Abstract
In this work, we studied the in vitro sensitivity of 24 strains of Actinomadura madurae to a new oxazolidinone (DA-7867), gatifloxacin, moxifloxacin, and garenoxacin by using a broth microdilution method. We observed that the A. madurae strains had a high level of sensitivity to all the antimicrobials tested. The most active drug was DA-7867, with a MIC at which 90% of the strains are inhibited (MIC90) of 0.125 μg/ml and a MIC50 of 0.06 μg/ml.
Mycetoma is a chronic subcutaneous infectious disease characterized by the tumefaction of the region affected and the production of cavitary abscesses which drain a seropurulent secretion containing the etiologic agent. This infectious disease can be produced by various causative agents that can be either true fungi or aerobic actinomycetes of the genera Nocardia, Actinomadura, or Streptomyces (14). Worldwide, about half of the cases are produced by eumycetes and half by bacteria, although the distribution of the species may vary from region to region, depending on local environmental conditions. Mycetoma cases caused by Actinomadura madurae or Actinomadura pelletieri have been reported in many regions of the world, particularly Venezuela, Senegal, West Bengal and Madras (India), and Mali (3, 6, 14). In Mexico, 10.2% of the total cases of mycetoma are produced by A. madurae (5). Although A. madurae has been isolated primarily from mycetoma lesions, it has also been found in sputum, blood, and brain samples (8).
Several drugs have been used in the treatment of mycetoma caused by Actinomadura species, including streptomycin, dapsone, amikacin, and trimethoprim-sulfamethoxazole (SXT) (14). In our clinical dermatology department, we have used SXT in combination with amikacin, obtaining excellent results (13). However, these drugs have to be taken for several months, and in some persons they can produce serious side effects, such as ototoxicity or nephrotoxicity in the case of amikacin and hemolytic and aplastic anemia, urticaria, photosensitivity, etc., in the case of the sulfonamides. Recently, due to the development of global bacterial resistance, new and more potent antimicrobials have been developed, and we consider it important to evaluate their activity against this microorganism in order to have other therapeutic alternatives for the treatment of mycetoma caused by A. madurae.
In the present study, we assayed the sensitivity of 24 A. madurae strains isolated from patients with mycetoma to two oxazolidinones (linezolid and DA-7867) and several quinolones, including gatifloxacin, moxifloxacin, and the recently developed quinolone garenoxacin (12). These antimicrobials were selected because of their reported activity against mycobacteria, nocardiae, or other gram-positive microorganisms (1, 2, 4, 10, 11). The activities of these compounds were compared to those of SXT and amikacin.
Linezolid was obtained from its manufacturers (Pharmacia and Upjohn, Kalamazoo, Mich.); sulfamethoxazole, trimethoprim, and amikacin were obtained from Sigma Chemical Products (St. Louis, Mo.). DA-7867 was provided by Dong-A Pharmaceutical Company, Limited, Yongin-Si, Korea. Garenoxacin compound was donated by Bristol-Myers Squibb, and the other quinolones, gatifloxacin and moxifloxacin, were obtained from commercial sources.
We used the broth microdilution method that has been previously described (1, 11). Briefly, we utilized fresh colonies on Sabouraud dextrose agar (7-days old) to prepare the inoculum. A cellular suspension was prepared by placing two loopfuls of the bacterial culture in a glass test tube and grinding it to suspend part of the bacterial mass. The ground colonies were suspended in 1 ml of saline solution and diluted with cation-adjusted Mueller-Hinton broth until turbidity matched that of the McFarland 0.5 standard. This suspension was diluted to obtain a final concentration of approximately 1 × 104 to 5 × 104 CFU per well, and 0.1 ml was added to microplate wells (Microtest Primaria; Becton Dickinson and Co., Franklin Lakes, N.J.) containing an equal volume of broth with serial dilutions of the drugs tested. For the control, we inoculated in the same way a well containing cation-adjusted Mueller-Hinton broth without the drug. After 3 days of incubation at 35°C, the plates were read and the MIC was determined as the lowest concentration of the drug totally inhibiting bacterial growth. The assays were run in triplicate. For the sulfonamides, we considered the MIC to be the lowest concentration that inhibits 80% of the growth compared to the amount of growth in the control well. As controls, we utilized Escherichia coli ATCC 25922, Streptomyces aureus ATCC 29213, and A. madurae ATCC 19425. All the antimicrobials except SXT were tested at concentrations of 64 to 0.015 μg/ml according to the guidelines of the National Committee for Clinical Laboratory Standards (9). The SXT combination (ratio, 1:20) was tested at trimethoprim/sulfamethoxazole concentrations of 0.29/0.015 to 152/8 μg/ml.
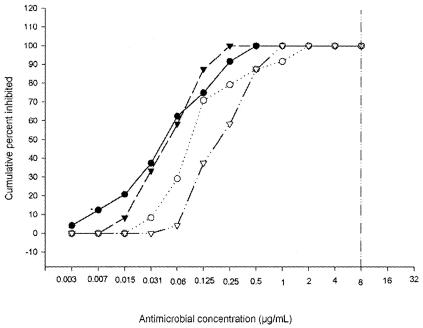
The MICs of DA-7867 and the other antimicrobial agents for the 24 clinical isolates of A. madurae are presented in Table 1. Based on the breakpoint of <8 μg/ml utilized for Nocardia spp. (1), all the strains were sensitive to amikacin. For about half of the strains, SXT MICs were above 32 μg/ml, which is the breakpoint at which Nocardia spp. are considered to be sensitive. All other drugs showed excellent activity against all the strains, with the top range value of 1 μg/ml (Fig. 1 and 2). DA-7867 presented the lowest values, with a MIC at which 50% of the strains are inhibited (MIC50) of 0.0625 and a MIC90 of 0.125 μg/ml. The amikacin, SXT, linezolid, DA-7867, gatifloxacin, moxifloxacin, and garenoxacin MICs for the control strains were 0.125, 2.3/0.125 (trimethoprim/sulfamethoxazole), 0.125, <0.03, 1, 1, and 0.5, respectively, for Nocardia asteroides ATCC 19247 and 0.0625, 9.5/0.5, 0.125, 0.03, 0.03, 0.125 and 0.015, respectively, for A. madurae ATCC 19425.
TABLE 1.
MICs of the drugs tested for the A. madurae strains
| Antimicrobial agent | MIC range | MIC50 | MIC90 |
|---|---|---|---|
| Amikacin | 0.03-2 | 0.125 | 1 |
| SXT | 9.5/0.5-152/8 | 38/2 | 76/4 |
| Linezolid | 0.03-0.25 | 0.125 | 0.25 |
| DA-7867 | 0.02-0.125 | 0.0625 | 0.125 |
| Gatifloxacin | 0.02-0.25 | 0.0625 | 0.25 |
| Moxifloxacin | 0.06-1 | 0.25 | 1 |
| Garenoxacin | 0.003-0.5 | 0.0625 | 0.25 |
FIG. 1.
Sensitivity of A. madurae to amikacin (○), moxifloxacin (▿), gatifloxacin (▾), and garenoxacin (•). The graph shows the cumulative percentage of A. madurae isolates inhibited by these drugs. The vertical line to the right represents the breakpoint used to determine the susceptibility to amikacin of a related actinomycete, N. brasiliensis.
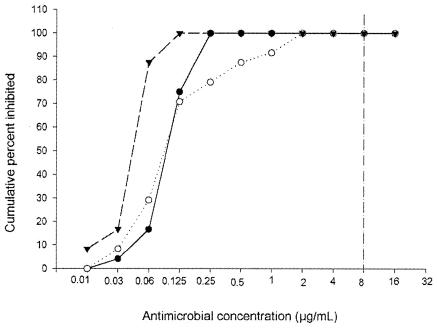
FIG. 2.
Sensitivity of A. madurae to amikacin (○), linezolid (•), and DA-7867 (▾). The vertical line to the right represents the breakpoint used to determine the susceptibility of N. brasiliensis to amikacin and linezolid.
DA-7867 has been demonstrated to be more active than linezolid against gram-positive organisms (K. Lee, J. H. Jum, et al., Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-1312, 2001), including the related aerobic actinomycete Nocardia brasiliensis. In our assays, we did not observe such a difference in the activities of these oxazolidinones against the A. madurae strains tested (Fig. 2).
Few studies on the in vitro sensitivity of A. madurae to antimicrobials have been published (8), none using the drugs assayed in this work. Although mycetoma produced by Actinomadura spp. is not very frequently seen in Mexico, in some countries Actinomadura spp. are commonly isolated and can produce as much as 50% of the total cases (5). Therefore, there is a need to search for more-potent drugs in order to avoid the amputation of limbs or other sequelae of this infection. All drugs used in these assays were quite active against the strains tested, and considering the levels in serum reached by these drugs, it is quite possible that they might be active in patients suffering from actinomycetoma.
One of these drugs, linezolid, has been tested against an actinomadura-related microorganism, N. brasiliensis, in an experimental murine model of mycetoma in which a statistically significant decrease in the formation of lesions in the group treated with linezolid compared to that for the animals treated with saline solution was observed (L. Vera-Cabrera, A. Gomez-Flores, et al., Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. B-509, 2001). The clinical utility of linezolid has been observed in a group of patients with nocardial infection, in which successful results were obtained in most of the cases (7). A. madurae showed in vitro sensitivity to linezolid very similar to that observed for the N. brasiliensis strains. However, it will be necessary to develop in vivo experimental assays as well as to conduct clinical trials with patients to demonstrate the utility of linezolid or any of the other antimicrobials assayed in this work in the treatment of actinomycetoma caused by A. madurae.
Acknowledgments
We acknowledge the support of the National Council for Science and Technology (CONACYT) (grant no. 31015-M, PAICYT, and SA-600-01).
REFERENCES
- 1.Brown-Elliott, B. A., S. C. Ward, C. J. Crist, L. B. Mann, R. W. Wilson, and R. J. Wallace, Jr. 2001. In vitro activities of linezolid against multiple Nocardia species. Antimicrob. Agents Chemother. 45:1295-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clemett D., and A. Markham. 2000. Linezolid. Drugs 59:815-827. [DOI] [PubMed] [Google Scholar]
- 3.Dieng, M. T., M. H. Sy, B. M. Diop, S. O. Niang, and B. Ndiaye. 2003. Mycetoma: 130 cases. Ann. Dermatol. Venereol. 130:16-19. (In French.) [PubMed] [Google Scholar]
- 4.Hoogkamp-Korstanje, J. A., and J. Roelofs-Willemse. 2000. Comparative in vitro activity of moxifloxacin against gram-positive clinical isolates. J. Antimicrob. Chemother. 45:31-39. [DOI] [PubMed] [Google Scholar]
- 5.López-Martínez, R., L. J. Méndez-Tovar, P. Lavalle, O. Welsh, A. Saul, and E. Macotela-Ruíz. 1992. Epidemiología del micetoma en México: estudio de 2105 casos. Gac. Med. Mex. 128:477-481. [PubMed] [Google Scholar]
- 6.Maiti, P. K., A. Ray, and S. Bandyopadhyay. 2002. Epidemiological aspects of mycetoma from a retrospective study of 264 cases in West Bengal. Trop. Med. Int. Health 7:788-792. [DOI] [PubMed] [Google Scholar]
- 7.Moylett, E. H., S. E. Pacheco, B. A. Brown-Elliott, T. R. Perry, E. S. Buescher, M. C. Birmingham, J. J. Schentag, J. F. Gimbel, A. Apodaca, M. A. Schwartz, R. M. Rakita, and R. J. Wallace, Jr. 2003. Clinical experience with linezolid for the treatment of nocardia infection. Clin. Infect. Dis. 36:313-318. [DOI] [PubMed] [Google Scholar]
- 8.McNeil, M. M., J. M. Brown, W. R. Jarvis, and L. Ajello. 1990. Comparison of species distribution and antimicrobial susceptibility of aerobic actinomycetes from clinical specimens. Rev. Infect. Dis. 12:778-783. [DOI] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard. Document M7—A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 10.Valera, L., E. Gradelski, E. Huczko, T. Washo, H. Yigit, and J. Fung-Tomc. 2002. In vitro activity of a novel des-fluoro(6) quinolone, garenoxacin (BMS-284756), against rapidly growing mycobacteria and Nocardia isolates. J. Antimicrob. Chemother. 50:140-142. [DOI] [PubMed] [Google Scholar]
- 11.Vera-Cabrera, L., A. Gomez-Flores, W. G. Escalante-Fuentes, and O. Welsh. 2001. In vitro activity of PNU-100766 (linezolid), a new oxazolidinone antimicrobial, against Nocardia brasiliensis. Antimicrob. Agents Chemother. 45:3629-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weller, T. M., J. M. Andrews, G. Jevons, and R. Wise. 2002. The in vitro activity of BMS-284756, a new des-fluorinated quinolone. J. Antimicrob. Chemother. 49:177-184. [DOI] [PubMed] [Google Scholar]
- 13.Welsh, O., E. Sauceda, J. González, and J. Ocampo. 1987. Amikacin alone and in combination with trimetoprim-sulfamethoxazole in the treatment of actinomycotic mycetoma. J. Am. Acad. Dermatol. 17:443-448. [DOI] [PubMed] [Google Scholar]
- 14.Welsh, O. 1991. Mycetoma: current concepts in treatment. Int. J. Dermatol. 30:387-398. [DOI] [PubMed] [Google Scholar]