Abstract
We present ‘dcGO’ (http://supfam.org/SUPERFAMILY/dcGO), a comprehensive ontology database for protein domains. Domains are often the functional units of proteins, thus instead of associating ontological terms only with full-length proteins, it sometimes makes more sense to associate terms with individual domains. Domain-centric GO, ‘dcGO’, provides associations between ontological terms and protein domains at the superfamily and family levels. Some functional units consist of more than one domain acting together or acting at an interface between domains; therefore, ontological terms associated with pairs of domains, triplets and longer supra-domains are also provided. At the time of writing the ontologies in dcGO include the Gene Ontology (GO); Enzyme Commission (EC) numbers; pathways from UniPathway; human phenotype ontology and phenotype ontologies from five model organisms, including plants; anatomy ontologies from three organisms; human disease ontology and drugs from DrugBank. All ontological terms have probabilistic scores for their associations. In addition to associations to domains and supra-domains, the ontological terms have been transferred to proteins, through homology, providing annotations of >80 million sequences covering 2414 complete genomes, hundreds of meta-genomes, thousands of viruses and so forth. The dcGO database is updated fortnightly, and its website provides downloads, search, browse, phylogenetic context and other data-mining facilities.
INTRODUCTION
Scientists are increasingly confronted with the grand challenge: how to convert sequenced genome information into higher-order knowledge on function (1), phenotype (2) and even human disease (3).
The domain-centric Gene Ontology (dcGO) database at http://supfam.org/SUPERFAMILY/dcGO is a comprehensive ontology resource that contributes to the aforementioned challenge through a new domain-centric strategy. Our method, dcGO (4), annotates protein domains with ontological terms. Ontologies are hierarchically organized controlled vocabularies/terms defined to categorize a particular sphere of knowledge (5). For example, ‘Gene Ontology’ (GO) was created to describe functions of proteins (6). Ontological labels are already available for full-length proteins, derived from experimental data for that protein. The dcGO approach takes the terms attached to full-length sequences, and combines them with the domain composition of the sequences on a large scale, to statistically infer for each term which domain is the functional unit responsible for it. The method has been formulated in a general way, enabling it to be applied to numerous ontologies. The dcGO database now contains a panel of ontologies from a variety of contexts: functions such as GO (6,7), enzymes (8), pathways (9) and keywords used by UniProt (10); phenotype and anatomy ontologies across major model organisms, including mouse (11), worm (12), yeast (13), fly (14), zebrafish (15), Xenopus (16) and Arabidopsis (17); human phenotypes (18), diseases (19) and drugs (20). In addition to complete sets of ontological terms, a collapsed subset (slim version) is also provided for each ontology. The automatically generated slim version of each ontology is based on annotation frequency (4), and it provides the user with a manageable and more coarse-grained list. This is analogous to the ‘GO slim’ provided by the Gene Ontology consortium, which has proven useful for enrichment analyses (21).
The domain definitions used in dcGO are taken from the structural classification of proteins (SCOP) (22) classified at both the superfamily and family levels. SCOP groups domains at the superfamily level if there is structure, sequence and function evidence for a common evolutionary ancestor. Some superfamilies are sub-divided into families, which often share a higher sequence similarity and a related function. In addition to individual domains at these two different levels, dcGO also offers annotations for combinations of domains. We use the concept of supra-domains to describe combinations of two or more successive domains of known structure. In addition to providing ontology for SCOP domains, the generality of the method has enabled us to also include Pfam (23) domains in dcGO.
Our domain-centric ontology derived from proteins with experimental evidence can be turned and used as a predictor on proteins of unknown function but where the domain content is known. The ‘dcGO Predictor’ provides pre-computed functional annotation [using SUPERFAMILY hidden Markov models (24)] of all sequences in UniProt (25), 2414 completely sequenced genomes, thousands of viral genomes and hundreds of meta-genomes. The dcGO website also has a facility for the user to submit their own sequences for function prediction. The dcGO Predictor took part in the recent Critical Assessment of Functional Annotation experiment (http://biofunctionprediction.org); hence, for comparison with other non-domain-centric predictors, we refer the reader to this independent evaluation of its performance. A key result from the experiment, however, was that dcGO performs significantly better than the most commonly used method for GO annotation, Basic Local Alignment Search Tool searches against UniProt (25).
In the main body of the article later we describe the database contents in detail, doing so separately for GO and for other biomedical ontologies (collectively denoted as ‘BO’ hereinafter). Then, we provide an overview of various utilities available through the website that may interest users. Finally, we conclude with planned future developments.
DATABASE CONTENTS
Algorithm summary
To fully understand the content of the dcGO database (Table 1), it is necessary to give a description of the algorithm that is used to build it. Without loss of generality, we take the GO as an exclusive example, but in principle, the applications to other ontologies are the same. For more detail, the reader is referred to a previous publication of the algorithm (4).
Table 1.
A summary of the dcGO database contents (on 15 August 2012)
| Ontology | Domains (superfamily level) |
Domains (family level) |
Supra-domains (superfamily level) |
Data source | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of terms a | Number of domains b | Number of annotations c | Number of terms a | Number of domains b | Number of annotations c | Number of terms a | Number of supra- domains b | Number of annotations c | ||
| Functions | ||||||||||
| Gene Ontology (GO) | High-quality version d | High-quality version d | UniProtKB-GOA (6) | |||||||
| 4761 | 481 | 27 833 | 4652 | 657 | 29 529 | |||||
| High-coverage version e | High-coverage version e | |||||||||
| 10 497 | 1265 | 139 958 | 10 032 | 2026 | 157 618 | 13 306 | 7761 | 587 917 | ||
| Diseases | ||||||||||
| Disease Ontology (DO) | 357 | 115 | 1345 | 364 | 145 | 1702 | 402 | 276 | 2765 | DO (19) |
| Phenotypes | ||||||||||
| Human Phenotype (HP) | 670 | 147 | 2079 | 605 | 141 | 1930 | 750 | 289 | 4104 | HPO (18) |
| Mammalian Phenotype (MP) | 1858 | 299 | 8555 | 2040 | 368 | 12 008 | 2202 | 844 | 23 149 | MGI (11) |
| Worm Phenotype (WP) | 556 | 296 | 4349 | 540 | 320 | 4572 | 571 | 507 | 6976 | WormBase (12) |
| Yeast Phenotype (YP) | 76 | 271 | 1070 | 72 | 256 | 1039 | 79 | 392 | 1529 | SGD (13) |
| Fly Phenotype (FP) | 64 | 140 | 268 | 62 | 167 | 314 | 69 | 283 | 557 | FlyBase (14) |
| Fly Anatomy (FA) | 502 | 191 | 3210 | 555 | 210 | 4183 | 551 | 349 | 8151 | FlyBase (14) |
| Zebrafish Anatomy (ZA) | 158 | 66 | 694 | 164 | 57 | 701 | 173 | 121 | 1316 | ZFIN (15) |
| Xenopus Anatomy (XA) | 243 | 474 | 8376 | 245 | 583 | 11 187 | 253 | 875 | 17 730 | Xenbase (16) |
| Arabidopsis Plant (AP) | 259 | 579 | 20 689 | 253 | 778 | 32 405 | 266 | 1093 | 45 311 | TAIR (17) |
| Others | ||||||||||
| Enzyme Commission (EC) | 1918 | 830 | 8483 | 1973 | 1565 | 10 278 | 1947 | 2958 | 21 028 | IntEnz (8) |
| DrugBank ATC_code (DB) | 964 | 143 | 2801 | 904 | 145 | 2659 | 984 | 230 | 3950 | DrugBank (20) |
| UniProtKB KeyWords (KW) | 857 | 1573 | 19 312 | 840 | 2798 | 25 841 | 866 | 5579 | 84 815 | UniProt (10) |
| UniPathway (UP) | 664 | 474 | 6332 | 626 | 796 | 7395 | 665 | 1356 | 12 918 | UniPathway (9) |
aThe total number of ontology terms used to annotate.
bThe number of annotatable domains (or supra-domains).
cThe number of domain-centric annotations.
dThis version is truly domain-centric, supported both by single-domain proteins and all proteins (including multi-domain proteins).
eThis version is only supported by all proteins, suitable for large-scale studies.
The GO is designed to annotate full-length proteins in a species-independent manner for generality. The most comprehensive protein-level annotations are maintained by the Gene Ontology Annotation (GOA) project (7). Motivated by a domain-centric viewpoint, we developed a general algorithm (4) for revealing functional signals carried by protein domains (and supra-domains in the multi-domain proteins). Using protein domain architectures from SUPERFAMILY and protein GO annotations from UniProKB-GOA (respecting the GO hierarchy), we first prepare a correspondence matrix between domains/supra-domains and GO terms. Each entry has the observed number of UniProt proteins that contain a domain/supra-domain (columns) and that can be annotated by a GO term (rows). With this correspondence matrix, we then use Fisher’s exact test to infer associations between the rows and columns. On top of this, we take advantage of the true path rule of the directed acyclic graph of the GO to determine the optimal level at which to make an association. We achieve this by comparing the significance of each term using two different backgrounds, one background using all analysable UniProt proteins (being annotatable by the GO), and one background using only those UniProt proteins annotated to direct parents of the term. If a GO term and its parent term are both significantly associated with a domain/supra-domain using the first background, and if the term is not significantly different from the parent term using the second background, then it is desirable to only associate the parent term. As a result of these dual constraints, only the most significant GO term associations to domains/supra-domains will be retained.
The significance of association is assessed by the method of false discovery rate (FDR) to account for multiple hypothesis tests, whereas the strength of association is measured by a hypergeometric distribution-based score. For a domain/supra-domain, the associated GO terms (i.e. direct annotations) are propagated to all ancestor terms (i.e. inherited annotations); together they constitute a complete GO annotation profile. Based on the information content of a GO term (i.e. negative logarithmic transformation of frequency of domains/supra-domains annotated to that term), a search procedure is applied to partition the directed acyclic graph structure of the GO, each partition reflecting the same or similar specificity but located in distinct paths. With four seeds of increasing information content, the procedure produces the ‘GO slim’ that contains GO terms classified into four levels of increasing granularity. These are highly general, general, specific and highly specific (Supplementary Table S1). The use of information content (as a measure of how specific and informative a term is) adds great value to the existing GO hierarchy for the user. The GO was created for annotating proteins, so some parts of GO structure are less valid for annotating domains/supra-domains than others. Rather than merely relying on the ontology graph depth to define the term specificity, our approach has taken into account actual usage of terms when determining the four-level depth classification of domains/supra-domains.
Domain-centric GO
Using the algorithm described earlier in the text, dcGO provides the user with two alternative versions of the GO associations with domains (Table 1). The high-quality version of associations includes only those that are supported by the unambiguous evidence (in terms of the causal domain) that comes from single-domain proteins of known function. The high-coverage associations also include those that are supported through statistical disambiguation from multi-domain proteins of known function. The high-quality associations are more reliable in their domain-centricity, but high-coverage associations are reliable enough for large-scale studies and provide a much greater coverage of function. Enrichment analyses are improved more by annotation coverage than by annotation quality; hence, there is a strong justification for using the high-coverage version in such studies. Restricting the annotations to GO slim (described earlier in the text) is also highly recommended for domain-based enrichment analyses.
In addition to individual domains, dcGO also associates GO terms with supra-domains (Table 1). In general, supra-domains are defined as recurring combinations of two or more successive domains that could function together. In dcGO, we only include completely assigned supra-domains, without any significant gaps between domains, that is, supra-domains with regions not assigned to a known domain are excluded (26). GO associations to supra-domains hold great promise for understanding how domain combinations contribute to functional diversification and also in predicting the functions of multi-domain proteins.
Domain-centric BO
In dcGO, the ‘BO’ refers to all other Biomedical Ontologies that are not GO. They mainly consist of phenotype ontologies that have been developed to classify and organize information on model organisms and human. Similarly to GO, the BO is hierarchical going from general terms at the top to more specific terms at the bottom. As with domain-centric GO, dcGO has the associations of the BO terms to individual domains and supra-domains; each has its own slim version of the ontology at four levels of increasing granularity based on information content. Unlike the GO, the BO does not have the high-quality version of the associations. This is largely because of an insufficient number of single-domain proteins with annotations, especially for species-specific ontologies. As listed in Table 1, currently dcGO has eight phenotype and/or anatomy ontologies covering seven major model organisms. They include Mouse/Mammalian Phenotypes (MP) from Mouse Genome Informatics (MGI) (11), Worm Phenotypes (WP) from WormBase (12), Yeast/Ascomycete Phenotype (YP) from Saccharomyces Genome Database (SGD) (13), Fly Phenotype (FP) and Fly Anatomy (FA) from FlyBase (14), Zebrafish Anatomy (ZA) from ZFIN (15), Xenopus Anatomy (XA) from Xenbase (16) and Arabidopsis Plant (AP) ontology from TAIR (17). In addition to model organisms, dcGO also contains three ontologies with specific relevance to humans, including Human Phenotype (HP) (18), Disease Ontology (DO) (19) and DrugBank ATC codes (DB). The remaining ontologies have a fixed-length or much-simplified hierarchy. These include Enzyme Commission (EC) (8), UniProtKB UniPathway (UP) (9) and UniProtKB KeyWords (KW) (10).
DATABASE WEBSITE
Downloading data
The underlying data summarized in Table 1 are available for download on the dcGO website. For each ontology, the full and slim versions are provided separately for individual domains (i.e. superfamilies and families) and supra-domains. In addition, the user can download the MySQL relational database tables along with detailed documentation. All downloadable files are free for academic or commercial use and are automatically updated fortnightly.
Searching dcGO
The faceted search on the dcGO website (Figure 1) is a mining hub for users, with additional bioinformatics tools hyperlinked from the search results. Full-text query is supported for SCOP domains, ontologies and genomes. Identifier or accession number lookup is supported for sequences. Ontologies and SCOP domains are linked to pages for browsing their respective hierarchies. Every genome is presented within its phylogenetic context by linking to a species tree of life (called sTOL, see ‘Analysing GO terms over the species tree of life’ section). There are also links from domains and ontological terms to the tree of life (to see their distribution across species). Search results returning BO terms are linked to a cross-ontology comparison tool, the phenotype similarity network (PSnet, see ‘Cross-linking similar phenotypes’ section). PSnet searches for terms from other ontologies with a similar profile of associations. For lookups returning a specific genome sequence, the user is provided with the facility to submit it automatically to the ‘dcGO Predictor’ for function, phenotype and disease prediction. In conclusion, the faceted search is designed for multi-tasking; it does not just provide search results but is intended to interconnect all the tools and cross-referencing abilities of dcGO.
Figure 1.
The dcGO website has the ‘Faceted Search’ interface as a hub to mine the resource. By searching against keywords of interest, the user can access the resource in an organized manner and can link to additional analysis tools.
Browsing the hierarchies
The ‘BROWSE’ navigation on the website (aforementioned) provides browsing for the SCOP, GO and various BO hierarchies. The hierarchy-like structure of the SCOP (or ontology) has a domain (or term) as a node and its relations to parental nodes as directed edges. To navigate this hierarchy, we display all the paths from the current node upwards to the root ordered by the shortest distances. Also, all direct children of the current node are listed underneath to enable browsing downwards. In addition to the hierarchy itself, a tabbed interface is used to aid the display of domain-centric annotations in a subject-specific manner. The SCOP-orientated hierarchy shows terms used to annotate a domain, and vice versa, the ontology-orientated hierarchy shows domains/supra-domains annotated by a term.
Analysing GO terms over the species tree of life
The dcGO website is integrated with a species tree of life (called sTOL), which is provided by SUPERFAMILY (27). The sTOL is a fully resolved binary tree of species of completely sequenced organisms providing a phylogenetic context. Within the sTOL, the presence/absence of domains and supra-domains are pre-computed and stored, both for extant genomes and for reconstructed ancestral genomes in eukaryotes. The integration enables cross-comparison between both resources for understanding the evolutionary context of the functional associations. The distribution of GO terms can be explored over the sTOL tree, which can be accessed either by links from the GO hierarchy pages or from the faceted search. GO term enrichment for extant and ancestral genomes (using domain-based GO enrichment analysis) can also be explored when browsing the tree. Thus, the sTOL adds an extra dimension to the dcGO resource utility.
Cross-linking similar phenotypes
Traditionally, phenotype ontologies are developed for within-species comparisons. Recently, many attempts have been made at cross-species comparisons (28–30), these studies mostly focusing on text mining and formal definitions. Within dcGO is a tool called ‘PSnet’ that cross-references terms between ontologies (mostly phenotypes in different model organisms). Given one phenotype, PSnet can be used to search for a similar candidate phenotype on the basis of their shared domain annotations (both at superfamily and family levels). The statistical significance of the shared domains versus the expected overlap by chance is evaluated by Fisher’s exact test. PSnet reports a Z-score for the strength of the overlap, and a P-value and false discovery rate (accounting for multiple hypothesis tests) for the significance. An information content-based similarity metric is used to rank the phenotype similarities; if a certain domain is more frequently annotated (less informative) than others, then its contribution to the phenotype similarity is less. In this way, for any given phenotype, PSnet will suggest the best-correlated terms from other ontologies.
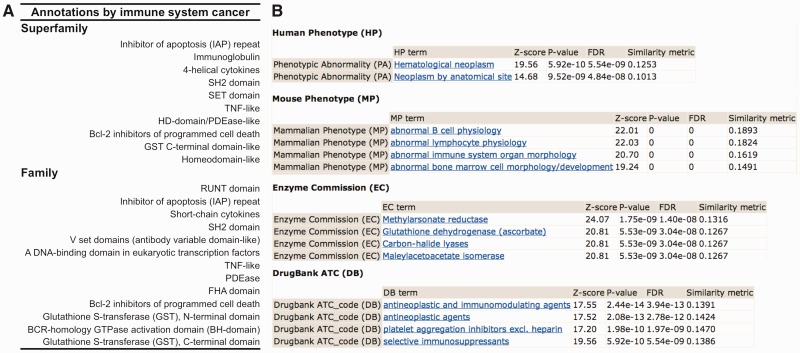
As a proof of principle, we consider the disease term ‘immune system cancer’ [DOID: 0 060 083] from the Disease Ontology (19). In Figure 2, we illustrate with this example, how PSnet displays cross-links between correlated terms, which facilitates the development of hypotheses. In dcGO, 10 superfamilies and 13 families are associated to this term (Figure 2A). Supplementary Figure S1B shows the numerical pathway of associating immune system cancer with the immunoglobulin superfamily, following the general procedure shown in Supplementary Figure S1A. Given this disease and its domain-centric annotation profile in Figure 2A, PSnet searches for phenotypes with similar domain annotations. As shown in Figure 2B, PSnet cross-references this disease term with closely related terms from the Human Phenotype ontology (18), suggests possible links to the abnormal counterparts in the Mouse Phenotype ontology (11), reveals the mechanisms by listing top enzymes from the Enzyme Commission (8) and implicates the treatment agents through DrugBank ATC codes (20). The multiple layers of information revealed by PSnet provide a powerful tool for hypothesis generation. PSnet brings additional understanding to the essential roles that protein domains can play in functions, phenotypes and diseases.
Figure 2.
Using ‘PSnet’ to cross-link phenotypes and other ontologies based on shared domain-centric annotations. (A) A list of superfamilies and families annotated by a disease term ‘immune system cancer’. (B) The top well-correlated ontological terms are returned for the disease term in this query.
Predicting functions, phenotypes and diseases for >80 million sequences
Using domain-centric GO annotations as a functional predictor, we entered the Critical Assessment of Function Annotation competition and came in the top 10 of >50 methods. Considering that only domain information is involved and natively used as a single direct prediction, its relative success validates the quality of this resource for widespread use. We provide pre-computed annotations for >80 million sequences (at the time of writing) stored in the SUPERFAMILY database that includes 2414 genomes, UniProt and hundreds of meta-genomes. Through the dcGO Predictor (Figure 3), functions and other higher-order knowledge (phenotypes, diseases and more) can be predicted for user-submitted sequences. The implementation is fairly straightforward: first the domain architecture of the query protein is determined, and then the ontological terms associated with its component domains/supra-domains are transferred to the query protein. A score is provided for ranking the confidence of such predictions/transfers. In addition to access through the faceted search, a batch query mode is provided, which allows the submission of up to 1000 sequences at a time (Figure 3A). The prediction results are summarized to give an overview of the prediction content and are also available for download (Figure 3B). Figure 3C shows the results for the example input sequence ‘Q01826’, i.e. special AT-rich sequence-binding protein 1 (SATB1). As a chromatin regulator, this protein has been reported to promote tumour growth and metastasis (31), which is consistent with the prediction. For a sequence to receive annotation, first there must be domains detected by the SUPERFAMILY hidden Markov model library search, and then those domains/supra-domains must have ontological associations in dcGO. Our coverage will improve as new structures are deposited in the Protein Data Bank (32) and as more sequences have ontological terms experimentally determined.
Figure 3.
Converting genome sequences to knowledge about function, phenotype and disease using the ‘dcGO Predictor’. (A) A batch query facility allows the user to upload up to 1000 sequences for the prediction on function, disease, phenotype and other information, such as enzyme classification, drugs and pathways. (B) The result page provides a summary of the prediction content. New predictions are supported by instantly switching to other ontologies. In addition to the download, the user can also explore predictions for each of the input sequences, such as Q01826 (human SATB1 protein; see next). (C) The domain architecture of the human SATB1 protein is graphically displayed using the SCOP domains at the superfamily level, whereas the bottom panel shows the predicted Disease Ontology terms.
CONCLUSION AND FUTURE DEVELOPMENTS
With the rate of growth of biological (e.g. sequence) data increasing rapidly, the only realistic way to analyse biology in a holistic way is computationally. Gene Ontology has become a widely adopted medium for handling biological concepts in a structured way that can be processed computationally. With this unique database ‘dcGO’, treating domains and supra-domains as functional units, we provide GO plus a growing number of other ontologies in a probabilistic framework. The results of the Critical Assessment of Function Annotation experiment show that this domain-centric approach performs significantly better than simple whole-sequence pair-wise homology on the task of labelling sequences of unknown function with GO terms; by ‘simple whole-sequence homology’, we mean the strategy of annotating a sequence with GO terms by searching it against UniProt using Basic Local Alignment Search Tool and transferring any GO terms associated with significant hits. Thus, the dcGO database, in providing full functional annotations of all completely sequenced genomes in addition to the domain-ontology associations themselves, makes a massive contribution to the body of computer-readable biological knowledge. In the future, the intention is to expand the ontologies included in the database, expand the domain collection as more domains are classified, and expand the collection of functionally annotated sequences as new genomes, meta-genomes and so forth are released.
In addition to the value of the large-scale raw annotations in the dcGO database, the anticipated potential for comparative analyses is already reflected in the sTOL evolutionary context and PSnet cross-referencing tools. Other than the data expansion aforementioned, other future developments will focus on introducing more comparative tools and increasing the use cases of the existing ones. These will include network-based infrastructures, for example, of domains and of terms spanning different ontologies. The construction of functional domain networks with respect to GO is already on the agenda.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online: Supplementary Table 1 and Supplementary Figure 1.
FUNDING
Funding for open access charge: Biotechnology and Biological Sciences Research Council [BB/G022771/1 to J.G.].
Conflict of interest statement. None declared.
ACKNOWLEDGEMENTS
The authors acknowledge the numerous providers of the primary ontologies. Special thanks go to all of the contributors to the SUPERFAMILY resource, which is tightly linked to the dcGO database.
REFERENCES
- 1.Friedberg I. Automated protein function prediction—the genomic challenge. Brief. Bioinform. 2006;7:225–242. doi: 10.1093/bib/bbl004. [DOI] [PubMed] [Google Scholar]
- 2.Thorisson GA, Muilu J, Brookes AJ. Genotype-phenotype databases: challenges and solutions for the post-genomic era. Nat. Rev. Genet. 2009;10:9–18. doi: 10.1038/nrg2483. [DOI] [PubMed] [Google Scholar]
- 3.Butler D. Human genome at ten: science after the sequence. Nature. 2010;465:1000–1001. doi: 10.1038/4651000a. [DOI] [PubMed] [Google Scholar]
- 4.de Lima Morais DA, Fang H, Rackham OJ, Wilson D, Pethica R, Chothia C, Gough J. SUPERFAMILY 1.75 including a domain-centric gene ontology method. Nucleic Acids Res. 2011;39:D427–D434. doi: 10.1093/nar/gkq1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith B, Ashburner M, Rosse C, Bard J, Bug W, Ceusters W, Goldberg LJ, Eilbeck K, Ireland A, Mungall CJ, et al. The OBO Foundry: coordinated evolution of ontologies to support biomedical data integration. Nat. Biotechnol. 2007;25:1251–1255. doi: 10.1038/nbt1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrell D, Dimmer E, Huntley RP, Binns D, O’Donovan C, Apweiler R. The GOA database in 2009—an integrated Gene Ontology Annotation resource. Nucleic Acids Res. 2009;37:D396–D403. doi: 10.1093/nar/gkn803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleischmann A, Darsow M, Degtyarenko K, Fleischmann W, Boyce S, Axelsen KB, Bairoch A, Schomburg D, Tipton KF, Apweiler R. IntEnz, the integrated relational enzyme database. Nucleic Acids Res. 2004;32:D434–D437. doi: 10.1093/nar/gkh119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgat A, Coissac E, Coudert E, Axelsen KB, Keller G, Bairoch A, Bridge A, Bougueleret L, Xenarios I, Viari A. UniPathway: a resource for the exploration and annotation of metabolic pathways. Nucleic Acids Res. 2012;40:D761–D769. doi: 10.1093/nar/gkr1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bairoch A, Apweiler R, Wu CH, Barker WC, Boeckmann B, Ferro S, Gasteiger E, Huang H, Lopez R, Magrane M, et al. The Universal Protein Resource (UniProt) Nucleic Acids Res. 2005;33:D154–D159. doi: 10.1093/nar/gki070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith CL, Eppig JT. The Mammalian Phenotype Ontology: enabling robust annotation and comparative analysis. Wiley Interdiscip. Rev. Syst. Biol. Med. 2009;1:390–399. doi: 10.1002/wsbm.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schindelman G, Fernandes JS, Bastiani CA, Yook K, Sternberg PW. Worm Phenotype Ontology: integrating phenotype data within and beyond the C. elegans community. BMC Bioinformatics. 2011;12:32. doi: 10.1186/1471-2105-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engel SR, Balakrishnan R, Binkley G, Christie KR, Costanzo MC, Dwight SS, Fisk DG, Hirschman JE, Hitz BC, Hong EL, et al. Saccharomyces Genome Database provides mutant phenotype data. Nucleic Acids Res. 2010;38:D433–D436. doi: 10.1093/nar/gkp917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grumbling G, Strelets V. FlyBase: anatomical data, images and queries. Nucleic Acids Res. 2006;34:D484–D488. doi: 10.1093/nar/gkj068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradford Y, Conlin T, Dunn N, Fashena D, Frazer K, Howe DG, Knight J, Mani P, Martin R, Moxon SA, et al. ZFIN: enhancements and updates to the Zebrafish Model Organism Database. Nucleic Acids Res. 2011;39:D822–D829. doi: 10.1093/nar/gkq1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowes JB, Snyder KA, Segerdell E, Jarabek CJ, Azam K, Zorn AM, Vize PD. Xenbase: gene expression and improved integration. Nucleic Acids Res. 2009;38:D607–D612. doi: 10.1093/nar/gkp953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ilic K, Kellogg EA, Jaiswal P, Zapata F, Stevens PF, Vincent LP, Avraham S, Reiser L, Pujar A, Sachs MM, et al. The plant structure ontology, a unified vocabulary of anatomy and morphology of a flowering plant. Plant Physiol. 2007;143:587–599. doi: 10.1104/pp.106.092825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson PN, Kohler S, Bauer S, Seelow D, Horn D, Mundlos S. The Human Phenotype Ontology: a tool for annotating and analyzing human hereditary disease. Am. J. Hum. Genet. 2008;83:610–615. doi: 10.1016/j.ajhg.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schriml LM, Arze C, Nadendla S, Chang YW, Mazaitis M, Felix V, Feng G, Kibbe WA. Disease Ontology: a backbone for disease semantic integration. Nucleic Acids Res. 2012;40:D940–D946. doi: 10.1093/nar/gkr972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knox C, Law V, Jewison T, Liu P, Ly S, Frolkis A, Pon A, Banco K, Mak C, Neveu V, et al. DrugBank 3.0: a comprehensive resource for ‘omics’ research on drugs. Nucleic Acids Res. 2011;39:D1035–D1041. doi: 10.1093/nar/gkq1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis MJ, Sehgal MS, Ragan MA. Automatic, context-specific generation of Gene Ontology slims. BMC Bioinformatics. 2010;11:498. doi: 10.1186/1471-2105-11-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreeva A, Howorth D, Chandonia JM, Brenner SE, Hubbard TJ, Chothia C, Murzin AG. Data growth and its impact on the SCOP database: new developments. Nucleic Acids Res. 2008;36:D419–D425. doi: 10.1093/nar/gkm993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Punta M, Coggill PC, Eberhardt RY, Mistry J, Tate J, Boursnell C, Pang N, Forslund K, Ceric G, Clements J, et al. The Pfam protein families database. Nucleic Acids Res. 2012;40:D290–D301. doi: 10.1093/nar/gkr1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gough J, Karplus K, Hughey R, Chothia C. Assignment of homology to genome sequences using a library of hidden Markov models that represent all proteins of known structure. J. Mol. Biol. 2001;313:903–919. doi: 10.1006/jmbi.2001.5080. [DOI] [PubMed] [Google Scholar]
- 25.The UniProt Consortium. Reorganizing the protein space at the Universal Protein Resource (UniProt) Nucleic Acids Res. 2012;40:D71–D75. doi: 10.1093/nar/gkr981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson D, Madera M, Vogel C, Chothia C, Gough J. The SUPERFAMILY database in 2007: families and functions. Nucleic Acids Res. 2007;35:D308–D313. doi: 10.1093/nar/gkl910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson D, Pethica R, Zhou Y, Talbot C, Vogel C, Madera M, Chothia C, Gough J. SUPERFAMILY—sophisticated comparative genomics, data mining, visualization and phylogeny. Nucleic Acids Res. 2009;37:D380–D386. doi: 10.1093/nar/gkn762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Washington NL, Haendel MA, Mungall CJ, Ashburner M, Westerfield M, Lewis SE. Linking human diseases to animal models using ontology-based phenotype annotation. PLoS Biol. 2009;7:e1000247. doi: 10.1371/journal.pbio.1000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoehndorf R, Schofield PN, Gkoutos GV. PhenomeNET: a whole-phenome approach to disease gene discovery. Nucleic Acids Res. 2011;39:e119. doi: 10.1093/nar/gkr538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mungall CJ, Gkoutos GV, Smith CL, Haendel MA, Lewis SE, Ashburner M. Integrating phenotype ontologies across multiple species. Genome Biol. 2010;11:R2. doi: 10.1186/gb-2010-11-1-r2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han HJ, Russo J, Kohwi Y, Kohwi-Shigematsu T. SATB1 reprogrammes gene expression to promote breast tumour growth and metastasis. Nature. 2008;452:187–193. doi: 10.1038/nature06781. [DOI] [PubMed] [Google Scholar]
- 32.Velankar S, Alhroub Y, Alili A, Best C, Boutselakis HC, Caboche S, Conroy MJ, Dana JM, van Ginkel G, Golovin A, et al. PDBe: Protein Data Bank in Europe. Nucleic Acids Res. 2011;39:D402–D410. doi: 10.1093/nar/gkq985. [DOI] [PMC free article] [PubMed] [Google Scholar]