1007/s00213-011-2385-2. [DOI] [PubMed] [Google Scholar]
- 20.Sadri-Vakili G, et al. Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. J Neurosci. 2010;30:11735–11744. doi: 10.1523/JNEUROSCI.2328-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berglind WJ, et al. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci. 2007;26:757–766. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- 22.Pierce RC, Bari AA. The role of neurotrophic factors in psychostimulant-induced behavioral and neuronal plasticity. Rev Neurosci. 2001;12:95–110. doi: 10.1515/revneuro.2001.12.2.95. [DOI] [PubMed] [Google Scholar]
- 23.Cazorla M, et al. Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice. J Clin Invest. 2011;121:1846–1857. doi: 10.1172/JCI43992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakuma Y. Gonadal steroid action and brain sex differentiation in the rat. J Neuroendocrinol. 2009;21:410–414. doi: 10.1111/j.1365-2826.2009.01856.x. [DOI] [PubMed] [Google Scholar]
- 25.Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McElligott ZA, Winder DG. Modulation of glutamatergic synaptic transmission in the bed nucleus of the stria terminalis. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1329–1335. doi: 10.1016/j.pnpbp.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung WC, Swaab DF, De Vries GJ. Apoptosis during sexual differentiation of the bed nucleus of the stria terminalis in the rat brain. J Neurobiol. 2000;43:234–243. [PubMed] [Google Scholar]
- 28.Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci. 2011;8:73–96. doi: 10.1007/7854_2010_93. [DOI] [PubMed] [Google Scholar]
- 30.Quinones-Jenab V, Jenab S. Progesterone attenuates cocaine-induced responses. Horm Behav. 2010;58:22–32. doi: 10.1016/j.yhbeh.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psychiatry. 2003;160:687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- 32.Tsuang MT, et al. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry. 1998;55:967–972. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- 33.Merikangas KR, et al. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973–979. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- 34.Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- 35.Graham DL, et al. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- 36.Whitfield TW, Jr, Shi X, Sun WL, McGinty JF. The suppressive effect of an intra-prefrontal cortical infusion of BDNF on cocaine-seeking is Trk receptor and extracellular signal-regulated protein kinase mitogen-activated protein kinase dependent. J Neurosci. 2011;31:834–842. doi: 10.1523/JNEUROSCI.4986-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maze I, Nestler EJ. The epigenetic landscape of addiction. Ann N Y Acad Sci. 2011;1216:99–113. doi: 10.1111/j.1749-6632.2010.05893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Misra AL, Giri VV, Patel MN, Alluri VR, Mule SJ. Disposition and metabolism of [3H] cocaine in acutely and chronically treated monkeys. Drug Alcohol Depend. 1977;2:261–272. doi: 10.1016/0376-8716(77)90004-7. [DOI] [PubMed] [Google Scholar]
- 39.Li H, George VK, Crossland WJ, Anderson GF, Dhabuwala CB. Characterization of cocaine binding sites in the rat testes. J Urol. 1997;158:962–965. doi: 10.1097/00005392-199709000-00079. [DOI] [PubMed] [Google Scholar]
- 40.Guerrero-Bosagna C, Settles M, Lucker B, Skinner MK. Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome. PLoS One. 2010;5:e13100. doi: 10.1371/journal.pone.0013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steger K, Cavalcanti MC, Schuppe HC. Prognostic markers for competent human spermatozoa: fertilizing capacity and contribution to the embryo. Int J Androl. 2010;34:513–527. doi: 10.1111/j.1365-2605.2010.01129.x. [DOI] [PubMed] [Google Scholar]
- 42.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Champagne DL, et al. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J Neurosci. 2008;28:6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weaver IC, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 45.Chen WG, et al. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- 46.Martinowich K, et al. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302:890–893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- 47.Sadri-Vakili G, et al. Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. J Neurosci. 2010;30:11735–11744. doi: 10.1523/JNEUROSCI.2328-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen-Plotkin AS, et al. Decreased association of the transcription factor Sp1 with genes downregulated in Huntington's disease. Neurobiol Dis. 2006;22:233–241. doi: 10.1016/j.nbd.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Braveman MW, Chen-Plotkin AS, Yohrling GJ, Cha JH. Chromatin immunoprecipitation technique for study of transcriptional dysregulation in intact mouse brain. Methods Mol Biol. 2004;277:261–276. doi: 10.1385/1-59259-804-8:261. [DOI] [PubMed] [Google Scholar]
- 50.Sadri-Vakili G, et al. Histones associated with downregulated genes are hypo-acetylated in Huntington's disease models. Hum Mol Genet. 2007;16:1293–1306. doi: 10.1093/hmg/ddm078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.