Abstract
Purpose
Carnitine is an essential cofactor for mitochondrial fatty acid oxidation that is actively reabsorbed by the luminal transporter Octn2 (Slc22a5). Since the nephrotoxic agent cisplatin causes urinary loss of carnitine in humans, we hypothesized that cisplatin may affect Octn2 function.
Experimental Design
Excretion of carnitine and acetylcarnitine was measured in urine collected from mice with or without cisplatin administration. The transport of carnitine was assessed in cells that were transfected with OCT1 or OCT2. The effect of cisplatin treatment on gene expression was analyzed using a mouse GeneChip array and validated using qRT-PCR.
Results
In wildtype mice, urinary carnitine excretion at baseline was about ~3 fold higher than in mice lacking the basolateral cisplatin transporters Oct1 and Oct2 [Oct1/2(−/−) mice], indicating that carnitine itself undergoes basolateral uptake into the kidney. Transport of carnitine by OCT2, but not OCT1, was confirmed in transfected cells. We also found that cisplatin caused an increase in the urinary excretion of carnitine and acetylcarnitine in wildtype mice but not in Oct1/2(−/−) mice, suggesting that tubular transport of cisplatin is a prerequisite for this phenomenon. Cisplatin did not directly inhibit the transport of carnitine by Octn2, but downregulated multiple target genes of the transcription factor Ppar-α, including Slc22a5, in kidney of wildtype mice that were absent in Oct1/2(−/−) mice.
Conclusion
Our study demonstrates a pivotal role of Oct1 and Oct2 in cisplatin-related disturbances in carnitine homeostasis. We postulate that this phenomenon is triggered by deactivation of Ppar-α and leads to deregulation of carnitine-shuttle genes.
Introduction
Cisplatin is among the most widely used anticancer agents, but its clinical use is limited by its potential to induce severe nephrotoxicity. The cisplatin concentration in proximal tubular cells is ~5 times the circulating blood level, and this disproportionate accumulation of cisplatin in kidney tissue is known to contribute to nephrotoxicity (1). Recent studies have indicated that the initial uptake of cisplatin into renal tubular cells is mediated by the organic cation transporter OCT2 (2), and that mice lacking the ortholog transporters Oct1 and Oct2 [Oct1/2(−/−) mice] are protected from experiencing severe cisplatin nephrotoxicity (3, 4). Furthermore, various physiologic hallmarks of cisplatin nephrotoxicity in humans, such as changes in blood urea nitrogen, serum creatinine, and glucose (5), were also specifically altered in wildtype mice undergoing cisplatin treatment (3, 4).
Decreased glucose levels (hypoglycemia) can occur when fatty acid oxidation is defective, leading to glucose consumption without regeneration via gluconeogenesis. Carnitine (also known as vitamin BT; β-hydroxy-γ-trimethylaminobutyric acid) is a hydrophilic zwitterion that plays an essential role in this process by the transfer of long-chain fatty acids inside mitochondria for β-oxidation, and as such is critical in the production of cellular energy (6). This pathway of carnitine-dependent transport of fatty acids to mitochondria consists of carnitine palmitoyltransferase I (Cpt1), carnitine-acylcarnitine translocase (Cact) and carnitine palmitoyltransferase II (Cpt2) (7) (Suppl. Figure S1). Carnitine is normally maintained at a steady level in the blood via a rescue mechanism that involves reabsorption in the kidney of filtered carnitine by the solute carrier OCTN2 (SLC22A5; CT1) (8). The existence of this reabsorption mechanism for carnitine, as well as the occurrence of mutations in OCTN2 that lead to primary systemic carnitine deficiency, strongly support the critical physiological importance of OCTN2 (9). Indeed, metabolic abnormalities similar to human primary systemic carnitine deficiency have been described in juvenile visceral steatosis mice (10), and demonstrated to be caused by a null mutation (1114T>G; L352R) in the mouse Octn2 gene, Slc22a5 (11). Furthermore, heterozygosity for OCTN2 mutations in humans is associated with an intermediate carnitine-deficiency phenotype, suggesting that even partial loss of transporter function may be detrimental. In this context, it is particularly noteworthy that cisplatin was previously reported to cause excessive urinary loss of carnitine in cancer patients (5), although the mechanism by which this occurs remains unknown. Against this background, we investigated the impact of cisplatin treatment on carnitine homeostasis in wildtype mice and Oct1/2(−/−) mice to (a) elucidate the molecular basis of the putative interaction of cisplatin with carnitine-shuttle genes, and to (b) further our understanding of treatment-related side effects associated with hypocarnitinemia.
Materials and Methods
Chemicals and reagents
[3H]Carnitine (80 Ci/mmol) and [14C]tetraethylammonium (55 mCi/mmol; TEA) were purchased from American Radiolabeled Chemicals Inc. Unlabeled carnitine, acetylcarnitine, TEA bromide, and cisplatin were purchased from Sigma. Deuterated carnitines were obtained from Cambridge Isotope Laboratories. Cisplatin-glutathione conjugates were produced by incubating glutathione (3.33 mM) with cisplatin (1.67 mM) in a buffer containing 154 mM sodium chloride and 10 mM sodium phosphate (pH 7.4) at 37 °C, as described (12). All other compounds used in this study were of reagent grade or better.
Animals
Adult (8–12 week old) male FVB wild-type mice (n=11) and Oct1/2(−/−) mice (n=12) were obtained from Taconic. The experimental protocols were reviewed and approved by the St. Jude Children’s Research Hospital Animal Care Committee in accordance with the “Guide for the Care and Use of Laboratory Animals”. The animals were acclimatized for at least 1 week before experimentation, fed with a standard National Institute of Nutrition diet, and allowed tap water ad libitum. For collection of urine, animals were housed in metabolic cages in a temperature controlled environment with a 12-h reverse light cycle. After a 24-h baseline urine sample was collected, mice were given a single intraperitoneal (i.p.) injection of cisplatin at a dose of 10 mg/kg. Urine was subsequently collected at 24, 48, and 72 h after administration.
Carnitine analysis
Quantitative determination of carnitine and acetylcarnitine in mouse urine was performed using liquid chromatography with tandem mass spectrometric detection. In brief, 40 μL-samples were diluted 5-fold with water containing d3-carnitine hydrochloride (10 μM) and d3-acetylcarnitine hydrochloride (2 μM) as internal standards, and 25 μL-aliquots were injected onto a Waters 2695 Separation Module. Chromatographic separation was achieved on a reversed phase column (150×2.1 mm, i.d.) packed with a 3-μm ODS-3 stationary phase (GL Sciences) using a mobile phase [an 85:15 (v/v) mixture of 0.2% of the ion-pairing agent IPCC-MS3 in water and 0.1% formic acid in acetonitrile] delivered at a flow rate of 0.3 mL/min. The column effluent was monitored using a Micromass Quattro LC triple-quadrupole mass-spectrometric detector equipped with an electrospray interface, and controlled by Masslynx v4.1 software. The observed mass transitions (m/z) in positive ion mode included 162.2→84.8 (carnitine), 165.2→102.7 (d3-carnitine), 204.2→84.8 (acetylcarnitine) and 207.2→84.7 (d3-acetylcarnitine). Samples were analyzed along with an 8-point calibration curve ranging from 1 to 200 μM (carnitine) or 0.25 to 50 μM (acetylcarnitine) and three quality control samples analyzed in quadruplicate, and results were interpolated on a quadratic regression curve with1/x2 weighing. The percent deviation from nominal values, within-run precision, and between-run precision for each analyte were always within ±3%, <10.7%, and <2.9%, respectively.
Gene expression analysis
RNA was extracted using the RNEasy mini kit (Qiagen). RNA samples were amplified from 4 animals per group [wildtype mice or Oct1/2(−/−) mice] both before and at 72 h after administration of cisplatin (10 mg/kg, i.p.) and then analyzed using the Mouse 430v2 GeneChip array (Affymetrix). Additional samples were obtained from wildtype mice at 24, 48, and 72 h after cisplatin administration. For real-time RT-PCR analyses, RNA was reverse transcribed in samples from 3 animals per group using SuperScript III first strand synthesis supermix for qRT-PCR (Invitrogen) according to manufacturer’s recommendations. The relative expression levels for mRNA were measured using Taqman probes, Universal Master Mix and Assay-on-Demand products from Applied Biosystems. Each reaction was carried out in triplicate, and transcripts of each sample were normalized to the housekeeping gene, Gapdh.
Nephrotoxicity analysis
After collection of kidneys, tissues from all animals were fixed overnight in 10% neutral-buffered formalin, embedded in paraffin, sectioned (4μm), and stained with hematoxylin and eosin (Lab Vision). Microscopic evaluation was performed independently by two experienced veterinary pathologists blinded to the composition of the groups. Nephrotoxicity was assessed from the percentage of observed damaged tubules and graded on a 5-point scale as 0 (<10%; absent), 1 (11–25%; minimal); 2 (26–50%; mild), 3 (51–75%; moderate), 4 (>75%; severe).
Assessment of tubular apoptosis
Caspase-3 analysis was performed on formalin-fixed, parrafin-embedded sections of kidney samples of 4 animals per group after deparaffinization in xylene. Heat-induced epitope-retrieval was performed by heating slides in a pressure cooker at 98°C for 30 min in Target Retrieval buffer, pH 9.0 (DAKO). Endogenous peroxidases were blocked by incubating slides for 5 min with 3% aqueous hydrogen peroxide, and nonspecific protein binding was blocked by incubating for 30 min with Superblock (Pierce). The primary antibody, rabbit anti-human cleaved caspase-3 (BioCare Medical) was used at 1:100 for 60 min, followed by incubation for 30 min with the polymer Rabbit on Rodent (BioCare Medical). Next, slides were incubated for with the chromagen 3,3′-diaminobenzidine (DAKO) for 5 min and counterstained with dilute hematoxylin. In each slide, 10 random high-power fields (40×) were selected for immunohistochemistry scoring. The number of cells with strong cytoplasmic or nuclear staining for caspase-3 was counted for each tissue to obtain the average number of positive cells per high power field for each kidney.
Cellular transport studies
HEK293 cells transfected with human OCTN1, human OCTN2, mouse Octn2, or an empty pcDNA3 vector were kindly provided by Dr. Akira Tsuji (Kanazawa, Japan). These cells were cultivatedin DMEM containing 10% fetal calfserum, 1 mg/mL G418, 100 U/mL penicillin, and 100μg/mL streptomycin. Stably transfected 293Flp-In cells expressing OCT2 or an empty pcDNA5 vector have been described previously (2). These cell lines were collected and reseeded in complete DMEM containing 10% FBS and hygromycin B (100 μg/mL). All uptake and inhibition assays were performed on monolayer cultures in 6-well plates in a humidified incubator at 37°C under 5% CO2 and 95% humidity, as described (2). For evaluating OCT1 function, Xenopus laevis oocytes (BD Biosciences) injected with transporter cRNA and water-injected controls were used, as described (13).
Computational OCTN2 docking analysis
An OCTN2 homology model was generated similar to that described previously for OCT1 (14). The sequence of human OCTN2 was taken from the SwissProt database (entry O76082; http://us.expasy.org/sprot/), and submitted to the meta-server 3D-Jury (15) to identify a protein template, and the template of GlpT (PDB entry: 1PW4) (16) was identified from this search. The homology module of InsightII (MSI Inc.) and the ClustalW algorithm (17) were then applied in sequence alignment, and the Blosum scoring matrix (18) was employed to obtain the best-fit alignment, which was selected according not only to the value of the alignment score, but also the reciprocal positions of residues that are important for the transport function of OCTN2 (19, 20). The resultant structure of OCTN2 was optimized using a molecular mechanics method with the following parameters: a distance-dependent dielectric constant of 1.0; non-bonded cutoff of 8 Å; Amber force field (20), and Kollman-all-atom charges; and conjugate gradient minimization until 0.05 kcal/(mol·Å). The minimized structure was validated using the PROCHECK, as described (21).
The putative active sites of OCTN2 were indicated based on our experimental data obtained in OCTN2-overexpressed HEK293 cells. The transmembrane domains (TMD) 1–7 were found to be responsible for organic cation transport and for sodium dependence in carnitine transport, and carnitine transport by OCTN2 requires the linkage between TMD 1–7 and TMD11 (19). Furthermore, the residues of Q180, Q207, S467, and P478 are known to be critical for the function of OCTN2 (20). Collectively, these prior studies have indicated that the putative active site of OCTN2 for carnitine recognition is identified as the cave between TMD1–7 and TMD11, which is near to the residues mentioned above. As a next step, automated docking studies were performed with GOLD 3.0 (Genetic Optimisation for Ligand Docking). A radius of 10 Å from the residue of S467, which is critical to the activity of the OCTN2, was used to design the binding site. 30 GA runs were performed in each docking calculation. For each of the genetic algorithm runs, a maximum number of 150,000 operations were performed on a population of 100 individuals with a selection pressure of 1.1. Operator weights for crossover, mutation, and migration were set to 95, 95, and 10, respectively. The GoldScore fitness function was used to identify the best-fitting binding mode. An in silico screen was performed on the DrugBank database (http://www.drugbank.ca/), which includes >1,480 FDA-approved small molecule drugs and >3,200 investigational agents.
Statistical analysis
All in vitro experiments were performed 3 times at least in triplicate. The animal experiments were done 3 times at least triplicate, and urinary excretion and histology data were available in 11 wildtype mice and 12 Oct1/2(−/−) mice. Four mice in each group were randomly selected from the total number for additional caspase-3 and microarray analyses, and real-time RT-PCR analyses were done in 3 of these animals. All data are presented as mean and standard error, unless otherwise stated. Statistical analyses were done using a two-tailed t test (2 groups) or a one-way ANOVA (multiple groups), after confirming normality using the Shapiro-Wilk W Test. P<0.05 was considered statistically significant. All statistical calculations were performed using the software package NCSS v 2004 (Number Cruncher Statistical System).
Results
Carnitine undergoes OCT2-mediated basolateral uptake in renal cells
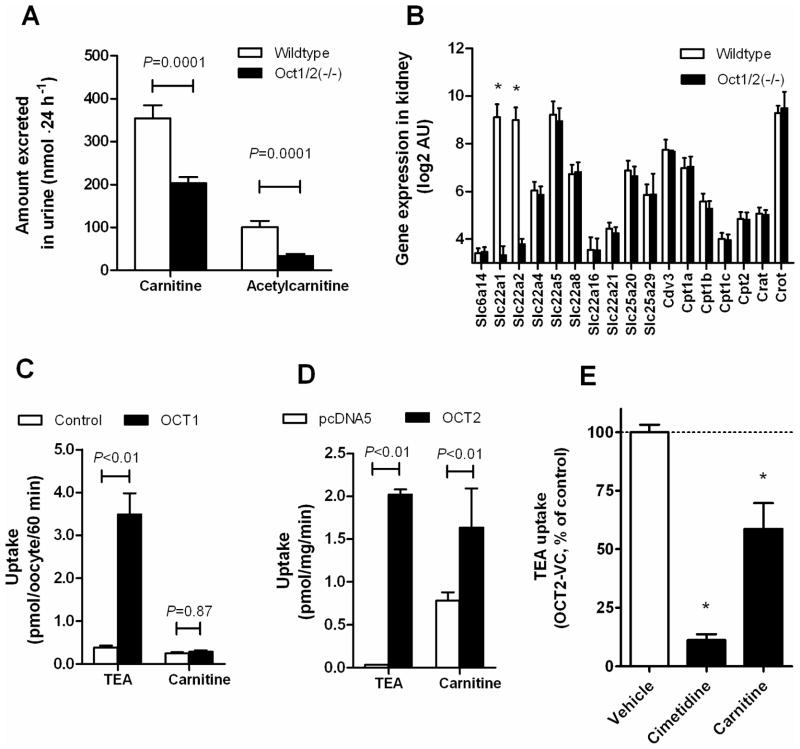
We recently reported that Oct1 and Oct2 in mice are key proteins involved in the transfer of cisplatin from the circulation into renal tubular cells (3). Based on this finding, we hypothesized that the ability of cisplatin to affect carnitine homeostasis is possibly indirectly influenced by Oct1/Oct2-deficiency. While addressing this question, we made the serendipitous observation that the baseline urinary excretion of carnitine and its acetylated form, acetylcarnitine, was about ~3 fold lower in Oct1/2(−/−) mice than in wildtype mice (Figure 1A), although the urine flow rate was the same (0.859±0.152 vs 1.11±0.093 mL·24 h−1; P=0.19). Analysis of kidneys from Oct1/2(−/−) mice revealed that the expression of genes previously implicated in renal carnitine handling, including Slc22a4 (Octn1), Slc22a5 (Octn2), Slc22a21 (Octn3), and Slc22a8 (Oat3) (22), was not changed compared with wildtype mice (Figure 1B). This suggests that carnitine itself may undergo carrier-mediated basolateral uptake in renal tubular cells of wildtype mice by Oct1 and/or Oct2. To confirm this possibility we assessed transport of carnitine in model systems overexpressing these proteins in vitro. We found that carnitine was not transported by OCT1 (Figure 1C), but that the intracellular uptake of carnitine was significantly increased in cells overexpressing OCT2 (Figure 1D). Furthermore, carnitine itself caused ~40% inhibition of OCT2 function as assessed by changes in transport of TEA (Figure 1E), a known OCT2 substrate.
Figure 1.
Involvement of organic cation transporter Oct2 in carnitine homeostasis. (A) Urinary excretion of carnitine and acetylcarnitine at baseline in wildtype mice (n=11) and Oct1/2(−/−) mice (n=12). (B) Expression of carnitine-related transporter genes [Slc6a14 (Atb0+), Slc22a1 (Oct1, Slc22a2 (Oct2), Slc22a4 (Octn1), Slc22a5 (Octn5), Slc22a8 (Oat3), Slc22a16 (Oct6), Slc22a21 (Octn3), Slc25a20 (Cact), Slc25a29 (Cacl; Ornt3)] and enzymes [Cdv3 (Tpp36), Cpt1a, Cpt1b, Cpt1c, Cpt2, Crat and Crot]. *, P<0.05 vs Oct1/2(−/−) mice. (C) Transport of tetraethylammonium (60 μM; TEA; 60-min incubations), a positive control, and carnitine (2 μM) in water-injected Xenopus laevis oocytes and oocytes injected with organic cation transporter 1 (OCT1) cRNA. (D) Transport of TEA (60 μM; 30-min incubations), a positive control, and carnitine (2 μM) in 293Flp-In cells transfected with an empty pcDNA5 vector or organic cation transporter 2 (OCT2). (E) Inhibition of OCT2-mediated transport of TEA (2 μM) by cimetidine (1 mM), a positive control, and carnitine (1 mM). *, P<0.05 vs vehicle control. All data represent mean (bars) and standard error (error bars).
Cisplatin-induced carnitine wasting is dependent on Oct1/Oct2
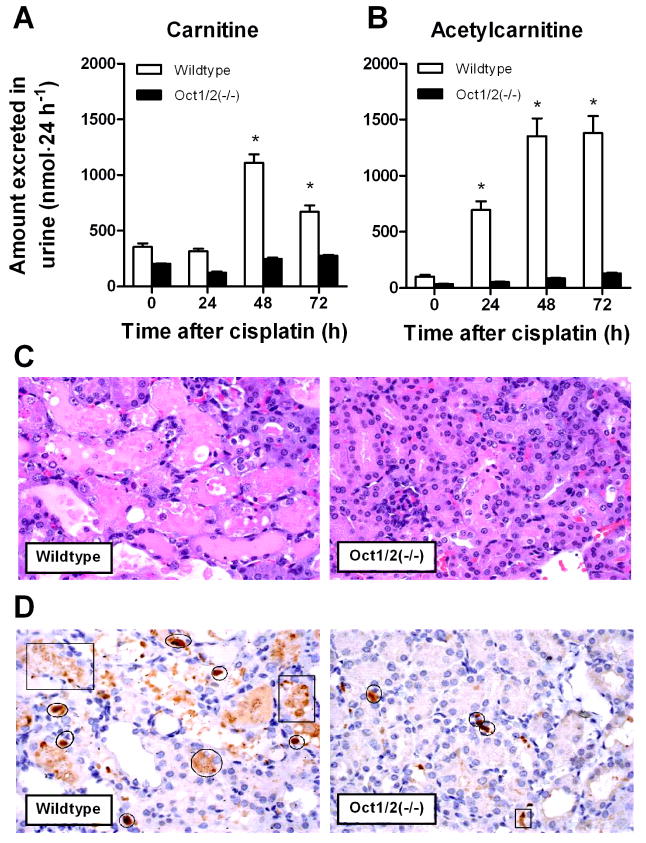
Experiments performed in wildtype mice indicated that even a single administration of cisplatin (10 mg/kg) resulted in significant increases in the urinary excretion of carnitine (Figure 2A) and acetylcarnitine (Figure 2B) within the first days after dosing. Within this time frame, cisplatin treatment in wildtype mice was not associated with significant changes in urinary creatinine (baseline, 0.47±0.21 mg·24 h−1; day 1, 0.46±0.02 mg·24 h−1; day 2, 0.50±0.15 mg·24 h−1; P=0.93), suggesting that membrane damage was not the cause for the concurrently observed urinary carnitine changes. Cisplatin-induced changes in urinary carnitines were absent in Oct1/2(−/−) mice (Figures 2A and 2B), suggesting that renal tubular transport of cisplatin by Oct1 and Oct2 is a prerequisite for treatment-related disturbances in carnitine excretion. All wildtype mice developed severe nephrotoxicity (>75% damaged tubules) by day 3, as exemplified by increases in the number of dilated tubules filled with necrotic epithelial cells (Figure 2C), and enhanced apoptosis (caspase-3 activity) compared to Oct1/2(−/−) mice (Figure 2D). The cumulative, baseline-corrected urinary loss of total carnitines within the first 3 days of cisplatin administration was dramatically increased in mice experiencing severe nephrotoxicity by day 3 compared to those that did not (4.61 μmol vs 0.424 μmol; P=0.014).
Figure 2.
Influence of cisplatin on carnitine homeostasis and nephrotoxicity. Effect of cisplatin (10 mg/kg, i.p.) on urinary carnitine (A) and acetylcarnitine (B) loss in wildtype mice and Oct1/2(−/−) mice within the first 3 days after treatment. Data represent mean (bars) and standard error (error bars). *, P<0.05 vs baseline (time zero). (C) Comparative cisplatin-related nephrotoxicity in wildtype mice and Oct1/2(−/−) mice 72 hours after the administration of cisplatin (10 mg/kg, i.p.) from representative animals. Severe renal tubular necrosis (>75% of the cortex affected per section), characterized by dilated tubules filled with necrotic tubular epithelial cells, was observed in kidneys of all wildtype mice (n=4) but in none of the Oct1/2(−/−) mice (n=4). (D) Immunohistochemistry for caspase-3, a marker of apoptosis. Quantification of the number of positively labeled cells within 10 high power fields (40×) from the outer cortex indicated an average value of 11.7±0.638 for wildtype mice and 4.95±0.330 for Oct1/2(−/−) mice (P=0.000082). The circles indicate representative positively labeled cells and squares represent areas of artifact.
Cisplatin does not directly inhibit OCTN2 activity
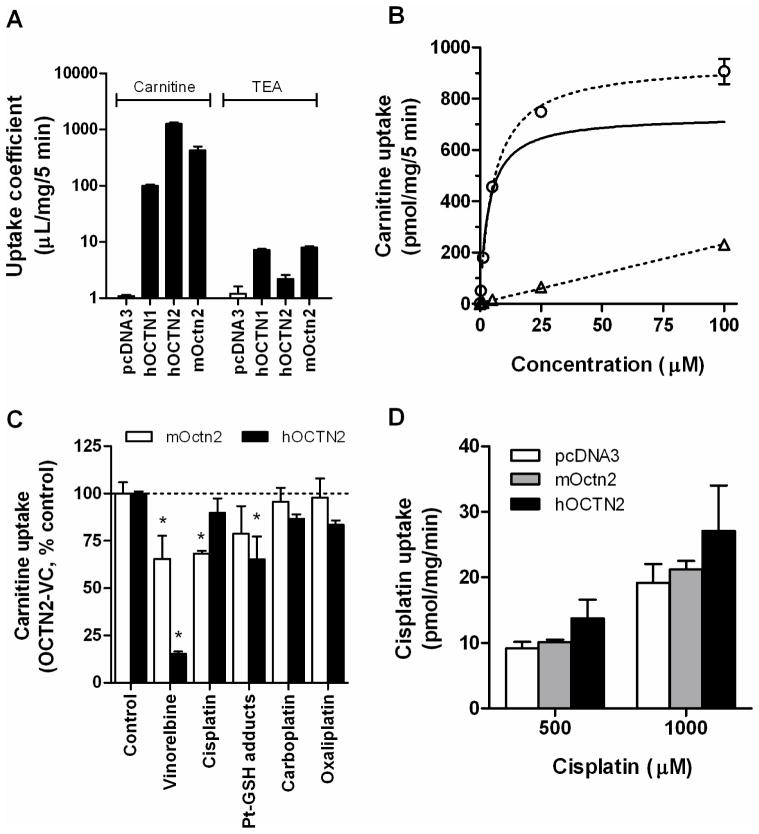
We next tested the possibility that cisplatin inhibits renal tubular transporters involved in the reabsorption of carnitine and acetylcarnitine. Functional characterization of transfected HEK293 cells confirmed that carnitine was more efficiently transported by OCTN2 than by the related solute carrier, OCTN1 (Figure 3A), although both transporters have a substrate preference for carnitine relative to the prototypical cation, TEA. In addition, we found that carnitine transport by mouse Octn2 was reduced compared with human OCTN2 (Figure 3A), and that transport by human OCTN2 was saturable with a Michaelis-Menten constant of 3.43 μM (Figure 3B). Subsequent studies focused specifically on the influence of cisplatin on the function of mouse Octn2 and human OCTN2. Although cisplatin slightly inhibited carnitine transport by both transporters in vitro (Figure 3C), the degree to which OCTN2 function was affected was less substantial than that observed in the presence of vinorelbine, used as a positive control.1 A similar lack of OCTN2-inhibitory ability was noted for carboplatin and oxaliplatin, consistent with the notion that neither cisplatin (Figure 3D) nor these two platinum analogues are themselves transported substrates (23). An in silico docking analysis on the carnitine binding site within the OCTN2 protein confirmed a relatively low predicted affinity for cisplatin (affinity score, 39.83) compared with the top-ranking agent in the DrugBank database, desloratadine (affinity score, 70.58), a known highly potent inhibitor of OCTN2 (24) (Suppl. Table 1).
Figure 3.
Influence of cisplatin on mouse Octn2 and human OCTN2 function. (A) Transport of carnitine (10 nM) and tetraethylammonium (60 μM; TEA) in HEK293 cells transfected with an empty pcDNA3 vector, human OCTN1 (hOCTN1), human OCTN2 (hOCTN2) and mouse Octn2 (mOctn2). (B) Concentration-dependence of carnitine transport in HEK293 cells transfected with an empty pcDNA3 vector (triangles) and human OCTN2 (circles). The solid line represented OCTN2-mediated carnitine transport, with an observed Michaelis-Menten (Km) constant of 3.43±0.38 μM. (C) Inhibition of carnitine (10 nM; 30-min) transport by mouse Octn2 (mOctn2) and human Octn2 (hOCTN2) in the presence of vinorelbine (60 μM), a positive control, cisplatin (100 μM), cisplatin-glutathione conjugates (Pt-GSH; 100 μM equivalent), carboplatin (100 μM) or oxaliplatin (100 μM). *, P<0.05 vs control. (D) Lack of cisplatin (30-min incubations) transport in HEK293 cells transfected with mouse Octn2 (mOctn2) and human OCTN2 (hOCTN2) compared with cells transfected with an empty pcDNA3 vector. All data represent mean (bars or symbols) and standard error (error bars).
In vivo, cisplatin is known to undergo extensive intracellular metabolic transformation following dissociation of the chlorides from the molecule, and can form glutathione adducts (12), which subsequently accumulate in urine (25). However, at equimolar concentrations (100 μM), the presence of cisplatin-glutathione adducts did not result in dramatically altered carnitine transport by OCTN2 compared with unconjugated cisplatin. To further rule out that in vivo cisplatin metabolites affect OCTN2 function, carnitine transport was assessed in the presence of urine extracts from patients treated with cisplatin (2). The extent of inhibition of OCTN2-mediated carnitine transport by these extracts was ~58%, but this inhibition was also noted in samples taken prior to cisplatin administration (data not shown). In line with a similar recent observation (26), this result suggests that the observed inhibition by human urine can be explained by carnitine(s) in the samples regardless of the presence of cisplatin and/or cisplatin metabolites.
Cisplatin-induced expression changes of carnitine genes
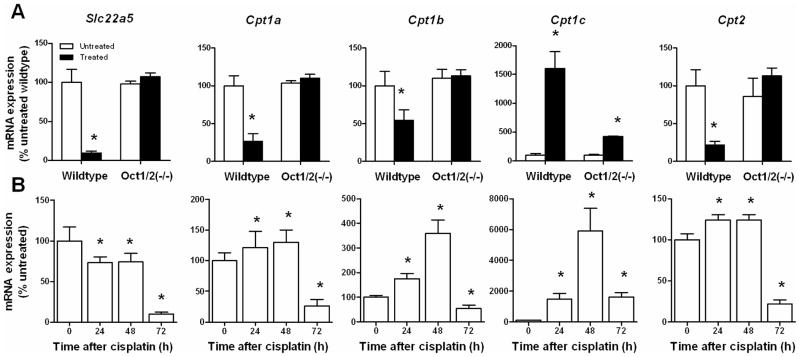
As a next step toward understanding the mechanism underlying the Oct1/Oct2-dependent changes in urinary carnitine and acetylcarnitine by cisplatin, we performed transcriptional profiling of kidney biopsies following cisplatin administration in vivo. Several well-characterized carnitine genes, such as Slc22a5 (Octn2) and carnitine-palmitoyltransferases (Cpt1a, Cpt1b, and Cpt2), showed strong repression in kidneys of treated wildtype mice by day 3, while their alteration was weaker or not detected in treated Oct1/2(−/−) mice (Figure 4A). Expression of the carnitine transporter genes Slc22a4 (Octn1) and Slc22a21 (Octn3) was not affected by cisplatin treatment (data not shown). A subsequent time-course analysis in samples obtained from wildtype mice revealed significant downregulation of Octn2 mRNA as early as 24 h after cisplatin administration (Figure 4B). In contrast to Octn2, we noted a temporal induction of some carnitine-palmitoyltransferases involved in the formation of acetylcarnitine, in particular Cpt1c mRNA (~60-fold by day 2), although expression normalized or even reduced by day 3 compared with baseline levels (Figure 4B).
Figure 4.
Influence of cisplatin on renal expression of carnitine-shuttle genes. (A) Real-time RT-PCR analyses for Slc22a5 (Octn2), Cpt1a, Cpt1b, Cpt1c, and Cpt2 performed on kidney biopsies taken before (untreated) and 72-h after cisplatin (10 mg/kg, i.p.; treated) administration to wildtype mice and Oct1/2(−/−) mice (n=4 per group). Not the difference in scale for the Y-axes. *, P<0.05 vs untreated. (B) Additional analyses were done on samples obtained from wildtype mice at baseline, 24 h, 48 h, and 72 h after cisplatin (10 mg/kg, i.p.) administration (n=4 per group). Not the difference in scale for the Y-axes. *, P<0.05 vs untreated. All data were normalized to the housekeeping gene, Gapdh, and expressed as a percentage relative to untreated wildtype, which was set at 100%. Data represent mean (bars) and standard error (error bars).
Discussion
This study demonstrates that the nephrotoxic agent cisplatin can dramatically affect urine levels of carnitine, an essential cofactor for mitochondrial fatty acid oxidation, via a process that involves downregulation of the expression of Octn2, a transporter involved in tubular reabsorption of filtered carnitine. In addition, we found that cisplatin altered the expression of Cpt1 isoforms, the rate-limiting carnitine-palmitoyltransferases required for mitochondrial fatty acid oxidation and energy production. The cisplatin-induced changes in urinary carnitine and gene expression were completely absent in Oct1/2(−/−) mice, suggesting that transport of cisplatin itself from the circulation into renal tubular cells is a prerequisite for treatment-related disturbances in carnitine homeostasis. The current study complements previous knowledge on the interaction of cisplatin with renal organic cation transporters, and provides further mechanistic insight into the role these proteins play in drug-related side effect profiles.
Over the last few decades, a number of studies have established that drug-induced alteration of the function of proteins involved in carnitine homeostasis can lead to deleterious phenotypic changes. An example of this has been recently reported for the nephrotoxic antibiotic, cephaloridine (27). In particular, these studies have demonstrated that cephaloridine increases the fractional renal excretion of carnitine, presumably due to interference with a reabsorption process in the kidney. Similar phenomena have been described with usage of the antiepileptic valproate (28), and the antibiotic pivampicillin (29). Several of these agents share a significant structural similarity with carnitine, in that they also contain quaternary nitrogens and exist as zwitterions under physiological conditions. Although not definitively established, it is plausible that in many instances, including cephaloridine (30), the underlying mechanism involved in these agents’ effects on carnitine homeostasis is related to direct inhibition of OCTN2 via a competitive mechanism (24).
Interestingly, in a previous study involving a small cohort of adult patients, treatment with cisplatin was also associated with a significant increase in the urinary excretion of carnitine, which eventually normalized about 7 days after discontinuing therapy (5). Similar observations have been made in patients undergoing combination chemotherapy with ifosfamide-doxorubicin-cisplatin (31), paclitaxel-carboplatin, or vinorelbine-carboplatin (32). In the case of cisplatin treatment, the increases in urinary carnitine were hypothesized to be due to inhibition of active tubular reabsorption of carnitine and acylcarnitines, with average losses amounting to as much as 1 mmol of total carnitine per day (5). The reported extent of urinary carnitine loss in patients undergoing cisplatin chemotherapy and the occurrence of peak changes on day 2 after drug administration closely match our current observations in wildtype mice. This finding supports the contention that the mouse is an appropriate animal model to further study the mechanisms and therapeutic implications of this phenomenon.
Although theoretically cisplatin could increase renal excretion of carnitine as a result of transport inhibition, this possibility is less likely considering the fact that urinary changes for carnitine itself were not observed before day 2 after drug administration, by which time the major fraction of the cisplatin dose (>95%) has already been excreted (3). Furthermore, using cellular models, we found that cisplatin did not substantially inhibit the direct transport of carnitine by mouse Octn2 or human OCTN2, and that cisplatin was not itself a transported substrate under the applied conditions. This lack of interaction of cisplatin with OCTN2 was further confirmed with a computational modeling analysis. In consideration of the specific time profile and extent of total carnitine loss associated with cisplatin treatment, it is also unlikely that gross cellular damage to renal tubular cells would be the principle contributing mechanism to the observed phenomena. For example, we found that the administration of cisplatin was not associated with substantial concurrent changes in urinary excretion of creatinine.
The observed changes in urinary carnitine excretion induced by cisplatin in wildtype mice are congruent with a previous observation that fatty acid oxidation is inhibited in a mouse model of cisplatin-induced renal failure (33). Since both carnitine and acetylcarnitine are transported substrates of Octn2 (34), it is likely that the observed cisplatin-induced downregulation of this transporter in wildtype mice, but not in Oct1/2(−/−) mice, contributes to the wasting of carnitines. In addition, the Oct1/Oct2-genotype dependent changes in the expression of carnitine-palmitoyltransferases following cisplatin administration are in line with the finding that cisplatin initially increases but then subsequently decreases the activity of both mitochondrial Cpt1 and peroxisomal acyl-coenzyme A oxidase (acyl-CoA) (33). Since Cpt1 is the rate-limiting enzyme in fatty acid oxidation, the observed temporal expression changes induced by cisplatin can explain why the initially occurring phenotypic alterations are noted with acetylcarnitine, and not with carnitine. Paradoxically, of the 3 known Cpt1 enzymes, we found that renal expression of Cpt1c, previously believed to be localized exclusively in the central nervous system (35), was exceptionally susceptible to cisplatin treatment. However, unlike Cpt1a and Cpt1b, Cpt1c is unable to catalyze the acyl transfer from fatty acyl-CoAs to carnitine (36), suggesting it may lack a direct role in the observed cisplatin-induced carnitine changes.
It is unclear at present whether the urinary carnitine changes induced by cisplatin and the subsequent inhibition of fatty acid oxidation constitutes an adaptive metabolic response to renal tubular injury or, alternatively, whether cisplatin nephrotoxicity is ultimately the result of reduced expression of Octn2 (and Cpt1), thereby affecting local carnitine stores and altering intracellular levels of toxic long-chain fatty acid intermediates. The possibility that the carnitine loss and the associated gene expression changes are coincidental and not causatively linked with nephrotoxicity is suggested by the finding that these phenomena are delayed relative to changes in urinary activity of N-acetyl-β-D-glucosaminidase, an early marker of renal tubular injury.2 We are planning additional studies involving carnitine-deficient rodent models, such as juvenile visceral steatosis mice that lack functional Octn2, in order to resolve this issue.
It is noteworthy in this context that a link between cisplatin toxicity and carnitine wasting has recently been suggested in a number of investigations demonstrating that exogenous carnitine supplementation alleviates cisplatin-induced injury of the kidney in mice (37) and rats (38–41). These prior studies have generally found that carnitine strongly inhibits cisplatin-induced injury to DNA, mitochondrial dysfunction, lipid peroxidation, and apoptosis of epithelial cells in the kidney, and these effects have been typically ascribed to intrinsic antioxidative, antiapoptotic, and anti-inflammatory properties of carnitine. Our current studies, however, provide support for an alternate mechanism by which exogenous carnitine supplementation can reduce cisplatin nephrotoxicity. In particular, we found that carnitine is both a substrate and an inhibitor of OCT2, which suggests that carnitine can inhibit transporter-mediated uptake of cisplatin in renal proximal tubular cells, and subsequently ameliorate cisplatin nephrotoxicity. Support for this possibility comes from our recent studies indicating that chemical inhibitors of OCT2, such as cimetidine, can be used as a strategy to prevent severe nephrotoxicity in mice receiving cisplatin (42). The previous observation that carnitine does not have an appreciable effect on the in vivo tumoricidal action of cisplatin (37) is in line with the notion that OCT2 is known to be either absent or detectable at very low levels in most tumors (3, 4), and that OCT2 inhibitors do not seem to affect the accumulation of cisplatin in tumor cells (42).
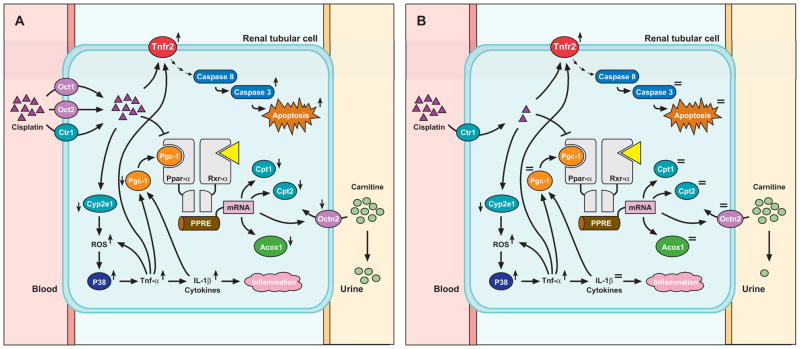
The mechanism by which cisplatin causes deregulation of Octn2 expression in the kidney of wildtype mice is not entirely clear. Recent studies have provided evidence that the expression of mouse Octn2 is directly governed by the transcription factor Ppar-α (peroxisome proliferator activated receptor α), and that this process is mediated via a functional peroxisome proliferator response element located in the first intron (43). Our proposed mechanism of action of cisplatin-induced downregulation of Octn2 and other genes regulating fatty acid catabolism is shown in Figure 5. Following cisplatin administration, the drug is taken up into renal tubular cells by Oct1 and Oct2, with perhaps a minor role of the copper transporter Ctr1 (44), where the interaction between cisplatin and Cyp2e1 results in the generation of reactive oxygen species (ROS) (45). The accumulation of ROS then triggers, directly or indirectly, activation of Mapk14 (p38 MAPK) (46), leading to increased production of tumor necrosis factor-α (TNF-α) (47). As a result, the tubular expression of multiple cytokines, including IL-1β, is increased (48), and this subsequently causes a decrease in the expression of Pargc-1α (PGC-1α), a tissue-specific transcriptional coactivator of Ppar-α and its obligate partner retinoid X receptor (Rxr-α) (49). The reduced availability of Pargc-1α, along with the ability of cisplatin to directly impair DNA binding activity to the Ppar-α/Rxr-α heterodimer (33), decreases the expression of Ppar-α targets such as Octn2 and ultimately leads to reduced reabsorptive capacity and loss of carnitines in urine. In support of this proposed pathway, we found in a preliminary analysis that the expression of key Tnf-α-related genes such as Cxcl2 (MIP-2) and known Ppar-α target genes such as Pargc-1α and Acox1 (acyl-CoA oxidase) are specifically affected in the kidney of wildtype mice (Suppl. Table 2).
Figure 5.
Proposed pathway of urinary carnitine loss by cisplatin in wildtype mice (A) but not in Oct1/2(−/−) mice (B). See Discussion for details. Abbreviations: Acox1, acyl-coenzyme A oxidase; Cpt, carnitine palmitoyltransferase; Ctr1, copper transporter 1 (Slc31a1); Cyp2e1, cytochrome P450 isoform 2e1; Il-1β, interleukin-1β; P38, P38 mitogen-activated protein kinase (P38 MAPK, Mapk14); Oct, organic cation transporter; Octn2, novel organic cation transporter 2; Ppar-α, peroxisome proliferator activated receptor-alpha; Pgc1, peroxisome proliferator activated receptor-gamma-coactivator-1 (Ppargc1α); PPRE, peroxisome proliferator response element; ROS, reactive oxygen species; Rxr-α, retinoid X receptor; Tnf-α, tumor necrosis factor-α; Tnfr2, tumor necrosis factor receptor 2 (Tnfrsf1a).
In conclusion, we report that cisplatin treatment is associated with excessive urinary loss of carnitines and that this process is dependent on renal tubular uptake of cisplatin by the basolateral organic cation transporters Oct1 and Oct2. Unlike other agents previously linked with hypocarnitinemia, cisplatin-induced carnitine wasting is associated with an unusual regulatory mechanism likely involving deactivation of the transcription factor Ppar-α and subsequent downregulation of the luminal carnitine transporter Octn2. Additional studies are in progress using Ppar-α-deficient mice to further understand the significance of this transcription factor in cisplatin-related carnitine wasting and its pharmacodynamic implications.
Supplementary Material
Statement of Translational Relevance.
Carnitine (vitamin BT), an essential cofactor for mitochondrial fatty acid oxidation, is maintained at steady levels in the blood by a rescue mechanism that involves renal tubular resorption of filtered carnitine by OCTN2, a luminal organic cation transporter. The physiological significance of OCTN2 has been confirmed by the identification of mutations that cause a potentially lethal autosomal recessive disease known as primary systemic carnitine deficiency. Furthermore, heterozygosity for OCTN2 mutations is associated with an intermediate carnitine-deficiency phenotype, suggesting that even partial loss of transporter function may be detrimental. We found that the widely used anticancer drug cisplatin causes a dramatic urinary loss of carnitine in mice in a manner that is dependent on transporter-mediated uptake of cisplatin in renal tubular cells. These cisplatin-related disturbances in carnitine homeostasis are proposed to be triggered by deactivation of the transcription factor PPAR-α, leading to deregulation of OCTN2 and a number of other carnitine-shuttle genes. Our study is of direct human relevance because interference with OCTN2 may cause reduced carnitine levels in patients treated with cisplatin and contribute to treatment-related complications.
Acknowledgments
Funding
This work was supported by the American Lebanese Syrian Associated Charities (ALSAC) and United States Public Health Service Cancer Center Support Grant (3P30CA021765).
We thank David Finkelstein, John Killmar, Kelli Boyd, Peter Vogel, and Laura Janke for expert assistance with data analysis, Sharyn Baker for critical review of the manuscript, and Akira Tsuji for providing the OCTN-overexpressing cell lines.
Footnotes
C. Hu, Z. Zuo, Z. Chen, and A. Sparreboom. Inhibition of OCTN2-mediated carnitine transport by anticancer drugs. Manuscript in preparation.
Disclosure of Potential Conflict of interest
The authors have no conflict of interest.
References
- 1.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 2.Filipski KK, et al. Interaction of cisplatin with the human organic cation transporter 2. Clin Cancer Res. 2008;14:3875–3880. doi: 10.1158/1078-0432.CCR-07-4793. [DOI] [PubMed] [Google Scholar]
- 3.Filipski KK, et al. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther. 2009;86:396–402. doi: 10.1038/clpt.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciarimboli G, et al. Organic cation transporter 2 mediates cisplatin-induced oto-and nephrotoxicity and is a target for protective interventions. Am J Pathol. 2010 doi: 10.2353/ajpath.2010.090610. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heuberger W, et al. Increased urinary excretion of carnitine in patients treated with cisplatin. Eur J Clin Pharmacol. 1998;54:503–508. doi: 10.1007/s002280050504. [DOI] [PubMed] [Google Scholar]
- 6.Longo N, Amat di San Filippo C, Pasquali M. Disorders of carnitine transport and the carnitine cycle. Am J Med Genet C Semin Med Genet. 2006;142:77–85. doi: 10.1002/ajmg.c.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nalecz KA, et al. Carnitine: transport and physiological functions in the brain. Mol Aspects Med. 2004;25:551–567. doi: 10.1016/j.mam.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Ohtani Y, Nishiyama S, Matsuda I. Renal handling of free and acylcarnitine in secondary carnitine deficiency. Neurology. 1984;34:977–979. doi: 10.1212/wnl.34.7.977. [DOI] [PubMed] [Google Scholar]
- 9.Scaglia F, et al. Defective urinary carnitine transport in heterozygotes for primary carnitine deficiency. Genet Med. 1998;1:34–39. doi: 10.1097/00125817-199811000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Koizumi T, et al. Infantile disease with microvesicular fatty infiltration of viscera spontaneously occurring in the C3H-H-2(0) strain of mouse with similarities to Reye’s syndrome. Laboratory animals. 1988;22:83–87. doi: 10.1258/002367788780746511. [DOI] [PubMed] [Google Scholar]
- 11.Lu K, et al. A missense mutation of mouse OCTN2, a sodium-dependent carnitine cotransporter, in the juvenile visceral steatosis mouse. Biochem Biophys Res Commun. 1998;252:590–594. doi: 10.1006/bbrc.1998.9708. [DOI] [PubMed] [Google Scholar]
- 12.Goto S, et al. Augmentation of transport for cisplatin-glutathione adduct in cisplatin-resistant cancer cells. Cancer research. 1995;55:4297–4301. [PubMed] [Google Scholar]
- 13.Hu S, et al. Interaction of imatinib with human organic ion carriers. Clin Cancer Res. 2008;14:3141–3148. doi: 10.1158/1078-0432.CCR-07-4913. [DOI] [PubMed] [Google Scholar]
- 14.Perry JL, et al. A three-dimensional model of human organic anion transporter 1: aromatic amino acids required for substrate transport. The Journal of biological chemistry. 2006;281:38071–38079. doi: 10.1074/jbc.M608834200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ginalski K, et al. 3D-Jury: a simple approach to improve protein structure predictions. Bioinformatics (Oxford, England) 2003;19:1015–1018. doi: 10.1093/bioinformatics/btg124. [DOI] [PubMed] [Google Scholar]
- 16.Huang Y, et al. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science (New York, NY. 2003;301:616–620. doi: 10.1126/science.1087619. [DOI] [PubMed] [Google Scholar]
- 17.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic acids research. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henikoff S, Henikoff JG. Amino acid substitution matrices from protein blocks. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:10915–10919. doi: 10.1073/pnas.89.22.10915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inano A, et al. Functional regions of organic cation/carnitine transporter OCTN2 (SLC22A5): roles in carnitine recognition. Drug Metab Pharmacokinet. 2004;19:180–189. doi: 10.2133/dmpk.19.180. [DOI] [PubMed] [Google Scholar]
- 20.Seth P, et al. Mutations in novel organic cation transporter (OCTN2), an organic cation/carnitine transporter, with differential effects on the organic cation transport function and the carnitine transport function. The Journal of biological chemistry. 1999;274:33388–33392. doi: 10.1074/jbc.274.47.33388. [DOI] [PubMed] [Google Scholar]
- 21.Laskowski RA, et al. AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. Journal of biomolecular NMR. 1996;8:477–486. doi: 10.1007/BF00228148. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi Y, et al. Renal transport of organic compounds mediated by mouse organic anion transporter 3 (mOat3): further substrate specificity of mOat3. Drug Metab Dispos. 2004;32:479–483. doi: 10.1124/dmd.32.5.479. [DOI] [PubMed] [Google Scholar]
- 23.Yonezawa A, et al. Cisplatin and oxaliplatin, but not carboplatin and nedaplatin, are substrates for human organic cation transporters (SLC22A1-3 and multidrug and toxin extrusion family) The Journal of pharmacology and experimental therapeutics. 2006;319:879–886. doi: 10.1124/jpet.106.110346. [DOI] [PubMed] [Google Scholar]
- 24.Diao L, Ekins S, Polli JE. Novel inhibitors of human organic cation/carnitine transporter (hOCTN2) via computational modeling and in vitro testing. Pharm Res. 2009;26:1890–1900. doi: 10.1007/s11095-009-9905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leone R, et al. Influence of glutathione administration on the disposition of free and total platinum in patients after administration of cisplatin. Cancer chemotherapy and pharmacology. 1992;29:385–390. doi: 10.1007/BF00686008. [DOI] [PubMed] [Google Scholar]
- 26.Haschke M, et al. Urinary excretion of carnitine as a marker of proximal tubular damage associated with platin-based antineoplastic drugs. Nephrol Dial Transplant. 25:426–433. doi: 10.1093/ndt/gfp456. [DOI] [PubMed] [Google Scholar]
- 27.Ganapathy ME, et al. beta-lactam antibiotics as substrates for OCTN2, an organic cation/carnitine transporter. The Journal of biological chemistry. 2000;275:1699–1707. doi: 10.1074/jbc.275.3.1699. [DOI] [PubMed] [Google Scholar]
- 28.Ohtani Y, Matsuda I. Valproate treatment and carnitine deficiency. Neurology. 1984;34:1128–1129. doi: 10.1212/wnl.34.8.1128. [DOI] [PubMed] [Google Scholar]
- 29.Holme E, et al. Carnitine deficiency induced by pivampicillin and pivmecillinam therapy. Lancet. 1989;2:469–473. doi: 10.1016/s0140-6736(89)92086-2. [DOI] [PubMed] [Google Scholar]
- 30.Kano T, et al. Carnitine/organic cation transporter OCTN2 (Slc22a5) is responsible for renal secretion of cephaloridine in mice. Drug Metab Dispos. 2009;37:1009–1016. doi: 10.1124/dmd.108.025015. [DOI] [PubMed] [Google Scholar]
- 31.Hockenberry MJ, et al. Carnitine plasma levels and fatigue in children/adolescents receiving cisplatin, ifosfamide, or doxorubicin. J Pediatr Hematol Oncol. 2009;31:664–669. doi: 10.1097/MPH.0b013e3181b259a7. [DOI] [PubMed] [Google Scholar]
- 32.Mancinelli A, et al. Urinary excretion of L-carnitine and its short-chain acetyl-L-carnitine in patients undergoing carboplatin treatment. Cancer chemotherapy and pharmacology. 2007;60:19–26. doi: 10.1007/s00280-006-0341-3. [DOI] [PubMed] [Google Scholar]
- 33.Portilla D, et al. Alterations of PPARalpha and its coactivator PGC-1 in cisplatin-induced acute renal failure. Kidney Int. 2002;62:1208–1218. doi: 10.1111/j.1523-1755.2002.kid553.x. [DOI] [PubMed] [Google Scholar]
- 34.Wu X, et al. Functional characteristics and tissue distribution pattern of organic cation transporter 2 (OCTN2), an organic cation/carnitine transporter. The Journal of pharmacology and experimental therapeutics. 1999;290:1482–1492. [PubMed] [Google Scholar]
- 35.Obici S, et al. Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production. Nat Med. 2003;9:756–761. doi: 10.1038/nm873. [DOI] [PubMed] [Google Scholar]
- 36.Wolfgang MJ, et al. The brain-specific carnitine palmitoyltransferase-1c regulates energy homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7282–7287. doi: 10.1073/pnas.0602205103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang B, et al. L-Carnitine inhibits cisplatin-induced injury of the kidney and small intestine. Arch Biochem Biophys. 2002;405:55–64. doi: 10.1016/s0003-9861(02)00342-9. [DOI] [PubMed] [Google Scholar]
- 38.Aleisa AM, et al. Reversal of cisplatin-induced carnitine deficiency and energy starvation by propionyl-L-carnitine in rat kidney tissues. Clin Exp Pharmacol Physiol. 2007;34:1252–1259. doi: 10.1111/j.1440-1681.2007.04714.x. [DOI] [PubMed] [Google Scholar]
- 39.Martinez G, et al. Cisplatin-induced kidney injury in the rat: L-carnitine modulates the relationship between MMP-9 and TIMP-3. Exp Toxicol Pathol. 2009;61:183–188. doi: 10.1016/j.etp.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Sayed-Ahmed MM, et al. Progression of cisplatin-induced nephrotoxicity in a carnitine-depleted rat model. Chemotherapy. 2004;50:162–170. doi: 10.1159/000080689. [DOI] [PubMed] [Google Scholar]
- 41.Tufekci O, et al. Evaluation of the effect of acetyl L-carnitine on experimental cisplatin nephrotoxicity. Chemotherapy. 2009;55:451–459. doi: 10.1159/000240020. [DOI] [PubMed] [Google Scholar]
- 42.Franke RM, et al. Influence of Oct1/Oct2-Deficiency on Cisplatin-Induced Changes in Urinary N-Acetyl-{beta}-D-Glucosaminidase. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-10-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wen G, Ringseis R, Eder K. Mouse OCTN2 is directly regulated by peroxisome proliferator-activated receptor alpha (PPARalpha) via a PPRE located in the first intron. Biochemical pharmacology. 2010;79:768–776. doi: 10.1016/j.bcp.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Pabla N, et al. The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. American journal of physiology. 2009;296:F505–511. doi: 10.1152/ajprenal.90545.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu H, Baliga R. Cytochrome P450 2E1 null mice provide novel protection against cisplatin-induced nephrotoxicity and apoptosis. Kidney Int. 2003;63:1687–1696. doi: 10.1046/j.1523-1755.2003.00908.x. [DOI] [PubMed] [Google Scholar]
- 46.Bragado P, et al. Apoptosis by cisplatin requires p53 mediated p38alpha MAPK activation through ROS generation. Apoptosis. 2007;12:1733–1742. doi: 10.1007/s10495-007-0082-8. [DOI] [PubMed] [Google Scholar]
- 47.Ramesh G, Reeves WB. p38 MAP kinase inhibition ameliorates cisplatin nephrotoxicity in mice. American journal of physiology. 2005;289:F166–174. doi: 10.1152/ajprenal.00401.2004. [DOI] [PubMed] [Google Scholar]
- 48.Ramesh G, Reeves WB. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest. 2002;110:835–842. doi: 10.1172/JCI15606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim MS, et al. Tumor necrosis factor and interleukin 1 decrease RXRalpha, PPARalpha, PPARgamma, LXRalpha, and the coactivators SRC-1, PGC-1alpha, and PGC-1beta in liver cells. Metabolism. 2007;56:267–279. doi: 10.1016/j.metabol.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.