Abstract
A central question in categorization research concerns the categories that animals and humans learn naturally and well. Here, the authors examined monkeys’ and humans’ learning of the important class of exclusive-or (XOR) categories. Both species exhibited—through a sustained level of ongoing errors—substantial difficulty learning XOR category tasks at three stimulus dimensionalities. Clearly, both species brought a linear-separability constraint to XOR category learning. This constraint illuminates the primate category-learning system from which that of humans arose, and it has theoretical implications concerning the evolution of cognitive systems for categorization. The present data also clarify the role of exemplar-specific processes in fully explaining XOR category learning, and suggest that humans sometimes overcome their linear-separability constraint through the use of language and verbalization.
Keywords: categorization, learning, primates, comparative psychology, exclusive-or relation
Categorization is a basic cognitive function that has been a sharp focus in animal and human research (Ashby & Maddox, 2005; Brooks, 1978; Chase & Heinemann, 2001; Feldman, 2000; Hampton, 2006; Huber, 2001; Jitsumori, 1994; Kruschke, 1992; Lea & Ryan, 1990; Malt, 1995; Murphy, 2003; Nosofsky, 1987; Pearce, 1994; Smith & Minda, 1998; Smith, Redford, & Haas, 2008; Thompson & Oden, 2000; Vauclair, 2002; Wasserman, Kiedinger, & Bhatt, 1988). A central question in both literatures concerns the category structures that organisms find coherent and learn naturally and well.
One possible intuition is that natural, learnable categories will obey the family-resemblance (FR) principle discussed by Rosch and others (e. g., Rosch & Mervis, 1975). FR category members have variable and probabilistic similarity relationships. A pair of members will share some but not all features, and the sources of similarity will vary from pair to pair. FR categories also have a graded structure, with highly typical members central to the category’s exemplar cloud and less typical members toward the cloud’s periphery. The image of this exemplar cloud—with a typicality gradient running from its center out to its periphery—is a common one in the literature. Finally, FR categories occupy a discrete and coherent region of stimulus space, a region that can potentially be separated away from related or opposing categories. For this reason, pairs of FR categories are also sometimes described as being linearly separable (LS) from one another. There has been substantial interest in the possibility that categorizers sometimes bring an FR cognitive expectation to category problems (Ashby, 1992; Ashby & Alfonso-Reese, 1995; Ashby & Maddox, 1992; Flannagan, Fried, & Holyoak, 1986; Fried & Holyoak, 1984; Myung, 1994; Rosch & Mervis, 1975), or that they are constrained to learn easily only LS categories. This possible constraint is known as the LS constraint in category learning.
However, that humans have an LS constraint in category learning has been questioned. For example, exemplar theory hypothesizes that learners store category exemplars as separate memory representations, compare new items to these, and grant the new items category membership if they have enough within-category exemplar similarity. By this theory, learners should be free of an LS constraint in category learning. They should not need coherent, FR-based clouds of category exemplars that are separable from other category clouds. They should only need the close similarity of new items to exemplars in memory. This similarity could arise no matter how the stored exemplars were distributed through perceptual space, and even if the exemplar clouds that represented the categories were overlapping or intertwined.
In fact, influential articles have shown that humans sometimes transcend an LS constraint in category learning. Medin and Schwanenflugel (1981) noted the dominance of the FR principle in the literature, the theoretical importance of an LS constraint if it did limit the range of natural/learnable category structures, and the lack of evidence on this issue. They compared LS/NLS category learning by humans. The LS categories obeyed the FR principle and they were separable from one another. The non-linearly separable (NLS) categories did not—they contained exceptional exemplars that had to be learned separately and individually. Yet humans learned LS and NLS categories equivalently.
Medin, Altom, Edelson, and Freko (1982) focused on the most theoretically important NLS category task—the Exclusive-Or (XOR) task. In a 2-dimensional XOR task, for example, Yellow Squares (logical stimulus 00) and Blue Circles (11) would be Category A members; Yellow Circles (01) and Blue Squares (10) would be Category B members. One cannot solve this category task using color or shape. Indeed, one must place completely dissimilar stimuli into the same category. These 2-exemplar category clouds are not linearly separable from one another. They are overlapping and coincident in feature space. Yet many humans did learn XOR categories.
However, contrasting data suggests that humans have difficulty learning NLS categories. Indeed, many subjects never reach a learning criterion (e.g., Medin, Dewey, & Murphy, 1983; Medin & E. Smith, 1981; Medin & Schaffer, 1978). In complementary research, Smith and Minda (1998) showed that humans pass through an extended stage of category learning that is consistent with an LS constraint. That is, humans performed below chance on exceptional category members because they placed those items incorrectly into their FR-appropriate categories. Blair and Homa (2001) extended these findings to the influential dot-distortion paradigm (Posner, Goldsmith, & Welton, 1967; Knowlton & Squire, 1993; Homa et al., 1981; Smith & Minda, 2002). They also found an LS-constrained phase of category learning.
Cook and Smith (2006) extended this research comparatively. They gave pigeons prototype-exception tasks that were constructed using the color-disk stimuli used in the present research. Pigeons, like humans, showed a clear psychological transition from abstraction-based processing early on to exemplar-based processing later on. The same multiple-process learning progression—embodying an initial LS constraint—was found in two species with more than 100 million years of phylogenetic separation.
Smith et al. (in press) gave monkeys prototype-exception tasks instantiated using Posner’s dot-distortion materials. Again, early in learning, the categories’ general structure was abstracted, causing typical items to be categorized accurately but exception items to be categorized below chance. Monkeys demonstrated the same initial LS constraint that humans and pigeons do.
Thus, there is a mixed empirical and theoretical picture regarding LS constraints in animal and human category learning. The goal of the present research was to clarify this picture in several ways.
First, this article represents the most systematic exploration of XOR category learning by nonhuman primates, to understand better the capabilities of the primate category-learning system from which that of humans emerged.
Second, this research compares, across tasks of several dimensionalities, the XOR category learning of monkeys and humans to evaluate how far humans have progressed toward learning NLS categories easily and naturally.
Third, this article draws attention to category learnability as an issue of foraging, safety, and fitness. This perspective has not been adopted sufficiently in the literature, though it raises crucial questions. For what category problems has evolution best prepared organisms? What category problems do organisms modally face in the natural world? How good is the fit between cognitive systems for categorization and these modal requirements? In our view, considering these questions is a constructive part of describing fully a category-learning system and explaining its origins.
Fourth, this article draws attention to the standards by which we judge category learnability. Laboratory research has emphasized the ultimate learnability of categories given extensive training and the safety of the laboratory that allows indefinitely many errors. By this ultimate-learnability criterion, humans sometimes transcend an LS constraint in category learning. However, this criterion may not apply to non-laboratory situations. Organisms do not learn real-world categories in laboratory safety. They are not allowed indefinite errors—that is, multiple food poisonings or multiple predator-recognition mistakes. A category will be effectively unlearnable for them if they generally die learning it. For this reason, ultimate learnability may not be what guided the evolution of brain systems for category learning. Accordingly, this article considers the utility of a complementary learning criterion that might be called adaptive learnability.
Experiment 1: Monkeys
Experiment 1 evaluates monkeys’ learning of 2-, 3-, and 4-dimensional XOR categories. Their performance provides a reference standard to which humans’ performance is compared in Experiment 2. It also illuminates the primate category-learning system that was the likely antecedent to the human system.
Method
Participants
Rhesus monkeys (Macaca mulatta) Luke (8 years old), Han (5), and Hank (24) were tested. They had been trained, using procedures described elsewhere (Rumbaugh, Richardson, Washburn, Savage-Rumbaugh, & Hopkins, 1989; Washburn & Rumbaugh, 1992) to respond to computer-graphic stimuli by manipulating a joystick. They were tested in their home cages at the Language Research Center of Georgia State University, with ad lib access to the test apparatus, working or resting as they chose during long sessions. The animals were neither food deprived nor weight reduced for the purposes of testing and they had continuous access to water.
Apparatus
The monkeys were tested using the Language Research Center’s Computerized Test System (Washburn & Rumbaugh, 1992), comprising a computer, a digital joystick, a color monitor, and a pellet dispenser. Monkeys manipulated the joystick through the mesh of their home cages, producing isomorphic movements of a computer-graphic cursor on the screen. Contacting appropriate computer-generated stimuli with the cursor brought them a 94-mg fruit-flavored chow pellet (Bio-Serve, Frenchtown, NJ) using a Gerbrands 5120 dispenser interfaced to the computer through a relay box and output board (PIO-12 and ERA-01; Keithley Instruments, Cleveland, OH). Correct responses were accompanied by a computer-generated whooping sound that bridged the monkeys to their reward. On incorrect responses, the screen froze with the wrong response visible, and there was a computer-generated buzzing sound and a 20 s timeout. The monkeys were experienced with the general methods and response modality of computer-based, joystick-controlled tasks. They had previously participated in numerous discrimination tasks. They had not participated in discrimination tasks using the color-disk stimuli used here.
Categorization stimuli
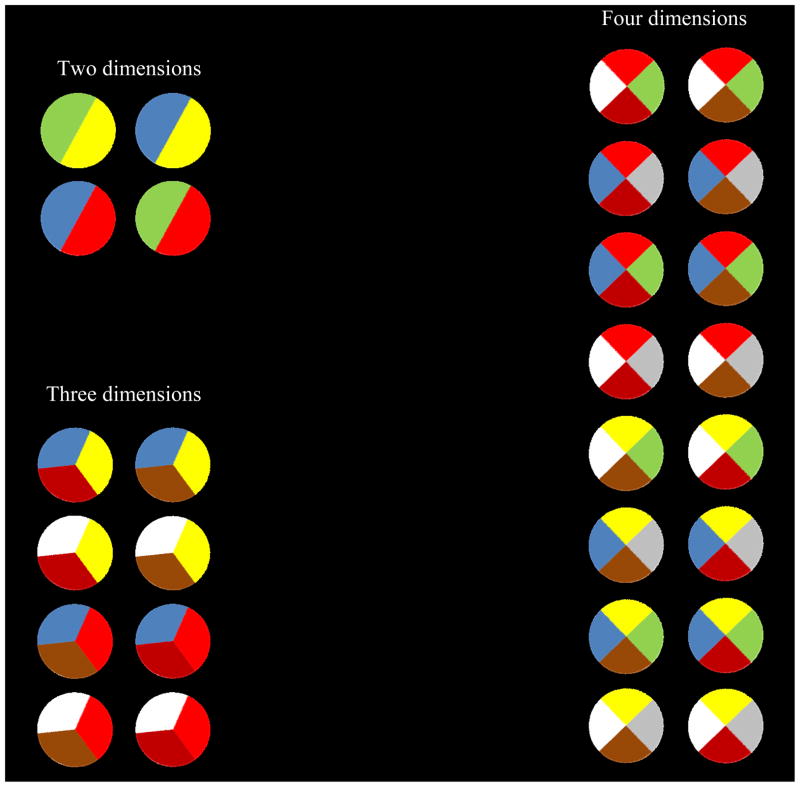
The stimuli were multi-colored circles divided into two 180° sectors, three 120° sectors, or four 90° sectors (Figure 1).
Figure 1.
The present article’s 2-, 3-, and 4-dimensional XOR categories illustrated. Category A and B stimuli are in the left and right column in each task. In the three tasks, respectively, Category A members had both red and blue sectors or neither, both red and brown sectors or neither, and both yellow and brown sectors or neither.
Categorization tasks
Table 1 describes the XOR tasks abstractly. Figure 1 concretizes these descriptions. The table’s dimensional columns would map to particular sectors in the figure’s disks. The 0s and 1s in the table signify that each sector could take on one of two colors. In Figure 1’s 2-dimensional task, the two sectors were green/blue or yellow/red. For all tasks, Category A members were defined by having either both or none of the critical XOR colors, whereas Category B members were defined by having only one critical color. In Figure 1’s 2-dimensional task, the critical XOR colors were blue and red.
Table 1.
Abstract Descriptions of 2-, 3-, and 4-Dimensional XOR Tasks.
| Category | D1 | D2 | D3 | D4 | Category | D1 | D2 | D3 | D4 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 Dimensions | ||||||||||
| A | 0 | 0 | B | 0 | 1 | |||||
| A | 1 | 1 | B | 1 | 0 | |||||
| 3 Dimensions | ||||||||||
| A | 0 | 0 | 0 | B | 0 | 1 | 0 | |||
| A | 0 | 0 | 1 | B | 0 | 1 | 1 | |||
| A | 1 | 1 | 0 | B | 1 | 0 | 0 | |||
| A | 1 | 1 | 1 | B | 1 | 0 | 1 | |||
| 4 Dimensions | ||||||||||
| A | 0 | 0 | 0 | 0 | B | 0 | 1 | 0 | 0 | |
| A | 0 | 0 | 0 | 1 | B | 0 | 1 | 0 | 1 | |
| A | 0 | 0 | 1 | 0 | B | 0 | 1 | 1 | 0 | |
| A | 0 | 0 | 1 | 1 | B | 0 | 1 | 1 | 1 | |
| A | 1 | 1 | 0 | 0 | B | 1 | 0 | 0 | 0 | |
| A | 1 | 1 | 0 | 1 | B | 1 | 0 | 0 | 1 | |
| A | 1 | 1 | 1 | 0 | B | 1 | 0 | 1 | 0 | |
| A | 1 | 1 | 1 | 1 | B | 1 | 0 | 1 | 1 | |
In the table, the XOR relation is always instantiated by the first two dimensional columns. In actuality, the 3- and 4-dimensional XOR tasks were run with the XOR relation variably instantiated. Luke completed the 3-, 4-, and 2-dimensional tasks, in that order, with the XOR relations instantiated using Dimensions 2–3, 1–3, and 1–2. Han completed the 2-, 3-, and 4-dimensional tasks, in that order, with the XOR relations instantiated using Dimensions 1–2, 1–3, and 2–3. Hank completed the 4-, 2-, and 3-dimensional tasks, in that order, with the XOR relations instantiated using Dimensions 1–4, 1–2, and 1–2. Table 1 shows that dimensions not involved in the XOR relation carried no category-relevant information. For different monkeys we used different color palates to produce a range of concrete tasks.
Note that XOR tasks are qualitatively different from the polymorphous (N out of M) categories used elegantly by others (e.g., Depy, Fagot, & Vauclair, 1997; Huber, 2001; Jitsumori, 1994). In polymorphous tasks, all features partially predict category membership, category members share an overall family resemblance, one can add up feature presences to decide category memberships (e.g., a 2 of 3 rule), and the categories are linearly separable from one another. In contrast, in XOR tasks single features do not predict category membership, category members do not share family resemblance, one cannot add up feature presences to decide category membership, and thus the categories are not linearly separable from one another. XOR tasks pose a theoretically crucial category problem because they require some kind of exemplar-based strategy or the discovery of a logical, rule-based solution.
Categorization trials
Each trial presented a to-be-categorized disk in the screen’s top center against a black background. Below each disk appeared screen icons (A,B) representing monkeys’ response options. Monkeys used the joystick-controlled cursor to select a response by touching an icon.
Trials were run in successive random permutations of the 4, 8, and 16 stimuli in the 2-, 3-, and 4-dimensional tasks. These permutations were presented with no break or punctuation that would have made them apparent to monkeys.
Categorization sessions
The monkeys completed 576-trial sessions, which represented 144 4-trial blocks (2-dimensional task), 72 8-trial blocks (3-dimensional task), and 36 16-trial-blocks (4-dimensional task). They received 10 sessions (5,760 trials) with each of three XOR tasks. Han’s 6th 3-dimensional session ran short by 32 trials because his session time expired at that point.
Results
The data were analyzed in 96-trial blocks (24, 12, and 6 trial blocks for the 2-, 3-, and 4-dimensional XOR tasks). The data were entered into SAS’s General Linear Model (GLM) procedure, with dimensionality and trial block as within-subject factors.
Monkeys achieved only modest overall proportions correct. They were .784, .685, and .617 correct overall in the 2-, 3-, and 4-dimensional XOR tasks, respectively. Because of the monkeys’ highly variable performances, this difference across dimensions was not significant, F (2, 4) = 3.640, MSerr = 0.350, ns. This performance variability underscores the difficulty they had in fully mastering XOR tasks.
As a converging window on dimensionality and task difficulty, we asked when monkeys reached a performance threshold of .75 on their tasks. This was an arbitrary level to reflect some general acquisition. Other threshold levels would produce the same general pattern. Monkeys reached a threshold of .75 correct performance after 16 96-trial blocks (1,536 trials) and 24 96-trial blocks (2,304 trials) in the 2- and 3- dimensional XOR tasks, respectively. Monkeys never reached the .75 threshold in a 96-trial block in the 4-dimensional XOR task, even after 5,760 trials.
There was an effect of trial block, F (59, 118) = 13.312, MSerr = 003, p < .0001. In the 2-, 3-, and 4-dimensional tasks, respectively, monkeys progressed from .573, .517, and .458 in their first block to .830, .764, and .656 in their last block. Substantial learning occurred in the XOR task at all dimensionalities, though Table 2 shows that the monkeys learned slowly in all three tasks.
Table 2.
Proportion correct achieved by 3 monkeys (M) performing 2-, 3-, and 4-dimensional (D) XOR tasks in their first 96-trial block and in every 5th 96-trial block (1–60).
| M | D | 1 | 5 | 10 | 15 | 20 | 25 | 30 | 35 | 40 | 45 | 50 | 55 | 60 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Luke | 2 | .521 | .635 | .719 | .625 | .708 | .771 | .917 | .917 | .927 | .844 | .594 | .698 | .750 |
| Luke | 3 | .500 | .500 | .510 | .500 | .760 | .698 | .698 | .583 | .823 | .854 | .823 | .823 | .875 |
| Luke | 4 | .552 | .656 | .594 | .594 | .510 | .667 | .677 | .635 | .698 | .615 | .563 | .615 | .531 |
| Han | 2 | .479 | .521 | .458 | .677 | .729 | .635 | .615 | .719 | .781 | .792 | .760 | .802 | .760 |
| Han | 3 | .583 | .625 | .708 | .656 | .635 | .677 | .875 | .625 | .573 | .708 | .719 | .573 | .719 |
| Han | 4 | .406 | .635 | .573 | .583 | .698 | .729 | .750 | .740 | .750 | .750 | .656 | .604 | .750 |
| Hank | 2 | .719 | .698 | .760 | .927 | .990 | .917 | .969 | 1.00 | .969 | .979 | .958 | .979 | .979 |
| Hank | 3 | .469 | .698 | .656 | .698 | .729 | .615 | .635 | .750 | .750 | .719 | .771 | .750 | .698 |
| Hank | 4 | .417 | .458 | .479 | .552 | .531 | .594 | .729 | .594 | .677 | .635 | .542 | .677 | .688 |
We also explored the process behind monkeys’ XOR-task learning. Their performance improvements might be associative. That is, stimulus features, feature combinations, or whole stimuli might come to trigger correct responses more often through the catalysis of positive reinforcement signals. If associations were gradually strengthening, monkeys should approach solution slowly with gently sloped acquisition curves. Another possibility, though, is that monkeys might at some point suddenly realize the XOR rule that solves the task. Rule discovery would produce a steeply accelerating acquisition curve. These possibilities motivated Zeaman and House’s (1963) analysis of children’s discrimination learning, Smith, Tracy, & Murray’s (1993) analysis of category learning during depression, and Shepard, Hovland, and Jenkins’s (1961) and Feldman’s (2000) surveys of diverse category problems.
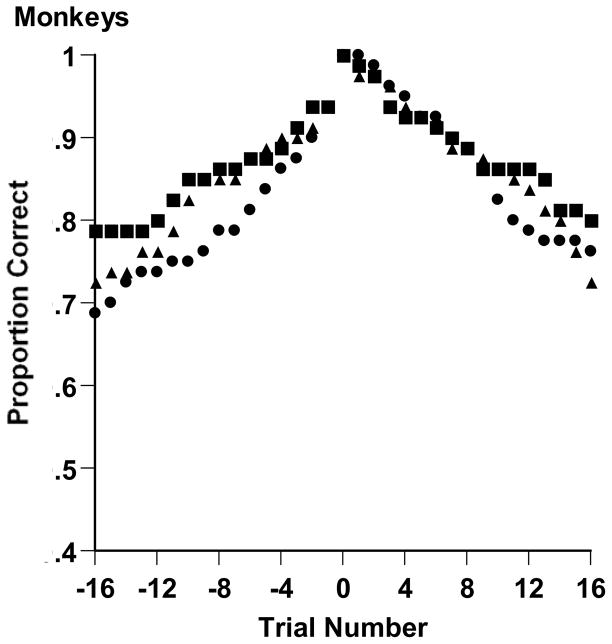
To distinguish these possibilities, we plotted backward learning curves for the monkeys’ 2-dimensional XOR tasks as follows. Backward learning curves align the criterion runs of subjects and summarize performance before or after that point. To produce backward learning curves, we found the first five instances in which each animal completed an errorless run of 16 trials in his two-dimensional task. We aligned the five arrivals at this criterion point for each animal at Trial 0. We plotted average performance backward and forward from those arrival points, with the performance level always summarizing performance over the preceding 16 trials. The crucial result (Figure 2) is that monkeys’ performance after the criterion runs brought them back down to about the level from which they began the runs. Their solutions did not stick. The one exception to this is that Hank mainly sustained his solution in the two-dimensional XOR task after about Trial 1,400 (Table 2, Block 15). Thus, nearly all the seeming “criterion runs” were actually just occasional binomial runs of successes occurring on some baseline of moderate performance. Accordingly, there is every indication that monkeys brought gradually strengthening associations and conditioning processes, but not rule discovery, to their XOR task solutions. Figure 2’s analysis of the two-dimensional XOR task is conservative, because it focused on the simplest 2-dimensional XOR task that required the fewest exemplar-response associations for successful performance.
Figure 2.
Backward learning curves for monkeys Luke (circles), Han (triangles), and Hank (squares) performing in the 2-dimensional XOR task of Experiment 1. Each curve shows the average results from 5 epochs within which the monkey completed a run of 16 consecutive correct trials. The 5 arrivals at this criterion point were aligned at Trial 0. Performance is shown before and after that arrival point. Each data point summarizes performance over the preceding 16 trials.
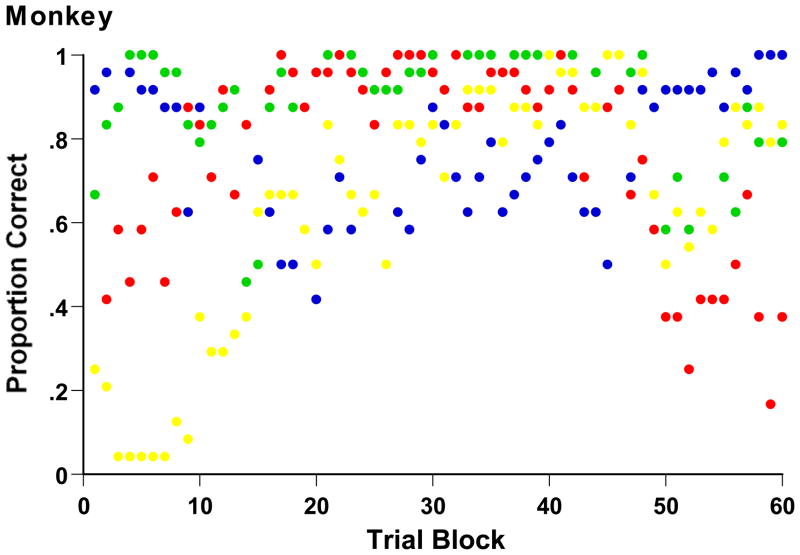
Figure 3 shows Luke’s full learning history in his 2-dimensional XOR task. The figure illustrates the learning dynamics that are generally evident in the data. Early on, the monkey’s Category A bias produced near-perfect performance on Stimuli A1 (logical stimulus 00, green symbols) and A2 (11, blue symbols), exact perceptual opposites, and near-zero performance on Stimulus B1 (01, yellow symbols). During this period, there was some exemplar learning of Stimulus B2 (10, red symbols). Next, the animal demonstrated strong mastery of Stimulus A1 (00) and B2 (10), indicating sharp attention to the first stimulus dimension that differentiates these stimuli. That reliance produced poor (chance) performance on the other stimuli in the task, though evidently some exemplar learning protected the monkey from below-chance performance on those items. Last, the animal mastered Stimulus A2 (11) to a high level, with the cost of very poor performance on Stimulus B3 (10). This indicates sharp attention to the first stimulus dimension, but now with logical value 1 assigned to Category A.
Figure 3.
Performance of Monkey Luke in his 2-dimensional XOR task, showing his percentage correct on each exemplar separately during each 96-trial block of the task. Green, Blue, Yellow, and Red symbols, respectively correspond to Stimulus A1 (logical stimulus 0 0), A2 (1 1), B1 (0 1), and B2 (1 0).
One probably sees in this learning narrative both whole-exemplar and single-feature response associations. One also sees in Figure 3 an animal that never completely mastered the simplest possible XOR task. To the contrary, there is a persistent complementarity to performance (some exemplars rise and others fall) that reveals a strong LS constraint in category learning.
Discussion
These XOR category problems were very difficult for monkeys to learn. Monkeys had 22%, 32%, and 38% error rates in their three tasks that produced 3,731, 5,452, and 6,608 20-s penalties. Even their terminal performance levels were modest. The experiment’s principal conclusion is that monkeys have a severe LS constraint in the category structures that they find naturally or easily learnable. Smith, Minda, and Washburn (2004) suggested this conclusion based on exploring one XOR task at one dimensionality. The present results allow this conclusion to be generalized to XOR tasks at several dimensionalities and probably to all XOR tasks.
These results suggest limits on the power or tuning sharpness of the associative system that monkeys brought to bear on these tasks. The capacity of this associative system to learn categorization tasks of this kind was surprisingly weak. This limitation is a factor to be considered in understanding monkeys’ overall categorization competence.
A common assumption would be that XOR performance predominantly depends on exemplar-memorization or exemplar-generalization processes. On adopting this assumption, one would conclude that monkeys do not remember exemplars well enough or categorize using them sensitively enough to master XOR tasks. From this perspective, the article evaluates negatively the exemplar system that monkeys bring to XOR tasks. Note that this negative evaluation is neither a rejection of exemplar processes in category learning nor a critique of exemplar theory. To the contrary, this article endorses that monkeys use exemplar processes in category tasks, while pointing out that they have significant limits on their ability to do so.
This negative evaluation would not imply that animals are generally poor exemplar memorizers. To the contrary, diverse species have demonstrated great competence in memorizing responses to hundreds or thousands of different pictorial stimuli (e.g., Cook & Fagot, 2009; Fagot & Cook, 2006). The narrower question under consideration here is the extent to which exemplar storage, exemplar recognition, and exemplar-response association serve correct categorization performance when the stimuli lie within a multidimensional, binary-feature space. The present results show that exemplar processes only provide insufficient and incomplete support to category learning in XOR tasks that involve NLS pairs of categories.
From an ecological perspective, viewing category learnability as an issue of foraging, safety, and fitness, this weakness and the ongoing errors could well be prohibitive. That is, XOR categories of substantial dimensionality would likely be adaptively unuseable by foraging monkeys. To our knowledge, this foraging reference standard has never been applied to the problem of XOR category learning, and doing so sheds a new light on monkeys facing this class of category problem and on the kinds of category structures that monkeys find naturally learnable. Monkeys had a severe constraint in learning the present XOR category problems. We believe this constraint would characterize their real-world, ecological categorization as well.
Some may question this extrapolation to nature and fitness, asking why monkeys’ categorization of laboratory colored disks should extend to their real-world categorization of natural kinds. One possible concern is that the color-disk stimuli are inherently difficult to differentiate and learn about. But this is not true. These stimuli were used in LS category tasks with other macaques by Couchman, Coutinho, and Smith (in press). The monkeys received new category tasks with new color palates each session. They sustained performance at 92.3% and 85.2%, respectively, over the 1,440 testing trials in each session. Most strikingly, they learned each new task in only about 100 trials. Color-disk stimuli were also categorized easily by pigeons in Cook and Smith (2006). The stimuli were no obstacle to learning here.
Another question is whether laboratory XOR tasks have ecological validity regarding XOR category problems in the natural world. The XOR task, in its laboratory or ecological forms, logically requires that exact perceptual opposites be treated as psychological equivalents, while closer perceptual similarities are treated contrastively (Table 1). There is no apparent reason why it matters whether the underlying features are sectors of color, or leaf shape and color, or fruit color and size, and so forth. In fact, in the history of experimental psychology, it has nearly always been the case that ecological cognitive processes and principles turn out to closely mirror the processes and principles established using experimental materials in the laboratory (Banaji & Crowder, 1989; Neisser, 1991; Roediger, 1991; Tulving, 1991).
A third possible concern is that XOR tasks have impoverished similarity structure and minimal family-resemblance within category, making them inherently difficult to learn. But this is not a concern. This is an important point of the article. The XOR task is a premier task in the literature. It provides the most compelling evidence favoring exemplar-based categorization processes, because it so elegantly precludes prototype-based processes. Thus, it is striking that monkeys in this premier task showed that they could not adaptively memorize just a few items that repeated hundreds of times. Of course this kind of failure would have serious consequences in their natural ecology.
Experiment 2: Humans
Experiment 2 evaluates humans’ performance in the same XOR tasks so that the performance of the two species can be compared. This comparison illuminates—in humans’ performance—the extent to which they have advanced quantitatively or qualitatively beyond the primate system of category learning.
Method
Participants
One hundred and eight undergraduates from the University at Buffalo, The State University of New York, participated in a session lasting about an hour to fulfill a course requirement. Our participant pool contained slightly more women (55%-45%). Participants were in their late teens or early twenties with apparently normal or corrected-to-normal visual acuity. The approximate racial mix of our participant pool was 67% Caucasian, 16% Asian, 7% African-American, and 10% Other. As a tool to increase performance motivation, the top scorers were awarded $10 cash prizes.
Categorization stimuli, tasks, trials
Humans were tested in a research carrel with a computer work station. Stimuli, tasks, and trials were produced and presented as already described (Table 1, Figure 1). The 3-dimensional task was run in counterbalanced fashion with the XOR relation instantiated by dimensions 1–2, 1–3, and 2–3. The 4-dimensional task was run in counterbalanced fashion with the XOR relation instantiated by dimensions 1–2, 1–3, 1–4, 2–3, 2–4, and 3–4. Three different color–pair systems were also run in counterbalanced fashion for the task at each dimensionality.
Humans responded by pressing labeled keyboard keys that mirrored the spatial layout of the response icons on the screen. Participants received a point and heard a 0.5 s computer-generated reward whoop for each correct response. They heard a 1-s computer-generated penalty buzz and lost a point for each error. In addition, after each trial, participants received a scorecard textbox on the screen that gave them a +1 or −1 for the trial and gave them their current point total. After the feedback, the screen cleared and the next trial was presented.
Humans completed, in the 2-, 3-, and 4-dimensional tasks, 64 blocks (256 trials), 48 blocks (384 trials), and 40 blocks (640 trials), respectively. These increases in task length with task dimensionality incorporated our assumption that the tasks would grow in difficulty and in the trials needed for humans to show robust category learning. Each participant completed one of the three tasks.
Instructions
Humans received the following instructions for their category tasks. “In this experiment you will learn about two groups of multicolored circles. Your task is to learn how to place each circle you see into its correct category. These were sparse and non-directive instructions relative to the existing human category-learning literature. Monkeys, based on their extensive experience with computer tasks, certainly entered their tasks with the equivalent knowledge that they should correctly assign one of two responses to the category stimuli. Even if the instructions somehow changed humans’ cognitive set or focused their attention in some way, this would only suggest that the humans would have performed more like monkeys without instructions. Therefore, the instructions—if anything—make conservative the results and conclusions that follow.
Results
The data from each participant were analyzed in 16-trial blocks (4, 2, and 1 trial blocks for the 2-, 3-, and 4-dimensional tasks). The data were entered into SAS’s GLM procedure with task dimensionality and trial block as between- and within-subject factors, respectively. There was an effect of task dimensionality, F (2, 105) = 33.834, MSerr = .211, p < .001. Humans were .909, .829, and .846 correct overall on the 2-, 3-, and 4-dimensional tasks. Difficulty increased for the higher-dimensionality tasks.
There was also an effect of trial block, F (39, 2695) = 135.474, MSerr = .011, p < .001. In the 2-, 3-, and 4-dimensional tasks, respectively, humans progressed from .528, .476, and .462 in their first block to .995, .983, and .981 in their last block. Substantial XOR learning occurred at all dimensionalities. By the criterion of ultimate learnability discussed above, humans did not have an LS constraint in solving XOR problems.
Finally, there was a dimensionality by block interaction, F (38, 2695) = 5.287, MSerr = .011, p < .001. Humans reached .90 correct performance after 80 trials, 208 trials, and 288 trials in the 2-, 3-, and 4-dimensional XOR tasks, respectively. Humans learned more slowly as the dimensionality of the XOR problem increased. Figure 4 illustrates and summarizes these results.
Figure 4.
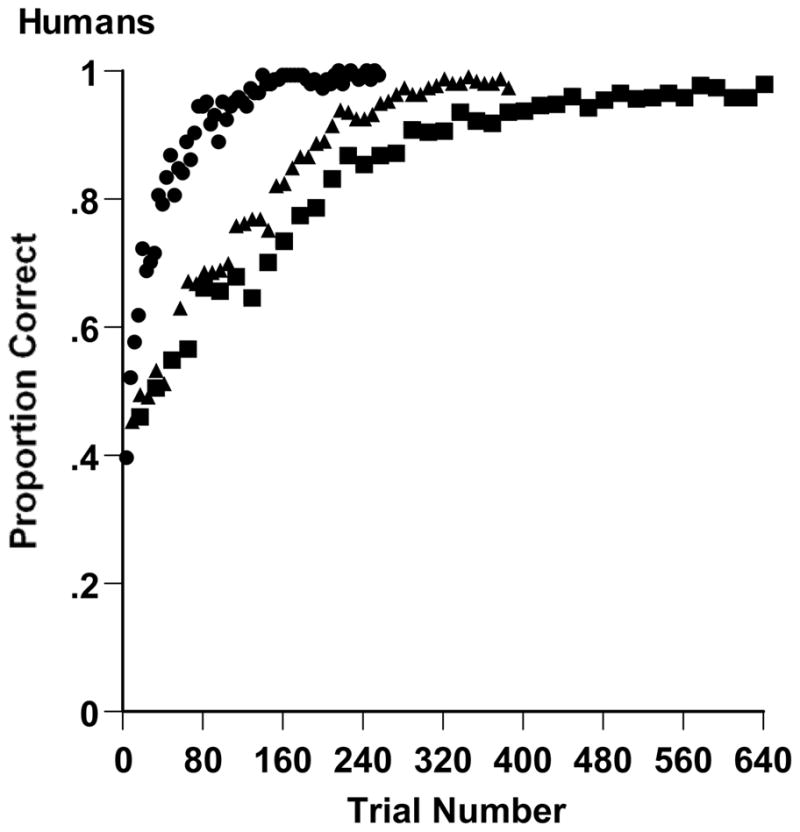
Proportion correct achieved over trials by humans learning 2-dimensional (circles), 3-dimensional (triangles), and 4-dimensional (squares) XOR tasks in Experiment 2.
Overall, these XOR category problems were surprisingly difficult for humans to learn. On average, it took participants 74, 178, and 236 trials, respectively, to achieve 16 consecutive correct responses in the 2-, 3-, and 4-dimensional tasks. (Unlike the blocked analyses just reported, these 16-trial runs could span trial blocks.) On average, participants made 21, 59, and 89 errors before making that criterion run.
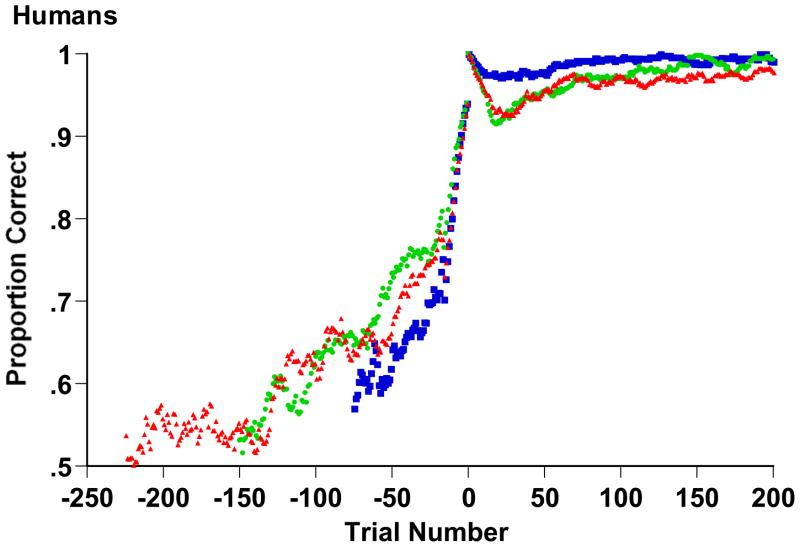
We used backward learning curves again to explore the process behind humans’ XOR-task solutions by pinpointing the moment of solution. These curves (Figure 5) were calculated as for monkeys except that we examined only humans’ first and crucial 16-trial run. The cognitive reorganization that produced the criterion run was sudden in all three cases. Errors suddenly ceased. The cognitive reorganization was lasting, too. The solution stuck and participants sustained performance at high levels thereafter.
Figure 5.
Backward learning curves for humans in Experiment 2’s 2-dimensional (blue symbols), 3-dimensional (green), and 4-dimensional (red) XOR tasks. Humans completed a run of 16 consecutive correct trials at Trial 0. In essence, they ceased making errors at trial -16, the start of the criterion run. Each data point summarizes performance over the preceding 16 trials.
This result suggests that humans used a process of rule discovery in the XOR task that produced their sudden and lasting correct performance. This conclusion is consistent with the conclusion of Shepard et al. (1961), Feldman (2000), Love (2002), and Smith et al. (2004), which was that humans discover and apply rules or reportable Boolean expressions for category problems of this kind rather than gradually associating responses with cues and rather than assigning category labels in a way that depends on stimulus generalization among similar stimuli. Other interpretations of this sudden performance shift are far less plausible. In fact, the principal existing categorization models (prototype models, exemplar models, etc.) have no natural way to predict or explain such a sudden performance shift.
Nonetheless, our results are consistent with exemplar learning prior to rule discovery. The early performance levels shown in Figure 5 are illuminating. Even after hundreds of trials, the performance levels were low and similar to those achieved by monkeys at mature performance. These levels suggest that humans’ associative or exemplar system was learning slowly and was in difficulty on the task (like monkeys’ equivalent system). Evidently, the capacity of the associative or exemplar system to learn XOR tasks is surprisingly weak in both species. This is an important result in the human literature because it restricts the comprehensiveness of the role that exemplar processes play in XOR learning. If humans lacked a rule-discovery process, XOR problems would be difficult to learn, just as they were for monkeys who lacked that rule-discovery process. Once again, this negative evaluation does not deny exemplar processes in categorization or reject exemplar theory. To the contrary, it is likely that humans used whole-exemplar processes early in learning, though we are also pointing out the limits on their ability to do so. We emphasize that we are considering the narrower issue of the exemplar processes that support category learning. Humans’ difficulty in categorizing by exemplars in the present tasks would not need to be one of encoding. The difficulty could arise in discriminating similar exemplars or in correctly mapping contrasting responses to similar stimuli. In other domains such as memory, humans, like pigeons and baboons, demonstrate highly sensitive and lasting exemplar encoding (Standing, 1973; Standing, Conezio, & Haber, 1970).
General Discussion
We examined humans’ and monkeys’ XOR category learning at three stimulus dimensionalities. Monkeys exhibited severe difficulty learning these tasks, making thousands of errors on the way to modest terminal performance.
One theoretical implication of this result is that nonhuman primates bring a severe LS constraint to learning XOR categories. Another implication concerns the exemplar process that is generally presumed to underlie the acquisition of XOR category relations. The idea behind exemplar theory is that participants store exemplars as separate, individuated memory representations. If monkeys used that exemplar system here, as they may well have done, it was clearly slow-learning and insensitive as a process supporting categorization. Hundreds of repetitions of just a few exemplars were still insufficient to let feedback signals produce strong exemplar-based categorization.
Smith et al. (in press) provide complementary support for these conclusions. Following on Smith and Minda (1998) and Blair and Homa (2001), they studied monkeys’ category learning when categories had exception items that did not fit the categories’ general FR structure. They found an initial stage of category learning that embodied an LS constraint and a strong resistance to the learning of exceptions. They also concluded that monkeys’ exemplar processes were weak and insensitive supports to categorization. This conclusion has profound implications in considering the appropriate theoretical framework for describing primates’ category learning.
The present conclusions may even extend more broadly to mammalian or vertebrate categorization systems. Cook and Smith (2006, also Wasserman et al., 1988) showed that pigeons also brought FR-based initial approaches to category tasks, had the same resistance to learning exceptional category members, and thus had the same LS constraint in category learning that humans and macaques do.
Humans also had substantial difficulty learning XOR tasks of higher dimensionality. They made scores of errors over hundreds of trials in achieving robust performance. Their early low performance levels were reminiscent of the monkeys’ acquisition curves, and may reflect humans’ use of similar early learning processes. These processes reveal an LS-constrained stage of category learning that fared poorly in the acquisition of XOR relations. If the early processes were exemplar based, then they reveal for humans the exemplar insensitivity that monkeys’ similar processes revealed.
For this reason, the human results bear on the status of exemplar processes in explaining humans’ categorization behavior. Humans’ learning of XOR categories has been held up as strong support for the idea that exemplar-based processes are what let humans transcend LS constraints in category learning. However, the present results suggest that humans do not finally, fully learn XOR categories by exemplar-based learning. Rather, humans’ XOR category learning supports the existence of a separate, declarative, rule-based category system that can transcend humans’ LS constraint. This support is separate from any support that XOR tasks provide for exemplar theory.
The conclusion about humans’ weak exemplar system explains extensive human categorization data. Humans often have great difficulty learning NLS categories. For example, in several classic articles, 30% up to 72% of participants failed to meet the learning criterion in different experiments (Medin & Schwanenflugel, 1981; Medin et al., 1983; Medin & E. Smith, 1981; Medin & Schaffer, 1978). Even those who met the criterion achieved only about 80% terminal performance.
Clearly, though, humans finally and suddenly achieved strong XOR performance—probably through a process of hypothesis testing and rule discovery. These results are consonant with research showing the power of humans’ conceptual systems when they self-construe or self-create categories or psychological equivalence classes (e.g., Barsalou, 1991; Malt & Sloman, 2007; Murphy & Medin, 1985). These results are also consonant with the multiple-systems theoretical perspective that has become an important part of the human categorization literature (Ashby & Ell, 2001; Ashby et al., 1998; Cook & Smith, 2006; Erickson & Kruschke, 1998; Homa et al., 1981; Love et al., 2004; Minda & Smith, 2001, Rosseel, 2002; Smith & Minda, 1998). The multiple-systems perspective distinguishes analytic, rule-based processes—that depend on working memory and executive attention and that provide conscious access and declarative reports of solutions to category problems—from nonanalytic, multidimensional processes that learn slowly, non-consciously, and non-declaratively to map responses to regions of perceptual space. Humans probably revealed in the present tasks another dissociation between these category-learning systems in the early and late stages of acquisition. In a sense, the monkeys did too, because they demonstrated the lack of a rule-based system that could finally, fully solve XOR problems.
It is interesting to consider when in phylogenetic development humans developed their rule-discovery system that was the profound difference here between their performances and those of the monkeys. This consideration might incorporate language as a vehicle for holding rules and hypotheses in mind, frontal areas as a brain-structural prerequisite for declarative rule use, or consciousness as conferring an ability to notice and deliberately pursue suspected environmental regularities. This consideration might also incorporate changes in the landscape of category problems, toward those with more NLS and XOR character, as complex social relationships, artifact categories, and the communication of logic and reasoning became more prominent.
However, it is equally important that humans, monkeys, and pigeons—spanning more than 100 million years of evolutionary separation—share an underlying LS constraint in category learning. This suggests the working hypothesis that primate, mammalian, or vertebrate systems of category learning are entrained to the category structures that have been featured in the natural histories of those species. If one examines monkeys’ important ecological categories (e.g., vervet monkeys, Cheney & Seyfarth, 1990), one sees that all of them (eagles, snakes, leopards, etc.) are FR categories in the sense described by Rosch and Mervis (1975). The LS constraint and insensitive exemplar processing as elements in their category-learning system are naturally explained if they reflect that cognitive evolution has granted privilege to a natural FR structure that organisms have often experienced.
In contrast, we know of no true XOR categories in the natural lives of nonhuman primates. That is, it seems never to be the case that a preferred food source is dimorphic (producing palatable orange, three-lobed berries and palatable blue, four-lobed berries) whereas an avoided food source is XOR dimorphic (producing toxic orange, four-lobed berries and toxic blue, three-lobed berries). It is a graceful and grateful state of nature that the genetics of biological kinds—fruits, nuts, and predators—creates well-behaved FR categories, not systematic, featural, XOR double-crosses. Indeed, even in cases of species mimicry, it is clear that mimicry functions to let one species benefit from another’s LS categorization imperative. It is adaptive to perceptually resemble a toxic moth or a poisonous snake because it felicitously allows inclusion within a to-be-avoided FR category.
Thus, the present research and discussion offer a simple organizing idea that encompasses a depth and breadth of the natural history of category learning. The idea is that natural kinds featured FR principles over hundreds of millions of years, and that through the cognitive shaping accomplished by this common affordance, FR category learning became the privileged, default process for many species. Even humans clearly give FR categories privilege in naming (Rosch et al., 1976) and in cognitive/language development (Anglin, 1970; Berlin, Breedlove, & Raven, 1973; Brown, 1958). Given this privilege, one would predict an LS constraint, poor XOR learning, the early systematic misclassification of exception items, and a weak exemplar system that would not be critically needed in an FR-dominant world. Then, in one or a few species, the ancestral category-learning system was augmented by an executive, rule-based system that transcends LS constraints and supports more robust XOR learning.
We understand that the human categorization literature has contained a long-lived, compelling narrative that says that FR categories are not especially natural, are not especially learnable, and that humans do not have an LS constraint in category learning. This narrative arose fairly from elegant research showing that humans—given extensive training and unlimited error safety—do not have an LS constraint by the criterion of the ultimate learnability of NLS categories. However, the alternative narrative expressed here—spanning more species, more data, and more evolutionary time—has received almost no theoretical attention. The underlying theoretical goal of this article is to allow these two narratives to be considered in parallel and in relation to one another as comparative research on animal and human categorization develops further.
Acknowledgments
The preparation of this article was supported by Grant HD-38051 from the National Institute of Child Health and Human Development
Contributor Information
J. David Smith, Department of Psychology and Center for Cognitive Science, University at Buffalo, The State University of New York.
Mariana V. C. Coutinho, Department of Psychology, University at Buffalo, The State University of New York
Justin J. Couchman, Department of Psychology, University at Buffalo, The State University of New York
References
- Anglin . Word, object, and conceptual development. New York: Norton; 1977. [Google Scholar]
- Ashby FG. Multidimensional models of categorization. In: Ashby FG, editor. Multidimensional models of perception and cognition. Hillsdale, NJ USA: Erlbaum; 1992. pp. 449–483. [Google Scholar]
- Ashby FG, Maddox WT. Human category learning. Annual Review of Psychology. 2005;56:149–178. doi: 10.1146/annurev.psych.56.091103.070217. [DOI] [PubMed] [Google Scholar]
- Ashby FG. Multidimensional models of categorization. In: Ashby FG, editor. Multidimensional models of perception and cognition. Hillsdale, NJ: Erlbaum; 1992. pp. 449–483. [Google Scholar]
- Ashby FG, Alfonso-Reese LA. Categorization as probability density estimation. Journal of Mathematical Psychology. 1995;39:216–233. [Google Scholar]
- Ashby FG, Alfonso-Reese LA, Turken AU, Waldron EM. A neuropsychological theory of multiple systems in category learning. Psychological Review. 1998;105:442–481. doi: 10.1037/0033-295x.105.3.442. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Ell SW. The neurobiology of human category learning. Trends in Cognitive Science. 2001;5:204–210. doi: 10.1016/s1364-6613(00)01624-7. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Maddox WT. Human category learning. Annual Review of Psychology. 2005;56:149–178. doi: 10.1146/annurev.psych.56.091103.070217. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Maddox WT. Complex decision rules in categorization: Contrasting novice and experienced performance. Journal of Experimental Psychology: Human Perception and Performance. 1992;18:50–71. [Google Scholar]
- Banaji MR, Crowder RG. On the bankruptcy of everyday memory. American Psychologist. 1989;44:1185–1193. [Google Scholar]
- Barsalou LW. Deriving categories to achieve goals. In: Bower GH, editor. The Psychology of Learning and Motivation. Vol. 27. New York: Academic Press; 1991. pp. 1–64. [Google Scholar]
- Berlin B, Breedlove DE, Raven PH. General principles of classification and nomenclature in folk biology. American Anthropology. 1973;75:214–242. [Google Scholar]
- Blair M, Homa D. Expanding the search for a linear separability constraint on category learning. Memory & Cognition. 2001;29:1153–1164. doi: 10.3758/bf03206385. [DOI] [PubMed] [Google Scholar]
- Blair M, Homa D. As easy to memorize as they are to classify: The 5–4 categories and the category advantage. Memory & Cognition. 2003;31:1293–1301. doi: 10.3758/bf03195812. [DOI] [PubMed] [Google Scholar]
- Brooks LR. Nonanalytic concept formation and memory for instances. In: Rosch E, Lloyd BB, editors. Cognition and categorization. Hillsdale, NJ: Erlbaum; 1978. pp. 169–211. [Google Scholar]
- Brown R. How shall a thing be called? Psychological Review. 1958;65:14–21. doi: 10.1037/h0041727. [DOI] [PubMed] [Google Scholar]
- Chase S, Heinemann EG. Exemplar memory and discrimination. Cook RG, editor. Avian visual cognition. 2001 [On-line]. www.pigeon.psy.tufts.edu/avc/chase/
- Cheney DL, Seyfarth RM. How monkeys see the world. Chicago: University of Chicago Press; 1990. [Google Scholar]
- Cook RG, Fagot J. First trial rewards promote 1-trial learning and prolonged memory in pigeon and baboon. Proceedings of the National Academy of Sciences of the United States of America. 2009 Jun;106:9530–9533. doi: 10.1073/pnas.0903378106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RG, Smith JD. Stages of abstraction and exemplar memorization in pigeon category learning. Psychological Science. 2006;17:1059 –1067. doi: 10.1111/j.1467-9280.2006.01833.x. [DOI] [PubMed] [Google Scholar]
- Couchman JJ, Coutinho MVC, Smith JD. Rules and resemblance: Their changing balance in the category learning of humans (Homo sapiens) and monkeys (Macaca mulatta) Journal of Experimental Psychology: Animal Behavior Processes. doi: 10.1037/a0016748. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depy D, Fagot J, Vauclair J. Categorisation of three-dimensional stimuli by humans and baboons: search for prototype effects. Behavioural Processes. 1997;39:299–306. doi: 10.1016/s0376-6357(96)00757-7. [DOI] [PubMed] [Google Scholar]
- Erickson MA, Kruschke JK. Rules and exemplars in category learning. Journal of Experimental Psychology: General. 1998;127:107–140. doi: 10.1037//0096-3445.127.2.107. [DOI] [PubMed] [Google Scholar]
- Fagot J, Cook RG. Evidence for large long-term memory capacities in baboons and pigeons and its implications for learning and the evolution of cognition. Proceedings of the National Academy of Sciences of the United States of America. 2006 Nov;103:17564–17567. doi: 10.1073/pnas.0605184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman J. Minimization of Boolean complexity in human concept learning. Nature. 2000 Oct;407:630–633. doi: 10.1038/35036586. [DOI] [PubMed] [Google Scholar]
- Flannagan MJ, Fried LS, Holyoak KJ. Distributional expectations and the induction of category structure. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1986;12:241–256. doi: 10.1037//0278-7393.12.2.241. [DOI] [PubMed] [Google Scholar]
- Fried LS, Holyoak KJ. Induction of category distributions: A framework for classification learning. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1984;10:234–257. doi: 10.1037//0278-7393.10.2.234. [DOI] [PubMed] [Google Scholar]
- Hampton JA. Concepts as prototypes. In: Ross BH, editor. The Psychology of Learning and Motivation: Advances in Research and Theory. Vol. 46. 2006. pp. 79–113. [Google Scholar]
- Homa D, Sterling S, Trepel L. Limitations of exemplar-based generalization and the abstraction of categorical information. Journal of Experimental Psychology: Human Learning and Memory. 1981;7:418–439. doi: 10.1037//0278-7393.10.4.638. [DOI] [PubMed] [Google Scholar]
- Huber L. Visual categorization in pigeons. In: Cook R, editor. Avian visual cognition. Medford, MA: Robert Cook and Comparative Cognition Press; 2001. [On-line]. www.pigeon.psy.tufts.edu/avc/huber/ [Google Scholar]
- Jitsumori M. Discrimination of artificial polymorphous categories in humans and nonhumans. In: Hayes SC, Hayes LJ, Sato M, Ono K, editors. Behavior analysis of language and cognition. Washington, DC USA: APA; 1994. pp. 91–106. [Google Scholar]
- Knowlton BJ, Squire LR. The learning of categories: Parallel brain systems for item memory and category knowledge. Science. 1993;262:1747–1749. doi: 10.1126/science.8259522. [DOI] [PubMed] [Google Scholar]
- Kruschke JK. ALCOVE: An exemplar-based connectionist model of category learning. Psychological Review. 1992;99:22–44. doi: 10.1037/0033-295x.99.1.22. [DOI] [PubMed] [Google Scholar]
- Lea SEG, Ryan CME. Unnatural concepts and the theory of concept discrimination in birds. In: Commons ML, Herrnstein RJ, Kosslyn SM, Mumford DB, editors. Quantitative analyses of behavior. VIII. Hillsdale, NJ USA: Erlbaum; 1990. pp. 165–185. [Google Scholar]
- Love BC. Comparing supervised and unsupervised category learning. Psychonomic Bulletin and Review. 2002;9:829–835. doi: 10.3758/bf03196342. [DOI] [PubMed] [Google Scholar]
- Love BC, Medin DL, Gureckis TM. SUSTAIN: A network model of category learning. Psychological Review. 2004;111:309–332. doi: 10.1037/0033-295X.111.2.309. [DOI] [PubMed] [Google Scholar]
- Malt BC. Category coherence in cross cultural perspective. Cognitive Psychology. 1995;29:85–148. [Google Scholar]
- Malt BC, Sloman SA. Artifact categorization: The good, the bad, and the ugly. In: Margolis E, Laurence S, editors. Creations of the Mind: Theories of Artifacts and Their Representation. Oxford University Press; 2007. [Google Scholar]
- Medin DL, Altom MW, Edelson SM, Freko D. Correlated symptoms and simulated medical classification. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1982;8:37–50. doi: 10.1037//0278-7393.8.1.37. [DOI] [PubMed] [Google Scholar]
- Medin DL, Schaffer MM. Context theory of classification learning. Psychological Review. 1978;85:207–238. [Google Scholar]
- Medin DL, Schwanenflugel PJ. Linear separability in human classification learning. Journal of Experimental Psychology: Human Learning & Memory. 1981;7:355–368. [Google Scholar]
- Medin DL, Dewey GI, Murphy TD. Relationships between item and category learning: Evidence that categorization is not automatic. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1983;9:607–625. [Google Scholar]
- Medin DL, Smith EE. Strategies and classification learning. Journal of Experimental Psychology: Human Learning and Memory. 1981;7:241–253. [Google Scholar]
- Minda JP, Smith JD. Prototypes in category learning: The effects of category size, category structure, and stimulus complexity. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27:775–799. [PubMed] [Google Scholar]
- Murphy GL. The big book of concepts. Cambridge, MA: MIT Press; 2003. [Google Scholar]
- Murphy GL, Medin DL. The role of theories in conceptual coherence. Psychological Review. 1985;92:289–316. [PubMed] [Google Scholar]
- Myung IJ. Maximum entropy interpretation of decision bound and context models of categorization. Journal of Mathematical Psychology. 1994;38:335–365. [Google Scholar]
- Neisser U. A case of misplaced nostalgia. American Psychologist. 1991;46:84–86. [Google Scholar]
- Nosofsky RM. Attention and learning processes in the identification and categorization of integral stimuli. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1987;13:87–108. doi: 10.1037//0278-7393.13.1.87. [DOI] [PubMed] [Google Scholar]
- Pearce JM. Discrimination and categorization. In: Mackintosh NJ, editor. Animal learning and cognition. New York: Academic Press; 1994. pp. 109–134. [Google Scholar]
- Posner MI, Goldsmith R, Welton KE. Perceived distance and the classification of distorted patterns. Journal of Experimental Psychology. 1967;73:28–38. doi: 10.1037/h0024135. [DOI] [PubMed] [Google Scholar]
- Roediger HL. They read an article? A commentary on the everyday memory controversy. American Psychologist. 1991;46:37–40. [Google Scholar]
- Rosch E, Mervis CB. Family resemblances: Studies in the internal structure of categories. Cognitive Psychology. 1975;7:573–605. [Google Scholar]
- Rosch E, Mervis CB, Gray WD, Johnson DM, Boyes-Braem P. Basic objects in natural categories. Cognitive Psychology. 1976;8:382–439. [Google Scholar]
- Rosseel Y. Mixture models of categorization. Journal of Mathematical Psychology. 2002;46:178–210. [Google Scholar]
- Rumbaugh DM, Richardson WK, Washburn DA, Savage-Rumbaugh ES, Hopkins WD. Rhesus monkeys (Macaca mulatta), video tasks, and implications for stimulus-response spatial contiguity. Journal of Comparative Psychology. 1989;103:32–38. doi: 10.1037/0735-7036.103.1.32. [DOI] [PubMed] [Google Scholar]
- Shepard RN, Hovland CI, Jenkins HM. Learning and memorization of classifications. Journal of Experimental Psychology. 1961;65:94–102. doi: 10.1037/h0043732. [DOI] [PubMed] [Google Scholar]
- Smith JD, Chapman WP, Redford JS. Stages of category learning in monkeys (Macaca mulatta) and humans (Homo sapiens) Journal of Experimental Psychology: Animal Behavior Processes. doi: 10.1037/a0016573. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Minda JP. Prototypes in the mist: The early epochs of category learning. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1998;24:1411–1436. [Google Scholar]
- Smith JD, Minda JP. Distinguishing prototype-based and exemplar-based processes in category learning. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2002;28:800–811. [PubMed] [Google Scholar]
- Smith JD, Minda JP, Washburn DA. Category learning in rhesus monkeys: A study of the Shepard, Hovland, and Jenkins tasks. Journal of Experimental Psychology: General. 2004;133:398–414. doi: 10.1037/0096-3445.133.3.398. [DOI] [PubMed] [Google Scholar]
- Smith JD, Redford JS, Haas SM. Prototype abstraction by monkeys (Macaca mulatta) Journal of Experimental Psychology: General. 2008;137:390–401. doi: 10.1037/0096-3445.137.2.390. [DOI] [PubMed] [Google Scholar]
- Smith JD, Tracy J, Murray MJ. Depression and categorization. Journal of Experimental Psychology: General. 1993;122:331–346. doi: 10.1037//0096-3445.122.3.331. [DOI] [PubMed] [Google Scholar]
- Standing L. Learning 10,000 pictures. Quarterly Journal of Experimental Psychology. 1973;25:207–222. doi: 10.1080/14640747308400340. [DOI] [PubMed] [Google Scholar]
- Standing L, Conezio J, Haber RN. Perception and memory for pictures: Single-trial learning of 2500 visual stimuli. Psychonomic Science. 1970;19:73–74. [Google Scholar]
- Thompson RKR, Oden DL. Categorical perception and conceptual judgments by nonhuman primates: The paleological monkey and the analogical ape. Cognitive Science. 2000;24:363–396. [Google Scholar]
- Tulving E. Memory research is not a zero-sum game. American Psychologist. 1991;46:41–42. [Google Scholar]
- Vauclair J. Categorization and conceptional behavior in nonhuman primates. In: Bekoff M, Allen C, editors. The cognitive animal: Empirical and theoretical perspectives on animal cognition. Cambridge, MA: MIT Press; 2002. pp. 239–245. [Google Scholar]
- Washburn DA, Rumbaugh DM. Testing primates with joystick-based automated apparatus: Lessons from the Language Research Center’s Computerized Test System. Behavior Research Methods, Instruments, and Computers. 1992;24:157–164. doi: 10.3758/bf03203490. [DOI] [PubMed] [Google Scholar]
- Wasserman EA, Kiedinger RE, Bhatt RS. Conceptual behavior in pigeons: categories, subcategories, and pseudocategories. Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:235–246. [Google Scholar]
- Zeaman D, House BJ. The role of attention in retardate discrimination learning. In: Ellis NR, editor. Handbook of mental deficiency. New York: McGraw-Hill; 1963. pp. 159–223. [Google Scholar]