Abstract
During animal development, cells undergo dynamic changes in position and gene expression. A collection of quantitative information about morphological dynamics under a wide variety of gene perturbations would provide a rich resource for understanding the molecular mechanisms of development. Here, we created a database, the Worm Developmental Dynamics Database (http://so.qbic.riken.jp/wddd/), which stores a collection of quantitative information about cell division dynamics in early Caenorhabditis elegans embryos with single genes silenced by RNA-mediated interference. The information contains the three-dimensional coordinate values of the outlines of nuclear regions and the dynamics of the outlines over time. The database provides free access to 50 sets of quantitative data for wild-type embryos and 136 sets of quantitative data for RNA-mediated interference embryos corresponding to 72 of the 97 essential embryonic genes on chromosome III. The database also provides sets of four-dimensional differential interference contrast microscopy images on which the quantitative data were based. The database will provide a novel opportunity for the development of computational methods to obtain fresh insights into the mechanisms of development. The quantitative information and microscopy images can be synchronously viewed through a web browser, which is designed for easy access by experimental biologists.
INTRODUCTION
One approach to understanding the molecular mechanisms involved in animal development is to analyse morphological changes in cell positions, movements and divisions when gene expression is perturbed. Because many genes are essential for animal development, a genome-wide gene perturbation analysis of morphological dynamics would provide useful information about the development. RNA-mediated interference (RNAi) and morpholino techniques can be used for genome-wide gene silencing analyses (1,2). In several organisms, large-scale gene-silencing analyses of morphological dynamics have been performed (3–10). To obtain quantitative information about morphological dynamics from microscopy images, methods based on computer-image processing have been developed (11–15). Such quantitative information enables highly objective comparisons of morphological dynamics and the generation of mathematical models and hypotheses pertaining to animal development, and it thus facilitates the attainment of insights into animal development. However, before this study, no collection of quantitative information about morphological dynamics under a wide variety of gene perturbations was available.
The nematode Caenorhabditis elegans is the only animal for which essential embryonic genes have been identified through genome-wide RNAi screenings (7,16). Here, we conducted RNAi experiments targeting the 97 essential embryonic genes on chromosome III, and analysed the resultant embryos by four-dimensional (4D) differential interference contrast (DIC) microscopy. We then applied our computational method (13) to the 4D DIC microscopy images to obtain quantitative information about cell division dynamics in early C. elegans embryos. We designed the Worm Developmental Dynamics Database (WDDD) to allow access to this information. The current version of WDDD includes 50 sets of quantitative data from wild-type embryos and 136 sets of quantitative data from RNAi experiments, where 72 of the 97 essential embryonic genes on chromosome III were individually silenced (i.e. one gene silenced per embryo).
METHODS
We first recorded three-dimensional (3D) time-lapse microscopy images of early C. elegans embryo by using a 4D DIC microscope, as described previously (17). At each time point, we recorded images of the embryo at 66 consecutive focal planes spaced at 0.5 μm intervals. Sets of images were recorded at 40 s intervals during the first three rounds of cell division or up to 2 h for embryos whose cell division was very slow.
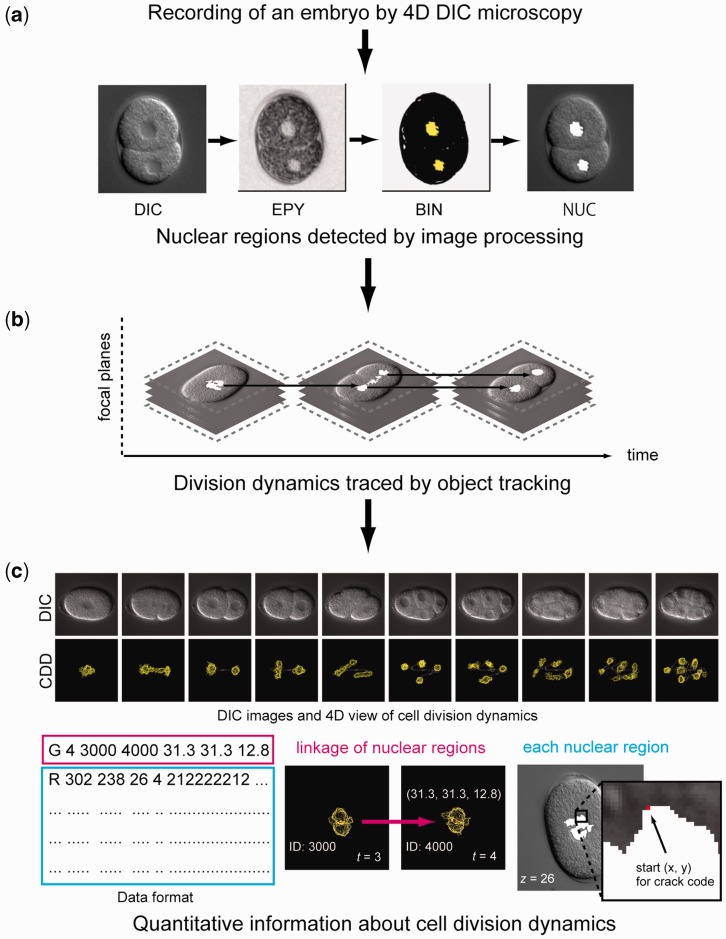
To obtain quantitative information about cell division dynamics from the microscopy images, we used the computational method that we developed previously (13). We automatically detected nuclear regions in the images by distinguishing the image texture in the nucleus from that in the cytoplasm (Figure 1a), and grouped nuclear regions corresponding to the same nucleus at each time point. Lastly, we traced nuclear movements and divisions over time by using an object tracking algorithm (Figure 1b) and obtained the 3D coordinate values of the outlines of nuclear regions and the dynamics of the outlines over time (Figure 1c). The errors of automated nuclear detection and nuclear tracking were manually corrected by visual inspection.
Figure 1.
Overview of our computational method for obtaining quantitative information about cell division dynamics. (a) Use of computer image processing to detect nuclear regions (white) in a DIC microscopy image. DIC, DIC microscopy image; EPY, local image entropy; BIN, low-entropy regions designated by binary thresholding, NUC, detected nuclear regions. (b) Use of object tracking algorithm to obtain division dynamics of detected nuclear regions. (c) Example of DIC microscopy images and quantitative information about cell division dynamics (top). DIC, DIC microscopy images; CDD, 4D view of quantitative information about cell division dynamics. Example of a data file (bottom). Data format of the file (left). The first character of each line, ‘G’ or ‘R’, indicates that the line contains information about nuclear tracking or information about the outline of the nuclear region. The numbers following the index ‘G’ represent time point, identification number (ID) of the ancestor of the nucleus at previous time point, unique ID of the nucleus and xyz positions of the centroid of the nucleus relative to the upper-left corner of the image and the top focal plane, where x and y positions are represented by converting 1 pixel to 0.105 µm (middle). The numbers following ‘R’ represent the start pixel point of the crack code relative to the upper-left corner of the image, focal plane, time point and the crack code that represents the outline of the nuclear region (right). Details of the data format are available at http://so.qbic.riken.jp/wddd/.
To obtain embryos in which the expression of individual genes was silenced, we used the RNAi method. Double-stranded RNAs (dsRNAs) homologous to the genes of interest were produced by using the same pairs of primers as those used in a previous genome-wide screen (16). Young adult worms were then separately injected with each dsRNA. We targeted all 97 genes on chromosome III that, when silenced, produced an embryonic lethal phenotype in 100% of offspring in the previous screen (16). Embryonic lethality in our RNAi experiments was checked both for the progeny on the plate laid by each injected worm and for the recorded embryo on the glass slide.
DATA SOURCE
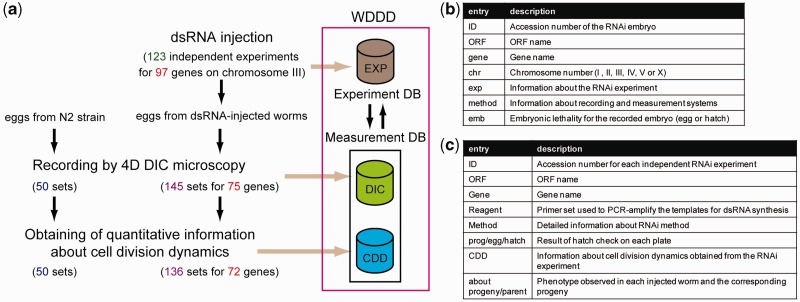
The current version of the WDDD provides information about cell division dynamics during the first three rounds of cell division (from the one-cell to eight-cell stage). It includes a total of 136 sets of information for RNAi embryos corresponding to 72 of the 97 essential embryonic genes on chromosome III (1–4 sets of information for each gene; Figure 2a) and 50 sets of information for wild-type embryos. The information for RNAi embryos corresponding to the remaining 15 genes was not obtained because the injected worms produced no embryos, the embryos did not undergo cell division or the embryos had abnormalities in either nuclear appearance or cytokinesis or in their size. The WDDD also provides all sets of 4D DIC microscopy images from RNAi and wild-type embryos at high resolution of 600 × 600 pixels (Figure 2a).
Figure 2.
Overview of the WDDD. (a) Contents of the current version of the WDDD. EXP, results of RNAi experiments; DIC, datasets of 4D DIC microscopy images; CDD, datasets of quantitative information about cell division dynamics. (b) Information provided in search results for each RNAi embryo. (c) Information provided in search results for each RNAi experiment.
For all 97 essential embryonic genes targeted, the WDDD provides information about embryonic lethality and phenotypic defects that were observed in the injected worms and their progeny.
SEARCH
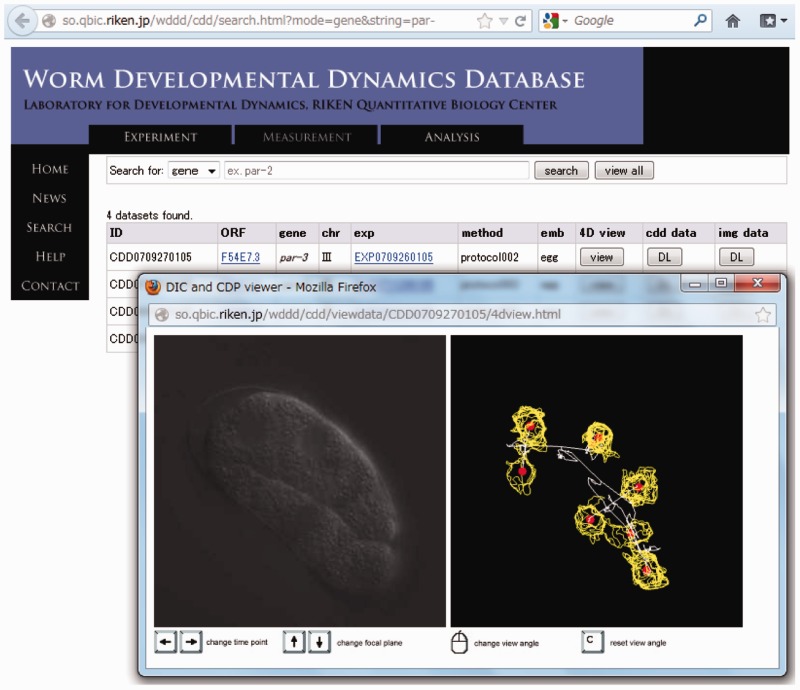
The WDDD is composed of two cross-linked databases: a database of quantitative information about cell division dynamics and a database of information about the RNAi experiments. The database of quantitative information about cell division dynamics, which also contains the 4D DIC microscopy images, can be searched based on the name of the open reading frame (ORF) or gene targeted by the RNAi, or based on the accession number of embryo. Search results provide the accession number, ORF name, gene name, chromosome number, information about the RNAi experiment (accession number of RNAi experiment and link to the information), information about recording and measurement systems (e.g. microscope system, temperature and version of our system) and the embryonic lethality of the recorded embryo (Figure 2b). Links to the WormBase (18) are available to rapidly obtain information about the gene. The quantitative information about cell division dynamics and the set of 4D DIC microscopy images for each embryo can be downloaded from the search results or viewed in a web browser (Figure 3). The viewer was implemented with the use of WebGL, HTML5 Canvas and JavaScript. In the viewer, focal planes and time points can be changed with arrow keys, and the view angle of the data can be changed by using the mouse to drag-and-move. Synchronous viewing of microscopy images and the related quantitative information enable us to easily understand cell positions, movements and divisions.
Figure 3.
Viewing 4D DIC microscopy images and quantitative information about cell division dynamics in a web browser. In this example, four datasets for par genes were found in the WDDD and viewed interactively through a web browser. The time point and focal plane could be changed by using the keyboard, and the view angle could be changed by using the mouse. The left and right panel in the viewer window show the DIC microscopy image and quantitative information about cell division dynamics, respectively. Yellow lines represent outlines of detected nuclear regions, and red points represent centroids of nuclear regions corresponding to each nucleus.
The database of RNAi experiments can also be searched based on the name of the ORF or gene targeted by the RNAi, or based on the accession number for each independent experiment. Search results provide the accession number, ORF name, gene name, primer set used to amplify the templates for dsRNA synthesis, detailed information about the RNAi method, descriptions of embryonic lethality and other phenotypes observed in the RNAi experiment and the information about cell division dynamics obtained from the RNAi experiment (accession number of embryo) (Figure 2c). These search results contain links to the WormBase to enable the user to obtain the information about the targeted gene and the primer set used to create the dsRNA.
DATA FORMAT
The information about cell division dynamics is numerically represented. The data format consists of two blocks: one provides information about nuclear tracking and the other provides information about outlines of nuclear regions (Figure 1c). Using the information, we can calculate parameters related to cell division dynamics such as cell position, movement and division. Details of the data format are available at http://so.qbic.riken.jp/wddd/.
FUTURE DIRECTIONS
We aim to obtain five sets of quantitative information about cell division dynamics for each essential embryonic gene in C. elegans. The current version of the WDDD includes only one or two sets of information for most of the genes examined. We are currently in the process of obtaining five sets of information for each essential embryonic gene not only on chromosome III but also on the other chromosomes.
We plan to extend the cell stages included in the database. By using our computational method (13), we can consistently obtain quantitative information from the one-cell stage to the 20–24-cell stage from 4D DIC microscopy images. If we can extend the methodology to later stages, the database could be used to understand various other developmental phenomena: e.g. gastrulation, which begins at the 26-cell stage in C. elegans. We anticipate that the extension of cell stages can be achieved by optimizing the parameters of our computational method.
We are currently performing further computational analyses of the sets of quantitative information stored in the WDDD. For example, we can perform computational phenotypic analysis (13), in which we mathematically define and calculate various kinds of phenotypic characters and then statistically compare these characters between wild-type and RNAi embryos. Because our collection has a feature of four dimensionality, the computational analysis will enable us to screen for 3D spatial and temporal phenotypes that are difficult to identify in manual analysis. Using the results of these analyses, we will create a database of RNAi-induced phenotypes that can be searched based on phenotype, such as the RNAiDB and the Phenobank (7,19).
DISCUSSION
In the WDDD, we have released a collection of quantitative information about cell division dynamics in wild-type and RNAi embryos. Because the information is numerically represented, various parameters related to cell division dynamics such as cell positions, movements and divisions can be computationally extracted. The sets of the information from wild-type embryos enable statistical comparison of cell division dynamics between wild-type and RNAi embryos. The information collection enables us to analyse cell division dynamics by various computational methods such as those using statistical and clustering techniques (13,19,20). The WDDD will provide a novel opportunity for computational biologists to develop computational methods to attain novel insights into the molecular mechanisms of animal development.
We expect that the WDDD can provide useful information for the broader community. For instance, the 4D DIC microscopy images of early embryos contain various kinds of morphological information, such as that pertaining to the cell membrane and cytoplasm. Such information will enable researchers to analyse many cellular mechanisms such as cytokinesis, and this information can be used by computer scientists to develop new quantitative morphological measures. Moreover, to make access simple for experimental biologists, the 4D DIC microscopy images and quantitative information about cell division dynamics can be viewed in a web browser without the use of a special software program.
We released the quantitative information about cell division dynamics in an original format, which is different from the formats used for several other sets of the quantitative information about morphological dynamics (12,14). If the formats of quantitative information about morphological dynamics were unified, computational methods developed to analyse such information could be applied to all sets without modification. Efforts will be made to establish a unified format for quantitative information about morphological dynamics.
FUNDING
KAKENHI (Grant-in-Aid for Scientific Research) on Priority Areas ‘Systems Genomics’ [17017038]; Special Coordination Funds for the Promotion of Science and Technology, from the Ministry of Education, Culture, Sports, Science and Technology of Japan. Funding for open access charge: National Bioscience Database Center, Japan Science and Technology Agency.
Conflict of interest statement. None declared.
ACKNOWLEDGEMENTS
Worm strains were provided by the Caenorhabditis Genetics Center, funded by the NIH National Center for Research Resources. The authors are grateful to members of the Onami laboratory for discussion.
REFERENCES
- 1.Fire A, Xu S, Montgomery MK, Kostas SA, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 2.Summerton J. Morpholino antisense oligomers: the case for an RNase H-independent structural type. Biochim. Biophys. Acta. 1999;1489:141–158. doi: 10.1016/s0167-4781(99)00150-5. [DOI] [PubMed] [Google Scholar]
- 3.Gönczy P, Echeverri C, Oegema K, Coulson A, Jones SJ, Copley RR, Duperon J, Oegema J, Brehm M, Cassin E, et al. Functional genomic analysis of cell division in C. elegans using RNAi of genes on chromosome III. Nature. 2000;408:331–336. doi: 10.1038/35042526. [DOI] [PubMed] [Google Scholar]
- 4.Piano F, Schetter AJ, Morton DG, Gunsalus KC, Reinke V, Kim SK, Kemphues KJ. Gene clustering based on RNAi phenotypes of ovary-enriched genes in C. elegans. Curr. Biol. 2002;12:1959–1964. doi: 10.1016/s0960-9822(02)01301-5. [DOI] [PubMed] [Google Scholar]
- 5.Yamada L, Shoguchi E, Wada S, Kobayashi K, Mochizuki Y, Satou Y, Satoh N. Morpholino-based gene knockdown screen of novel genes with developmental function in Ciona intestinalis. Development. 2003;130:6485–6495. doi: 10.1242/dev.00847. [DOI] [PubMed] [Google Scholar]
- 6.Reddien PW, Bermange AL, Murfitt KJ, Jennings JR, Sanchez Alvarado A. Identification of genes needed for regeneration, stem cell function, and tissue homeostasis by systematic gene perturbation in planaria. Dev. Cell. 2005;8:635–649. doi: 10.1016/j.devcel.2005.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sönnichsen B, Koski LB, Walsh A, Marschall P, Neumann B, Brehm M, Alleaume AM, Artelt J, Bettencourt P, Cassin E, et al. Full-genome RNAi profiling of early embryogenesis in Caenorhabditis elegans. Nature. 2005;434:462–469. doi: 10.1038/nature03353. [DOI] [PubMed] [Google Scholar]
- 8.Pickart MA, Klee EW, Nielsen AL, Sivasubbu S, Mendenhall EM, Bill BR, Chen E, Eckfeldt CE, Knowlton M, Robu ME, et al. Genome-wide reverse genetics framework to identify novel functions of the vertebrate secretome. PLoS One. 2006;1:e104. doi: 10.1371/journal.pone.0000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rana AA, Collart C, Gilchrist MJ, Smith JC. Defining synphenotype groups in Xenopus tropicalis by use of antisense morpholino oligonucleotides. PLoS Genet. 2006;2:e193. doi: 10.1371/journal.pgen.0020193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 11.Onami S, Hamahashi S, Nagasaki M, Miyano S, Kitano H. Automatic acquisition of cell lineage through 4D microscope and analysis of early C. elegans embryogenesis. In: Kitano H, editor. Foundations of Systems Biology. Cambridge: MIT Press; 2001. pp. 39–55. [Google Scholar]
- 12.Bao Z, Murray JI, Boyle T, Ooi SL, Sandel MJ, Waterston RH. Automated cell lineage tracing in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA. 2006;103:2707–2712. doi: 10.1073/pnas.0511111103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamahashi S, Kitano H, Onami S. A system for measuring cell division patterns of early Caenorhabditis elegans embryos by using image processing and object tracking. Systems Comput. Jpn. 2007;38:12–24. [Google Scholar]
- 14.Keller PJ, Schmidt AD, Wittbrodt J, Stelzer EH. Reconstruction of zebrafish early embryonic development by scanned light sheet microscopy. Science. 2008;322:1065–1069. doi: 10.1126/science.1162493. [DOI] [PubMed] [Google Scholar]
- 15.Jaensch S, Decker M, Hyman AA, Myers EW. Automated tracking and analysis of centrosomes in early Caenorhabditis elegans embryos. Bioinformatics. 2010;26:i13–i20. doi: 10.1093/bioinformatics/btq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- 17.Hamahashi S, Onami S, Kitano H. Detection of nuclei in 4D Nomarski DIC microscope images of early Caenorhabditis elegans embryos using local image entropy and object tracking. BMC Bioinformatics. 2005;6:125. doi: 10.1186/1471-2105-6-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yook K, Harris TW, Bieri T, Cabunoc A, Chan J, Chen WJ, Davis P, de la Cruz N, Duong A, Fang R, et al. WormBase 2012: more genomes, more data, new website. Nucleic Acids Res. 2012;40:D735–D741. doi: 10.1093/nar/gkr954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunsalus KC, Yueh WC, MacMenamin P, Piano F. RNAiDB and PhenoBlast: web tools for genome-wide phenotypic mapping projects. Nucleic Acids Res. 2004;32:D406–D410. doi: 10.1093/nar/gkh110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohya Y, Sese J, Yukawa M, Sano F, Nakatani Y, Saito TL, Saka A, Fukuda T, Ishihara S, Oka S, et al. High-dimensional and large-scale phenotyping of yeast mutants. Proc. Natl Acad. Sci. USA. 2005;102:19015–19020. doi: 10.1073/pnas.0509436102. [DOI] [PMC free article] [PubMed] [Google Scholar]