Abstract
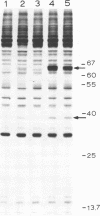
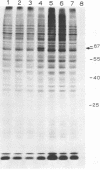
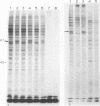
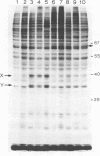
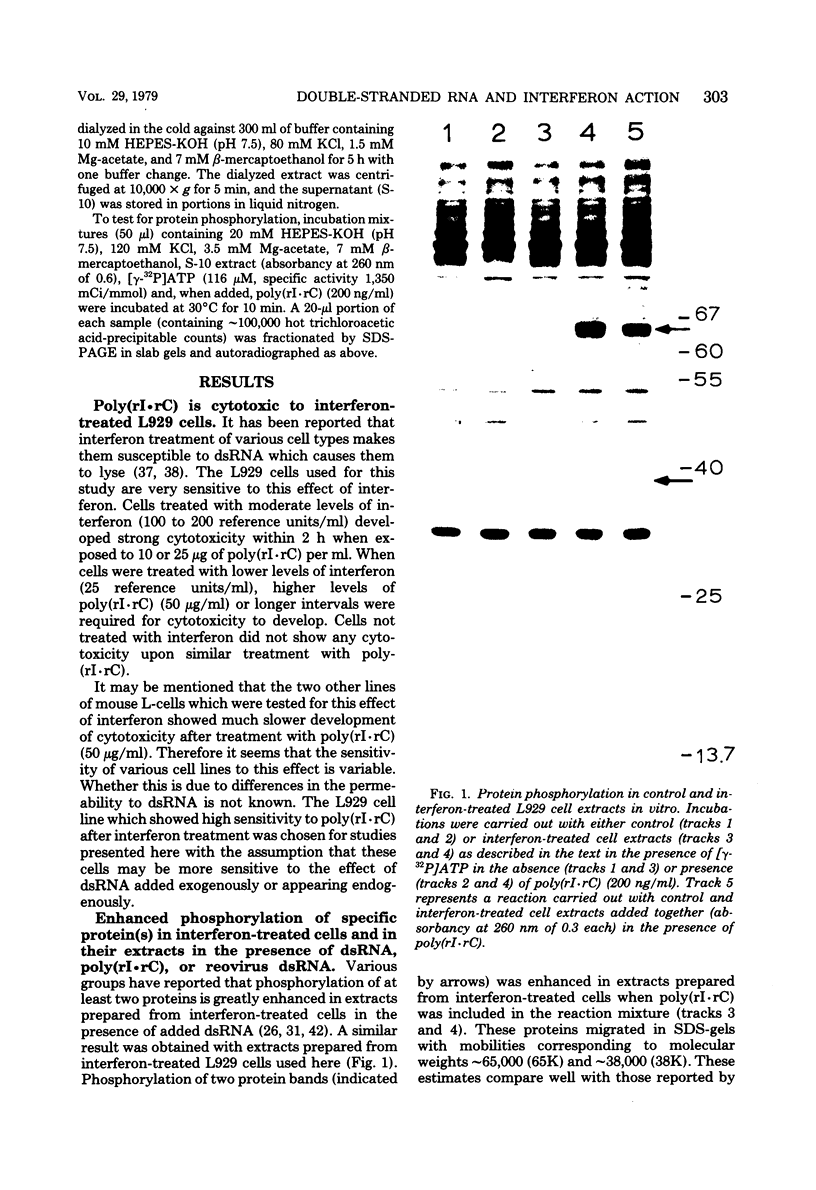
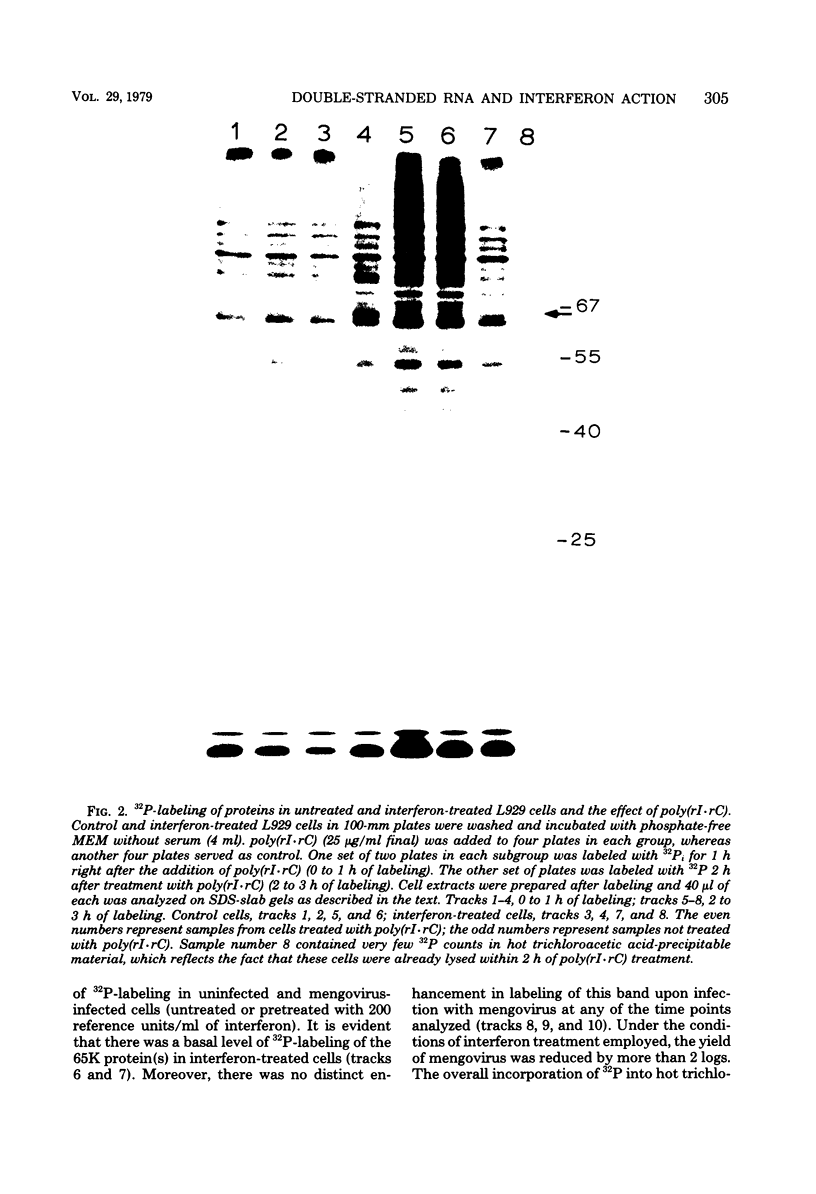
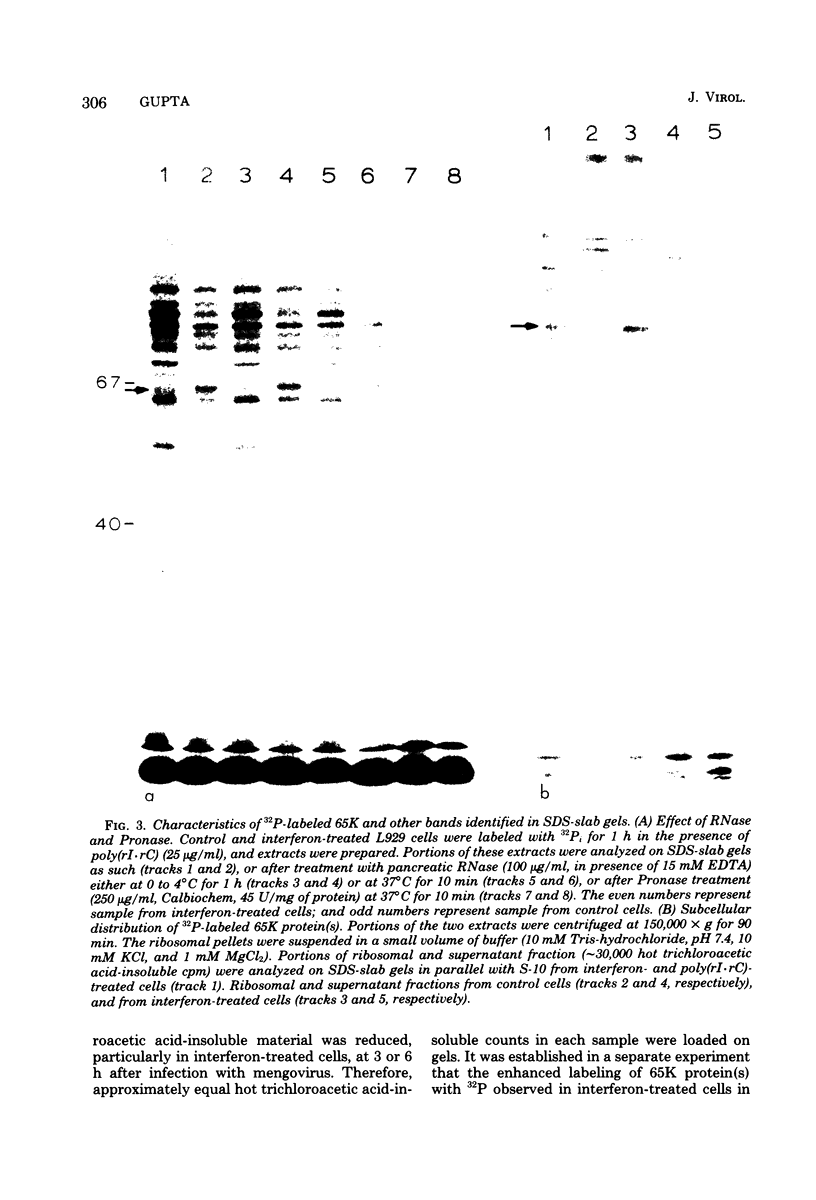
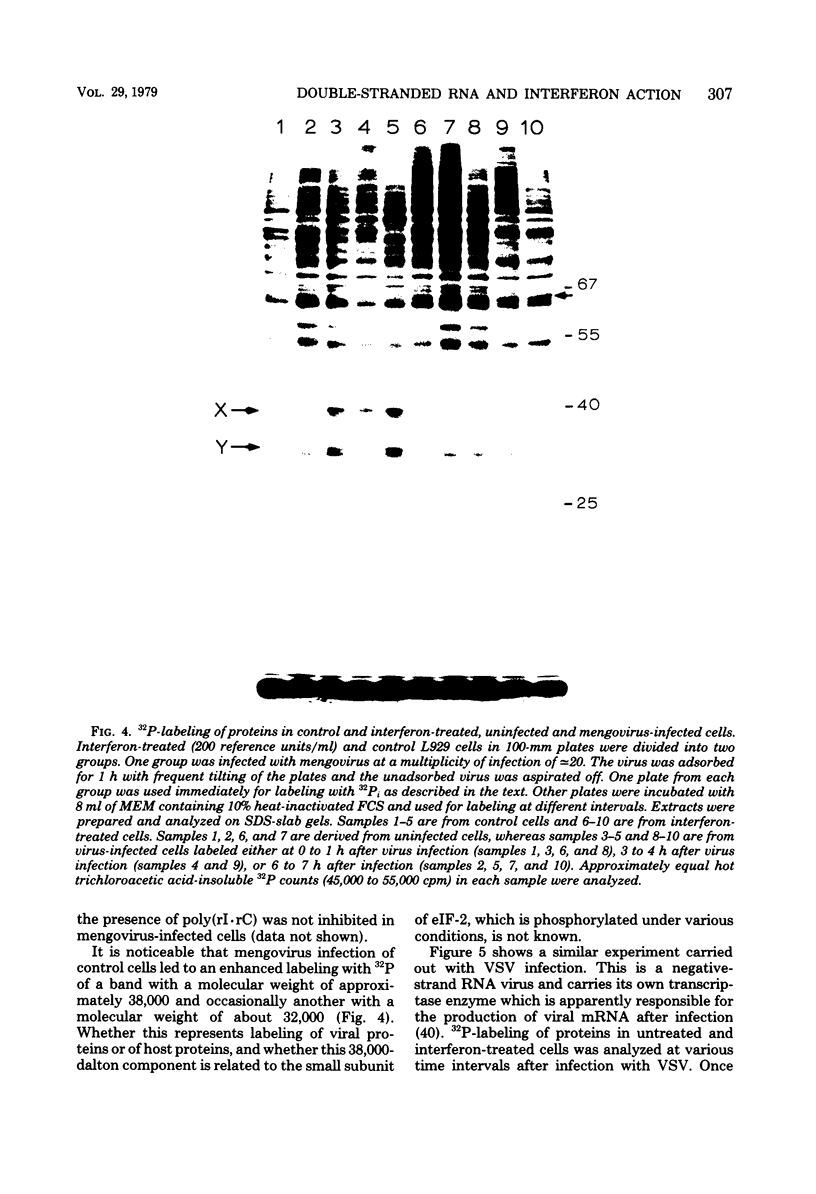
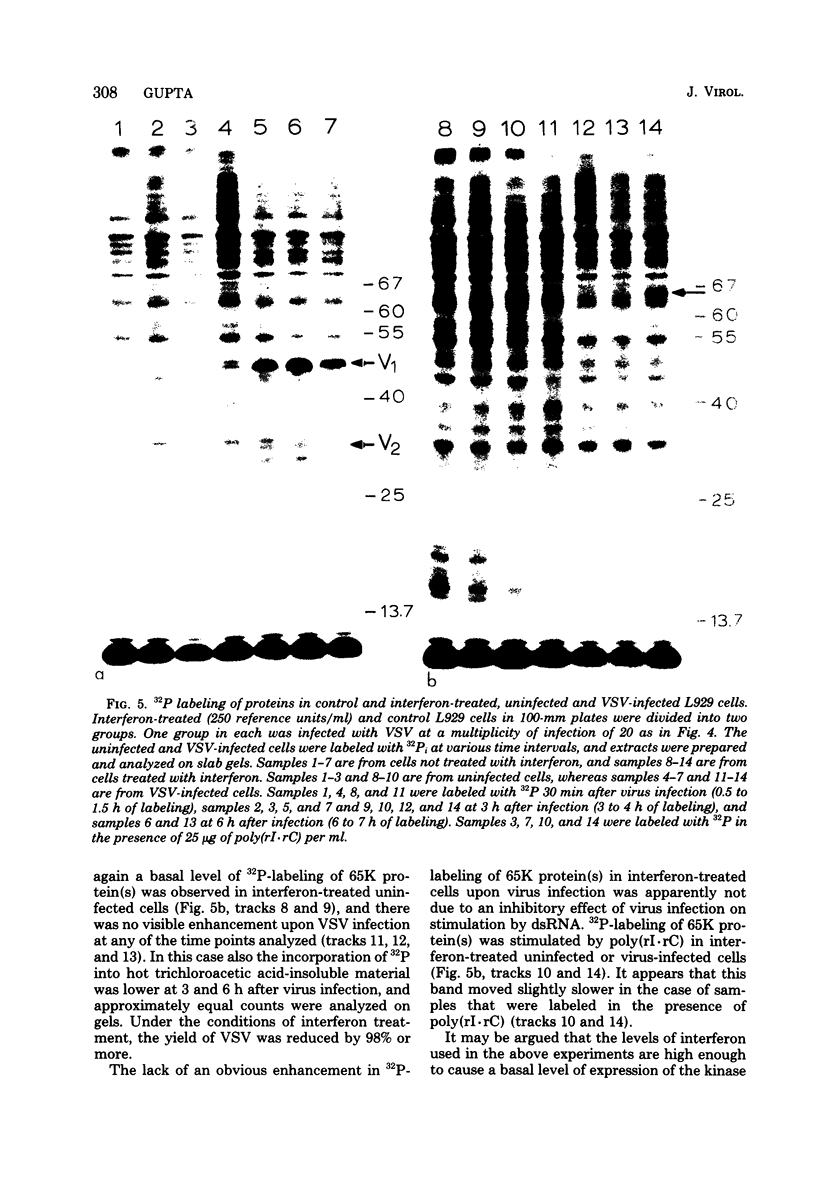
The enhanced phosphorylation of specific protein(s) observed in extracts from interferon-treated cells (in the presence of ATP and double-stranded [ds] RNA) was also seen in intact mouse L929 cells upon treatment with dsRNA, polyriboinosinic.polyribocytidylic acid [poly(rI.rC)] or reovirus dsRNA, using 32Pi as radiolabel. Labeling of a 65,000-dalton protein(s) with 32P was greatly increased in interferon-treated cells in the presence of added dsRNA, suggesting that the expression in vivo of the kinase activity involved is regulated by dsRNA. This was used as a test system to investigate whether the activity of interferon-induced enzyme(s) is stimulated following virus infection, possibly owing to the accumulation of dsRNA. No obvious increase in 32P-labeling of 65,000-dalton protein(s) was observed upon infection of interferon-treated cells with mengovirus or vesicular stomatitis virus. A basal level of 32P-labeling of the 65,000-dalton protein(s) was detected in interferon-treated cells in the absence of added dsRNA, indicating a basal level of expression of the kinase activity involved. The possible implications of these results are discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clemens M. J., Williams B. R. Inhibition of cell-free protein synthesis by pppA2'p5'A2'p5'A: a novel oligonucleotide synthesized by interferon-treated L cell extracts. Cell. 1978 Mar;13(3):565–572. doi: 10.1016/0092-8674(78)90329-x. [DOI] [PubMed] [Google Scholar]
- Colby C., Jurale C., Kates J. R. Mechanism of synthesis of vaccinia virus double-stranded ribonucleic acid in vivo and in vitro. J Virol. 1971 Jan;7(1):71–76. doi: 10.1128/jvi.7.1.71-76.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby C., Morgan M. J. Interferon induction and action. Annu Rev Microbiol. 1971;25:333–360. doi: 10.1146/annurev.mi.25.100171.002001. [DOI] [PubMed] [Google Scholar]
- Colby C., Penhoet E. E., Samuel C. E. A comparison of transfer RNA from untreated and interferon-treated murine cells. Virology. 1976 Oct 1;74(1):262–264. doi: 10.1016/0042-6822(76)90153-7. [DOI] [PubMed] [Google Scholar]
- Content J., Lebleu B., Zilberstein A., Berissi H., Revel M. Mechanism of the interferon-induced block of mRNA translation in mouse L cells: reversal of the block by transfer RNA. FEBS Lett. 1974 Apr 15;41(1):125–130. doi: 10.1016/0014-5793(74)80970-1. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr The origin of mRNA and the structure of the mammalian chromosome. Harvey Lect. 1973;(69):1–47. [PubMed] [Google Scholar]
- Falcoff E., Falcoff R., Lebleu B., Revel M. Correlation between the antiviral effect of interferon treatment and the inhibition of in vitro mRNA translation in noninfected L cells. J Virol. 1973 Sep;12(3):421–430. doi: 10.1128/jvi.12.3.421-430.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Friedman R. M. Antiviral activity of interferons. Bacteriol Rev. 1977 Sep;41(3):543–567. doi: 10.1128/br.41.3.543-567.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Metz D. H., Esteban R. M., Tovell D. R., Ball L. A., Kerr I. M. Mechanism of interferon action: inhibition of viral messenger ribonucleic acid translation in L-cell extracts. J Virol. 1972 Dec;10(6):1184–1198. doi: 10.1128/jvi.10.6.1184-1198.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. L., Graziadei W. D., 3rd, Weideli H., Sopori M. L., Lengyel P. Selective inhibition of viral protein accumulation in interferon-treated cells; nondiscriminate inhibition of the translation of added viral and cellular messenger RNAs in their extracts. Virology. 1974 Jan;57(1):49–63. doi: 10.1016/0042-6822(74)90107-x. [DOI] [PubMed] [Google Scholar]
- Gupta S. L., Sopori M. L., Lengyel P. Inhibition of protein synthesis directed by added viral and cellular messenger RNAs in extracts of interferon-treated Ehrlich ascites tumor cells. Location and dominance of the inhibitor(s). Biochem Biophys Res Commun. 1973 Sep 18;54(2):777–783. doi: 10.1016/0006-291x(73)91491-5. [DOI] [PubMed] [Google Scholar]
- Gupta S. L., Sopori M. L., Lengyel P. Release of the inhibition of messenger RNA translation in extracts of interferon-treated Ehrlich ascites tumor cells by added transfer RNA. Biochem Biophys Res Commun. 1974 Apr 8;57(3):763–770. doi: 10.1016/0006-291x(74)90612-3. [DOI] [PubMed] [Google Scholar]
- Harel L., Montagnier L. Homology of double stranded RNA from rat liver cells with cellular genome. Nat New Biol. 1971 Jan 27;229(4):106–108. doi: 10.1038/newbio229106a0. [DOI] [PubMed] [Google Scholar]
- Horak I., Jungwirth C., Bodo G. Poxvirus specific cytopathic effect in interferon-treated L cells. Virology. 1971 Aug;45(2):456–462. doi: 10.1016/0042-6822(71)90345-x. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G., Brown R. E., Kerr I. M. Synthesis of low molecular weight inhibitor of protein synthesis with enzyme from interferon-treated cells. Nature. 1977 Aug 11;268(5620):537–540. doi: 10.1038/268537a0. [DOI] [PubMed] [Google Scholar]
- Imblum R. L., Wagner R. R. Protein kinase and phosphoproteins of vesicular stomatitis virus. J Virol. 1974 Jan;13(1):113–124. doi: 10.1128/jvi.13.1.113-124.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joklik W. K., Merigan T. C. Concerning the mechanism of action of interferon. Proc Natl Acad Sci U S A. 1966 Aug;56(2):558–565. doi: 10.1073/pnas.56.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakita M., Cabrer B., Taira H., Rebello M., Slattery E., Weideli H., Lengyel P. Purification of interferon from mouse Ehrlich ascites tumor cells. J Biol Chem. 1978 Jan 25;253(2):598–602. [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E., Ball L. A. Increased sensitivity of cell-free protein synthesis to double-stranded RNA after interferon treatment. Nature. 1974 Jul 5;250(461):57–59. doi: 10.1038/250057a0. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E. pppA2'p5'A2'p5'A: an inhibitor of protein synthesis synthesized with an enzyme fraction from interferon-treated cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):256–260. doi: 10.1073/pnas.75.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramerov D. A., Ryskov A. P., Georgiev G. P. The structural organization of nuclear pre-mRNA. II. Very long double-stranded structures in nuclear pre-mRNA. Biochim Biophys Acta. 1977 Apr 4;475(3):461–475. doi: 10.1016/0005-2787(77)90062-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lebleu B., Sen G. C., Shaila S., Cabrer B., Lengyel P. Interferon, double-stranded RNA, and protein phosphorylation. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3107–3111. doi: 10.1073/pnas.73.9.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D., London I. M. Regulation of protein synthesis: activation by double-stranded RNA of a protein kinase that phosphorylates eukaryotic initiation factor 2. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1121–1125. doi: 10.1073/pnas.75.3.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer S. A., Summers D. F. Phosphorylation of vesicular stomatitis virus in vivo and in vitro. J Virol. 1974 Feb;13(2):455–465. doi: 10.1128/jvi.13.2.455-465.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L., Sen G. C., Brown G. E., Lebleu B., Kawakita M., Cabrer B., Slattery E., Lengyel P. Interferon, double-stranded RNA and RNA degradation. Characteristics of an endonuclease activity. Eur J Biochem. 1977 Oct 3;79(2):565–577. doi: 10.1111/j.1432-1033.1977.tb11841.x. [DOI] [PubMed] [Google Scholar]
- Ratner L., Wiegand R. C., Farrell P. J., Sen G. C., Cabrer B., Lengyel P. Interferon, double-stranded RNA and RNA degradation. Fractionation of the endonucleaseINT system into two macromolecular components; role of a small molecule in nuclease activation. Biochem Biophys Res Commun. 1978 Apr 14;81(3):947–954. doi: 10.1016/0006-291x(78)91443-2. [DOI] [PubMed] [Google Scholar]
- Roberts W. K., Hovanessian A., Brown R. E., Clemens M. J., Kerr I. M. Interferon-mediated protein kinase and low-molecular-weight inhibitor of protein synthesis. Nature. 1976 Dec 2;264(5585):477–480. doi: 10.1038/264477a0. [DOI] [PubMed] [Google Scholar]
- Samuel C. E., Joklik W. K. A protein synthesizing system from interferon-treated cells that discriminates between cellular and viral messenger RNAs. Virology. 1974 Apr;58(2):476–491. doi: 10.1016/0042-6822(74)90082-8. [DOI] [PubMed] [Google Scholar]
- Sen G. C., Gupta S. L., Brown G. E., Lebleu B., Rebello M. A., Lengyel P. Interferon treatment of Ehrlich ascites tumor cells: effects on exogenous mRNA translation and tRNA inactivation in the cell extract. J Virol. 1975 Jan;17(1):191–203. doi: 10.1128/jvi.17.1.191-203.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen G. C., Lebleu B., Brown G. E., Kawakita M., Slattery E., Lengyel P. Interferon, double-stranded RNA and mRNA degradation. Nature. 1976 Nov 25;264(5584):370–373. doi: 10.1038/264370a0. [DOI] [PubMed] [Google Scholar]
- Sokol F., Clark H. F. Phosphoproteins, structural components of rhabdoviruses. Virology. 1973 Mar;52(1):246–263. doi: 10.1016/0042-6822(73)90413-3. [DOI] [PubMed] [Google Scholar]
- Stern R., Friedman R. M. Ribonucleic acid synthesis in animal cells in the presence of actinomycin. Biochemistry. 1971 Sep 28;10(20):3635–3645. doi: 10.1021/bi00796a001. [DOI] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, De Clercq E., Billiau A., Desmyter J., De Somer P. Increased susceptibility of cells treated with interferon to the toxicity of polyriboinosinic-polyribocytidylic acid. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1851–1854. doi: 10.1073/pnas.69.7.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, De Clercq E., De Somer P. Specificity of interferon-induced enhancement of toxicity for double-stranded ribonucleic acids. J Gen Virol. 1973 Mar;18(3):237–246. doi: 10.1099/0022-1317-18-3-237. [DOI] [PubMed] [Google Scholar]
- Taylor J. Inhibition of interferon action by actinomycin. Biochem Biophys Res Commun. 1964;14:447–451. doi: 10.1016/0006-291x(64)90084-1. [DOI] [PubMed] [Google Scholar]
- Yakobson E., Prives C., Hartman J. R., Winocour E., Revel M. Inhibition of viral protein synthesis in monkey cells treated with interferon late in simian virus 40 lytic cycle. Cell. 1977 Sep;12(1):73–81. doi: 10.1016/0092-8674(77)90186-6. [DOI] [PubMed] [Google Scholar]
- Zilberstein A., Federman P., Shulman L., Revel M. Specific phosphorylation in vitro of a protein associated with ribosomes of interferon-treated mouse L cells. FEBS Lett. 1976 Sep 15;68(1):119–124. doi: 10.1016/0014-5793(76)80418-8. [DOI] [PubMed] [Google Scholar]