Abstract
Eight unrelated clinical Acinetobacter baumannii isolates resistant to all commonly used antibiotics were subjected to three-dimensional checkerboard microtiter plate dilution and time-kill studies at one-fourth of their MICs of polymyxin B, imipenem, and rifampin. Synergy was demonstrated with combinations of polymyxin B and imipenem, polymyxin B and rifampin, and polymyxin B, imipenem, and rifampin. Double combinations of polymyxin B and imipenem and of polymyxin B and rifampin were bactericidal for seven of eight isolates, and triple combinations were bactericidal for all isolates within 24 h. Future clinical studies using double and triple therapy with these antibacterials may provide an effective option against potentially lethal infection due to multiresistant Acinetobacter baumannii.
In recent years, Acinetobacter baumannii has emerged as one of the more ubiquitous antibiotic-resistant gram-negative nosocomial pathogens among critically ill patients (4, 23). Although the carbapenems, ampicillin-sulbactam, and amikacin have retained excellent in vitro and clinical activities against susceptible strains of A. baumannii, a growing number of reports have documented resistance to these antibacterials (1, 2, 10, 12-14, 16). As a result nontraditional agents, including polymyxin B and colistin, have been used to treat patients infected with multiresistant A. baumannii (6, 9, 15). However, pulmonary infections have not responded well to such monotherapy, and resistance has occurred among strains that have persisted during treatment (6, 15, 24).
In this communication, we describe the in vitro double and triple interactions of polymyxin B, imipenem, and rifampin against eight unique strains of multidrug-resistant A. baumannii using checkerboard microdilution and time-kill methods.
MATERIALS AND METHODS
Eight multidrug-resistant clinical strains of A. baumannii were obtained from the Clinical Microbiology Laboratory at New York Hospital Queens. All isolates had different pulsed-field gel electrophoresis patterns and were considered unrelated according to the criteria established by Tenover et al. (21). MICs of selected individual antibiotics were then determined using microdilution and E-test methods (AB Biodisk North America Inc., Piscataway, N.J.) by the Infectious Disease Research Laboratory. E-test metallo-beta-lactamase (E-test MBL) strips were used to detect the presence of molecular class B beta-lactamases (25). Rifampin and polymyxin B were obtained from Sigma (St. Louis, Mo.), and imipenem powder was supplied by Merck and Co. (Rahway, N.J.). MIC determinations were performed using checkerboard microdilution methods with Mueller-Hinton broth and a final inoculum of approximately 5 × 105 CFU/ml. Microdilution plates for evaluation of triple drug combinations were performed in the following manner. The first microtiter plate contained no imipenem and increasing concentrations of rifampin ranging from 0 to 32 μg/ml on the x axis and increasing concentrations of polymyxin B ranging from 0 to 32 μg/ml on the y axis. Each of the subsequent six plates contained a fixed concentration of imipenem ranging from 1 to 32 μg/ml with increasing concentrations of rifampin ranging from 0 to 32 μg/ml on the x axis and increasing concentrations of polymyxin B ranging from 0 to 32 μg/ml on the y axis. MICs and fractional inhibitory concentrations (FICs) were determined after 24 h of growth. The MIC was defined as the well in the microtiter plate with the lowest drug combination at which no visible growth was observed. A bactericidal effect was defined as ≥99.9% killing of the starting inoculum. FICs of <1.0, 1.0, and >1.0 were used to define synergy, addition, and antagonism, respectively, according to previously published methods (3, 27). Time-kill studies were also performed according to earlier described techniques (7, 17). Escherichia coli strain ATCC 25922 was used as a control for all experiments. The limit of detection in these studies was 2 log10 CFU/ml. Three-dimensional isobolograms were generated using Surfer 8 (Golden Software, Inc., Golden, Colo.).
RESULTS
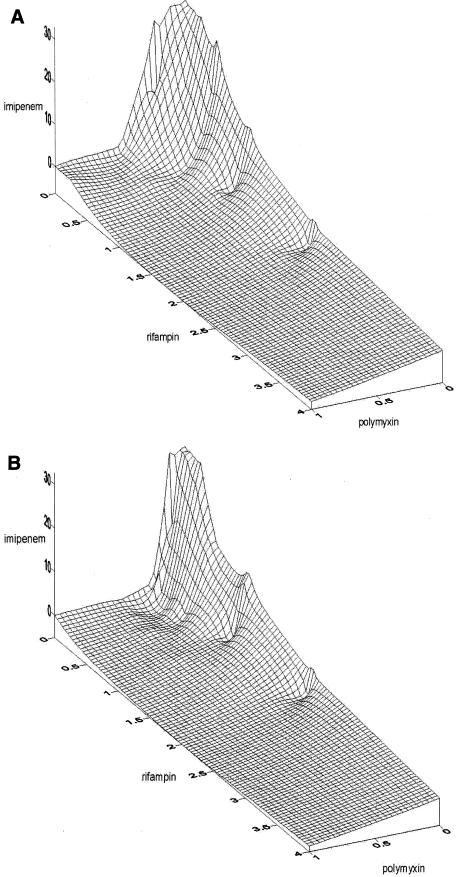
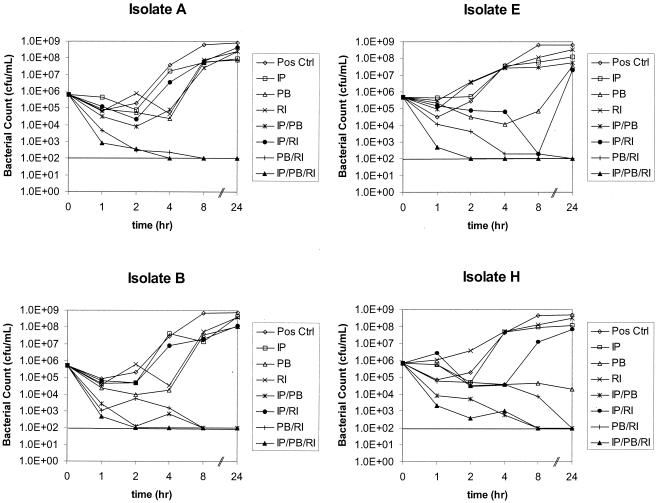
The MICs of the three antibiotics studied for all eight isolates are shown in Table 1. All eight isolates with imipenem MICs of 32.0 μg/ml or >32.0 μg/ml did not demonstrate an eightfold MIC reduction in the presence of EDTA using the E-test MBL procedure and were presumed not to possess metallo-beta-lactamases, according to the manufacturer's specifications. The results of time-kill experiments using 0.25 μg of polymyxin B/ml, 0.5 μg of rifampin/ml, and 8.0 μg of imipenem/ml for the eight unique clinical isolates of A. baumannii tested in this study are also summarized in Table 1. All isolates were killed within 24 h using polymyxin B-imipenem-rifampin, one isolate showed regrowth with imipenem plus polymyxin B (isolate E), and one isolate showed regrowth with polymyxin B plus rifampin (isolate A). Using three-dimensional checkerboard microdilution with polymyxin B, rifampin, and imipenem, the combined concentrations of each antibiotic showing synergy and their sum FICs (ΣFICs) of less than 1.0 against isolates A and B are presented in Table 2. Double combinations of polymyxin B (0.5 μg/ml) and imipenem (2.0 to 8 μg/ml) were synergistic against isolate A. Double combinations of polymyxin B (0.25 to 0.5 μg/ml) and imipenem (0.25 to 8.0 μg/ml) were synergistic against isolate B. Synergy was observed with a combination of polymyxin B and rifampin at concentrations of 0.5 μg/ml of each drug against isolate A. Double combinations of polymyxin B (0.25 to 0.5 μg/ml) and rifampin (0.03 to 0.5 μg/ml) were synergistic. Triple combinations of polymyxin B (0.12 to 0.5 μg/ml), rifampin (0.03 to 1.0 μg/ml), and imipenem (1.0 to 16.0 μg/ml) were also synergistic against isolates A and B. These results are also shown as three-dimensional isobolograms for isolates A and B (Fig. 1). The areas of concavity correspond to the aforementioned concentration ranges of double and triple combinations. The graphs of time-kill experiments using one-fourth MICs of each agent (0.25 μg of polymyxin B/ml, 0.5 μg of rifampin/ml, 8.0 μg of imipenem/ml) alone and in combination are shown for four isolates (A, B, E, and H) in Fig. 2.
TABLE 1.
MICs of each antibiotic alone and time-kill results for the eight clinical A. baumannii isolates
| A. baumannii isolate | MIC (μg/ml)
|
Time (h) and antibacterial effecta
|
||||
|---|---|---|---|---|---|---|
| IPM | PB | RIF | IPM+PB+RIF | IPM+PB | PB+RIF | |
| A | 32 | 1 | 2 | 2, bactericidal | 4, bactericidal | 8, regrowth |
| B | 32 | 1 | 2 | 4, bactericidal | 2, bactericidal | 2, bactericidal |
| C | >32 | 2 | 8 | 2, bactericidal | 8, bactericidal | 8, bactericidal |
| D | >32 | 2 | 12 | 4, bactericidal | 24, bactericidal | 8, bactericidal |
| E | >32 | 1 | 5 | 8, bactericidal | 2, regrowth | 24, bactericidal |
| F | >32 | 1 | 5 | 8, bactericidal | 8, bactericidal | 24, bactericidal |
| G | >32 | 8 | 1 | 8, bactericidal | 8, bactericidal | 24, bactericidal |
| H | >32 | 1.5 | >32 | 24, bactericidal | 8, bactericidal | 24, bactericidal |
Time-kill results are for experiments using double and triple combinations of imipenem (IPM), polymyxin B (PB), and rifampin (RIF) at 8, 0.25, and 0.5 μg/ml, respectively.
TABLE 2.
Individual and sum total FICs of the three antibiotics
| FIC (μg/ml) for isolate A
|
ΣFIC (μg/ml) of PB+RIF+IPM | FIC (μg/ml) for isolate B
|
ΣFIC (μg/ml) of PB+RIF+IPM | ||||
|---|---|---|---|---|---|---|---|
| PB | RIF | IPM | PB | RIF | IPM | ||
| 0.5 | 0.25 | 0 | 0.750 | 0.25 | 0.015 | 0 | 0.265 |
| 0.5 | 0.25 | 0 | 0.750 | 0.5 | 0.03 | 0 | 0.530 |
| 0.5 | 0.015 | 0.0313 | 0.546 | 0.5 | 0.06 | 0 | 0.560 |
| 0.5 | 0.03 | 0.0313 | 0.561 | 0.5 | 0.125 | 0 | 0.625 |
| 0.5 | 0.06 | 0.0313 | 0.591 | 0.5 | 0.25 | 0 | 0.750 |
| 0.5 | 0.125 | 0.0313 | 0.656 | 0.5 | 0.015 | 0.0313 | 0.546 |
| 0.25 | 0.25 | 0.0313 | 0.531 | 0.5 | 0.03 | 0.0313 | 0.561 |
| 0.12 | 0.5 | 0.0313 | 0.651 | 0.25 | 0.06 | 0.0313 | 0.341 |
| 0.5 | 0.015 | 0.0625 | 0.578 | 0.25 | 0.125 | 0.0313 | 0.406 |
| 0.5 | 0.03 | 0.0625 | 0.593 | 0.25 | 0.25 | 0.0313 | 0.531 |
| 0.5 | 0.06 | 0.0625 | 0.623 | 0.12 | 0.5 | 0.0313 | 0.651 |
| 0.5 | 0.125 | 0.0625 | 0.688 | 0.5 | 0.015 | 0.0625 | 0.578 |
| 0.5 | 0.25 | 0.0625 | 0.813 | 0.5 | 0.03 | 0.0625 | 0.593 |
| 0.25 | 0.5 | 0.0625 | 0.813 | 0.5 | 0.06 | 0.0625 | 0.623 |
| 0.5 | 0.015 | 0.1250 | 0.640 | 0.5 | 0.125 | 0.0625 | 0.688 |
| 0.5 | 0.03 | 0.1250 | 0.655 | 0.25 | 0.25 | 0.0625 | 0.563 |
| 0.5 | 0.06 | 0.1250 | 0.685 | 0.25 | 0.5 | 0.0625 | 0.813 |
| 0.5 | 0.125 | 0.1250 | 0.750 | 0.5 | 0.015 | 0.1250 | 0.640 |
| 0.25 | 0.25 | 0.1250 | 0.625 | 0.5 | 0.03 | 0.1250 | 0.655 |
| 0.25 | 0.5 | 0.1250 | 0.875 | 0.5 | 0.06 | 0.1250 | 0.685 |
| 0.25 | 0.015 | 0.2500 | 0.515 | 0.5 | 0.125 | 0.1250 | 0.750 |
| 0.5 | 0.03 | 0.2500 | 0.780 | 0.25 | 0.25 | 0.1250 | 0.625 |
| 0.5 | 0.06 | 0.2500 | 0.810 | 0.25 | 0.5 | 0.1250 | 0.875 |
| 0.25 | 0.125 | 0.2500 | 0.625 | 0.25 | 0.015 | 0.2500 | 0.515 |
| 0.12 | 0.25 | 0.2500 | 0.620 | 0.25 | 0.03 | 0.2500 | 0.530 |
| 0.06 | 0.5 | 0.2500 | 0.810 | 0.25 | 0.06 | 0.2500 | 0.560 |
| 0.25 | 0.015 | 0.5000 | 0.765 | 0.5 | 0.125 | 0.2500 | 0.875 |
| 0.25 | 0.03 | 0.5000 | 0.780 | 0.25 | 0.25 | 0.2500 | 0.750 |
| 0.25 | 0.06 | 0.5000 | 0.810 | 0.12 | 0.5 | 0.2500 | 0.870 |
| 0.12 | 0.125 | 0.5000 | 0.745 | 0.12 | 0.015 | 0.5000 | 0.635 |
| 0.06 | 0.25 | 0.5000 | 0.810 | 0.12 | 0.03 | 0.5000 | 0.650 |
| 0.03 | 0.25 | 0.5000 | 0.780 | 0.25 | 0.06 | 0.5000 | 0.810 |
| 0.5 | 0 | 0.0625 | 0.563 | 0.12 | 0.125 | 0.5000 | 0.745 |
| 0.5 | 0 | 0.1250 | 0.625 | 0.06 | 0.25 | 0.5000 | 0.810 |
| 0.5 | 0 | 0.2500 | 0.750 | 0 | 0.25 | 0.5000 | 0.750 |
| 0.5 | 0 | 0.0078 | 0.508 | ||||
| 0.5 | 0 | 0.0625 | 0.563 | ||||
| 0.25 | 0 | 0.1250 | 0.375 | ||||
| 0.25 | 0 | 0.2500 | 0.500 | ||||
| 0.25 | 0 | 0.5000 | 0.750 | ||||
Abbreviations: PB, polymyxin B; RIF, rifampin; IPM, imipenem.
FIG. 1.
(A) Three-dimensional isobologram for A. baumannii isolate A. The areas on the isobologram showing depressions (concave sinks) that approach zero represent the greatest synergy (ΣFIC ≤ 1.0). (B) Results for isolate B.
FIG. 2.
Time-kill experiments using one-fourth the MIC of each agent (0.25 μg of polymyxin B/ml, 0.5 μg of rifampin/ml, and 8.0 μg of imipenem/ml) alone and in combination for isolates A, B, E, and H. The dotted lines indicate the lower limit of detection of 2 log10 CFU/ml.
DISCUSSION
Several in vitro and animal model studies have demonstrated synergy with polymyxin B plus rifampin or imipenem and with polymyxin B plus meropenem against multidrug-resistant A. baumannii (23, 26; N. X. Chin, B. Scully, and P. Della-Latta, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-56, 1998). Another study has shown synergy between polymyxin B and rifampin against Serratia marcescens isolates from infected patients, even though the isolates were resistant to each antibiotic alone (19). In vitro triple synergistic studies with polymyxin B, trimethoprim, and sulfonamide have shown efficacy against multidrug-resistant Burkholderia cepacia, as has the combination of polymyxin E (methane sulfonate, colistimethate), trimethoprim, and sulfamethoxazole against S. marcescens (20, 22).
In the present study, double combinations of polymyxin B plus imipenem and polymyxin B plus rifampin were bactericidal against seven out of eight isolates tested. The triple combination of polymyxin B-rifampin-imipenem was synergistic against all isolates by checkerboard and time-kill studies. In these studies FICs (concentrations or MICs of the antibiotics in the combination divided by the MIC of the antibiotic alone) were calculated for two- and three-drug combinations (Table 2). All MICs have been graphically represented in a three-dimensional isobologram (Fig. 1). Synergy for three antibiotics has been defined by several investigators as a fractional sum of <1.0 (ΣFIC of <1.0) (3, 27). Plotted values showing concave surface areas on the isobolograms correspond to synergy, and convex areas represent antagonism (3, 27). The probable role of polymyxin B in such synergy is its rapid permeabilization of the outer membrane, allowing enhanced penetration and activity of both imipenem and rifampin. Synergy between polymyxin B or other peptide antibacterials and the carbapenems would be expected when carbapenem resistance is due to porin protein defects. Synergy may not be evident if the organism possesses significant carbapenemase activity due to class B beta-lactamases. Metallo-carbapenemases were most likely not contributing to imipenem resistance in the eight isolates, since EDTA using MBL strips did not reverse resistance. Numerous mechanisms other than class B enzymes have been implicated in carbapenem resistance in Acinetobacter, including other beta-lactamase classes, porin protein losses or their reduced expression, and penicillin-binding protein changes (8, 23).
Although the use of two or more agents is accepted as appropriate treatment of patients with tuberculosis or human immunodeficiency virus infection, this approach has not been tested in controlled clinical trials against multidrug-resistant Acinetobacter. However, it is known that the selection and isolation of mutants from existing populations of cells depend on a number of variables, including the bacterial inoculum at the site of infection, antibacterial agent(s) used, and mechanisms of resistance associated with targeted bacteria. It therefore seems reasonable to use at least two agents for treatment of selected patients infected with multidrug-resistant pathogens that have demonstrated mutation-related progressive resistance during single-drug therapy. Others have also suggested combination therapy as a tool to prevent the emergence of bacterial resistance and for improved outcomes in severely ill patients infected with gram-negative bacteria known to develop resistance on single therapy (5, 18).
Our studies showed that the combination of polymyxin B plus imipenem was as effective as polymyxin B plus rifampin and may provide an alternative for treatment even when Acinetobacter isolates are resistant to the carbapenems due to mechanisms other than metallo-carbapenemases. The addition of rifampin as a third component remains controversial. Synergy between colistin and rifampin has been reported previously in vitro (11). However, all except one of our eight isolates were killed within 24 h by imipenem and polymyxin B. Clinical trials will be necessary to determine whether combination therapy with two or three drugs is more effective than use of a single agent.
Acknowledgments
This work was supported by the BMA Medical Foundation, the Beatrice Snyder Foundation, and Agnes Varis.
REFERENCES
- 1.Afzal-Shah, M., and D. M. Livermore. 1998. Worldwide emergence of carbapenem-resistant Acinetobacter. J. Antimicrob. Chemother. 41:576-577. [DOI] [PubMed] [Google Scholar]
- 2.Appleman, M. D., H. Belzberg, D. M. Citron, P. N. R. Heseltine, A. E. Yellin, J. Murray, and T. V. Berne. 2000. In vitro activities of non-traditional antimicrobials against multiresistant Acinetobacter baumannii strains isolated in an intensive care breakout. Antimicrob. Agents Chemother. 44:1035-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berenbaum, M. C. 1978. A method for testing for synergy with any number of agents. J. Infect. Dis. 137:122-130. [DOI] [PubMed] [Google Scholar]
- 4.Bergogne-Berezin, E., and K. J. Towner. 1996. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin. Microbiol. Rev. 9:148-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow, J. W., and V. L. Yu. 1999. Combination antibiotic therapy versus monotherapy for gram-negative bacteraemia: a commentary. Int. J. Antimicrob. Agents 11:7-12. [DOI] [PubMed] [Google Scholar]
- 6.Craig, A. W., and A. Reboli. 1993. Infections caused by imipenem-resistant Acinetobacter calcoaceticus biotype anitratus. J. Infect. Dis. 168:1602-1603. [DOI] [PubMed] [Google Scholar]
- 7.Eliopoulos, G. M., and R. C. Mollering, Jr. 1996. Antimicrobial combinations, p. 330-396. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. Williams & Wilkins, Baltimore, Md.
- 8.Fernandez-Cuenca, F., L. Martinez-Martinez, M. C. Conejo, J. A. Ayala, E. J. Perea, and A. Pascual. 2003. Relationship between β-lactamase production, outer membrane protein and penicillin-binding protein profiles on the activity of carbapenems against clinical isolates of Acinetobacter baumannii. J. Antimicrob. Chemother. 51:565-574. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Viladrich, P., X. Corbella, L. Corral, F. Tubau, and A. Mateu. 1999. Successful treatment of ventriculitis due to carbapenem-resistant Acinetobacter baumannii with intraventricular colistin sulfomethate sodium. Clin. Infect. Dis. 28:916-917. [DOI] [PubMed] [Google Scholar]
- 10.Giacometti, A., O. Cirioni, M. S. Del Prete, F. Barchiesi, A. Mataloni Paggi, E. Petrelli, and G. Scalise. 2000. Comparative activities of polycationic peptides and clinically used antimicrobial agents against multidrug-resistant nosocomial isolates of Acinetobacter baumannii. J. Antimicrob. Chemother. 46:807-810. [DOI] [PubMed] [Google Scholar]
- 11.Giamarellos-Bourboulis, E. J., E. Xiruchaki, and H. Giamarellou. 2001. Interactions of colistin and rifampin on multidrug-resistant Acinetobacter baumannii. Diagn. Microbiol. Infect. Dis. 40:117-120. [DOI] [PubMed] [Google Scholar]
- 12.Go, E., C. Urban, J. Burns, B. Kreiswirth, W. Eisner, N. Mariano, K. Mosinka-Snipas, and J. J. Rahal. 1994. Clinical and molecular epidemiology of Acinetobacter only to polymyxin B and sulbactam. Lancet 344:1329-1332. [DOI] [PubMed] [Google Scholar]
- 13.Karlowsky, J. A., D. C. Draghi, M. E. Jones, C. Thornsberry, I. R. Friedland, and D. F. Sahm. 2003. Surveillance for antimicrobial susceptibility among clinical isolates of Pseudomonas aeruginosa and Acinetobacter baumannii from hospitalized patients in the United States, 1998 to 2001. Antimicrob. Agents Chemother. 47:1681-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levin, A. 2002. Multi-resistant Acinetobacter infections: a role for sulbactam combinations in overcoming an emerging worldwide problem. Eur. J. Clin. Microbiol. Infect. Dis. 8:144. [DOI] [PubMed] [Google Scholar]
- 15.Levin, A. S., A. A. Barone, J. Penco, M. V. Santos, I. S. Marinho, E. A. G. Arruda, E. I. Manrique, and S. F. Costa. 1999. Intravenous colistin as therapy for nosocomial infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Clin. Infect. Dis. 28:1008-1011. [DOI] [PubMed] [Google Scholar]
- 16.Manikul, V. M., D. Landman, G. Saurina, E. Oydna, H. Lai, and J. Quale. 2000. Endemic carbapenem resistant Acinetobacter species in Brooklyn, New York: citywide prevalence, inter-institutional spread, and relation to antibiotic usage. Clin. Infect. Dis. 31:101-106. [DOI] [PubMed] [Google Scholar]
- 17.Moody, J. A. 1992. Synergy testing: broth microdilution checkerboard and broth macrodilution methods, p. 5.18.1-5.18.23. In H. D. Eisenberg (ed.), Clinical microbiology procedures handbook. American Society for Microbiology, Washington, D.C.
- 18.Mouton, J. W. 1999. Combination therapy as a tool to prevent emergence of bacterial resistance. Infection 27:S24-S28. [DOI] [PubMed] [Google Scholar]
- 19.Ostenson, R. C., B. T. Fields, and C. M. Nolan. 1977. Polymyxin B and rifampin: new regimens for multi-resistant Serratia marcescens infections. Antimicrob. Agents Chemother. 12:655-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahal, J. J., M. S. Simberkoff, and P. J. Hyams. 1973. Pseudomonas cepacia tricuspid endocarditis: treatment with trimethoprim, sulfonamide and polymyxin B. J. Infect. Dis. 128:S762-S767. [DOI] [PubMed] [Google Scholar]
- 21.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas, F. E., Jr., J. M. Leonard, and R. H. Alford. 1976. Sulfamethoxazole-trimethoprim-polymyxin therapy of serious multiply drug-resistant Serratia infections. Antimicrob. Agents Chemother. 9:201-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urban, C., S. Segal-Maurer, and J. J. Rahal. 2003. Considerations in control and treatment of nosocomial infection due to multi-drug-resistant Acinetobacter baumannii. Clin. Infect. Dis. 36:1268-1274. [DOI] [PubMed] [Google Scholar]
- 24.Urban, C., N. Mariano, J. J. Rahal, E. Tay, C. Ponio, T. Koprivnjak, and J. Weiss. 2001. Polymyxin B-resistant Acinetobacter baumannii clinical isolates susceptible to recombinant BPI21 and cecropin P1. Antimicrob. Agents Chemother. 45:994-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh, T. R., A. Bolmström, A. Qwärnström, and A. Gales. 2002. Evaluation of a new E-test for detecting metallo-β-lactamases in routine clinical testing. J. Clin. Microbiol. 40:2755-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolff, M., M. L. Joly-Guillou, R. Farinotti, and C. Carbon. 1999. In vivo efficacies of combinations of β-lactams, β-lactamase inhibitors, and rifampin against Acinetobacter baumannii in a mouse pneumonia model. Antimicrob. Agents Chemother. 43:1406-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu, V. L., T. P. Felegie, R. B. Yee, A. W. Pasculle, and F. H. Taylor. 1980. Synergistic interaction in vitro with use of three antibiotics simultaneously against Pseudomonas maltophilia. J. Infect. Dis. 142:602-607. [DOI] [PubMed] [Google Scholar]