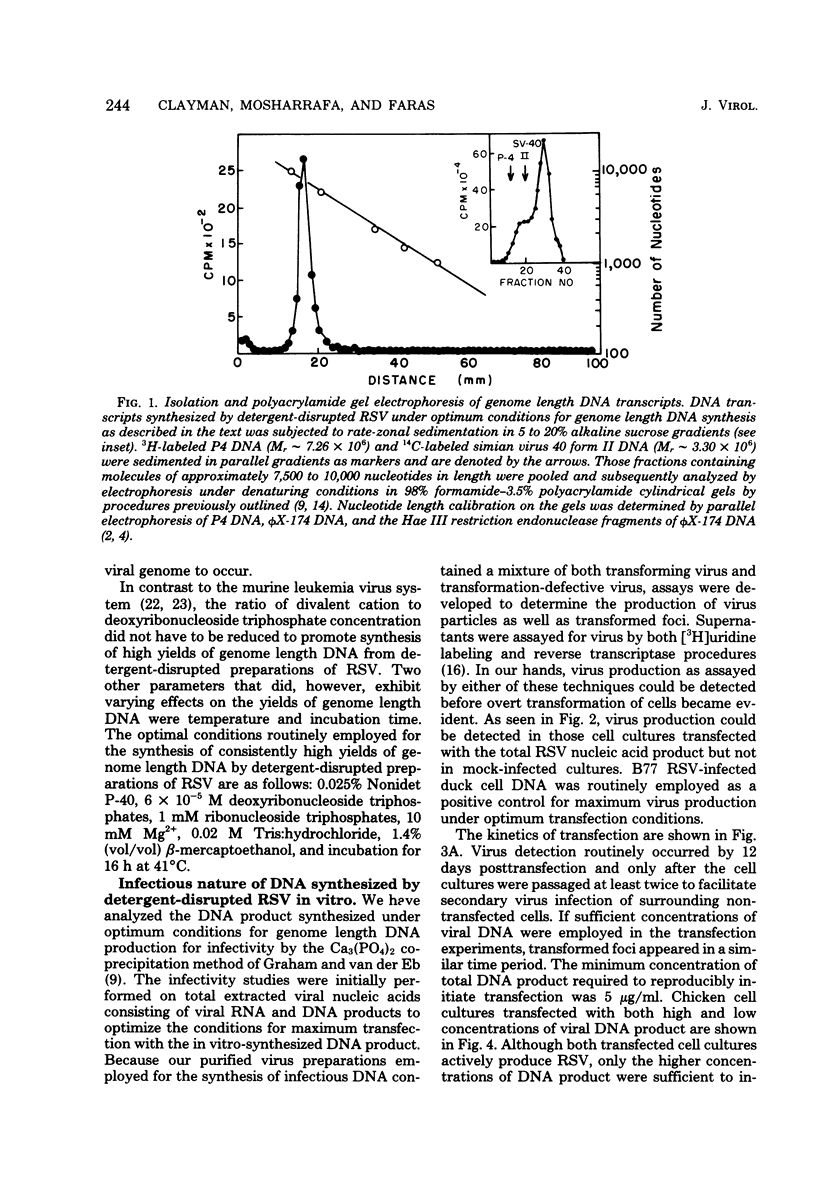
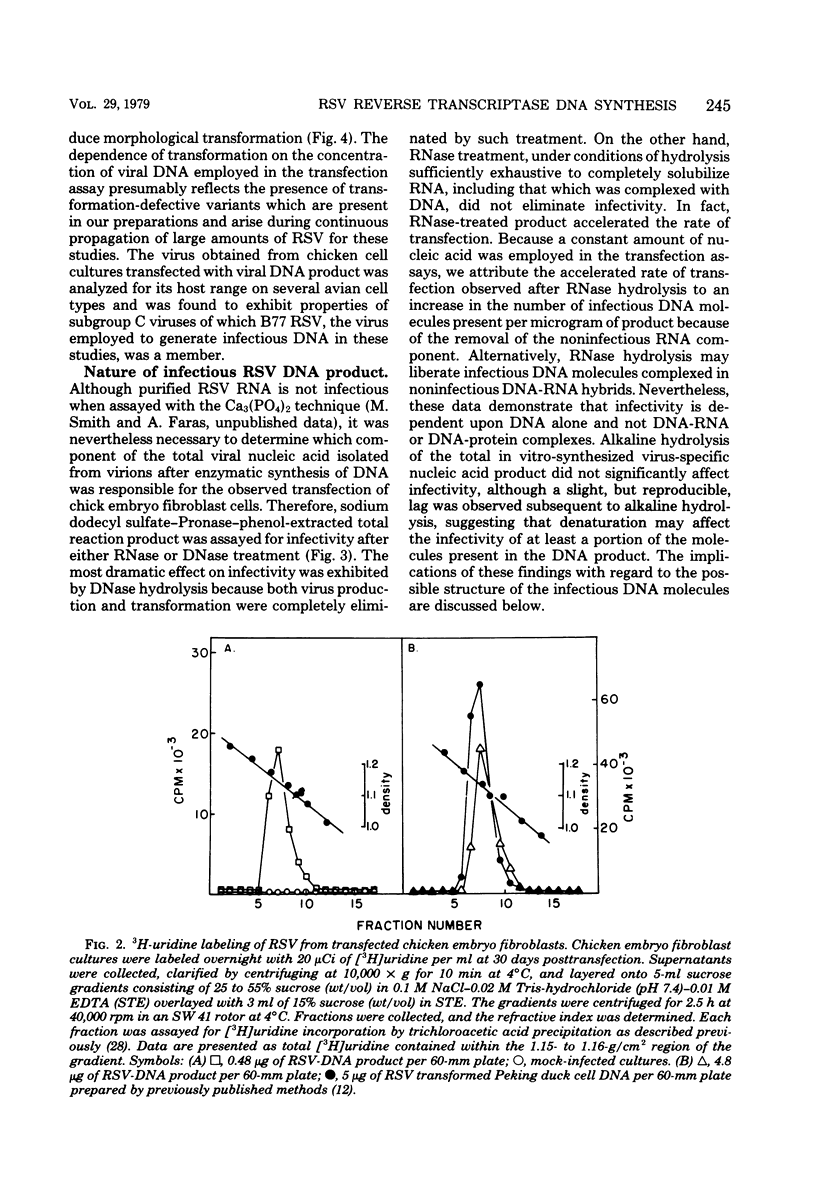
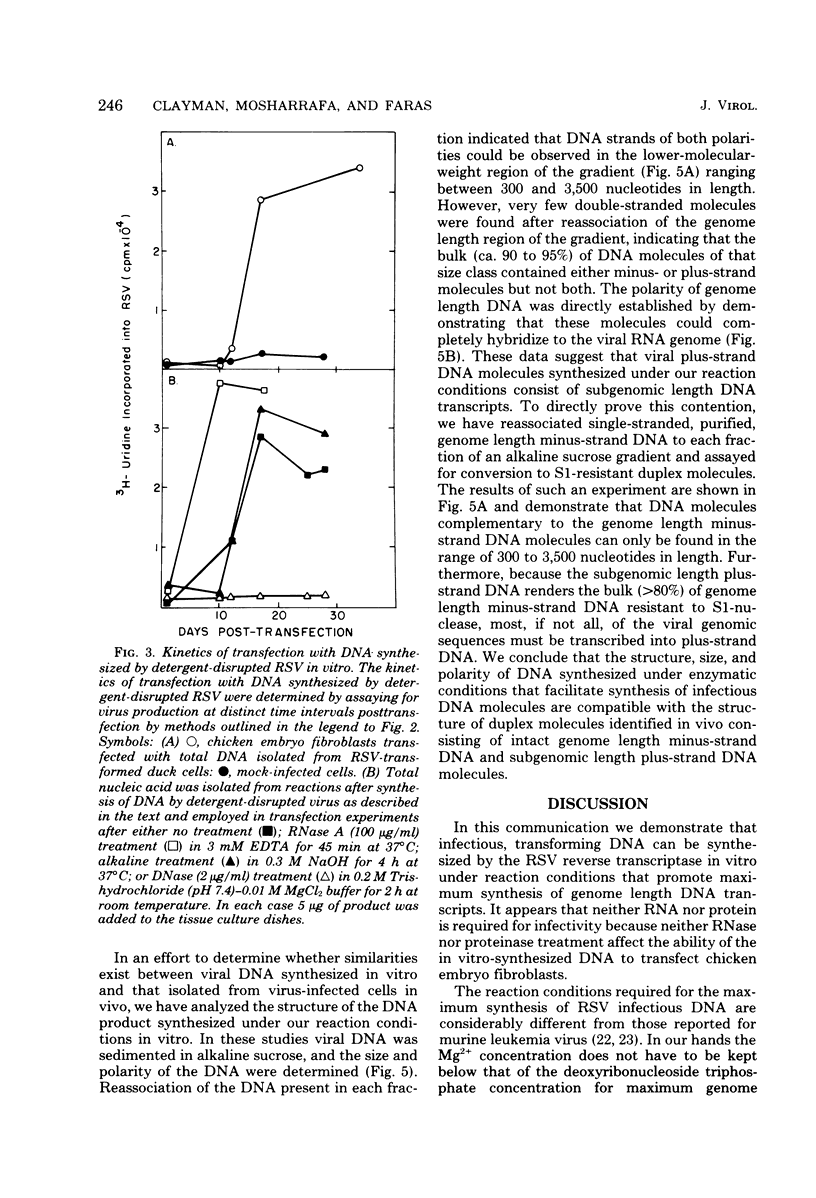
Abstract
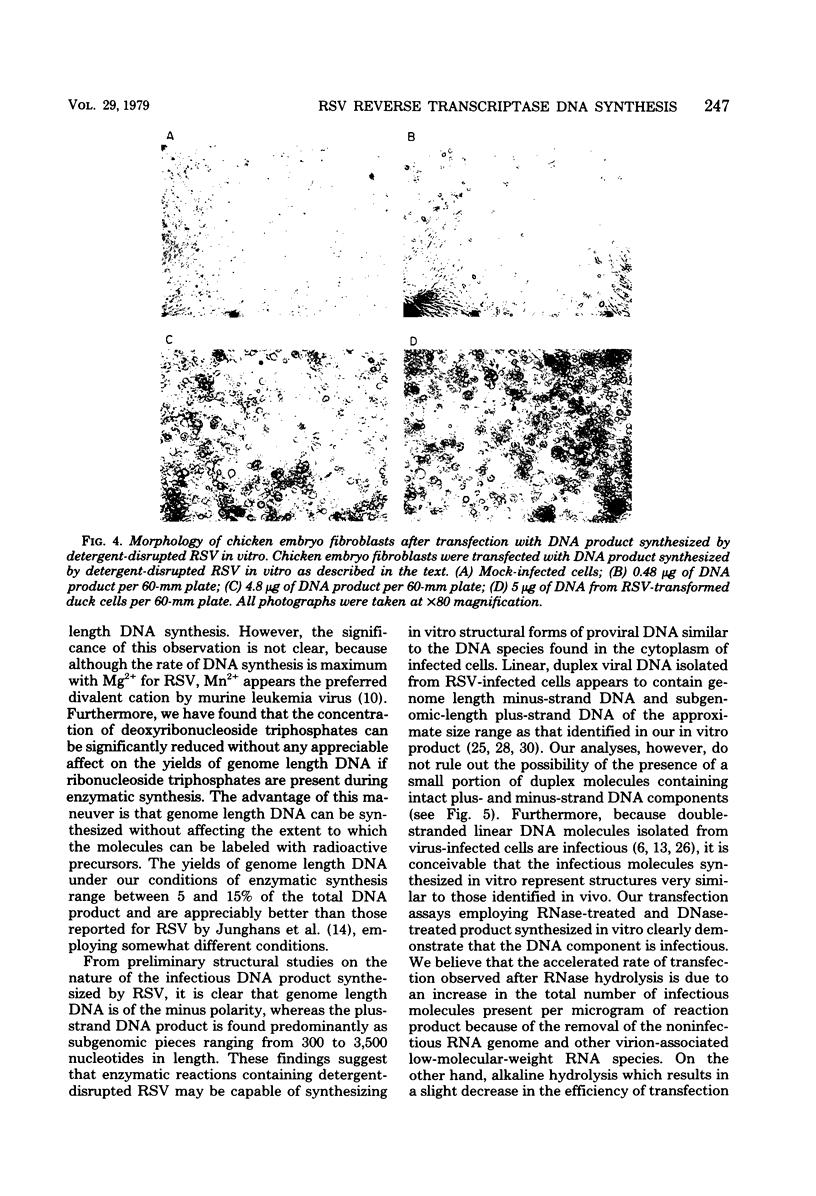
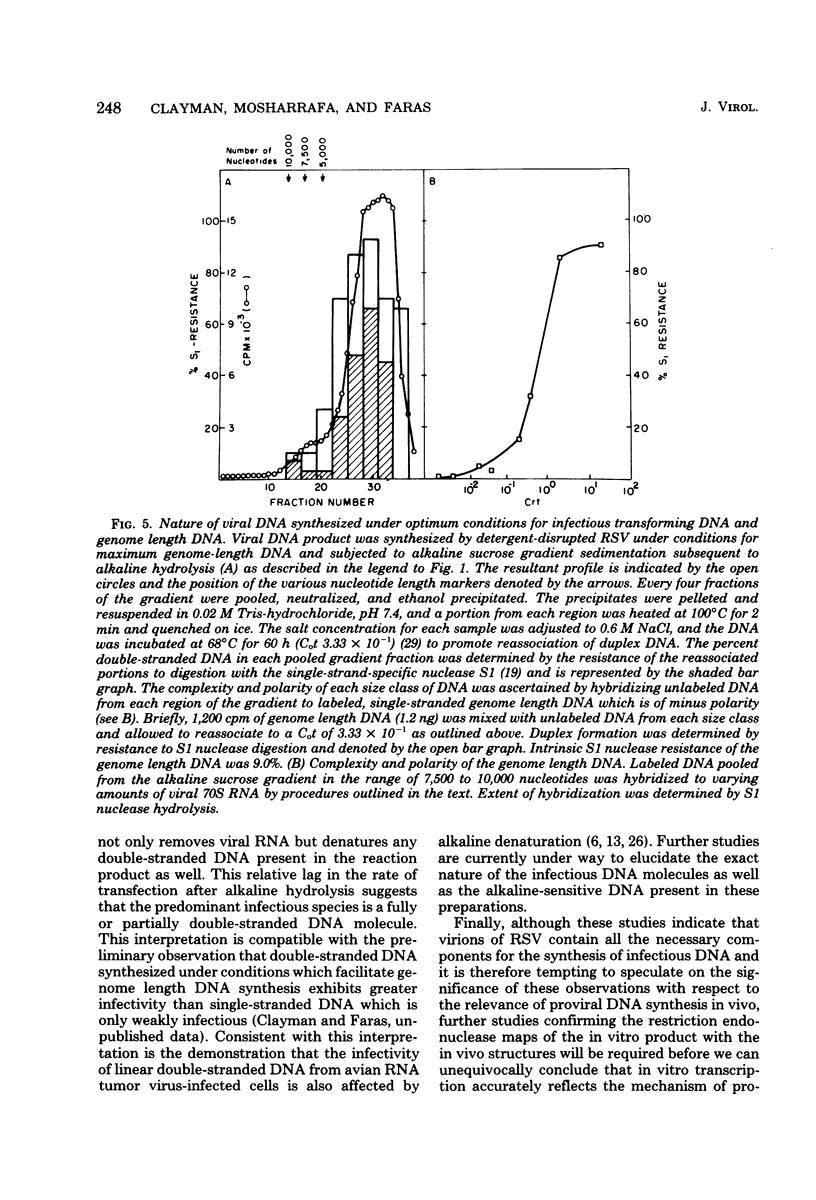
Infectious DNA molecules, capable of transforming chicken embryo fibroblasts, can be synthesized by the Rous sarcoma virus-associated reverse transcriptase in vitro. The optimal enzymatic conditions employed for infectious DNA synthesis also facilitate maximum synthesis of genome length DNA. Analysis of the DNA product synthesized by detergent-disrupted Rous sarcoma virus under these conditions indicates that DNA complementary to viral RNA (minus-strand DNA) is genome length in size, whereas DNA complementary to genome length minus-strand DNA (plus-strand DNA) appears as subgenomic-length molecules ranging between 300 and 3,500 nucleotides in length. These features of the DNA product synthesized by the Rous sarcoma virus reverse transcriptase in vitro are similar to those identified in the cytoplasm of cells shortly after infection and lend credence to studies of the mechanism of reverse transcription in vitro and their significance to proviral DNA synthesis in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M., Levinson W. E., Quintrell N., Sullivan D., Fanshier L., Jackson J. The low molecular weight RNAs of Rous sarcoma virus. I. The 4 S RNA. Virology. 1970 Sep;42(1):182–195. doi: 10.1016/0042-6822(70)90251-5. [DOI] [PubMed] [Google Scholar]
- Clayman C. H., Collett M. S., Faras A. J. In vitro transcription of the avian oncornavirus genome by the RNA-directed DNA polymerase: effect of actinomycin D on the extent of transcription. J Virol. 1977 Jul;23(1):209–212. doi: 10.1128/jvi.23.1.209-212.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Faras A. J. In vitro transcription of 70S RNA by the RNA-directed DNA polymerase of Rouse sarcoma virus: lack of influence of RNase H. J Virol. 1975 Jan;17(1):291–295. doi: 10.1128/jvi.17.1.291-295.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Faras A. J. In vitro transcription of theavian oncornavirus genome by the RNA-directed DNA polymerase: analysis of DNA transcripts synthesized in reconstructed enzymatic reactions. J Virol. 1977 Apr;22(1):86–96. doi: 10.1128/jvi.22.1.86-96.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch E., Temin H. M. Formation and structure of infectious DNA of spleen necrosis virus. J Virol. 1977 Jan;21(1):119–130. doi: 10.1128/jvi.21.1.119-130.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni A. M., Smotkin D., Weinberg R. A. Murine leukemia virus: detection of unintegrated double-stranded DNA forms of the provirus. Proc Natl Acad Sci U S A. 1975 Feb;72(2):447–451. doi: 10.1073/pnas.72.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni A. M., Weinberg R. A. Partially single-stranded form of free Moloney viral DNA. Nature. 1975 Jun 19;255(5510):646–648. doi: 10.1038/255646a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Green M., Gerard G. F. RNA-directed DNA polymerase--properties and functions in oncogenic RNA viruses and cells. Prog Nucleic Acid Res Mol Biol. 1974;14(0):187–334. [PubMed] [Google Scholar]
- Guntaka R. V., Richards O. C., Shank P. R., Kung H. J., Davidson N. Covalently closed circular DNA of avian sarcoma virus: purification from nuclei of infected quail tumor cells and measurement by electron microscopy and gel electrophoresis. J Mol Biol. 1976 Sep 15;106(2):337–357. doi: 10.1016/0022-2836(76)90090-5. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Traynor B. L., Ventura P. E., Alling D. W. Infectivity of visna virus DNA. Virology. 1976 Mar;70(1):65–79. doi: 10.1016/0042-6822(76)90236-1. [DOI] [PubMed] [Google Scholar]
- Hillova J., Goubin G., Hill M. Transfection des fibroblastes de poule par l'acide désoxyribonucléique dénaturé de cellules transformées de Rous. C R Acad Sci Hebd Seances Acad Sci D. 1972 Mar 27;274(13):1970–1973. [PubMed] [Google Scholar]
- Junghans R. P., Duesberg P. H., Knight C. A. In vitro synthesis of full-length DNA transcripts of Rous sarcoma virus RNA by viral DNA polymerase. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4895–4899. doi: 10.1073/pnas.72.12.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen R., Sedat J., Ziff E. Orientation of the complementary strands of polyoma virus DNA with respect to the DNA physical map. J Virol. 1975 Jan;17(1):212–218. doi: 10.1128/jvi.17.1.212-218.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieras R. M., Faras A. J. DNA polymerase of reticuloendotheliosis virus: inability to detect endogenous RNA-directed DNA synthesis. Virology. 1975 Jun;65(2):514–523. doi: 10.1016/0042-6822(75)90056-2. [DOI] [PubMed] [Google Scholar]
- Krzyzek R. A., Lau A. F., Faras A. J., Spector D. H. Post-transcriptional control of avian oncornavirus transforming gene sequences in mammalian cells. Nature. 1977 Sep 8;269(5624):175–179. doi: 10.1038/269175a0. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Hu S. S. In vitro synthesis and characterisation of full- and half-genome length complementary DNA from avian oncoviruses. Nature. 1978 Feb 2;271(5644):481–483. doi: 10.1038/271481a0. [DOI] [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson W. Fragmentation of the nucleus in Rous sarcoma virus-infected chick embryo cells. Virology. 1967 May;32(1):74–83. doi: 10.1016/0042-6822(67)90254-1. [DOI] [PubMed] [Google Scholar]
- Myers J. C., Spiegelman S., Kacian D. L. Synthesis of full-length DNA copies of avian myeloblastosis virus RNA in high yields. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2840–2843. doi: 10.1073/pnas.74.7.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Baltimore D. Increased length of DNA made by virions of murine leukemia virus at limiting magnesium ion concentration. J Virol. 1977 Jan;21(1):168–178. doi: 10.1128/jvi.21.1.168-178.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Baltimore D. Synthesis of long, representative DNA copies of the murine RNA tumor virus genome. J Virol. 1975 Jan;17(1):168–174. doi: 10.1128/jvi.17.1.168-174.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Smotkin D., Baltimore D., Weinberg R. A. In vitro synthesis of infectious DNA of murine leukaemia virus. Nature. 1977 Sep 8;269(5624):122–126. doi: 10.1038/269122a0. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Varmus H. E. Virus-specific DNA in the cytoplasm of avian sarcoma virus-infected cells is a precursor to covalently closed circular viral DNA in the nucleus. J Virol. 1978 Jan;25(1):104–104. doi: 10.1128/jvi.25.1.104-104.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda J., Hlozánek I., Mach O., Michlová A., Rïman J., Urbánková M. Transfection of chicken fibroblasts with single exposure to DNA from virogenic mammalian cells. J Gen Virol. 1973 Oct;21:47–55. doi: 10.1099/0022-1317-21-1-47. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Faras A. J., Varmus H. E., Levinson W. E., Bishop J. M. Ribonucleic acid directed deoxyribonucleic acid synthesis by the purified deoxyribonucleic acid polymerase of Rous sarcoma virus. Characterization of the enzymatic product. Biochemistry. 1972 Jun 6;11(12):2343–2351. doi: 10.1021/bi00762a021. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Heasley S., Linn J., Wheeler K. Use of alkaline sucrose gradients in a zonal rotor to detect integrated and unintegrated avian sarcoma virus-specific DNA in cells. J Virol. 1976 May;18(2):574–585. doi: 10.1128/jvi.18.2.574-585.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Levinson W. E., Bishop J. M. Extent of transcription by the RNA-dependent DNA polymerase of Rous sarcoma virus. Nat New Biol. 1971 Sep 1;233(35):19–21. doi: 10.1038/newbio233019a0. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Shank P. R. Unintegrated viral DNA is synthesized in the cytoplasm of avian sarcoma virus-transformed duck cells by viral DNA polymerase. J Virol. 1976 May;18(2):567–573. doi: 10.1128/jvi.18.2.567-573.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M. Genome organization of RNA tumor viruses. I. In vitro synthesis of full-genome-length single-stranded and double-stranded viral DNA transcripts. J Virol. 1978 Jun;26(3):615–629. doi: 10.1128/jvi.26.3.615-629.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]