Abstract
Purpose
Subsequent malignant neoplasms (SMNs) are a dominant cause of morbidity and mortality in children treated for Hodgkin's disease (HD). We evaluated select demographic and therapeutic factors associated with SMNs, specifically gender and radiation dose.
Methods and Materials
A total of 930 children treated for HD at five institutions between 1960 and 1990 were studied. Mean age at diagnosis was 13.6 years, and mean follow-up was 16.8 years (maximum, 39.4 years). Treatment included radiation alone (43%), chemotherapy alone (9%), or both (48%).
Results
We found that SMNs occurred in 102 (11%) patients, with a 25-year actuarial rate of 19%. With 15,154 patient years of follow-up, only 7.18 cancers were expected (standardized incidence ratio [SIR] = 14.2; absolute excess risk [AER] = 63 cases/10,000 years). The SIR for female subjects, 19.93, was significantly greater than for males, 8.41 (p < 0.0001). After excluding breast cancer, the SIR for female patients was 15.4, still significantly greater than for male patients (p = 0.0012). Increasing radiation dose was associated with an increasing SIR (p = 0.0085). On univariate analysis, an increased risk was associated with female gender, increasing radiation dose, and age at treatment (12–16 years). Using logistic regression, mantle radiation dose increased risk, and this was 2.5-fold for female patients treated with more than 35 Gy primarily because of breast cancer.
Conclusions
Survivors of childhood HD are at risk for SMNs, and this risk is greater for female individuals even after accounting for breast cancer. Although SMNs occur in the absence of radiation therapy, the risk increases with RT dose.
Keywords: Pediatric Hodgkin's disease, Breast cancer, Subsequent malignancy, Gender, Age
INTRODUCTION
The challenge in treating children with Hodgkin's disease (HD) is to continue progress in curability while diminishing risk for toxic events that compromise quality of life and survival. Pediatric HD is highly curable, with a 10-year actuarial survival rate of 85% to 97% in early-stage disease and 70% to 90% in advanced-stage disease (1–6). Reports from large cohorts demonstrate a 7- to 18-times higher risk of subsequent malignant neoplasms (SMNs) compared with that in the general population (7–10). Children may be more prone than adults to develop SMNs because of their growth potential and endogenous hormonal factors (11). In contrast to adults, in whom the risk for SMNs is reportedly gender neutral or greater in men (12–21), girls with HD are more prone to develop SMNs than boys (8, 9, 22–28), which is thought to be caused by the frequent occurrence of secondary breast cancer (7, 11, 14, 15, 18, 24, 25, 29–31).
Controversies persist that include the relevance of gender, age, radiation dose, chemotherapy, and splenectomy. Several reports attempt to address these issues but are limited by patient numbers, completeness and quality of data, and length of follow-up (7–10, 15, 22, 24–26, 32–35). Motivated by the goal of addressing these controversies, investigators from five institutions comprehensively reviewed all children and adolescents (<19 years of age) treated for HD between 1960 and 1990. Both the standardized incidence ratio (SIR) and absolute excess risk (AER) were determined, the latter because a high relative risk does not independently reflect the clinically relevant risk of developing a SMN if the expected risk is low. Up to 30 variables related to demographic characteristics, treatment factors, and outcome measures were determined for 930 patients followed for 39 years or less.
The identification of relevant patient-related parameters, both inherent and iatrogenic, may suggest alterations in therapy of the initial cancer that may modify the risk for a SMN, or direct the appropriate screening and intervention. Should a gender-associated predisposition be demonstrated, investigations into the genesis of secondary cancers in this population would be warranted.
METHODS AND MATERIALS
Patients
Five institutions participated in the study, including the University of Rochester Medical Center, the Boston Children's Hospital and the Dana Farber Cancer Institute, St. Jude Children's Research Hospital, University of Florida Medical Center, and the Sidney Kimmel Cancer Center at Johns Hopkins. The cohort consisted of children and adolescents less than 19 years of age at HD diagnosis who received their primary treatment between 1960 and 1990. Follow-up was continued through 2002. Eligibility criteria excluded any patient with a history of cancer before HD diagnosis. This study was approved by the institutional review board of each center.
Data abstraction was performed at each institution and included the following: (1) gender, race/ethnicity, date of birth; (2) date of diagnosis, initial clinical or pathologic stage (splenectomy), disease histology, date of last follow-up, disease status at last follow-up, date of death, reason for death; (3) radiation therapy (dates, fields, doses), chemotherapy (dates, agents, doses); (4) date of first or any subsequent relapse and therapy; and (5) date of diagnosis for each SMN (histology, site, location relative to radiation field, and treatment outcome).
Subsequent malignancies included all invasive malignancies as well as ductal carcinoma in situ. Basal and squamous cell carcinomas of the skin were the only malignancies not considered in this analysis, as accurate population-based incidence rates are not available in the United States.
Radiation therapy volume and dose determination
Each patient received a score reflecting the volume of the body that was treated. Specifically, regional HD treatment fields were scored as follows: mantle = 2, para-aortic/spleen = 1.5, para-aortic/splenic pedicle = 1, and pelvis = 1. When involved fields were treated, a score was assigned that reflected volume, generally 0.5. Scores were summed for statistical analysis. The location of each solid SMN was determined relative to the boundaries of the radiation field (in-field, outside-field, or within 2 cm from field edge). For any malignancy where the exact location was not specifically known, such as the site of a breast cancer within the breast, then the tumor was assumed to have been in-field.
Chemotherapy agent and dose determination
The total absolute and per square meter dose for each alkylator and anthracycline was determined. The alkylating class included mechlorethamine, cyclophosphamide, ifosfamide, carboplatin, cisplatin, busulfan, chlorambucil, procarbazine, nitrosoureas, triethylenemelamine, thiotepa, and dacarbazine. The anthracycline class included daunorubicin, doxorubicin, and idarubicin. Other chemotherapeutic agents administered included vinblastine, vincristine, prednisone, and etoposide. The highest cumulative dose for each chemotherapeutic agent given to any subject was tabulated and quartile values were calculated to determine the score for the doses of agents received by each patient. Scores of 1 to 4 were assigned depending on the quartile for each agent and were summed for each patient.
Statistical analyses
The survival time was defined as the date of HD diagnosis to date of death or last available contact for patients remaining alive. The endpoint of SMN was calculated as date of HD diagnosis to documented date of SMN. Subjects who did not develop a SMN were censored at date of death or last available contact. Some patients had a third malignancy; however, only the earliest relevant neoplasm was used in the analyses.
For SMNs, the SIRs were calculated as the ratio of observed cases to expected cases. The expected number was calculated by applying age-, gender-, and site-specific incidence rates from the registry of the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute to the respective person years of follow-up in each group using a standard time period (36). Confidence intervals (CIs) for the incidence ratios were determined by Poisson distribution. Univariate analyses to identify patient, disease, and treatment characteristics associated with the development of SMN were conducted using the Chi-square test, Mantel-Haenszel test for trend, and the Student's t test, respectively. Similarly, univariate associations with time to the development of SMN were examined using Kaplan-Meier techniques.
Significant variables included in multivariate logistic and proportional hazards regression models were age at HD diagnosis (<12 vs. 12–16 vs. >16 years), gender, and mantle radiation dose. For all analyses, a two-sided a level of 0.05 determined statistical significance.
RESULTS
Between 1960 and 1990, a total of 930 children were treated for HD at participating institutions: 532 were male and 398 were female (ratio, 1.3:1). Mean age at diagnosis was 13.6 years (range, 0.3–18.9), and mean follow-up was 16.8 years (range, 1 month to 39.4 years; Table 1). Stage distribution at diagnosis was known for 929 of the 930 patients: Stage I to II (571 patients) and Stage III to IV (358 patients). Primary treatment was radiation therapy alone (401 patients, 43%), chemotherapy alone (82 patients, 9%), and combined modality therapy (447 patients, 48%). After primary therapy, 227 patients relapsed and 120 of this group died (67 of HD, 14 of SMN, 19 of other toxic events, 20 unknown). Of the 703 patients who did not relapse, 80 died (10 of HD, 23 of SMN, 39 of other events, 8 unknown). At 25 years of follow-up, the survival rate was 74%. At the time that data were abstracted, contact was documented for 89% of patients within the previous 5 years and for 59% of patients within the previous 2 years.
Table 1.
Characteristics of patient population
| Characteristic | All patients n (%) | Patients with secondary malignancy n (%) | Patients without secondary malignancy n (%) | p Value |
|---|---|---|---|---|
| Total patients | 930 | 102 (11.0)* | 828 (89.0) | |
| Gender | <0.0001 | |||
| Male | 532 (57.2) | 30 (29.4) | 502 (60.6) | |
| Female | 398 (42.8) | 72 (70.6) | 326 (39.4) | |
| Stage of Hodgkin's disease | NS | |||
| I to II | 571 (61.4) | 70 (68.6) | 501 (60.5) | |
| III to IV | 358 (38.5)† | 32 (31.4) | 326 (39.4) | |
| Histology | NS | |||
| NS | 586 (63.0) | 66 (11.3) | 520 (88.7) | |
| MC | 226 (24.3) | 26 (11.5) | 200 (88.5) | |
| LP | 70 (7.5) | 5 (7.1) | 65 (92.9) | |
| other | 48 (5.0) | 5 (10.4) | 43 (89.6) | |
| Treatment | ||||
| RT alone | 401 (43.1) | 47 (46.1) | 354 (42.8) | NS |
| CT alone | 82 (8.8) | 6 (5.9) | 76 (9.2) | |
| RT + CT | 447 (48.1) | 49 (48.0) | 398 (48.1) | |
| Splenectomy‡ | ||||
| Yes | 624 (67.1) | 72 (70.6) | 552 (66.7) | NS |
| No | 305 (32.8) | 29 (28.4) | 276 (33.3) | |
| Relapse | ||||
| Yes | 227 (24.4) | 30 (27.4) | 197 (13.8) | NS |
| No | 703 (75.6) | 72 (70.6) | 631 (76.2) | |
| Vital status | ||||
| Alive | 730 (78.5) | 61 (59.8) | 669 (80.8) | <0.0001 |
| Deceased | 200 (21.5) | 41 (40.2) | 159 (19.2) | |
| Age (y), mean | ||||
| Age at Hodgkin's disease diagnosis | 13.6 | 14.1 | 13.5 | NS |
| Range | 0.3–18.9 | 0.3–18.9 | 0.8–18.9 | |
| At SMN diagnosis | 29.5 | |||
| Range | 14.5–54.5 | |||
| Follow-up (y), mean | 16.8 | 19.5 | 16.4 | 0.0003 |
| Range | 1 mo to 39.4 y | 2.9–36.2 | 0.6–39.4 |
Abbreviations: CT = chemotherapy; NS = not significant; RT = radiation therapy; SMN = subsequent malignant neoplasm.
Does not include third neoplasms (n = 8).
Stage unknown for 1 patient.
Splenectomy status unknown for 1 patient.
Treatment
Of the 401 patients treated with radiation alone, 87 received mantle alone, 234 received mantle and para-aortic (including the spleen or pedicle), 50 received total lymphoid, 14 received para-aortic and pelvic (inverted Y), and 16 were treated to other volumes. For individual treatment fields, radiation dose ranged from 6 to 49.8 Gy (mean, 37.1 Gy). Of the 447 patients treated with combined chemotherapy and radiation, 96 received mantle alone, 175 received mantle and para-aortic (including the spleen or pedicle), 135 received total lymphoid, 7 received para-aortic and pelvic, and 34 were treated to other volumes. For individual treatment fields, radiation dose ranged from 2 to 50 Gy (mean, 32.9 Gy).
Of the 616 patients treated with chemotherapy, 594 patients received an alkylator, 269 received an anthracycline, and 265 received both. Only 82 patients received chemotherapy alone. The most common regimens included MOPP^, MOPP^ + ABVD, ABVD, ABV/MOPP^, COP(P^), COP(P^)/ABVD.
Subsequent malignancies
SMNs developed in 102 patients (Table 1). With 15154 patient years of follow-up, the expected number of SMNs was 7.18, resulting in an SIR of 14.21 (95% CI, 11.62–17.33) and an AER of 63 cases per 10,000 person years (Table 2). The actuarial rate of SMNs at 25 years of follow-up was 19%. A third malignancy developed in 8 patients.
Table 2.
Risk of second malignancy
| Characteristic | No. of patients | Patient-years | Expected | Observed | SIR (95% CI) | AER |
|---|---|---|---|---|---|---|
| Entire cohort | 930 | 15154 | 7.18 | 102 | 14.21 (11.62–17.33) | 62.57 |
| Gender | ||||||
| Male | 532 | 8804 | 3.81 | 30 | 8.41 (5.68–12.03) | 30.02 |
| Female | 398 | 6349 | 3.61 | 72 | 19.93 (15.65–25.32) | 107.71 |
| Age (y) | ||||||
| <12 | 271 | 5010 | 1.61 | 23 | 14.30 (9.06–21.45) | 42.70 |
| 12–16 | 354 | 5827 | 2.94 | 47 | 16.00 (11.66–21.44) | 75.61 |
| >16 | 305 | 4316 | 2.63 | 32 | 12.16 (8.21–17.38) | 68.04 |
| Treatment | ||||||
| RT alone | 401 | 6884 | 3.31 | 47 | 14.20 (10.35–19.03) | 63.46 |
| CT alone | 82 | 1057 | 0.46 | 6 | 13.16 (4.83–28.47) | 52.47 |
| RT + CT | 447 | 7212 | 3.41 | 49 | 14.36 (10.47–19.24) | 63.21 |
| Relapse | ||||||
| Yes | 227 | 2776 | 1.22 | 30 | 24.68 (16.66–35.30) | 103.68 |
| No | 703 | 12377 | 5.96 | 72 | 12.07 (9.48–15.33) | 53.35 |
Abbreviations: AER = absolute excess risk; CI = confidence interval; CT = chemotherapy; RT = radiation therapy; SIR = standardized incidence ratio; SMN = subsequent malignant neoplasm.
Gender and age
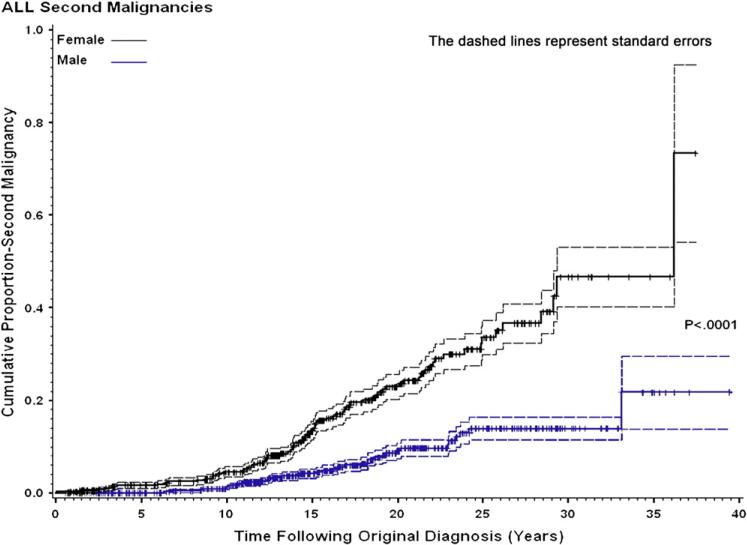
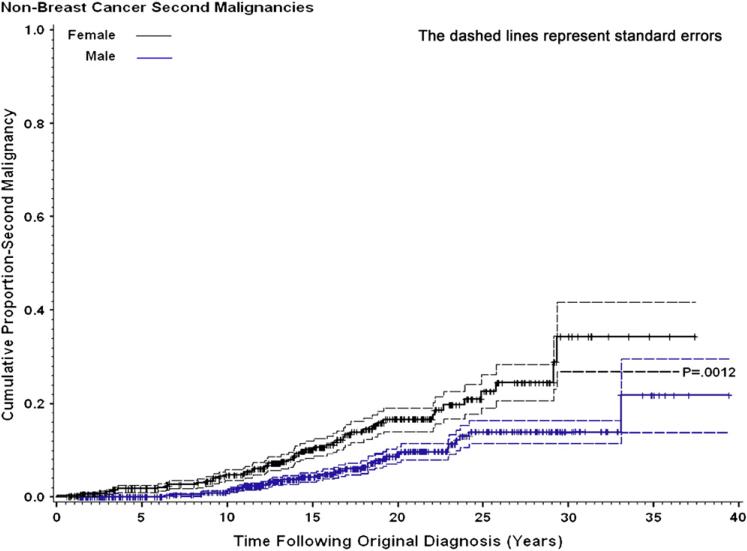
Of the 102 children who developed a SMN, 30 (29%) were male and 72 (71%) were female (ratio, 0.42:1). Age at diagnosis of HD did not differ between male (12.5 ± 4.2) and female (14.5 ± 3.3) patients. The SIR for females, 19.93 (95% CI, 15.65–25.32), was significantly greater than for males, 8.41 (95% CI, 5.68–12.03; p < 0.0001; Table 2). Cumulative incidence of SMNs by gender is shown in Fig. 1. Most cancer types occurred more frequently in females (Table 3). If breast cancer is excluded from risk analysis, the SIR for females falls from 19.93 to 15.14 (95% CI, 9.71–22.0) (p = 0.0012 compared with males). The impact of breast cancer on cumulative incidence is shown in Fig. 2. If thyroid cancer is excluded from risk analysis, the SIR for females falls from 19.28 to 17.28 (95% CI, 13.31–22.48), whereas the risk for males falls from 8.41 to 7.44 (95% CI, 4.90–10.86), which is still significantly different (p < 0.001). However when both breast and thyroid cancers are excluded, the female SIR is 10.0 (6.75–14.3), and the male SIR is 7.44 (4.90–10.86), which is not significantly different (p = 0.256). The mean age at diagnosis of HD for patients who developed a SMN was 14.1 years (not significantly different from patients who did not develop a SMN [mean, 13.5 years]). However, there were significant differences in risk of SMNs by age grouping, with greatest risk for those treated between ages 12 and 16 years, compared with those younger or older (p = 0.03).
Fig. 1.
Cumulative proportion of second malignancies according to gender, with standard errors.
Table 3.
Percentage of all subsequent malignant neoplasms (SMNs) according to site and gender
| All patients (N = 930) |
Female patients (n = 398) |
Male patients (n = 532) |
|
|---|---|---|---|
| SMN site | n (%) | n (% of females) | n (% of males) |
| Breast | 29 (3.1%) | 29 (7.3%) | 0 (0.0%) |
| (% Females vs. males) | 100.0% | 0.0% | |
| Thyroid | 14 (1.5%) | 11 (2.8%) | 3 (0.6%) |
| (% Females vs. males) | 78.6% | 21.4% | |
| Gastrointestinal | 13 (1.4%) | 6 (1.5%) | 7 (1.3%) |
| (% Females vs. males) | 46.2% | 53.8% | |
| Leukemia | 9 (1.0%) | 5 (1.3%) | 4 (0.8%) |
| (% Females vs. males) | 55.6% | 44.4% | |
| Soft tissue sarcoma | 9 (1.0%) | 6 (1.5%) | 3 (0.6%) |
| (% Females vs. males) | 66.7% | 33.3% | |
| Bone | 8 (0.9%) | 6 (1.5%) | 2 (0.4%) |
| (% Females vs. males) | 75.0% | 25.0% | |
| Lymphoma | 6 (0.6%) | 2 (0.5%) | 4 (0.8%) |
| (% Females vs. males) | 33.3% | 66.7% | |
| Central nervous system | 4 (0.4%) | 2 (0.5%) | 2 (0.4%) |
| (% Females vs. males) | 50.0% | 50.0% | |
| Head and neck | 3 (0.3%) | 2 (0.5%) | 1 (0.2%) |
| (% Females vs. males) | 66.7% | 33.3% | |
| Lung | 2 (0.2%) | 0 (0.0%) | 2 (0.4%) |
| (% Females vs. males) | 0.0% | 100.0% | |
| Other | 5 (0.5%) | 3 (1.3%) | 2 (0.4%) |
| (% Females vs. males) | 60% | 40.0% | |
| All secondary malignancies | 102 (11.0%) | 72 (18.1%) | 30 (5.6%) |
| 70.6% | 29.4% | ||
| No secondary malignancy | 828 (89.0%) | 326 (81.9%) | 502 (94.4%) |
| 39.1% | 60.9% |
For the data cells comparing female and male patients, the top value indicates number (n) and percentage (%) of all patients, and the bottom value indicates site-specific percentage (%) of occurrence in female vs. male patients.
Fig. 2.
Cumulative proportion of second malignancies according to gender, with standard errors, after excluding breast cancer.
Radiation and chemotherapy parameters
The risk of SMN was similar regardless of disease stage at diagnosis or primary therapy. For patients receiving radiation, increasing dose was significantly associated with increasing risk of SMNs by Mantel-Haenszel analysis (p = 0.0085). The SIR for patients treated with no radiation or with mantle radiation less than 25 Gy, 25 to less than 35 Gy, and 35 Gy or more was 13.16, 11.7, 12.1, and 16.5, respectively (AER, 63.3, 38.8, 50.8, and 77.8, respectively). Considering the entire patient population, mean radiation dose was greater (32.5 Gy) for patients who developed SMN compared with those who did not (29.2 Gy; p = 0.0099). Comparing the population who received radiation therapy and developed SMN vs. those who did not, mean doses were 35.3 Gy and 32.6 Gy, respectively (p = 0.0003). When only solid SMNs were analyzed, the trend remained significant. Of the SMNs, 77% occurred within the radiation field (85% when AML was excluded), 9% occurred outside the radiation field, 3% at a margin (within 2 cm), and 9% were indeterminate. Although most solid tumors occurred within a radiation field, radiation volume was not associated with risk. Of note, only 1 of 98 (1.0%) girls who received irradiation to the pelvis developed breast cancer vs. 28 of 272 (10.3%) girls who did not receive pelvic irradiation (p = 0.0032). The dose of alkylator, anthracycline, or both was not significantly different for patients who did vs. did not develop SMNs.
Subsequent malignancy type and interval to subsequent malignancy
Exclusive of skin cancers and one ovarian cancer patient, no solid malignancy developed before 8.2 years after therapy (median, 15.2 years) (Table 4). Of 9 leukemias, 7 occurred within 6.6 years after combined chemoradiation therapy. The SIR and AER for time to SMN is shown in Table 5. The probabilities of a SMN were 7%, 7%, 6%, and 6% at less than 15 years, 15 to less than 20, 20 to less than 25, and 25 to less than 30 years post-HD diagnosis, respectively.
Table 4.
Time from diagnosis to second malignancies
| Time (y) |
||||||
|---|---|---|---|---|---|---|
| SMN site | No. | Median | Minimum | Maximum | SIR | AER |
| Breast | 29 | 17.2 | 9.4 | 36.1 | 37.25 | 18.62 |
| Thyroid | 14 | 14.4 | 8.5 | 23.0 | 25.14 | 8.87 |
| Gastrointestinal | 13 | 15.8 | 10.0 | 33.1 | 27.15 | 8.26 |
| Leukemia | 9 | 6.4 | 2.4 | 17.8* | 21.49 | 5.66 |
| Soft-tissue sarcoma | 9 | 15.6 | 8.7 | 18.2 | 50.35 | 5.82 |
| Bone | 8 | 11.6 | 9.2 | 23.4 | 53.47 | 5.18 |
| Lymphoma | 6 | 15.3 | 1.4 | 23.9 | 5.20 | 3.20 |
| Central nervous system | 4 | 11.3 | 8.2 | 29.3 | 9.68 | 2.37 |
| Head and neck | 3 | 16.4 | 15.1 | 19.9 | 12.87 | 1.83 |
| Lung | 2 | 13.4 | 10.6 | 16.2 | 4.55 | 1.03 |
| Other | 5 | 11.5 | 0.03 | 24.9 | 1.78 | 1.45 |
Abbreviations: AER = absolute excess risk; SIR = standardized incidence ratio; SMN = subsequent malignant neoplasm.
Two cases of chronic leukemia occurred at 14.3 and 17.8 years, respectively. No cases of acute leukemia occurred after 7 years.
Table 5.
Second malignancies, by follow-up time
| Time(y) | Patient-years | No. | SIR (95% CI) | AER |
|---|---|---|---|---|
| <15 | 11,530 | 51 | 14.48 (10.75–19.12) | 41.18 |
| 15–<20 | 1,995 | 29 | 19.94 (13.36–28.71) | 138.10 |
| 20–<25 | 1,061 | 15 | 12.84 (7.19–21.19) | 130.39 |
| 25–<30 | 447 | 5 | 7.03 (2.28–16.37) | 95.91 |
| >30 | 121 | 2 | 6.19 (0.75–22.34) | 139.01 |
Abbreviations: AER = absolute excess risk; SIR = standardized incidence ratio.
Relapse and survival
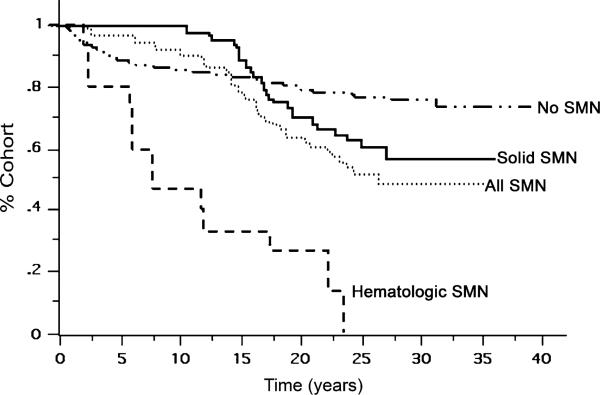
Patients who relapsed had similar risk of SMN compared with patients who did not. Estimated 20-year survival for patients who developed SMNs was 65% (72% for those with a solid tumor), compared with 80% for those with did not (p < 0.001). Among children who developed a hematologic malignancy, 20-year survival was 13% (Fig. 3). At 15 years, the likelihood of dying from a SMN is greater than the likelihood of dying from HD.
Fig. 3.
Overall survival of patients according to whether they developed no secondary malignancy, a solid secondary malignancy, a hematopoietic secondary malignancy, or any secondary malignancy.
On univariate analysis, an increased risk of SMNs was associated with female gender, age, and increasing radiation dose. Splenectomy, radiation volume, anthracycline score, alkylator score, time since treatment, and relapse did not have statistically significant associations with risk. For multivariate analysis (Table 6), a series of models were developed using gender, age at diagnosis (<12 years, 12–16 years, >16 years), and radiation dose (none, and <35 Gy vs. ≥35 Gy). Both logistic and Cox regression techniques were used to model risk and time to SMN. Only gender was significantly associated with risk of SMN (threefold increase for girls). Using logistic regression, both female gender (odds ratio, 3.6) and increased mantle RT (OR, 2.1) were significantly associated with occurrence of SMN (Table 6), and mantle radiation 35 Gy or more increased the risk 2.5-fold for girls. Stratified analyses demonstrated no significant risk factors in male patients.
Table 6.
Factors predictive of time to second malignancy: Proportional hazards model*
| Variable | Parameter estimate | Hazard ratio | p Value |
|---|---|---|---|
| Gender | 1.047 | 2.90 | <0.0001 |
| Total RT (<35 Gy, ≥35 Gy) | –0.200 | 0.82 | NS |
| Mantle RT (<35 Gy, ≥35 Gy) | 0.495 | 1.64 | NS |
| Age at diagnosis (y) (<12, 12–16, >16) years | 0.053 | 1.05 | NS |
Abbreviations: RT = radiation therapy.
Using logistic regression modeling, both female gender (odds ratio, 3.6) and increased mantle RT (odds ratio, 2.1) were significantly associated with occurrence of second malignancy.
DISCUSSION
The risk for SMNs in HD has been extensively studied, but most reports are compromised by incomplete data acquisition, selectivity of patients leading to reporting bias, and limited details on therapy (34). Typically SIRs range from 7.0 to 18 and are highest for secondary leukemias and breast cancer (8–10, 15, 22, 24–26).
We examined risk factors for SMN in pediatric HD patients, focusing on gender and radiation dose. In this investigation, all children treated at five institutions during accrual intervals were analyzed to minimize bias in patient selection.
Association with gender
Even after eliminating breast cancer from risk analysis, the SIR for female patients of 15.14 (19.93 including breast cancer) is still significantly greater than that for male patients (8.41), suggesting that females are indeed more vulnerable than males.
Some studies support our observation of gender difference but attribute the difference to frequent occurrence of breast cancer or do not examine its contribution to gender discrepancy (7, 22, 24–26, 35). Conversely other reports have not shown greater risk for females. In a study of 5,925 children with HD from 16 population-based international cancer registries, the SIRs for females and males were 8.8 and 6.3, respectively (8). Among 1,641 children in five Nordic countries, the SIRs for females and males were 8.9 (95% CI, 6.2–12) and 6.5 (95% CI, 4.3–9.6), respectively (9). In the Stanford University Medical Center (SUMC) report of 694 children, the SIRs for females and males were 15.4 (95% CI, 10.6–21.5) and 10.6 (95% CI, 6.6–16), respectively (25), and among 182 children treated at Roswell Park Cancer Institute (RPCI), the SIRs for females and males were 10.16 (95% CI, 5.56–17.05) and 9.39 (95% CI, 4.05–18.49), respectively (26). The CIs overlapped in these studies, and in the SUMC series the increased female risk (hazard ratio, 1.8) was due to breast cancers. The excessive frequency of thyroid cancer in our female patients has been observed in some studies but not in others (37–39). Regardless, no obvious explanation for gender association with secondary thyroid cancer has been identified. When considering large cohort studies assessing the risk for SMNs in children with any primary malignancy, a gender-based difference is not identified except for secondary breast cancer (23, 28, 40). The low background rate of breast cancer in males precludes any assessment of treatment-associated gender difference.
The risk of SMNs in adults treated for HD is much smaller than for children and demonstrates an overall male predominance (or an equal gender distribution) in most, but not all, reports (13, 14–22, 41). In an international collaborative report of 12,411 HD patients aged 14 years or more, the SIR for females was 2.6 and for males it was 3.0. (19). In an Italian study of 1,524 patients, male gender was associated with increased cumulative probability of solid SMNs (18% vs. 8.9%, p = 0.02) (21). No explanation for a gender-based difference for SMNs in adults, beyond that reflecting a gender-based difference in background incidence, was identified.
Association with radiation dose and volume
High, but not low, radiation doses were related to risk for SMN in our study. Increasing dose was associated with an increasing SIR (p = 0.0085), and the mean dose was greater for patients who developed a SMN (p = 0.0099). About 75% of SMNs occurred within a radiation field. However, we did not observe a relationship between the volume of body irradiated and the development of SMNs. Such a relationship might be expected for secondary sarcomas; however, the number of sarcomas in our study was small, obviating our ability to demonstrate a relationship. Since the 98 girls who had pelvic radiation therapy appeared to be protected against breast cancer, data on this subgroup would also undermine any association between volume and SMNs. In a recent report in adults with HD that compared subtotal nodal vs. involved field radiation (both combined with chemotherapy), the 3 patients who developed a SMN were in the subtotal nodal group (42).
As in most other studies, patients treated only with radiation did not have an increased risk for hematologic malignancy (17, 25, 36, 43). The SIR for those treated with only chemotherapy has limited precision: only 82 subjects were included in this group. Data for radiation dose was associated with a significant increase in risk. Most reports addressing radiation dose and location of malignancy are adult based but support our observations (13, 17, 27, 29).
Chemotherapeutic agents
The association between alkylators and SMNs, particularly leukemia and bone sarcomas, has been extensively described (22, 27, 43–45). In our study, alkylators were not independently associated with risk. A possible explanation is our large number of breast cancers and recent data that show an inverse association between alkylating therapy and breast cancer (16, 45, 46). However, some investigators have found increased risk for breast cancer when alkylating agents are combined with radiation (13, 25). Although association between doxorubicin and the development of SMN has been reported in survivors of Wilms’ tumor (47), anthracyclines are generally considered to have less carcinogenic potential. No association between anthracyclines and SMNs was observed in our study.
Association with age at treatment
In our study, children treated between the ages of 12 and 16 years were more likely to develop SMNs than children who were either younger or older (p = 0.03). This finding is at odds with the recent report from the Late Effects Study Group (LESG), in which SMNs were independently associated with younger age at diagnosis of HD (p = 0.02) (7). Age was significant on univariate analysis and was entered into the models; however, similar to the findings of the LESG, we found that age was not statistically predictive of SMN after adjustment for the other variables in the model. The univariate association with peri-pubertal age in our report may relate to the large number of girls who later developed breast cancer, a subgroup who also had an age-related risk (data reported separately). It is also possible that younger patients were less aggressively treated, accounting for an increased risk for SMNs in older children.
The explanation for a greater likelihood of SMNs in children as compared with adults is not unequivocally known. However, it is probable that the developing tissues of children are more susceptible to mutational events than the more static tissues in adults. This may relate to some combination of the proliferative rate and genomic instability, but hormonal differences in these populations may also be relevant (11). Finally, the natural incidence of malignancies in adults, such as breast and lung cancer, may account for some of the difference between adults and children (32, 48).
Interval to development of subsequent malignancy
The interval to SMN was related to its histopathologic type, consistent with other reports (10, 13, 15, 24–26, 30, 49). Acute myelocytic leukemias occurred within 2 to 7 years, whereas two cases of chronic leukemia, which followed radiation alone, occurred at 14 and 17 years. No solid tumors occurred before 8 years. Breast cancers most commonly occurred 10 to 15 years after HD diagnosis, and sarcomas between 15 and 20 years after HD diagnosis. Of note, solid tumors continue to appear over extended time intervals, a common finding in reports of pediatric HD survivors.
Treatment modality and relapse
Patients treated with radiation alone or combined chemoradiation had a similar risk for the occurrence of SMN. Too few patients were treated with chemotherapy alone to allow us to determine any association. Similar risk for SMN after radiation and combined modality therapy was also reported by the St. Jude Children's Research Hospital (SJCRH) and SUMC groups (24, 25), and in the latter study risk was similar for children treated with chemotherapy alone. The association of recurrent HD with risk (24, 25, 48) presumably relates to additional therapeutic burden. A recent report, however, does not support this observation (50). In our investigation, relapse was significant by univariate analysis for the entire cohort, but not by multivariate analysis, possibly relating to patient numbers.
Breast cancer
Breast cancer was the most common SMN in our study, with a SIR of 37.25 (95% CI, 24.96–53.64; AER, 18.62). This is within the range reported in other studies (note variability): LESG 55.5, SUMC 26.2, SJCRH 33.2, Nordic countries 17, and RPCI 7.77. An explanation for this broad range is unclear. High estimates could be attributed to less complete follow-up of healthy patients in multi-institutional surveys. It is interesting that the recently reported SIR from the LESG is less than that reported in their previous analysis (75.3; 95% CI, 44.9–118.4) in which the follow-up interval was shorter. Differences in treatment technique, and in particular radiation dose (51), have been suggested as explanations. Optimistically, tailored treatment volumes should reduce the likelihood of breast cancer in future survivors of pediatric HD.
Splenectomy
Splenectomy has been observed to be associated with SMN, specifically leukemia (22), in some reports (23, 26) but not others (24, 25). In our investigation, splenectomy was not significant.
Survival after development of subsequent malignancy
Our study confirms the dire fact that essentially all children who develop leukemia as a secondary malignancy will die as a consequence. Overall, risk of death from SMN surpasses risk of death from HD in this subgroup of patients (22, 25, 50, 52, 53).
Study limitations
Limitations of this study include the retrospective analysis of a patient cohort from five institutions in which treatment decision making was non-uniform. However, when comparing children who did or did not develop a SMN, no difference was seen between the various treatment approaches. We also could not adjust for lifestyle risk factors or for medication or hormonal use that could influence the occurrence of SMNs. Follow-up for the group of children who developed a SMN was 3.1 years longer than for those who did not, which could be a source of bias; however, this difference is not likely to obviate these findings.
CONCLUSION
Tremendous strides have been made in treating children with HD, both in terms of cure and reduction of toxicity. When Donaldson et al. initiated pediatric HD trials using chemotherapy and low-dose irradiation (54), the primary motivation was to reduce the musculoskeletal growth inhibition that accompanied treatment modeled after that used in adults. The identified role of radiation therapy and specific chemotherapeutic agents in causing SMN prompted modifications of therapy, which have had an impact. Genetic susceptibilities and hormonal influences will be critical to explore and are underscored by our observation that females are more susceptible than males (28, 55). Despite SMNs, the focus of treatment for children with HD should be to cure the primary malignancy. Minimizing the occurrence and impact of SMN will require a multifaceted attack, which fortunately is in progress.
Acknowledgment
The authors thank Amy K. Huser for thoughtful research, writing, and editing contributions.
Footnotes
Conflict of interest: none.
REFERENCES
- 1.Donaldson SS, Link MP. Combined modality treatment with low-dose radiation and MOPP chemotherapy for children with Hodgkin's disease. J Clin Oncol. 1987;5:742–749. doi: 10.1200/JCO.1987.5.5.742. [DOI] [PubMed] [Google Scholar]
- 2.Hutchinson R, Fryer C, Davis P, et al. MOPP or radiation in addition to ABVD in the treatment of pathologically staged advanced Hodgkin's disease in children: Results of the Children's Cancer Group Phase III Trial. J Clin Oncol. 1998;16:897–906. doi: 10.1200/JCO.1998.16.3.897. [DOI] [PubMed] [Google Scholar]
- 3.Landman-Parker J, Pacquement H, Leblanc T, et al. Localized childhood Hodgkin's disease: Response-adapted chemotherapy with etoposide, bleomycin, vinblastine, and prednisone before low-dose radiation therapy-results of the French Society of Pediatric Oncology Study MDH90. J Clin Oncol. 2000;18:1500–1507. doi: 10.1200/JCO.2000.18.7.1500. [DOI] [PubMed] [Google Scholar]
- 4.Nachman J, Sposto R, Herzog P, et al. Randomized comparison of low-dose involved-field radiotherapy and no radiotherapy for children with Hodgkin's disease who achieve a complete response to chemotherapy. J Clin Oncol. 2002;20:3765–3771. doi: 10.1200/JCO.2002.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Rühl U, Albrecht M, Dieckmann K, et al. Response-adapted radiotherapy in the treatment of pediatric Hodgkin's disease: An interim report at 5 years of the German GPOH-HD 95 trial. Int J Radiat Oncol Biol Phys. 2001;51:1209–1218. doi: 10.1016/s0360-3016(01)01798-9. [DOI] [PubMed] [Google Scholar]
- 6.Donaldson S, Hudson M, Lamborn K, et al. VAMP and low-dose, involved-field radiation for children and adolescents with favorable, early-stage Hodgkin's disease: Results of a prospective clinical trial. J Clin Oncol. 2002;20:3081–3087. doi: 10.1200/JCO.2002.12.101. [DOI] [PubMed] [Google Scholar]
- 7.Bhatia S, Yasui Y, Robison L, et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin's disease: Report from the Late Effects Study Group. J Clin Oncol. 2003;21:4386–4394. doi: 10.1200/JCO.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 8.Metayer C, Lynch C, Clarke E, et al. Second cancers among long-term survivors of Hodgkin's disease diagnosed in childhood and adolescence. J Clin Oncol. 2000;18:2435–2443. doi: 10.1200/JCO.2000.18.12.2435. [DOI] [PubMed] [Google Scholar]
- 9.Sankila R, Garwicz J, Olsen H, et al. Risk of subsequent malignant neoplasms among 1,641 Hodgkin's disease patients diagnosed in childhood and adolescence: A population-based cohort study in five Nordic countries. J Clin Oncol. 1996;14:1442–1446. doi: 10.1200/JCO.1996.14.5.1442. [DOI] [PubMed] [Google Scholar]
- 10.Hudson M, Poquette C, Lee J, et al. Increased mortality after successful treatment for Hodgkin's disease. J Clin Oncol. 1998;16:3592–3600. doi: 10.1200/JCO.1998.16.11.3592. [DOI] [PubMed] [Google Scholar]
- 11.Abrahamsen JF, Andersen A, Hannisdal E, et al. Second malignancies after treatment of Hodgkin's disease: The influence of treatment, follow-up time, and age. J Clin Oncol. 1993;11:255–261. doi: 10.1200/JCO.1993.11.2.255. [DOI] [PubMed] [Google Scholar]
- 12.Henry-Amar M. Second cancer after the treatment for Hodgkin's disease: A report from the International Database on Hodgkin's disease. Ann Oncol. 1992;3(Suppl 4):117–128. doi: 10.1093/annonc/3.suppl_4.s117. [DOI] [PubMed] [Google Scholar]
- 13.Kaldor J, Day N, Clarke E, et al. Leukemia following Hodgkin's disease. N Eng J Med. 1990;322:7–13. doi: 10.1056/NEJM199001043220102. [DOI] [PubMed] [Google Scholar]
- 14.van Leeuwen F, Klokman W, Hagenbeek A, et al. Second cancer risk following Hodgkin's disease: A 20-year follow-up study. J Clin Oncol. 1994;12:312–325. doi: 10.1200/JCO.1994.12.2.312. [DOI] [PubMed] [Google Scholar]
- 15.van Leeuwen F, Klokman W, van't Veer M, et al. Long-term risk of second malignancy in survivors of Hodgkin's disease treated during adolescence or young adulthood. J Clin Oncol. 2000;18:487–497. doi: 10.1200/JCO.2000.18.3.487. [DOI] [PubMed] [Google Scholar]
- 16.Hancock S, Tucker M, Hoppe R. Breast cancer after treatment of Hodgkin's disease. J Natl Cancer Inst. 1993;85:25–31. doi: 10.1093/jnci/85.1.25. [DOI] [PubMed] [Google Scholar]
- 17.Boivin JF, Hutchison GB, Zauber AG, et al. Incidence of second cancers in patients treated for Hodgkin's disease. J Natl Cancer Inst. 1995;87:732–741. doi: 10.1093/jnci/87.10.732. [DOI] [PubMed] [Google Scholar]
- 18.Mauch P, Kalish L, Marcus K, et al. Second malignancies after treatment for laparotomy staged IA-IIIB Hodgkin's disease: Long-term analysis of risk factors and outcome. Blood. 1996;87:3625–3632. [PubMed] [Google Scholar]
- 19.Kaldor J, Day N, Band P, et al. Second malignancies following testicular cancer, ovarian cancer and Hodgkin's disease: An international collaborative study among cancer registries. Int J Cancer. 1987;39:571–585. doi: 10.1002/ijc.2910390506. [DOI] [PubMed] [Google Scholar]
- 20.Tucker M, Coleman C, Cox R, et al. Risk of second cancers after treatment for Hodgkin's disease. N Engl J Med. 1988;318:76–81. doi: 10.1056/NEJM198801143180203. [DOI] [PubMed] [Google Scholar]
- 21.Cellai E, Magini S, Masala G, et al. the risk of second malignant tumors and its consequences for the overall survival of Hodgkin's disease patients and for the choice of their treatment at presentation: Analysis of a series of 1524 cases consecutively treated at the Florence University Hospital. Int J Radiat Oncol Biol Phys. 2001;49:1327–1337. doi: 10.1016/s0360-3016(00)01513-3. [DOI] [PubMed] [Google Scholar]
- 22.Bhatia S, Robison L, Oberlin O, et al. Breast cancer and other second neoplasms after childhood Hodgkin's disease. N Engl J Med. 1996;334:745–751. doi: 10.1056/NEJM199603213341201. [DOI] [PubMed] [Google Scholar]
- 23.Tarbell N, Gelber R, Weinstein H, et al. Gender differences in risk of second malignant tumours after Hodgkin's disease in childhood. Lancet. 1993;341:1428–1432. doi: 10.1016/0140-6736(93)90880-p. [DOI] [PubMed] [Google Scholar]
- 24.Beaty O, Hudson M, Greenwald C, et al. Subsequent malignancies in children and adolescents after treatment for Hodgkin's disease. J Clin Oncol. 1995;13:603–609. doi: 10.1200/JCO.1995.13.3.603. [DOI] [PubMed] [Google Scholar]
- 25.Wolden S, Lamborn K, Cleary S, et al. Second cancers following pediatric Hodgkin's disease. J Clin Oncol. 1998;16:536–544. doi: 10.1200/JCO.1998.16.2.536. [DOI] [PubMed] [Google Scholar]
- 26.Green D, Hyland A, Barcos M, et al. Second malignant neoplasms after treatment for Hodgkin's disease in childhood or adolescence. J Clin Oncol. 2000;18:1492–1499. doi: 10.1200/JCO.2000.18.7.1492. [DOI] [PubMed] [Google Scholar]
- 27.Neglia J, Friedman D, Yasui Y, et al. Second malignant neoplasms in five-year survivors of childhood cancer: Childhood Cancer Survivor Study. J Nat Cancer Inst. 2001;93:618–629. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 28.Armstrong G, Sklar C, Hudson M, et al. Long-term health status among survivors of childhood cancer: Does gender matter? J Clin Oncol. 2007;25:4477–4489. doi: 10.1200/JCO.2007.11.2003. [DOI] [PubMed] [Google Scholar]
- 29.Ng A, Bernardo M, Weller E, et al. Second malignancy after Hodgkin disease treated with radiation therapy with or without chemotherapy: Long-term risks and risk factors. Blood. 2002;100:1989–1996. doi: 10.1182/blood-2002-02-0634. [DOI] [PubMed] [Google Scholar]
- 30.Foss Abrahamsen A, Andersen A, Nome O, et al. Long-term risk of second malignancy after treatment of Hodgkin's disease: The influence of treatment, age and follow-up time. Ann Oncol. 2002;13:1786–1791. doi: 10.1093/annonc/mdf289. [DOI] [PubMed] [Google Scholar]
- 31.Dores G, Metayer C, Curtis R, et al. Second malignant neoplasms among long-term survivors of Hodgkin's disease: A population-based evaluation over 25 years. J Clin Oncol. 2002;20:3484–3494. doi: 10.1200/JCO.2002.09.038. [DOI] [PubMed] [Google Scholar]
- 32.Travis L, Gospodarowicz M, Curtis R, et al. Lung cancer following chemotherapy and radiotherapy for Hodgkin's disease. J Natl Cancer Inst. 2002;94:182–192. doi: 10.1093/jnci/94.3.182. [DOI] [PubMed] [Google Scholar]
- 33.Travis LB, Hill D, Dores GM, et al. Cumulative absolute breast cancer risk for young women treated for hodgkin lymphoma. J Natl Cancer Inst. 2005;97:1428–1437. doi: 10.1093/jnci/dji290. [DOI] [PubMed] [Google Scholar]
- 34.Hill DA, Gilbert E, Dores GM, et al. Breast cancer risk following radiotherapy for hodgkin lymphoma: Modification by other risk factors. Blood. 2005;106:3358–3365. doi: 10.1182/blood-2005-04-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bassal M, Mertens AC, Taylor L, et al. Risk of selected subsequent carcinomas in survivors of childhood cancer: A report from the childhood cancer survivor study. J Clin Oncol. 2006;24:476–483. doi: 10.1200/JCO.2005.02.7235. [DOI] [PubMed] [Google Scholar]
- 36.Ries L, Kosary C, Hankey B, et al. SEER Cancer Statistics Review, 1973–1996. National Cancer Institute; Bethesda, MD: 1999. [Google Scholar]
- 37.Ron E, Lubin J, Shore R, et al. Thyroid cancer after exposure to external radiation: A pooled analysis of seven studies. Radiat Res. 1995;141:259–277. [PubMed] [Google Scholar]
- 38.Sigurdson A, Ronckers C, Mertens A, et al. Primary thyroid cancer after a first tumour in childhood (the Childhood Cancer Survivor Study): A nested case-control study. Lancet. 2005;365:2014–2023. doi: 10.1016/S0140-6736(05)66695-0. [DOI] [PubMed] [Google Scholar]
- 39.Tucker M, Jones PH, Boice J, et al. Therapeutic radiation at a young age is linked to secondary thyroid cancer. The Late Effects Study Group. Cancer Res. 1991;51:2885–2888. [PubMed] [Google Scholar]
- 40.Cardous-Ubbink M, Heinen R, Bakker P, et al. Risk of second malignancies in long-term survivors of childhood cancer. Eur J Cancer. 2007;43:351–362. doi: 10.1016/j.ejca.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Hodgson D, Gilbert E, Dores G, et al. Long-term solid cancer risk among 5-year survivors of Hodgkin's lymphoma. J Clin Oncol. 2007;25:1489–1497. doi: 10.1200/JCO.2006.09.0936. [DOI] [PubMed] [Google Scholar]
- 42.Bonadonna G, Bonfante V, Viviani S, et al. ABVD plus subtotal nodal versus involved-field radiotherapy in early-stage Hodgkin's disease: Long-term results. J Clin Oncol. 2004;22:2835–2841. doi: 10.1200/JCO.2004.12.170. [DOI] [PubMed] [Google Scholar]
- 43.Donaldson S, Hancock S. Second cancers after Hodgkin's disease in childhood. N Eng J Med. 1996;334:792–794. doi: 10.1056/NEJM199603213341210. [DOI] [PubMed] [Google Scholar]
- 44.Josting A, Wiedenmann S, Franklin J, et al. Secondary myeloid leukemia and myelodysplastic syndromes in patients treated for Hodgkin's disease: A report from the German Hodgkin's Lymphoma Study Group. J Clin Oncol. 2003;21:3440–3446. doi: 10.1200/JCO.2003.07.160. [DOI] [PubMed] [Google Scholar]
- 45.Tucker M, Coleman C, Cox R, et al. Risk of second cancers after treatment for Hodgkin's disease. N Eng J Med. 1988;318:76–81. doi: 10.1056/NEJM198801143180203. [DOI] [PubMed] [Google Scholar]
- 46.Tucker MA, Meadows AT, Boice JD, Jr, et al. Leukemia after therapy with alkylating agents for childhood cancer. J Natl Cancer Inst. 1987;78:459–464. [PubMed] [Google Scholar]
- 47.Breslow N, Takashima J, Whittam J, et al. Second malignant neoplasms following treatment for Wilms’ tumor: A report from the Wilms’ Tumor Study Group. J Clin Oncol. 1995;13:1851–1859. doi: 10.1200/JCO.1995.13.8.1851. [DOI] [PubMed] [Google Scholar]
- 48.Travis L, Hill D, Dores G, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA. 2003;290:465–475. doi: 10.1001/jama.290.4.465. [DOI] [PubMed] [Google Scholar]
- 49.van Leeuwen F, Klokman W, Stovall M, et al. Roles of radiation dose, chemotherapy, and hormonal factors in breast cancer following Hodgkin's disease. J Nat Cancer Inst. 2003;95:971–980. doi: 10.1093/jnci/95.13.971. [DOI] [PubMed] [Google Scholar]
- 50.Forrest D, Hogge D, Nevill T, et al. High-dose therapy and autologous hematopoetic stem-cell transplantation does not increase the risk of second neoplasms for patients with Hodgkin's lymphoma: A comparison of conventional therapy alone versus conventional therapy followed by autologous hematopoietic stem-cell transplantation. J Clin Oncol. 2005;23:7994–8002. doi: 10.1200/JCO.2005.01.9083. [DOI] [PubMed] [Google Scholar]
- 51.Koletsky A, Bertion J, Farber L, et al. Second neoplasms in patients with Hodgkin's disease following combined modality therapy—the Yale experience. J Clin Oncol. 1986;4:311–317. doi: 10.1200/JCO.1986.4.3.311. [DOI] [PubMed] [Google Scholar]
- 52.Dietrich PY, Henry-Amar M, Cosset J, et al. Second primary cancers in patients continuously disease-free from Hodgkin's disease: A protective role for the spleen? Blood. 1994;84:1209–1215. [PubMed] [Google Scholar]
- 53.Ng A, Bernardo M, Weller E, et al. Long-term survival and competing causes of death in patients with early-stage Hodgkin's disease treated at age 50 or younger. J Clin Oncol. 2002;20:2101–2108. doi: 10.1200/JCO.2002.08.021. [DOI] [PubMed] [Google Scholar]
- 54.Donaldson SS, Glatstein E, Rosenberg SA, et al. Pediatric Hodgkin's disease. II. Results of therapy. Cancer. 1976;37:2436–2447. doi: 10.1002/1097-0142(197605)37:5<2436::aid-cncr2820370537>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 55.Mertens A, Mitby P, Radloff G, et al. XRCC1 and glutathione-S-transferase gene polymorphisms and susceptibility to radiotherapy-related malignancies in survivors of Hodgkin disease: A report from the Childhood Cancer Survivor Study. Cancer. 2004;101:1463–1472. doi: 10.1002/cncr.20520. [DOI] [PubMed] [Google Scholar]