Abstract
Current theories of human categorization differentiate an explicit, rule-based system of category learning from an implicit system that slowly associates regions of perceptual space with response outputs. The researchers extended this theoretical differentiation to the category learning of New World primates. Four capuchins learned categories of circular sine-wave gratings that varied in bar spatial frequency and orientation. The rule-based and information-integration tasks, respectively, had one-dimensional and two-dimensional solutions. Capuchins, like humans, strongly dimensionalized the stimuli and learned the rule-based task more easily. The results strengthen the suggestion that nonhuman primates have some structural components of humans’ capacity for explicit categorization, which in humans is linked to declarative cognition and consciousness. The results also strengthen the primate contrast to other vertebrate species that may lack the explicit system. Therefore, the results raise important questions about the origins of the explicit categorization system during cognitive evolution and about its overall phylogenetic distribution.
Keywords: explicit categorization, primate cognition, comparative cognition, category learning, capuchin monkeys
1. Introduction
Learning and using categories—behavioral or psychological equivalence classes—is a basic cognitive function for animals and humans. For example, predator categories confer a fitness advantage by allowing predator recognition and avoidance. For this reason, categorization is a sharp focus in animal research (e.g., Herrnstein, Loveland, & Cable, 1976; Jitsumori, 1994; Lea & Wills, 2008; Lazareva & Wasserman, 2010; Pearce, 1994; Smith, Redford, & Haas; 2008; Thompson & Oden, 2000; Vauclair, 2002) and human research (e.g., Ashby & Maddox, in press; Brooks, 1978; Feldman, 2000; Knowlton & Squire, 1993; Medin & Schaffer, 1978; Murphy, 2003; Nosofsky, 1987; Rosch & Mervis, 1975; Smith & Minda, 1998).
Categorization is apparently an important enough capacity to receive redundant expression within cognition. Researchers have described several interactions and tradeoffs among different representational systems in categorization. For example, different processes dominate categorization at early and late stages of category learning (Cook & Smith, 2006; Reed, 1978; Smith, Chapman, & Redford, 2010; Wasserman et al. 1988), when categories have small or large exemplar-set sizes (Blair & Homa, 2003; Homa, Sterling, & Trepel, 1981; Minda & Smith, 2001), and when the categorization rule is easy or difficult to describe verbally (Ashby & Maddox, 2005).
Based on these interactions and tradeoffs, there is a growing consensus in the human categorization literature that a comprehensive description of categorization requires a multiple-system theoretical perspective that grants humans multiple categorization capacities that specialize in different aspects of category learning (Ashby, Alfonso-Reese, Turken, & Waldron, 1998; Ashby & Ell, 2001; Erickson & Kruschke, 1998; Homa et al., 1981; Minda & Smith, 2001; Rosseel, 2002; Smith & Minda, 1998). The multiple-system perspective has promoted rapid theoretical development within the human literature, but it has barely been extended to comparative categorization research (Herbranson, Fremouw, & Shimp, 1999; Smith, Beran, Crossley, Boomer, Ashby, 2010; Smith, Ashby, et al., 2011). One goal of the present research is to further this extension.
An influential multiple-system theory (Ashby et al., 1998; Ashby & Ell, 2001; Ashby, Ennis, & Spiering, 2007) distinguishes implicit and explicit categorization systems. The implicit system is thought to derive response outputs using nonanalytic, multidimensional processes that learn slowly to map responses to general regions of perceptual space. The explicit system is thought to derive explicit dimensional rules using analytic, often unidimensional processes that depend on working memory and executive attention (see also, Miles and Minda, 2011).
Supporting the implicit-explicit distinction, Brooks (1978) found that incidental and intentional categorizations by humans were, respectively, nonanalytic and analytic. Kemler Nelson (1984) found that incidental and intentional category learners, respectively, solved category problems using multi-dimensional similarity or single-dimensional rules. Other researchers have shown that a cognitive load (Waldron & Ashby, 2001) or depression (Smith, Tracy, & Murray, 1993) leave multi-dimensional category learning intact while disrupting rule-based category learning.
The implicit-explicit distinction is also grounded in cognitive neuroscience. Humans’ implicit/nonanalytic system probably relies on the striatum and is based on the reinforcement-mediated strengthening of dopamine-related synapses (Ashby et al., 1998; Ashby, Ennis, & Spiering, 2007). Humans’ explicit/analytic system probably relies on a broad neural network that includes the anterior cingulate gyrus, prefrontal cortex, the head of the caudate nucleus, and medial temporal lobe structures that also serve declarative memory. This system is also related to the neural complex that affords the executive control of attention (Rossi, Pessoa, Desimone, & Ungerleider, 2009).
Evidence for these dissociable learning systems comes from rule-based (RB) and information-integration (II) category tasks as shown in Figure 1. The exemplars in the tasks illustrated are circular sine-wave gratings that vary in the spatial frequency and orientation of the bars. In the present research, there are 300 exemplars for each category in each task. Figure 1 shows only a few illustrative stimuli. The participant must learn to make correct Category A or B decisions through the successive presentation of single exemplars with feedback following each stimulus-response pair.
Figure 1.
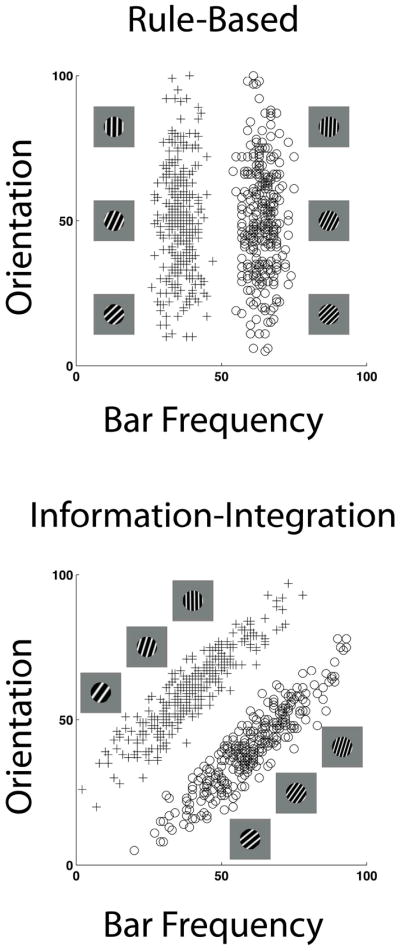
Examples of rule-based and information-integration category structures. The stimuli are sine-wave disks varying in bar spatial frequency and orientation. For each task, three illustrative Category A and Category B stimuli are provided. In addition, the open circles and pluses illustrate the distribution of the experiment’s stimuli as represented in an abstract space. The text specifies how these abstract values were converted into physically realized stimuli. The pluses and circles, respectively, are Category A and Category B exemplars. In the top panel, only variation in bar frequency carries diagnostic category information, so optimal performance would be governed by a one-dimensional, bar-frequency rule (narrowly vs. widely spaced bars). In the lower panel, bar frequency and orientation carry useful but insufficient category information—information from both dimensions would have to be integrated into a category decision.
In Figure 1 (top), the vertical category boundary shows that only bar frequency carries information that supports a category decision. Bar orientation varies equivalently across its whole range within both categories. This is an example of an analytic, rule-based task because the category bound can be discovered through stimulus analysis and a unidimensional rule that is easily verbalized (narrowly vs. widely spaced bars).
In contrast, in Figure 1 (bottom), the diagonal category boundary shows that bar frequency and bar orientation carry useful category information, but that neither carries sufficient category information. Here the participant must learn to integrate the information offered by both stimulus dimensions to make a correct category decision, and accuracy will be maximized only to the extent that this multidimensional integration is successful. There is no simple way to verbally describe this category boundary.
The RB and II tasks are matched in category size, within-category exemplar similarity, between-category exemplar separation, the a priori perceptual difficulty of the categorization problem, and the maximum proportion correct achievable by an ideal observer. The tasks represent a strong mutually-controlling pair within cognitive science, because they differ only in the analytic-nonanalytic/unidimensional-bidimensional aspects that are crucial to their theoretical framework and to the present research.
Humans show contrastive behavioral profiles within RB and II tasks (Ashby & Maddox, 2005, 2010). They strongly dimensionalize these stimuli in the sense of treating the dimensions analytically and separably. They learn RB category tasks quickly through explicit reasoning and rule-based processes. They declare verbally their task solution. In contrast, humans integrate poorly across dimensions within II tasks. They learn II category tasks more slowly. They cannot describe their solution verbally.
These different behavioral profiles raise many comparative or cross-species questions. Is the multiple-system, implicit-explicit organization uniquely human? Is the explicit system dependent on language and verbal rules, or on propositional/logical mental representations that could be languageless and that might be possessed by animals as well as humans? What was the phylogenetic origin of these multiple category systems during cognitive evolution, and what is the phylogenetic depth of explicit categorization in particular? What does the phylogenetic map of the multiple-system organization look like, and what is the phylogenetic breadth of explicit categorization in particular? Is it a human thing, an ape thing, a primate thing, a mammal thing, a vertebrate thing?
These comparative questions highlight the inferential power of the matched and diagnostic RB and II tasks. These tasks support the evaluation of rule-based and nonanalytic task solutions within a controlled and well-understood empirical framework. By rotating the dimensional axis of category tasks, from II to RB, one can ask whether the minds of different species are dimensionally polarized. If so, then the dimensional task orientation will admit strong and rapid learning, just as a polarizing filter will strongly admit light when it finds the axis of the light’s polarization. Human minds are dimensionalized in this way. What about the minds of other species?
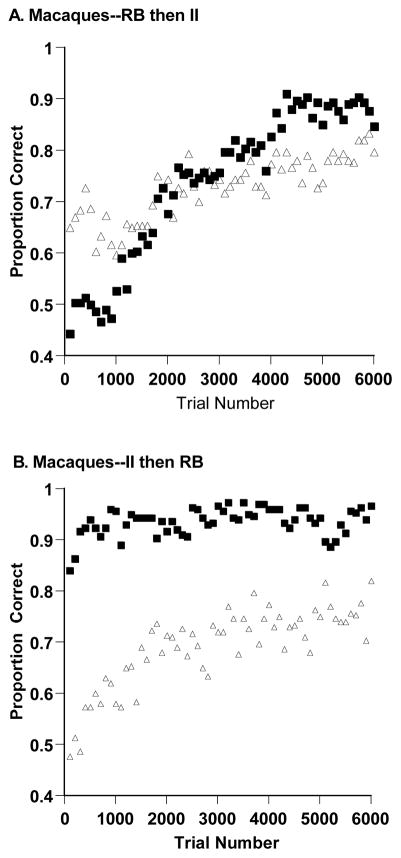
In Smith et al. (2010), a group of six rhesus macaques (Macaca mulatta) participated in matched RB and II category-learning tasks with learning order counterbalanced. As is true for humans, macaques strongly dimensionalized the frequency-orientation stimuli shown in Figure 1 and learned the RB task more quickly. Figure 2 shows that this result was obtained whether the RB task was experienced first or second. These results demonstrated an important empirical continuity between human and nonhuman primate cognition. They suggest that nonhuman primates may have some structural components of humans’ capacity for explicit cognition, though of course not necessarily all of those components. They suggest preliminary answers to some of the comparative questions posed above.
Figure 2.
A. Proportion of correct responses in each trial block for three macaques who performed 6,000 trials of a rule-based (RB) and information-integration (II) category task in that order. B. Proportion of correct responses in each 100-trial block for three macaques who performed 6,000 trials of an II and RB category task in that order. Square and triangle symbols, respectively, denote performance in RB and II tasks.
In Smith et al. (2011), two large groups of pigeons participated in matched RB and II category-learning tasks with learning order counterbalanced. These pigeons were run in laboratories in New Zealand (NZ-Canterbury; R. Grace) and the United States (USA-Tufts, R. Cook). Whereas humans strongly dimensionalize these stimuli and learn RB tasks more quickly than II tasks, pigeons learned the two tasks equally quickly to the same level. These results were obtained despite differences in experimental procedures across the two laboratories, underscoring the convergence and robustness of the common findings. Smith et al. suggested that pigeons illustrate a cognitive system in which the commitment to dimensional analysis and category rules was not strongly made, and that pigeons’ performance reflects the character of the ancestral vertebrate categorization system from which that of primates emerged.
The primary empirical purpose of this article is to illuminate further the distribution across the vertebrates of a multiple-system organization to categorization. Macaques, as Old World primates, cannot alone support the inference that the implicit-explicit organization is broadly represented within the primate order. To the contrary, there is growing evidence that Old World and New World primates may differ sharply along just the dimension of explicit-to-implicit cognition that is at issue within the present article. For example, it has been shown that macaques have a well-developed capacity for uncertainty monitoring, a basic form of metacognition that appears to show similarities to humans’ executive-attentional uncertainty processes (e.g., Kornell, 2009; Smith, 2009). However, research with several samples of capuchin monkeys in several standard animal-metacognition tasks have thus far shown that their metacognitive capacities are more poorly developed or absent (Basile, Hampton, Suomi, & Murray, 2009; Beran & Smith, 2011; Beran, Smith, Coutinho, Couchman, & Boomer, 2009; Fujita, 2009; Paukner, Anderson, & Fujita, 2006). Thus, there is a parallel need to evaluate broadly across the primates the capacity for explicit, rule-based categorization.
We tested capuchins monkeys (Cebus apella)—a New World primate. This group separated from the Old World primates about 40 million years ago. They can provide a second crucial data point in determining whether primates generally possess the implicit-explicit organization. We gave capuchin monkeys the RB and II category tasks illustrated in Figure 1. We asked whether RB category learning would proceed faster than II category learning, producing empirical support for a primate-broad dissociation between explicit and implicit systems of category learning.
2. Method
2.1 Participants
Four capuchin monkeys (Cebus apella) were tested: Logan (male, 5 years old), Lily (female, 13 years old), Liam (male, 7 years old), and Nala (female, 8 years old). All monkeys had been trained to respond to computer-generated stimuli using a joystick-response input (Evans, Beran, Chan, Klein, & Menzel, 2008).
2.2 Apparatus
The monkeys were tested using the Language Research Center’s Computerized Test System—LRC-CTS (described in Rumbaugh, Richardson, Washburn, Savage-Rumbaugh, & Hopkins, 1989; Washburn & Rumbaugh, 1992)—comprising a personal computer, a digital joystick, a color monitor, and a pellet dispenser. Monkeys manipulated the joystick to produce corresponding movements of a computer-graphic cursor on the screen. Contacting appropriate computer-generated stimuli with the cursor brought them a 45-mg fruit-flavored chow pellet using a pellet dispenser interfaced to the computer through a digital I/O board (KPCI-PDISO8A; Keithley Instruments, Cleveland, OH).
2.3 Stimuli
Each category exemplar was a circular sine-wave grating that varied on two dimensions: bar frequency and bar orientation. The disks subtended 4.77 degrees of visual angle, viewed on a 17-inch screen with an 800 × 600 pixel resolution from a distance of about 24 inches. In the present experiments, spatial frequency varied from 0.366 cycles per degree of visual angle to 1.408 cycles per degree. Orientation varied from 0.307 radians to 1.925 radians. Exemplars were created using the randomization technique developed by Ashby and Gott (1988). In accordance with this method, categories were first defined by bivariate normal distributions along the two stimulus dimensions that each ranged along a normalized 0-to-100 scale. Each stimulus was created by drawing a random sample (x,y) from the Category A or Category B distribution. To control for statistical outliers, the random sample was discarded if its Mahalanobis distance (Mahalanobis, 1936) was greater than 3.0. This process was repeated until 300 Category A and 300 Category B exemplars had been generated. The population parameters defining the Category A and Category B exemplar distributions in the RB and II tasks are given in Table 1.
Table 1.
Parameters Used to Generate the RB and II Category Tasks
| Category A | Category B | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| μx | μy | σx | σy | cov | μx | μy | σx | σy | cov | |
| Task | ||||||||||
| RB | 35.86 | 50 | 16.33 | 355.55 | 0 | 64.14 | 50 | 16.33 | 355.55 | 0 |
| II | 40 | 60 | 185.94 | 185.94 | 169.91 | 60 | 40 | 185.94 | 185.94 | 169.91 |
In the end, the 300 chosen Category A and Category B exemplars in the two tasks were slightly adjusted so that their sample means and sample covariance matched the desired population values for the two categories in the tasks as shown in Table 1. Finally, a linear transformation was applied to each stimulus coordinate-pair to map its values from the original 0-to-100 scale to a space representing actual values of spatial frequency (cycles per degree) and orientation (radians) used in the experiment. These mappings were: Spatial Frequency = 1.0 + X/30.0; Orientation = y * (pi/200) + pi/9.
2.4 Categorization trials
Each trial consisted of one disk presented in the center-top of a computer screen against a gray background. A trial began with a black square presented in the same position as the to-be-categorized stimulus. Animals moved their cursor to touch this square as a trial-start response, indicating their readiness. The black square released to the disk, and the two response icons were illuminated. The response icons were located on the screen’s lower-left and lower-right. To avoid any confusion with past response icons used by the animals, the A-response icon was a mirror-imaged TB. The B-response icon was a mirror-imaged QC. These were both novel response stimuli. Monkeys responded by using the joystick to move a small, red cursor to touch one of the response icons on the screen. For correct responses, they received a computer-generated bridging auditory signal (a whoop) and the food reward already described. For incorrect responses, they received a computer-generated penalty sound (a buzz) and a 20-s timeout period, during which time the monkeys were not able to move the cursor or get a new trial.
In the experiment’s pilot run of 6,000 trials, both correct and incorrect responses were simply followed by the next randomly selected trial. However, it appeared that monkeys were treating all the sine-wave stimuli indifferently and equivalently, as sometimes occurs when monkeys encounter a distinctively new stimulus domain. Accordingly, we transitioned to a method based on correction trials. By this method, time-out periods were followed by correction trials in which the monkeys were presented the same stimulus from the previous trial, but their responses were not followed by reward or penalty. Correct responses for correction trials were followed by the next regular trial. Incorrect responses for correction trials were followed by a repetition of the correction trial. Only experimental trials (not correction trials) were used in the data analyses.
We report data from 6,000 RB trials and 6,000 II trials for monkeys Logan, Liam, and Nala. Through experimenter error, Lily was given only 5,675 trials in her II condition. To address this situation, her data are graphed and were analyzed using only 5,675 trials from both her RB and II tasks. Accordingly, her 57th trial block in both tasks contained 75 trials, not 100 as for all other trial blocks for all other monkeys.
In all cases, the trials were successive, random permutations of the 300 Category A and 300 Category B stimuli available for a task. To allow this succession of permutations, stimuli were sampled without replacement until the supply of 600 stimuli was exhausted, and then the 600 stimuli were re-introduced.
All monkeys were given—in counterbalanced order—the RB task with a vertical decision bound and the II task with a positive-diagonal decision bound (Figure 1).
2.5 Categorization modeling
The following models were fit to the last 1,000 trials of each monkey’s performance in each task, thus allowing their mature performance strategy to be analyzed. The trials modeled were 5,001–6,000 for Logan, Liam, and Nala, and 4,676–5,675 for Lily. More details of the modeling procedures in this article can be found in Maddox and Ashby (1993).
The Rule-Based Model assumed that the participant set a decision criterion on one stimulus dimension (either bar frequency or orientation). The outcome of modeling was to specify the vertical or horizontal line drawn through the stimulus space that would most systematically partition the participant’s Category A responses from his or her Category B responses. This model had two parameters (a criterion on the relevant dimension and perceptual noise variance).
The Information-Integration Model assumed a general linear classifier strategy in which participants divided the stimulus space using a linear decision bound. The outcome of modeling was to specify the line drawn through the stimulus space, of any slope and intercept, that would most systematically partition the participant’s Category A responses from his or her Category B responses. This model had 3 parameters: the slope and intercept of the linear decision bound and a perceptual noise variance.
Finally, two random-response models assumed random guessing. One model assumed unbiased guessing and thus had zero parameters. The other guessing model assumed biased guessing and thus had one bias parameter.
The procedures for selecting the best-fitting model were as follows. Parameters were estimated using the method of maximum likelihood. That is, modeling evaluated which model would, with maximum likelihood, have created the distribution within the stimulus space of Category A and B responses that the participant actually produced. Then the Bayesian Information Criterion (Schwarz, 1978) determined model selection:
where r is the number of free parameters, N is the sample size, and L is the likelihood of the model given the data.
3. Results
3.1 Logan and Lily (task order RB-II)
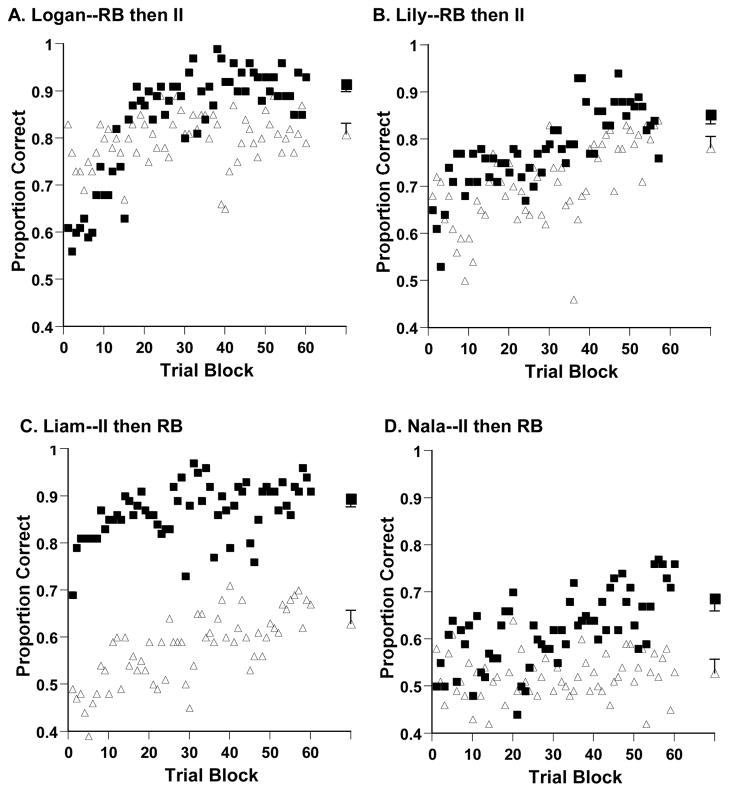
Figure 3A and 3B, respectively, show the results from Logan’s and Lily’s RB and II tasks over 60 and 57 trial blocks. Though the RB task was their first task, and they may have still been familiarizing themselves with the stimulus domain, they showed a strong acquisition of the RB task, with initial low performance levels that soon rose above 90% and 85%, respectively. Over their last 2,000 trials (Logan--Blocks 41–60) or 1,975 trials (Lily—Blocks 38–57), they were 91.4% and 85.4% correct, respectively.
Figure 3.
A–D. Proportion of correct responses in each trial block for the four capuchin monkeys. Square and triangle symbols, respectively, denote performance in RB and II tasks. The larger symbols at the graphs’ far right reflect the animals’ average performance over their last 2,000 trials (Logan, Liam, Nala) or 1,975 trials (Lily). For the RB average, we calculated the p=.01 lower confidence interval using procedures described in Hays (1981, pp. 224–226). For the II task, we calculated the p=.01 upper confidence interval in the same way.
By the time of their II (second) task, stimulus familiarization was complete, and Logan and Lily could (and did) perform above chance from the beginning. It is an interesting feature of Logan’s and Lily’s data that their performance dipped in the early phases of their II (second) task. They seem to have become confused, as though they were processing that the stimulus-reinforcement contingencies of the task had changed for them. Nonetheless, despite this possible recognition, their performance hardly improved across thousands of trials. Over the last 2,000 trials (Logan--Blocks 41–60) or 1,975 trials (Lily—Blocks 38–57) in the II task, Logan and Lily, respectively, were 80.8% and 78.2% correct, respectively, a 10.6% and 7.2% performance disadvantage compared to the RB task. For both animals, we calculated the p=.01 lower confidence interval for the RB task, and the p=.01 upper confidence interval for the II task, using procedures described in Hays (1981, pp. 224–226). The two confidence intervals were non-overlapping as shown in Figures 3A,B, confirming that there is almost no chance that their RB and II performances were underlyingly equivalent.
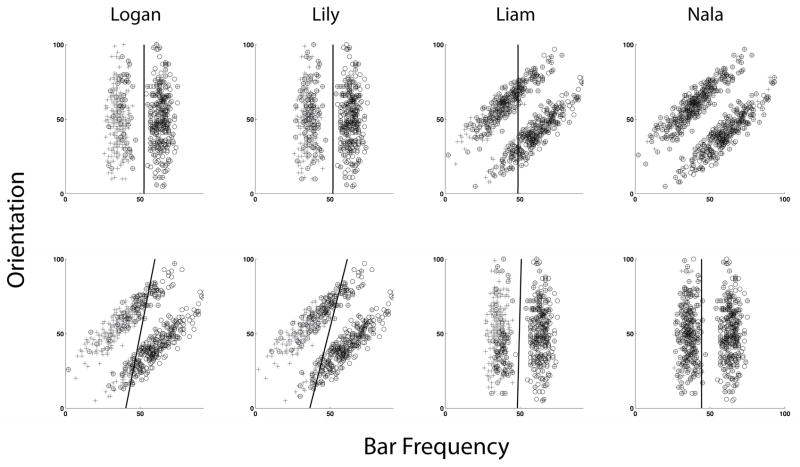
The performance of both animals in their last 2,000 trials was modeled using the procedures described above. They placed their RB decision boundary optimally (Figure 4), choosing the vertical decision boundary that best differentiated the Category A and B stimulus classes. Figure 4 uses plus and circle symbols, respectively, to indicate an animal’s Category A or Category B responses for stimuli that are plotted veridically within the spatial frequency-orientation stimulus space The best-fitting decision boundaries in the RB task accounted for 91.4% of Logan’s responses and 85.6% of Lily’s responses in the RB task. In Logan’s case, for example, this means that 1,806 of his 2,000 modeled trials produced responses consistent with his rule-based decision bound. One sees in Figure 4’s top-left panel that there were only a few “circle” (Category B) responses made for stimuli to the left of his boundary, and only a few “plus” (Category A) responses made for stimuli to the right of his boundary.
Figure 4.
The decision bounds that provided the best fits to the last 1,000 responses of the four capuchin monkeys in the rule-based (RB) and information-integration (II) tasks. Plus and circle symbols, respectively, indicate an animal’s Category A or Category B responses for stimuli plotted veridically within the spatial frequency-orientation stimulus space. The line represents the best-fitting decision boundary as determined by the modeling procedures described in the text. The animals’ first and second acquisitions, respectively, are shown in the top and bottom row. The absence of a decision bound for Nala’s first acquisition indicates that her purely chance performance led to the superior fit of a random-guessing model.
Logan’s and Lily’s II data were best fit by the Information-Integration model, and this best-fitting decision boundary accounted, respectively, for 89.1% and 84.4% of their responses in the II task. Therefore, both animals showed true II learning and an ability to integrate information across the two dimensions in the task. However, the parameters of both model fits were nonoptimal because the decision boundaries were closer to vertical. This implies that even though Logan and Lily showed information integration in the task, they continued to dimensionalize the stimuli to the detriment of their II performance. This is another confirmation of the psychological hold that dimensional foci exert on capuchins’ categorization performance.
It is not a viable interpretation of the present results to say that the stimulus space was asymmetrically salient, so that one overridingly salient dimension was in control of perception. To illustrate this, we went on to give Logan a third task, the RB task with a horizontal optimal decision boundary through the spatial frequency-orientation stimulus space. Now, Logan showed a flexible shift in his attention to emphasize the orientation dimension. His performance quickly rose above 90%, and, over his last 2,000 trials, he performed at 92.9%. Modeling revealed that he placed his RB decision boundary optimally, using essentially the horizontal decision boundary that best differentiated the new Category A and B stimulus classes.
Accordingly, the present results are not consistent with salience asymmetries within the stimulus space, or with associative inertia from task to task. To the contrary, the results are consistent with the preference and psychological privilege of rule-based processes that bring capuchins selective performance advantages within RB tasks. Logan easily and flexibly moved his decisional boundary into the next dimensional polarity. What he and Lily did not do nearly as well was move their decision boundary optimally into an information-integration, diagonal polarity.
3.2 Liam and Nala (task order II-RB)
Figure 3C shows the results from Liam’s II and RB tasks. Now the II task was Liam’s first acquisition, so possibly some residual familiarization with the stimuli was still occurring. Liam’s II acquisition was very weak, only carrying him from chance performance early on to performance just above 60% later on. Over the last 2,000 trials (Blocks 41–60) in the II task, Liam was 62.9% correct.
In sharp contrast, Liam’s appreciation of the RB task appeared to be almost immediate, and he learned to high levels by the RB task’s end. Over the last 2,000 trials (Blocks 41–60) in the RB task, Liam was 89.4% correct, a 26.5% performance advantage compared to the II task. As before, we calculated the p=.01 confidence intervals for both tasks. The two confidence intervals were non-overlapping (Figure 3C), confirming that the two performances were samples drawn from very different populations.
Liam’s performance for his last 2,000 trials was modeled using the procedures already described. Liam placed his II decision boundary vertically (Figure 4), confirming that he performed the task as a rule-based task even though it was not so constituted. The best-fitting decision boundary accounted for 68.3% of his responses in the II task. This vertical decision boundary illustrates again the psychological privilege with which capuchins apply rule-based frameworks to category tasks. Eventually a near-vertical decision boundary came to serve Liam well, when he transitioned into his second, RB task. Now the best-fitting decision boundary accounted for 89.5% of Liam’s responses.
Nala was the weakest learner of the four capuchins. Figure 3D shows the results from her II and RB tasks. The II task was Nala’s first acquisition, and she may have still been acclimating to the stimuli during it. Nonetheless, her II acquisition was extremely weak—she essentially never learned anything. Over the last 2,000 trials (Blocks 41–60) in the II task, Nala was 52.8% correct.
In contrast, Nala did make progress with her RB acquisition. Over the last 2,000 trials (Blocks 41–60) in the RB task, she was 68.6% correct, a 15.8% performance advantage compared to the II task. As before, we calculated the p=.01 confidence intervals for both tasks. They were non-overlapping (Figure 3D), confirming that the two performances were sampled from very different populations.
Nala’s performance for her last 2,000 trials was modeled as already described. Because Nala learned nothing in her II (first) acquisition, the random-guessing model fit her data best. For this reason, a decision boundary is not shown in Figure 4 for Nala’s II performance, because she did not learn to apply a systematic strategy in her II task. In her RB (second) acquisition, Nala was able to place her decision boundary vertically, appropriately to the structure of that task. The best-fitting decision boundary accounted for 68.5% of her responses in the RB task. Even though Nala was not the strongest performer, for her, too, the RB task was more psychologically approachable.
Because Nala learned nothing within her first II task, we thought it was important to give her a second opportunity to master the II category structure after she had fully familiarized herself with the stimulus space. Accordingly, Nala participated in a third task, the complementary II task to that shown in Figure 1B, in which the Category A and Category B stimulus ellipses followed the negative diagonal of the stimulus space and there was an optimal decisional boundary extending from 10 o’clock down to 4 o’clock. She was able to perform generally above chance in this task, and performed at 70.5% correct over her last 2,000 trials. However, modeling revealed that she did so by maintaining her vertical RB boundary, treating the task as rule-based even though it was not so constituted. That Nala failed her first II task, succeeded with her RB task, and construed her second II task analytically are all demonstrations of this article’s main finding, which is that capuchins, like humans, approach RB tasks with psychological privilege and greater learning potential.
4. Discussion
Four capuchin monkeys participated in RB and II category-learning tasks using an established methodology. All animals experienced difficulty learning tasks that required perceptual integration over two dimensions to make a categorization decision. Their learning in these tasks was slower to lower terminal performance levels. All four animals dimensionalized the spatial frequency-orientation perceptual space, attended well to single dimensions, and learned RB category tasks faster to higher terminal performance levels. Dimensional rules clearly have an important role in human categorization (Ahn & Medin, 1992; Ashby & Ell, 2001; Erickson & Kruschke, 1998; Medin, Wattenmaker, & Hampson, 1987; Nosofsky, Palmeri, & McKinley, 1994; Regehr & Brooks, 1995; Smith et al., 2004). Here, capuchins demonstrated their use of dimensional categorization processes that appear in humans to be explicit, rule-based, conscious, and declarative. The results join the results from Smith et al. (2010) to demonstrate a new continuity between nonhuman primate and human cognition.
Our results demonstrate continuity in the analytic, dimensional-rule aspects of explicit categorization, not necessarily in the awareness or consciousness aspects of explicit categorization. However, it is an exciting prospect that the present findings might eventually lead to paradigms that explore more fully monkeys’ declarative systems of categorization. For example, current theory suggests that explicit category rules held in working memory should be robust to delays in the reinforcement signal following category trials, because the animal could hold in mind its rule, and the response it had made, and bridge the temporal gap so that the reinforcement was still meaningful. In fact, Maddox, Ashby, and Bohil (2003) showed this robustness of humans’ RB learning. In contrast, II learning was disrupted even if reinforcement was only delayed by 2.5 s. Demonstrating this result in nonhuman primates would ground further the idea that the primates broadly share important elements of humans’ explicit category-learning system.
Likewise, if category rules are evaluated in immediate memory using executive attention, they should be disrupted if the subject is denied the time to deliberately process the reinforcement. In fact, Maddox, Ashby, Ing, and Pickering (2004) showed that RB category learning in humans was disrupted by limitations in the time to process the feedback signal. Demonstrating this result in nonhuman primates would suggest that they also deliberately process the reinforcement given. Though the present results do not allow one to infer that nonhuman primates share all aspects of humans’ capacity for explicit categorization, they ground a research program that may be able to illuminate this issue more fully.
The present results also shed light on the sufficient conditions for aspects of explicit categorization in monkeys and humans. In early formulations of multiple-system theories, from Shepard et al. (1961) to Ashby et al. (1998), there was some conflation of the construct of explicit categorization and rules with language and verbal descriptions of those rules. The emerging results from nonhuman primates show that this conflation must be qualified. Neither verbal rules nor language are necessary for the privilege of unidimensional category rules to develop. (Recent discussions of implicit-explicit categorization have avoided this error—see Ashby & Valentin, 2005). Nonetheless, it is possible that there are synergistic interactions between explicit category rules and verbal coding. Verbal coding could augment the privilege of explicit rules in cognition, by facilitating their formulation or evaluation, or by facilitating their maintenance across trials. Conversely, and this could be an important point about cognitive evolution, the pre-existing privilege of unidimensional attention and category rules could have generally promoted the development of the verbal coding and language communication of those rules.
The results from capuchin monkeys were not predetermined. From the perspective of task difficulty, RB and II tasks are carefully matched to one another to the point that they are mutual controls. This is their empirical elegance and theoretical power. The categories in the RB and II tasks have identical within-category similarity relationships. The exemplar clouds in each task are spread out in stimulus/perceptual space to exactly the same degree. Moreover, the categories in the RB and II tasks have identical between-category similarity relationships. The exemplar clouds in each task are separated in stimulus/perceptual space to exactly the same degree. Therefore, the two category tasks are matched in every aspect relating to the inherent perceptual difficulty of the categorization problem and the maximum proportion correct achievable by an ideal observer. Pothos and Close (2008) took an alternative formal approach toward showing that—to an organism that was not dimensionally tuned or focused—the RB and II tasks would be equally difficult and learnable. For this reason, one might have expected equivalent RB and II performance by capuchins. Of course they did not show this equivalence.
In considering this result, one must treat the concepts of task difficulty and performance difficulty psychologically and theoretically. All of the essential sources of difficulty are equated between the RB and II tasks except one—the tasks’ dimensional alignment. Therefore, the only explanation for the performance difficulty by capuchins in the II task, compared to the RB task, is that the RB task is dimensionally tuned or aligned, and that the capuchins respond to this alignment with strong psychological privilege. The II task is not difficult. It is dimensionally difficult, a very different psychological conclusion. One’s theoretical explanation must explain not just that RB learning occurred, but that RB learning occurred more quickly and that RB strategies were even sometimes favored in the II condition.
From a neuroscience perspective, there is evidence that II learning is managed in humans (and perhaps in monkeys) by an implicit-striatal system that uses a form of procedural learning. This implicit system relies on the low-level association of responses to locations or regions in perceptual space. Because it relies on such nonanalytic, multidimensional processes, that system would be indifferent to the rotation of the task in perceptual space and to the dimensional alignment of the task’s axes. Capuchins could have learned both II and RB tasks using this system, and then one would have predicted equal RB and II performance. Smith et al. (2011) provided—in pigeons—a concrete example of a vertebrate species that appears to have only a unitary system for category learning that treats RB and II tasks equivalently in the sense of learning both category tasks to the same level at the same speed. In contrast to pigeons, capuchins have some category-learning system that is sharply tuned dimensionally.
From a perceptual-representation perspective, capuchins might have perceived multidimensional stimuli less separably and more integrally than humans (Foard & Kemler Nelson, 1984; Garner, 1974; Garner & Felfoldy, 1970; Handel & Imai, 1972; Lockhead, 1972). This would also leave the processes of categorization indifferent to the rotation of the task in perceptual space and to the dimensional alignment of the task’s axes. There is also a concrete model for this—young human children sometimes perceive multidimensional stimulus combinations more integrally than adult humans do (Shepp & Swartz, 1976; Shepp, Burns, & McDonough, 1980; L. Smith & Kemler 1977, 1978; Smith & Kemler Nelson, 1984; Ward, 1983). From the perspective of comparative psychology, the configural theory of Pearce (1987, 1994)—that presumes that multidimensional stimulus compounds in their entirety enter into associations with outcomes and responses—would also allow one to predict equivalent RB and II performance.
Indeed, there might even be inherent advantages to having a unitary category learning system based in the nonanalytic integration of multiple dimensions. There could be a neural economy that might especially suit nervous systems constrained in size. Organisms could reduce strategy competition during category learning that arises from multiple systems engaging the same task, and avoid the adventitious rules that humans sometimes obey during category learning (Jitsumori, 1993). A unitary, nonanalytic system might also be especially adept at learning non-linear category boundaries that would defeat a rule-based system. And, if natural kinds are normally multidimensionally organized, with instances presenting task-relevant information along diverse and changing dimensions, then broad or configural attention would be adaptive for reducing the chance that attention would be misdirected away from relevant information.
For all the foregoing reasons, capuchins might have been found to possess a unitary category-learning system of parsimony, generality, and power that always, simply associated responses to stimuli, without overlaying axes, dimensions, and rules. But that is not the category-learning system revealed by the present results. Though one study can never demonstrate conclusively that nonhuman primates have an exact match to humans’ explicit category-learning system, the present results, viewed together with results from macaques, and contrastively to results from pigeons, indicate strongly that nonhuman primates have at least the beginnings of such a system.
Therefore, the capuchin results that were actually obtained raise important theoretical questions regarding the phylogenetic distribution of the multiple-system, implicit-explicit categorization system. Who has the overlay of axes and dimensions? When did it emerge in cognitive evolution? Why?
The results from pigeons show clearly that the multiple-system organization is not a vertebrate-wide cognitive adaptation. The results from capuchins, macaques, and humans suggest that this organization could be a primate-wide cognitive adaptation. Therefore, one likely possibility is that the privilege of rule-based systems emerged as part of the cognitive repertoire of the primates.
However, comparative research in this area is only beginning, and therefore we point out that there are other possibilities as well. Pigeons are not the rocket scientists of the class Aves—crows and parrots are. In addition, some marine-mammal species (dolphins, sea lions) are known to be cognitively sophisticated though they lie outside the order Primates. So, it remains possible that explicit, rule-based cognition could arise generally as the last frost that settles on the highest peaks of cognitive sophistication, including primates, marine mammals, corvids, and, of course, journal reviewers. Our research would naturally be followed up with crows or dolphins, providing critical tests of the evolutionary breadth of explicit category learning. If rule-based cognition is a primate invention, that would have profound implications for considering the cognitive evolution of the primates. If rule-based cognition is a broader-based, ultimate achievement of mind, that would be equally interesting in a different way.
Finally, we address the “why” of the emergence of an explicit categorization utility. Explicit categorization also has distinct advantages. It allows for economical, quickly learned, easy to maintain, and easy-to-generalize category representations (i.e., rules). It brings cognitive flexibility and attentional agility arising from dimensional analysis. Perhaps most important, it opens up the possibilities for cognitive analysis, rules, inferences, symbolic representations, and eventually even language. Therefore, the processing preference and privilege that developed for dimensional analysis and category rules may have been one of the premier adaptations that fostered cognitive evolution within the primate-hominid lineage.
Acknowledgments
The preparation of this article was supported by Grant 3P01HD038051-10S1 (ARRA administrative supplement), Grant HD-060563, Grant R01 MH3760-2 from NIMH, and by support from the U.S. Army Research Office through the Institute for Collaborative Biotechnologies under grant W911NF-07-1-0072.
We thank Betty Chan and Ted Evans for their assistance with data collection.
References
- Ahn W, Medin DL. A two-stage model of category construction. Cognitive Science. 1992;16:81–121. [Google Scholar]
- Ashby FG, Alfonso-Reese LA, Turken AU, Waldron EM. A neuropsychological theory of multiple systems in category learning. Psychological Review. 1998;105:442–481. doi: 10.1037/0033-295x.105.3.442. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Ell SW. The neurobiology of human category learning. Trends in Cognitive Science. 2001;5:204–210. doi: 10.1016/s1364-6613(00)01624-7. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Ennis JM, Spiering BJ. A neurobiological theory of automaticity in perceptual categorization. Psychological Review. 2007;114:632–656. doi: 10.1037/0033-295X.114.3.632. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Gott RE. Decision rules in the perception and categorization of multidimensional stimuli. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1988;14:33–53. doi: 10.1037//0278-7393.14.1.33. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Maddox WT. Human category learning. Annual Review of Psychology. 2005;56:149–178. doi: 10.1146/annurev.psych.56.091103.070217. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Maddox WT. Human category learning 2.0. Annals of the New York Academy of Sciences. 2010;1224:147–161. doi: 10.1111/j.1749-6632.2010.05874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby FG, Valentin VV. Multiple systems of perceptual category learning: Theory and cognitive tests. In: Cohen H, Lefebvre C, editors. Handbook of categorization in cognitive science. New York USA: Elsevier; 2005. pp. 547–572. [Google Scholar]
- Basile BM, Hampton RR, Suomi SJ, Murray EA. An assessment of memory awareness in tufted capuchin monkeys (Cebus apella) Animal Cognition. 2009;12:169–180. doi: 10.1007/s10071-008-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Smith JD. Information seeking by rhesus monkeys (Macaca mulatta) and capuchin monkeys (Cebus apella) Cognition. 2011;120:90–105. doi: 10.1016/j.cognition.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Smith JD, Coutinho MVC, Couchman JC, Boomer J. The psychological organization of “uncertainty” responses and “middle” responses: A dissociation in capuchin monkeys (Cebus apella) Journal of Experimental Psychology: Animal Behavior Processes. 2009;35:371–381. doi: 10.1037/a0014626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair M, Homa D. As easy to memorize as they are to classify: The 5-4 categories and the category advantage. Memory & Cognition. 2003;31:1293–1301. doi: 10.3758/bf03195812. [DOI] [PubMed] [Google Scholar]
- Brooks LR. Nonanalytic concept formation and memory for instances. In: Rosch E, Lloyd BB, editors. Cognition and categorization. Hillsdale, NJ USA: Erlbaum; 1978. pp. 169–211. [Google Scholar]
- Cook RG, Smith JD. Stages of abstraction and exemplar memorization in pigeons’ category learning. Psychological Science. 2006;17:1059–1067. doi: 10.1111/j.1467-9280.2006.01833.x. [DOI] [PubMed] [Google Scholar]
- Erickson MA, Kruschke JK. Rules and exemplars in category learning. Journal of Experimental Psychology: General. 1998;127:107–140. doi: 10.1037//0096-3445.127.2.107. [DOI] [PubMed] [Google Scholar]
- Evans TA, Beran MJ, Chan B, Klein ED, Menzel CR. An efficient computerized testing method for the capuchin monkey (Cebus apella): Adaptation of the LRC-CTS to a socially housed nonhuman primate species. Behavior Research Methods. 2008;40:590–596. doi: 10.3758/brm.40.2.590. [DOI] [PubMed] [Google Scholar]
- Feldman J. Minimization of Boolean complexity in human concept learning. Nature. 2000;407:630–633. doi: 10.1038/35036586. [DOI] [PubMed] [Google Scholar]
- Foard CF, Kemler Nelson DG. Holistic and analytic modes of processing: The multiple determinants of perceptual analysis. Journal of Experimental Psychology: General. 1984;113:94–111. doi: 10.1037//0096-3445.113.1.94. [DOI] [PubMed] [Google Scholar]
- Fujita K. Metamemory in tufted capuchin monkeys (Cebus apella) Animal Cognition. 2009;12:575–585. doi: 10.1007/s10071-009-0217-0. [DOI] [PubMed] [Google Scholar]
- Garner WR. The processing of information and structure. Potomac, MD USA: Erlbaum; 1974. [Google Scholar]
- Garner WR, Felfoldy FL. Integrality of stimulus dimensions in various types of information processing. Cognitive Psychology. 1970;1:225–241. [Google Scholar]
- Handel S, Imai S. The free classification of analyzable and unanalysable stimuli. Perception and Psychophysics. 1972;12:108–116. [Google Scholar]
- Hays WL. Statistics. New York: Holt, Rinehart, and Winston; 1981. [Google Scholar]
- Herbranson WT, Fremouw T, Shimp CP. The randomization procedure in the study of categorization of multidimensional stimuli by pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1999;25:113–135. [PubMed] [Google Scholar]
- Herrnstein RJ, Loveland DH, Cable C. Natural concepts in pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1976;2:285–301. doi: 10.1037//0097-7403.2.4.285. [DOI] [PubMed] [Google Scholar]
- Homa D, Sterling S, Trepel L. Limitations of exemplar-based generalization and the abstraction of categorical information. Journal of Experimental Psychology: Human Learning and Memory. 1981;7:418–439. doi: 10.1037//0278-7393.10.4.638. [DOI] [PubMed] [Google Scholar]
- Jitsumori M. Category discrimination of artificial polymorphous stimuli based on feature learning. Journal of Experimental Psychology, Animal Behavior Processes. 1993;19:244–254. doi: 10.1037//0097-7403.22.4.405. [DOI] [PubMed] [Google Scholar]
- Jitsumori M. Discrimination of artificial polymorphous categories in humans and nonhumans. In: Hayes SC, Hayes LJ, Sato M, Ono K, editors. Behavior analysis of language and cognition. Washington, DC USA: APA; 1994. pp. 91–106. [Google Scholar]
- Kemler Nelson DG. The effect of intention on what concepts are acquired. Journal of Verbal Learning and Verbal Behavior. 1984;23:734–759. [Google Scholar]
- Knowlton BJ, Squire LR. The learning of categories: Parallel brain systems for item memory and category knowledge. Science. 1993;262:1747–1749. doi: 10.1126/science.8259522. [DOI] [PubMed] [Google Scholar]
- Kornell N. Metacognition in humans and animals. Current Directions in Cognitive Science. 2009;18:11–15. [Google Scholar]
- Lazareva OF, Wasserman EA. Category learning and concept learning in birds. In: Mareschal D, Quinn PC, Lea SEG, editors. The making of human concepts. Oxford: Oxford University Press; 2010. [Google Scholar]
- Lea SEG, Wills AJ. Use of multiple dimensions in learned discriminations. Comparative Cognition and Behavior Reviews. 2008;3:115–133. [Google Scholar]
- Lockhead GR. Processing dimensional stimuli: A note. Psychological Review. 1972;79:410–419. doi: 10.1037/h0033129. [DOI] [PubMed] [Google Scholar]
- Maddox WT, Ashby FG. Comparing decision bound and exemplar models of categorization. Perception and Psychophysics. 1993;53:49–70. doi: 10.3758/bf03211715. [DOI] [PubMed] [Google Scholar]
- Maddox WT, Ashby FG, Bohil CJ. Delayed feedback effects on rule-based and information-integration category learning. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29:650–662. doi: 10.1037/0278-7393.29.4.650. [DOI] [PubMed] [Google Scholar]
- Maddox WT, Ashby FG, Ing AD, Pickering AD. Disrupting feedback processing interferes with rule-based but not information-integration category learning. Memory & Cognition. 2004;32:582–591. doi: 10.3758/bf03195849. [DOI] [PubMed] [Google Scholar]
- Mahalanobis PC. On the generalised distance in statistics. Proceedings of the National Institute of Sciences of India. 1936;2:49–55. Retrieved 2008-11-05. [Google Scholar]
- Medin DL, Schaffer MM. Context theory of classification learning. Psychological Review. 1978;85:207–238. [Google Scholar]
- Medin DL, Wattenmaker WD, Hampson SE. Family resemblance, conceptual cohesiveness, and category construction. Cognitive Psychology. 1987;19:242–279. doi: 10.1016/0010-0285(87)90012-0. [DOI] [PubMed] [Google Scholar]
- Miles SJ, Minda JP. The effects of concurrent verbal and visual tasks on category learning. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2011;37:588–607. doi: 10.1037/a0022309. [DOI] [PubMed] [Google Scholar]
- Minda JP, Smith JD. Prototypes in category learning: The effects of category size, category structure, and stimulus complexity. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27:775–799. [PubMed] [Google Scholar]
- Murphy GL. The big book of concepts. Cambridge, MA USA: MIT Press; 2003. [Google Scholar]
- Nosofsky RM. Attention and learning processes in the identification and categorization of integral stimuli. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1987;13:87–108. doi: 10.1037//0278-7393.13.1.87. [DOI] [PubMed] [Google Scholar]
- Nosofsky RM, Palmeri TJ, McKinley SK. Rule-plus exception model of classification learning. Psychological Review. 1994;101:53–79. doi: 10.1037/0033-295x.101.1.53. [DOI] [PubMed] [Google Scholar]
- Paukner A, Anderson JR, Fujita K. Redundant food searches by capuchin monkeys (Cebus apella): A failure of metacognition? Animal Cognition. 2006;9:110–117. doi: 10.1007/s10071-005-0007-2. [DOI] [PubMed] [Google Scholar]
- Pearce JM. A model of stimulus generalization for Pavlovian conditioning. Psychological Review. 1987;94:61–73. [PubMed] [Google Scholar]
- Pearce JM. Similarity and discrimination: A selective review and a connectionist model. Psychological Review. 1994;101:587–607. doi: 10.1037/0033-295x.101.4.587. [DOI] [PubMed] [Google Scholar]
- Pothos EM, Close J. One or two dimensions in spontaneous classification: A simplicity approach. Cognition. 2008;107:581–602. doi: 10.1016/j.cognition.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Reed SK. Category vs. item learning: implications for categorization models. Memory & Cognition. 1978;6:612–621. doi: 10.3758/bf03198251. [DOI] [PubMed] [Google Scholar]
- Regehr G, Brooks LR. Category organization in free classification: The organizing effect of an array of stimuli. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1995;21:347–363. [Google Scholar]
- Rosch E, Mervis CB. Family resemblances: Studies in the internal structure of categories. Cognitive Psychology. 1975;7:573–605. [Google Scholar]
- Rosseel Y. Mixture models of categorization. Journal of Mathematical Psychology. 2002;46:178–210. [Google Scholar]
- Rossi AF, Pessoa L, Desimone R, Ungerleider LG. The prefrontal cortex and the executive control of attention. Experimental Brain Research. 2009;192:489–497. doi: 10.1007/s00221-008-1642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbaugh DM, Richardson WK, Washburn DA, Savage-Rumbaugh ES, Hopkins WD. Rhesus monkeys (Macaca mulatta), video tasks, and implications for stimulus-response spatial contiguity. Journal of Comparative Psychology. 1989;103:32–38. doi: 10.1037/0735-7036.103.1.32. [DOI] [PubMed] [Google Scholar]
- Schwarz G. Estimating the dimension of a model. Annals of Statistics. 1978;6:461–464. [Google Scholar]
- Shepard RN, Hovland CI, Jenkins HM. Learning and memorization of classifications. Journal of Experimental Psychology. 1961;65:94–102. doi: 10.1037/h0043732. [DOI] [PubMed] [Google Scholar]
- Shepp BE, Swartz K. Selective attention and the processing of integral and nonintegral dimensions. A developmental study. Journal of Experimental Child Psychology. 1976;22:73–85. doi: 10.1016/0022-0965(76)90091-6. [DOI] [PubMed] [Google Scholar]
- Shepp BE, Burns B, McDonough D. The relation of stimulus structure to perceptual and cognitive development: Further tests of a separability hypothesis. In: Becker J, Wilkening F, editors. The integration of information by children. Hillsdale, NJ USA: Erlbaum; 1980. pp. 113–145. [Google Scholar]
- Smith JD. The study of animal metacognition. Trends in Cognitive Sciences. 2009;13:389–396. doi: 10.1016/j.tics.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Smith JD, Ashby FG, Berg ME, Murphy MS, Spiering B, Cook RG, Grace RC. Pigeons’ categorization may be exclusively nonanalytic. Psychonomic Bulletin and Review. 2011;18:422–428. doi: 10.3758/s13423-010-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Beran MJ, Crossley MJ, Boomer J, Ashby FG. Implicit and explicit category learning by macaques (Macaca mulatta) and humans (Homo sapiens) Journal of Experimental Psychology: Animal Behavior Processes. 2010;36:54–65. doi: 10.1037/a0015892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Chapman WP, Redford JS. Stages of category learning in monkeys (Macaca mulatta) and humans (Homo sapiens) Journal of Experimental Psychology: Animal Behavior Processes. 2010;36:39–53. doi: 10.1037/a0016573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Kemler Nelson DG. Overall similarity in adults’ classification: The child in all of us. Journal of Experimental Psychology: General. 1984;113:137–159. [Google Scholar]
- Smith JD, Minda JP. Prototypes in the mist: the early epochs of category learning. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1998;24:1411–1436. [Google Scholar]
- Smith JD, Minda JP, Washburn DA. Category learning in rhesus monkeys: A study of the Shepard, Hovland, and Jenkins tasks. Journal of Experimental Psychology: General. 2004;133:398–414. doi: 10.1037/0096-3445.133.3.398. [DOI] [PubMed] [Google Scholar]
- Smith JD, Redford JS, Haas SM. Prototype abstraction by monkeys (Macaca mulatta) Journal of Experimental Psychology: General. 2008;137:390–401. doi: 10.1037/0096-3445.137.2.390. [DOI] [PubMed] [Google Scholar]
- Smith JD, Tracy J, Murray MJ. Depression and categorization. Journal of Experimental Psychology: General. 1993;122:331–346. doi: 10.1037//0096-3445.122.3.331. [DOI] [PubMed] [Google Scholar]
- Smith JD, Redford JS, Haas SM. Prototype abstraction by monkeys (Macaca mulatta) Journal of Experimental Psychology: General. 2008;137:390–401. doi: 10.1037/0096-3445.137.2.390. [DOI] [PubMed] [Google Scholar]
- Smith LB, Kemler DG. Developmental trends in free classification: Evidence for a new conceptualization. Journal of Experimental Child Psychology. 1977;24:279–298. [Google Scholar]
- Smith LB, Kemler DG. Levels of experienced dimensionality in children and adults. Cognitive Psychology. 1978;10:502–532. doi: 10.1016/0010-0285(78)90009-9. [DOI] [PubMed] [Google Scholar]
- Thompson RKR, Oden DL. Categorical perception and conceptual judgments by nonhuman primates: The paleological monkey and the analogical ape. Cognitive Science. 2000;24:363–396. [Google Scholar]
- Vauclair J. Categorization and conceptional behavior in nonhuman primates. In: Bekoff M, Allen C, editors. The cognitive animal: Empirical and theoretical perspectives on animal cognition. Cambridge, MA: MIT Press; 2002. pp. 239–245. [Google Scholar]
- Waldron EM, Ashby FG. The effects of concurrent task interference on category learning: Evidence for multiple category learning systems. Psychonomic Bulletin & Review. 2001;8:168–176. doi: 10.3758/bf03196154. [DOI] [PubMed] [Google Scholar]
- Ward TB. Response tempo and separable-integral responding: Evidence for an integral-to-separable processing sequence in visual perception. Journal of Experimental Psychology: Human Perception and Performance. 1983;9:103–112. doi: 10.1037//0096-1523.9.1.103. [DOI] [PubMed] [Google Scholar]
- Washburn DA, Rumbaugh DM. Testing primates with joystick-based automated apparatus: Lessons from the Language Research Center’s Computerized Test System. Behavior Research Methods, Instruments, and Computers. 1992;24:157–164. doi: 10.3758/bf03203490. [DOI] [PubMed] [Google Scholar]
- Wasserman EA, Kiedinger RE, Bhatt RS. Conceptual behavior in pigeons: categories, subcategories, and pseudocategories. Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:235–246. [Google Scholar]